Abstract
The high-light-induced alterations in photosynthetic performance of photosystem II (PSII) and photosystem I (PSI) as well as effectiveness of dissipation of excessive absorbed light during illumination for different periods of time at room (22 °C) and low (8–10 °C) temperature of leaves of Arabidopsis thaliana, wt and lut2, were followed with the aim of unraveling the role of lutein in the process of photoinhibition. Photosynthetic parameters of PSII and PSI were determined on whole leaves by PAM fluorometer and oxygen evolving activity—by a Clark-type electrode. In thylakoid membranes, isolated from non-illuminated and illuminated for 4.5 h leaves of wt and lut2 the photochemical activity of PSII and PSI and energy interaction between the main pigment–protein complexes was determined. Results indicate that in non-illuminated leaves of lut2 the maximum rate of oxygen evolution and energy utilization in PSII is lower, excitation pressure of PSII is higher and cyclic electron transport around PSI is faster than in wt leaves. Under high-light illumination, lut2 leaves are more sensitive in respect to PSII performance and the extent of increase of excitation pressure of PSII, ΦNO, and cyclic electron transport around PSI are higher than in wt leaves, especially when illumination is performed at low temperature. Significant part of the excessive light energy is dissipated via mechanism, not dependent on ∆pH and to functioning of xanthophyll cycle in LHCII, operating more intensively in lut2 leaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the life cycle, plants are sensing different environmental stress conditions as high or low temperatures, drought, high salt concentrations, high-light intensities that have negative impact on their development and productivity. For performing effective photosynthetic process, higher plants rely on absorbing sun light but exposure to light intensities that prevail energetic demands of plants or their capacity to dissipate the excessive light energy results in over excitation of PSII expressed in increased excitation pressure on photosystems II (PSII) (1 − qP) (Huner et al. 1996, 1998; Öguist and Huner 2003) and results in reduction of photosynthetic efficiency. The extent of excitation pressure of PSII is increased when plants are exposed either to high-light illumination and/or to low, chilling temperatures. At the same time, exposure of plants to low temperature reduces the rates of enzymatic reactions and the sinks for the absorbed excitation energy, especially of CO2 fixation and photorespiration (Allen and Ort 2001).
Under conditions of high-light that exceed the energetic demands for performing effective photosynthetic process photooxidative damage including attack of D1 protein in the reaction center of PSII (photoinhibition of PSII) (Powles 1984; Aro et al. 1993; Long et al. 1994) and over-reduction of the acceptor side of photosystem I (PSI) (photoinhibition of PSI) (Sonoike et al. 1997; Ivanov et al. 1998; Miyake 2010) takes place due to excessive production of damaging reactive oxygen species. At chilling temperatures, the extent of damage of photosynthetic process is higher (Sonoike 1998).
To protect the photosynthetic machinery from excessive light, plants have developed different mechanisms for reduction of excitation pressure on PSII and protection from photoinhibition. The main PSII photoprotective mechanism that dissipates the excessive absorbed light as harmless heat, taking place in the light-harvesting complex of PSII (LHCII), is considered to be the ∆pH- and zeaxanthin-dependent non-photochemical quenching (NPQ) (Demming-Adams and Adams 1992; Horton et al. 1996; Niyogi 1999; Ort 2001). It had been shown, however, that only half of the dissipated light energy is realized by NPQ (Gilmore 1997). Decrease of excitation pressure of PSII under conditions of high-light illumination and/or low temperature can be achieved as well by diverting the excess absorbed light energy from PSII to PSI via state transition that is realized in the time scale of several minutes (Huner et al. 1996). State transitions have been also implicated as an effective adaptive mechanism which may play substantial role in protecting PSII from overexcitation in all oxygenic photosynthetic organisms (Allen 1995; Haldrup et al. 2001; Rochaix 2004).
An earlier study has suggested that high-light-induced conversion of photochemically active, fluorescent, closed PSII reaction centers into photochemically inactive, non-fluorescent PSII reaction centers may serve as an effective mechanism for energy dissipation (Giersch and Krause 1991). Indeed, it has been demonstrated that PSIIß-centers (monomers) (Delrieu 1998) and photoinactivated and/or damaged PSII reaction center complexes may function as effective strong quenchers of excess light excitation, via non-radiative charge recombination within the PSII reaction center (Lee et al. 2001; Matsubara and Chow 2004). More recently, PSII reaction center quenching was suggested as an alternative additional mechanism for non-radiative dissipation of excess light energy when the operation of ∆pH-dependent xanthophylls cycle is thermodynamically restricted (Ivanov et al. 2003, 2008; Sane et al. 2003).
The altered PSII/PSI stoichiometry under photoinhibitory conditions could induce highly imbalanced photosynthetic electron flow, which may result in an enhancement of PSI-dependent cyclic electron flow (CEF) over the linear electron transport (Baker et al. 1983; Brestic et al. 2014, 2015). The induction of CEF has been considered to play an important role in photoprotection of both PSII and PSI under conditions of high-light illumination (Munekage et al. 2002, 2004; Miyake et al. 2005; Johnson 2011). CEF decreases the electron flow through linear electron transport chain at the level of PSII (Miyake et al. 2005; Huang et al. 2017; Wei et al. 2017) and diverts the excessive electrons from the acceptor side of PSI (Brestic et al. 2015; Laisk et al. 2010; Yamori et al. 2016). The key protective role of CEF against PSI photoinhibition has been demonstrated in Arabidopsis plants under conditions of high-light illumination and low temperature (Munekage et al. 2004).
Intrinsic component of photosynthetic pigment–protein complexes is the photosynthetic pigments, chlorophylls, and carotenoids, which are exclusively bound to the light-harvesting complexes of the thylakoid membranes and each of the LHCII and LHCI polypeptides has a unique xanthophyll composition (Peter and Thornber 1991; Bassi et al. 1993; Horton et al. 1996). Carotenoids represent a vast and diverse group of pigments that perform multiple functions in photosynthesis ranging from light harvesting (Yruela et al. 1998), performing role in formation and stabilization of the trimeric structure of light-harvesting complexes of cyanobacteria and higher plants (Plumley and Schmidt 1987), assembling the functional structure of PSII (Moskalenko and Karapetyan 1996), performing a key role in the process of NPQ for dissipation of excess absorbed light (Havaux and Niyogi 1999) and scavenging the stress-generated reactive oxygen species (Frank and Cogdell 1996).
For determination of the exact localization and specificity of binding sides of different xanthophylls to the LHC monomers as well as their structural role in assembly of antenna complexes and their participation in energy capture, transfer and quenching variety of xanthophyll biosynthesis mutants of Arabidopsis thaliana are used. For the mutant chy1chy2lut5 (all xanthophylls are substituted with lutein) and chy1chy2lut2 (contain only xanthophylls from the β-brunch of carotenoid synthesis) had been shown to be completely depleted of qE, to be extremely photosensitive even at low light intensities, and to demonstrate high level of lipid peroxidation (DallÓsto et al. 2007). When all xanthophylls were substituted with zeaxanthin (lut2npq2), the structure of LHCII was completely compromised thus suppressing state transition and limiting the photochemical efficiency in low light (Havaux et al. 2004). With the use of three Arabidopsis mutants that are lacking minor light-harvesting complexes (CP24, CP26, and CP29) has been shown that these complexes play a key role for functioning and macroorganization of PSII supercomplex (DallOsto et al. 2014). On comparing the in vivo photoprotection in Arabidopsis xanthophyll mutants (lut2—does not contain lutein, npq2—violaxanthin, aba4npq1lut2—all xanthophylls are represented by violaxanthin, in npq2lut2—all xanthophylls are represented by zeaxanthin and in chy1chy2lut5—by lutein) has been shown that binding of every respective xanthophyll to its proper binding site in light-harvesting proteins is the governing factor for the antenna macro organization and effectiveness in photoprotection (Ware et al. 2016).
The xanthophyll lutein is the most abundant carotenoid in higher plants constituting half of the total carotenoid content (Jahns and Holzwarth 2012; Morosinotto et al. 2003). Although the role of lutein in photosynthetic process is intensively studied its functional importance is far from completely understood. In the mutant lut2 of A. thaliana that does not contain lutein due to the block of lycopene ε-cyclase gene, its lack is compensated by increased levels of the xanthophylls from the brunch of β-carotene synthesis from lycopene (Pogson et al. 1996). Substitution of missing lutein by the violaxanthin cycle xanthophylls in Arabidopsis mutants lut1 and lut2 compromises the trimeric structure of LHCII that impact negatively NPQ (DallOsto et al. 2007) but the light-harvesting functions are not affected (Lokstein et al. 2002; Pogson et al. 1996; Dall’Osto et al. 2006). In addition to the structural and light-harvesting function of lutein its role in quenching of triplet excited states of chlorophyll is recognized in isolated and recombinant LHCII (Groce et al. 1999; Peterman et al. 1995). Recently, it has been shown that the lack of lutein accelerates the degree of bleaching of photosynthetic pigments under high-light illumination of thylakoid membranes, isolated from lut2, demonstrating that lutein performs a key role in photoprotection (Dobrev et al. 2016). Earlier observations have suggested that the photoprotective mechanisms of lutein may involve a change in L1 binding domain in the major LHCII (Ilioaia et al. 2011). This finding is also in agreement with earlier reported data that the lack of lutein leads to a less effective quenching of reactive oxygen species, increased photoinhibition and degradation of LHCII proteins under conditions of high-light illumination (Jahns and Holzwarth 2012; Dall’Osto et al. 2006). The role of lutein in protecting plants against severe oxidative stress has also been reported (Huang et al. 2010) and that it might play a key role in photoprotection as a secondary barrier (Peng and Gilmore 2003). It has been suggested that the mutants of Arabidopsis and Chlamydomonas that lack lutein and/or zeaxanthin are more photosensitive than the respective wt, but that lutein alone is unable to provide effective photoprotection (DallOsto et al. 2007). However, it was shown that lutein accumulation in the absence of zeaxanthin can effectively restore the capacity for NPQ in the npq1 mutant of Arabidopsis (Li et al. 2009). In contrast, for lutein-deficient mutants of A. thaliana (Pogson et al. 1996) and Chlamydomonas reinhardtii (Niyogi et al. 1997) had been shown that lutein does not play an essential role in the photosynthetic process and that its structural role in organization of LHCII can be fulfilled by the substituting xanthophylls.
In this report, we present data about alterations in the photosynthetic performance under conditions of high-light illumination, performed at normal (22 °C) and low (8–10 °C) temperature in detached leaves of A. thaliana, wt (Col-0) and lut2 mutant, and on isolated thylakoid membranes from control and illuminated leaves with the aim to evaluate the role of lack of lutein in functioning and organization of photosynthetic apparatus under conditions of high-light illumination with a special attention on mechanisms of dissipation of excess light energy.
Materials and methods
Plant growth conditions
Plants of A. thaliana, wt and mutant lut2, were grown under controlled conditions with 12-h photoperiod (100 µmol photons m−2 s−1 PFD) on perlite-containing soil and day/night temperature of 20 °C/18 °C. For all experiments, leaves of fully developed plants (after 3–4 weeks growth) were used.
High-light treatment
Detached leaves were placed on d. H2O and illuminated up to 4.5 h with 1400 µmol photons m−2 s−1 PFD white light at room (22 °C) or at low (8–10 °C) temperature. Photosynthetic parameters were determined by PAM fluorоmeter after illumination of leaves for 0, 1.5, 3, and 4.5 h. Thylakoid membranes were isolated from high-light treated for 4.5 h at the respective temperature wt and lut2 leaves. Non-illuminated leaves, kept at room or low temperature at dim light, were used аs controls.
Isolation of thylakoid membranes
Thylakoid membranes were isolated from fully expanded leaves of wt and lut2 plants, control or high-light illuminated for 4.5 h at room or low temperature, as described in (Velichkova and Popova 2005). Leaves were grounded in a buffer containing 0.33 M sucrose, 5 mM MgCl2, and 20 mM TES (pH 7.5). After centrifugation at 4500×g for 5 min, the pellet was resuspended in the above buffer, diluted 1:20. After centrifugation at 6000×g for 5 min, the pellet was resuspended in a buffer containing 0.33 M sucrose, 2 mM MgCl2, 1 mM NH4Cl, 2 mM EDTA, and 50 mM TES (pH 7.5) to a final concentration of 1 mg chl/ml. The whole procedure was performed at 4 °C in dark. Chlorophyll concentration was spectrometrically determined in 80% acetone extract using the formulas of Lichtenthaler (1987).
Pulse-amplitude-modulated-chlorophyll fluorescence
Determination of the photosynthetic activity of PSII was performed using PAM 101-103 fluorоmeter (Heinz Walz GmbH, Effeltrich, Germany). Before every measurement, leaves were dark adapted for 15 min and measurements were performed at room temperature and ambient O2 and CO2 conditions. The initial chlorophyll fluorescence at all PSII centers open (Fo) was registered at illumination with weak modulated (1.6 kHz) light (0.120 µmol photons m−2 s−1 PFD) and the maximum fluorescence at all PSII centers closed (Fm) was induced by a saturating white light pulse of 3000 µmol photons m−2 s−1 PFD with duration of 0.8 s and modulation frequency of 100 kHz. Photosynthetic process was initiated by illumination for 5 min with actinic light of 100 µmol photons m−2 s−1 PFD, corresponding to the plants’ light growth conditions and every minute a light saturation pulse was given for determination of maximal fluorescence level in light-adapted state (\(F^{\prime}_{{\text{m}}}\)) and minimal fluorescence level in light-adapted state (\(F^{\prime}_{{\text{o}}}\)) was measured after switching off the actinic light. For characterization of photosynthetic activity of PSII, the formulas of van Kooten and Snell (1990) were applied. Maximal quantum yield of PSII in dark-adapted state was calculated as (Fv/Fm = (Fm − Fo)/Fm), PSII excitation pressure (relative reduction state of PSII)—as 1 − qP = 1 − ([\(F^{\prime}_{{\text{m}}}\) – F]/[ \(F^{\prime}_{{\text{m}}}\) – \(F^{\prime}_{{\text{o}}}\)]). The non-photochemical quenching was calculated as NPQ = (Fm − \(F^{\prime}_{{\text{m}}}\))/\(F^{\prime}_{{\text{m}}}\) and the quantum efficiency of ∆pH- and/or xanthophylls-dependent non-photochemical dissipation processes within the PSII antennae as ΦNPQ = (Fs/\(F^{\prime}_{{\text{m}}}\) − Fs/Fm). Constitutive non-photochemical energy dissipation and fluorescence were evaluated as ΦNO = Fs/Fm (Hendrickson et al. 2004; Ivanov et al. 2012). The PSII electron transport rate in whole leaves, determined by the fluorescence parameters, was calculated as ETR = PAR × ΦPSII × 0.84 × 0.5 (Genty et al. 1989). Quantum yield of photochemical efficiency of PSII was calculated as ΦPSII = (\(F^{\prime}_{{\text{m}}}\) − Fs)/\(F^{\prime}_{{\text{m}}}\). Mean values ± SE were calculated from eight independent experiments with four independent repetitions in every time point.
Measurement of redox state of P700
The oxidation–reduction kinetics of P700 was determined on control and photoinhibited at respective temperature fully expanded detached leaves of wt and lut2 after 15-min dark adaptation using a PAM-101/103 modulated fluorometer (Heinz Walz GmbH, Effeltrich, Germany) equipped with an ED-800T emitter-detector unit (Klughammer and Schreiber 1991) as described in detail by Ivanov et al. (1998). Measurements were performed under room temperature and ambient O2 and CO2 conditions. Detached leaves were illuminated with far-red light (FR, λmax = 715 nm) provided by a photodiode (102-FR, Heinz Walz GmbH, Effeltrich, Germany). The redox state of P700 was determined by FR light-induced absorbance change around 820 nm in a custom-designed cuvette. The capacity of CEF around PSI was determined by the half time of dark re-reduction of P700+ (t1/2) signal after switching off the FR light (Ravenel et al. 1994; Klughammer and Schreiber 1991; Ivanov at al. 1998). Six independent experiments were performed with four independent repetitions in every time point.
Photosynthetic O2 evolution
Net photosynthesis of control and high-light-treated plants was measured polarographically as CO2-saturated O2 evolution of leaf discs at room temperature using a Clark-type O2 electrode (model DW1, Hansatech Instruments, King’s Lynn, Norfolk, UK) equipped with a LD1/2 leaf-disc electrode chamber as described in detail previously (Gray et al. 1996). Every measurement was performed on 8 leaf discs with a total area of 10 cm2 in a saturating atmosphere of CO2, provided by 200 µl 1 M NaHCO3, at room temperature (22 °C). Leave discs were dark adapted for 5 min before the measurements. Irradiance-response curves were obtained by using 10 irradiance values over the range of 0 to 1400 µmol photons m−2 s−1 PFD provided by an array of red light-emitting (650 nm) diodes (Model LH36/2R, Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK) and were corrected for the levels of dark respiration. The rate of oxygen evolution ΦO2 was calculated by regression analysis of points in the linear, light-limiting range of the irradiance-response curves. The maximum rate of net photosynthesis (Pnmax) was obtained at saturating light intensity of 1400 µmol photons m−2 s−1 PFD. Light compensation points (LCP) were determined as in Bravo et al. (2007). Three independent experiments were performed and for every type of treatment at least 4 parallel samples were measured.
Low-temperature (77 K) fluorescence measurements
Thylakoid membranes, isolated from non-illuminated (control) and illuminated for 4.5 h at room (22 °C) and low (8–10 °C) temperature, were used to record chlorophyll fluorescence emission spectra at 77 K. Three independent experiments were performed and every sample was measured in duplicate. Thylakoid membranes were suspended in 0.33 М sucrose, 5 mM MgCl2, 10 mM NaCl, and 20 mM MES (pH 6.5) to a final chlorophyll concentration 15 µg chl/ml and transferred into a quartz tube for fluorescence measurements and immediately frozen in liquid nitrogen. Chlorophyll fluorescence emission spectra were recorded by a Jobin Yvon spectrofluorometer JY3 (Division d’Instruments S.A., Longjumeau, France) equipped with a red-sensitive photomultiplier and a low-temperature device. Fluorescence was excited either at 436 nm (excitation of chlorophyll a) or at 472 nm (excitation of chlorophyll b) and emission spectra were recorded in the spectral region 660–780 nm (Velichkova and Popova 2005). The width of the slits was 4 nm. Spectra were digitized by an in-built A/D converter and transferred to an online IBM-compatible computer for further retrieval and analysis. The spectra were analyzed by Origin 8 after subtraction of the baseline.
Measurement of oxygen production reactions
Determination of initial oxygen burst was performed on isolated thylakoid membranes from control and high-light treated at room and low temperature for 4.5 h whole leaves of wt and lut2 plants by using a home-constructed fast oxygen rate electrode equipped with a system for continuous illumination permitting estimation of oxygen production reactions as described in details by Zeinalov (2002). The volume of every sample was 100 µl with chlorophyll concentration of 300 µg chl/ml. Samples were pre-illuminated with 25 flashes followed by a 5-min dark adaptation before measurement. For continuous illumination measurements, a cold light source (LED LXHLLW3C, Luxeon, Philips Lumileds Lighting Company, San Jose, USA) providing irradiation on the surface of the sample (420 µmol photons m−2 s−1 PFD) was used. Data were digitized by a built-in A/D converter and transferred to an online IBM-compatible computer for further analysis. The initial oxygen burst was registered during illumination with a saturated continuous white light and the decay kinetics after the initial oxygen burst was analyzed using Microcal Origin software applying two exponential decay fitting. Details for the experimental setup were described in (Popova et al. 2007). Thylakoid membranes were suspended in a medium containing 0.33 M sucrose, 5 mM MgCl2, 10 mM NaCl, 20 mM KCl, and 20 mM MES (pH 6.5). No artificial electron acceptors or acceptors were added. Three independent experiments were performed. Every sample was measured in duplicate.
Photochemical activity of PSII and PSI
Photochemical activity of both photosystems, PSII and PSI, in isolated thylakoid membranes from control and high-light treated at normal and low temperature for 4.5 h whole leaves was polarographically determined by a Clark-type oxygen electrode (Hansatech DW1) in a temperature-controlled cuvette under saturating white light intensity at room temperature. The photochemical activity of PSII was determined by the rate of oxygen evolution in the presence of 0.4 mM exogenous electron acceptor BQ in a reaction medium 0.33 M sucrose, 5 mM MgCl2, 10 mM NaCl, 20 mM MES (pH 6.5). The activity of PSI was determined by the degree of oxygen uptake in a reaction medium containing 0.33 M sucrose, 5 mM MgCl2, 10 mM NaCl, 20 mM TRICINE (pH 7.5), and 0.4 μM DCMU, 0.5 mM NH4Cl, 5 mM NaN3 in the presence of exogenous electron donor 0.1 mM DCPIP reduced by 4 mM Na ascorbate and electron acceptor − 0.1 mM MV. Thylakoid membranes were equivalent to 25 µg chl/ml (Popova et al. 2007). Three independent experiments were performed and every sample was measured in duplicate.
SDS–PAGE electrophoresis and western immunoblotting
The alterations in the relative abundance of PSII and PSI reaction centers proteins as a result of high-light treatment of leaves from A. thaliana plants, wt and lut2, were analyzed by SDS–PAGE according to Laemmli (1970), using 4% (w/v) stacking and 15% (w/v) separating gels in the presence of 4 M urea in the resolving gel. Thylakoid membranes were incubated with sample buffer (3:1) for 1 h in dark at room temperature for solubilization. Equal volumes of thylakoid membranes, isolated from non-illuminated and illuminated for 4.5-h leaves were loaded in every line. Immunoblotting was performed by electrophoretically transferring the proteins from SDS–PAGE gel to PVDF membrane. Thereafter, proteins were probed with commercial antibodies raised against the reaction center protein of PSII, PsbA(D1) (AS05 084-10, 1:2000) and the reaction center proteins of PSI, PsaB (AS10 695, 1:2000). Both antibodies were supplied from Agrisera, Sweden. Alkaline Phosphatase Conjugate Substrate Kit (Bio-Rad, Hercules, CA) was used as a secondary antibody. Densitometric scanning and analysis of each replicate immunoblot was performed with Phoretix image analysis software (Phoretix International, Newcastle upon Tyne, UK). Immunoblot was carried out twice with thylakoid membranes from two different experiments. In both experiments, very similar results were obtained.
Statistics
Data were presented as mean values ± SE. Mean values were calculated from at least four independent experiments with four parallel samples for each time point. Statistically significant changes were determined by Student’s t-test and indicated as follows—(*P < 0.05, **P < 0.01, ***P < 0.001).
Results
Oxygen evolving activity in leaves of wt and lut2
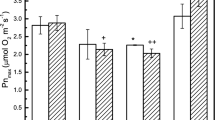
Light response curves of photosynthetic oxygen evolution of wt and lut2 were registered on leave discs of control (just detached) leaves, on detached leaves kept for 4.5 h under dim light at room (22 °C) or low (8–10 °C) temperature and on detached leaves, photoinhibited with high-light intensity (1400 µmol photons m−2 s−1 PFD) for 4.5 h at both temperatures (Fig. 1). The net photosynthetic oxygen evolution at increasing light intensities does not differ significantly in control and in leaves kept at dim light at both temperatures, for wt and lut2. The maximum rate of net photosynthesis (Pnmax) in control leaves of lut2 is 15% lower than for control wt leaves, being 8.1 ± 0.12 and 9.55 ± 0.14 µmol O2 m−2 s−1, respectively (Fig. 2b). Exposure of wt leaves to high-light (HL) treatment leads to a decrease in the maximum rate of photosynthetic oxygen evolution in a comparable degree irrespective of the temperature of HL treatment (Figs. 1a, 2b). In contrast, lut2 leaves exhibited differential, temperature-dependent response of Pnmax to the photoinhibitory treatment—the inhibition is much stronger, with 75% at low temperature (Figs. 1b, 2b).
Light response curves of photosynthetic oxygen evolution of A. thaliana leave discs of wt (a) and lut2 (b) measured at room temperature (22 °C) and CO2 saturated atmosphere. Oxygen evolution was determined by illumination with red (> 650 nm) light. Every point of the curves was calculated as means ± SE from at least 3 samples. Samples—control (just detached leaves), leaves under dim light at room (22 °C), and low (8–10 °C) temperature for 4.5 h and leaves, illuminated with 1400 µmol photons m–2 s–1 at room and low temperature for 4.5 h
Photosynthetic parameters of wt and lut2—control, leaves at dim light, and illuminated for 4.5 h with high-light intensity (1400 µmol m–2 s–1) at room (22 °C) and low (8–10 °C) temperature, calculated from light response curves in Fig. 1. a Quantum yield of oxygen evolution (ΦO2), b Maximum rate of net photosynthesis (Pnmax) and c LCP. Presented values (means ± SE) were calculated from at least three independent experiments. Statistically significant differences between non-illuminated wt and lut2 leaves, are marked by plus (+P < 0.05, ++P < 0.01, +++P < 0.001)
The quantum yields of oxygen evolution (ΦO2) for all treatments of wt and lut2 leaves calculated from the slopes of light response curves at low light intensities (Fig. 1) are presented in Fig. 2a. The values of ΦO2 are not significantly different between control leaves of wt and lut2 but are decreased after HL treatment in a temperature-dependent manner the extent of decline being stronger in lut2 leaves. The HL-induced decrease of ΦO2 for wt and lut2 leaves at room temperature is 30 and 45% in comparison with the control leaves, respectively, and 70 and 80%, respectively, at low temperature (Fig. 2a).
In addition, photoinhibitory treatment of wt and lut2 leaves causes a significant increase of LCP, determined from the light response curves that are related to the increased dark respiration and represent the light intensity at which the oxygen evolution prevails over O2 consumption. For wt and lut2 leaves, control and kept at dim light at both temperatures, LCP is comparable, ranging from 23 to 28 µmol photons m−2 s−1. In HL-treated leaves of wt, irrespective of the temperature, and for lut2 leaves, photoinhibited at room temperature, LCP is more than twice higher than in non-illuminated leaves, 75, 80, and 70 µmol photons m−2 s−1, respectively, while HL treatment at low temperature leads to a much stronger increase of LCP in mutant leaves, reaching 128 µmol photons m−2 s−1 (Fig. 2c).
Photochemical performance of PSII in vivo
Exposure of wt and lut2 leaves for 4.5 h to HL illumination at different temperatures reflects the photosynthetic performance of PSII, as determined by the maximum quantum efficiency of PSII (Fv/Fm) and excitation pressure on PSII (1 − qP) and results are presented in Fig. 3a, b, respectively. Fv/Fm is gradually decreased for wt and lut2 leaves with increase of time of illumination. For wt leaves, exposed to illumination at room temperature for 4.5 h Fv/Fm represents 77% of the value of control leaves, it decreases from 0.835 ± 0.002 to 0.645 ± 0.009. At room temperature, the illumination-induced decline in Fv/Fm proceeds faster for lut2 leaves than for wt and after 4.5 h of illumination is with 50% lower than for control leaves. When illumination is performed at low temperature both types of leaves show much faster decline in Fv/Fm than at room temperature. It is worth mentioning that the decline curve of Fv/Fm for wt leaves, illuminated at low temperature, follows the same trend as for lut2 leaves, photoinhibited at room temperature. On comparing the values of Fv/Fm of illuminated for 4.5 h leaves of wt and lut2, it is demonstrated that the decline at low temperature is with around 25% stronger expressed than at room temperature (Fig. 3a).
Time-dependent photoinhibition-induced alterations in the maximum quantum yield of PSII (Fv/Fm) (a), excitation pressure (1 − qP) (b), non-photochemical quenching (NPQ) (c) and quantum yield of non-regulated energy dissipation (ΦNO) (d) of wt (circles) and lut2 (triangles) leaves, photoinhibited at room (22 °C) (solid symbols) or low (8–10 °C) (empty symbols) temperature. Mean values ± SE were calculated from eight independent experiments with four parallel samples at every time point
When the absorbed light exceeds the energetic demands of plants, photoinhibition can occur that can be determined by the increase of the excitation pressure on PSII (1 − qP). The time-dependent increase in excitation pressure on PSII with HL is presented in Fig. 3b. For the wt HL treated leaves, till the third hour of illumination, the excitation pressure is only moderately increased at low temperature and much stronger for the longest period of illumination. For non-illuminated leaves of lut2, the excitation pressure is nearly twice higher than in the wt. The extent of increase of 1 − qP for the mutant is much stronger expressed and starts to increase earlier (after 1.5 h of illumination) than for wt. For both types of leaves, the excitation pressure on PSII is higher when illumination is performed at low temperature.
The ability of photosynthetic apparatus to dissipate the excessive absorbed light as harmless heat in wt and lut2 leaves under photoinhibitory treatment at normal and low temperature was assessed by comparing the dynamics of NPQ (Fig. 3c). Leaves of wt and lut2 show a strong decrease in the NPQ values during the first 1.5 h of illumination that proceeds slowly till the end of HL treatment period − 4.5 h. In Fig. 3d, the time-dependent trends of alterations in dissipation of absorbed light by mechanism that is not related to ∆pH and not dependent on the functioning of the xanthophyll cycle (ΦNO) in wt and lut2 leaves exposed to high-light treatment at room and low temperature are arranged. With increase of time of illumination, the extent of energy dissipation ΦNO is accelerated, stronger expressed in mutant leaves in comparison with the wt. The extent of ΦNO increase proceeds faster with increase of time of HL treatment when illumination is performed at low than at room temperature.
In order to evaluate the alterations in the proportions of absorbed light energy that is utilized in PSII for photochemistry (ΦPSII) and that dissipated as harmless heat in LHCII either by zeaxanthin-dependent (ΦNPQ) or by non-regulated mechanism (ΦNO) the respective energy proportions were evaluated at every time point of treatment of detached leaves with HL intensity at different temperatures and results are presented in Fig. 4, a and c at room and b and d at low temperature. Assuming that the absorbed light energy is either utilized or dissipated then relative yields will be equal to 1 (ΦPSII + ΦNPQ + ΦNO = 1). The quantum efficiency of PSII (ΦPSII) in illuminated leaves of wt declines with increase of time of illumination, better expressed at low temperature (Fig. 4b) than at room temperature (Fig. 4a). The same tendency is observed for lut2 leaves (Fig. 4c, d) but the extent of HL-induced reduction is to a higher extent than for wt leaves (Fig. 4a, b). It has to be mentioned that the values of ΦPSII in control, non-illuminated leaves of the mutant are with around 20% lower than in the control leaves of wt. With increase of time of illumination the quantum efficiency of NPQ (ΦNPQ) is also negatively influenced in both type of leaves and temperatures. At the same time, the excess energy, dissipated by a non-zeaxanthin-dependent mechanism (ΦNO), is increased to a higher extent in lut2 leaves and better expressed when illumination is performed at low temperature, valid for both types of leaves, wt and lut2.
Alterations in energy partitioning at PSII with increase of time of illumination of wt (a and b) and of lut2 leaves (c and d). Efficient quantum yield of PSII (ΦPSII), quantum yield of ΔpH- and/or zeaxanthin-dependent non-photochemical quenching (ΦNPQ) and quantum yield of non-regulated dissipation of light energy (ΦNO). Illumination was performed at room (22 °C) (a and c) or at low (8–10 °C) temperature (b and d). Mean values were calculated from eight independent experiments with four parallel samples at every time point
In Fig. 5, are arranged the rates of electron transport rates (ETR), of control, non-illuminated and illuminated with high-light intensity at both temperatures for 4.5 h whole leaves of wt and lut2, determined by the PAM fluorescence parameters. Similar to alterations in the quantum yield of oxygen evolution (ΦO2) (Fig. 2a), the ETR in control, non-illuminated leaves of the mutant is lower than in the control wt leaves. For the leaves, illuminated for the longest period of time (4.5 h) at normal temperature, the values of ETR are decreased with 25 and 52% for the wt and lut2, respectively. When illumination is performed at low temperature, the decline in ETR is stronger, with 55 and 79% for the wt and lut2, respectively.
Oxidation/re-reduction state of PSI in vivo
Alterations in the oxidation state of P700, as determined by the FR light-induced absorbance change at 820 nm, were followed in order to reveal changes in PSI redox state during time course of light illumination. In Fig. 6, are included the normalized traces of FR-induced oxidation of P700—in non-illuminated and illuminated with HL intensity for 4.5 h at room and low temperature leaves of wt (a) and of lut2 (b), determined in vivo. The kinetics of oxidation of P700 is delayed under photoinhibitory treatment in wt leaves, more pronounced at low temperature. In lut2 leaves, the delay in the oxidation rate of P700 as a result of illumination is less expressed than in wt leaves and in a similar manner for control as well as for illuminated at room and low temperature (Fig. 6a, b). The alterations in FR light-induced oxidation state of P700 (P700+) of wt and lut2 after illumination at different temperatures, expressed as percent of the P700+ in the respective non-illuminated leaves, are included in Fig. 6c. With increase of time of illumination, the amount of P700+ is decreased, to a similar extent for wt, illuminated at room and low temperature, and for lut2, illuminated at room temperature. The P700+ in lut2 leaves, illuminated at low temperature, declines faster with increase of time of illumination. From the traces of P700 oxidation/re-reduction was determined the half time of dark decay kinetics to the steady state (re-reduction of P700+) after switching off the FR light (t1/2) of wt and lut2 leaves subjected to HL treatment at different temperatures that is believed to characterize the capacity for cyclic electron transport around PSI (Maxwell and Biggins 1976; Johnson 2011; Savitch et al. 2011; Millaleo et al. 2013) and values are included in Table 1. In control leaves of lut2, the t1/2 is lower (1.438 s) than in wt non-illuminated leaves (2.194 s), indicating that the CEF around PSI is operating faster in the mutant. The reduction kinetic of P700+ after turning off the FR light depends on the electron donation to oxidized P700 that could be supplied by cyclic electron flow (as after dark adaptation the electrons from electron chain were exhausted and under FR no excitation of PSII occurred) and from stromal reductants as it has been demonstrated earlier (Bukhov et al. 2002). Deconvolution of dark reduction kinetics curves of P700+ into several components provides evidences for different rates of electron fluxes to P700+—CEF and alternative sources (Fan et al. 2008). Bukhov et al. (2002) showed that the complex kinetics of P700+ reduction manifest two types of PSI units in barley leaves differing in electron input from stromal reductants. With increase of time of illumination, t1/2 declines gradually, faster in wt leaves, valid for both temperatures of illumination and at the longest time of illumination the values for t1/2 are comparable for wt and lut2.
Typical normalized traces of in vivo measurements of P700 photo-oxidation by far-red (FR) light in fully expanded leaves of wt (a) and lut2 mutant (b) of control A. thaliana leaves (solid lines) and leaves exposed to high-light treatments for 4.5 h at room (22 °C) (dashed lines) or low (8–10 °C) (dotted lines) temperatures. Arrows indicate the application of FR light source. The measurements were performed at room temperature and ambient O2 and CO2 concentrations. c Time-dependent effects of high-light treatments at room and low temperatures on FR-induced steady state levels of P700 photo-oxidation (P700+) in wt and lut2 leaves. All results are expressed as percentage from the values of P700+ in control (non-high-light treated) leaves. Mean values ± SE were calculated from three independent experiments with four parallel samples. Circles—wt, triangles—lut2 leaves, photoinhibited at room (22 °C) (solid symbols) or low (8–10 °C) (empty symbols) temperature
Photochemical activity of PSII and PSI
For a more detailed characterization of the alterations in functioning of complexes of PSII and PSI after photoinhibitory treatment at room and low temperature, thylakoid membranes were isolated from control and illuminated for 4.5 h wt and lut2 leaves and the photochemical activity of both photosystems was evaluated (Fig. 7). The PSII-mediated electron transport, determined in the presence of artificial electron acceptor BQ (H2O → BQ), is negatively influenced in wt and lut2 under high-light illumination. The decline in photochemical activity of PSII in thylakoid membranes of illuminated wt leaves is with around 30% in comparison with non-illuminated ones, while for lut2 thylakoids are with 10% stronger affected. The illumination-induced decrease of photochemical activity of PSII for both types of leaves is not significantly influenced by the temperature of illumination (Fig. 7a). The photochemical activity of PSI (DCPIP/Na ascorbate → MV) is slightly affected by illumination with high-light intensity ranging from 3 to 7%, for lut2 and wt, respectively (Fig. 7b). It is worth noting that the photochemical activity of PSII in thylakoid membranes isolated from non-illuminated lut2 leaves is with 30% lower than in thylakoids isolated from control wt leaves, while the photochemical activity of PSI in lut2 control leaves is with 20% higher than in the respective ones of wt as judged from the absolute values of photochemical activity of PSII and PSI of thylakoid membranes isolated from non-treated wt and lut2 leaves (see Fig. 7 legend).
Effect of photoinhibitory treatment at room (22 °C) and low temperature (8–10 °C) on photochemical activity of PSII (a) and PSI (b) in thylakoid membranes, isolated from non-illuminated and illuminated for 4.5-h leaves of wt and lut2. Data are presented as percent from the activity of thylakoid membranes isolated from non-illuminated leaves. Mean values ± SE are calculated from three independent experiments with two parallel samples. 100% of PSII—for wt—24.08 ± 1.51 µmol O2/mg chl/ml and for lut2—16.01 ± 0.53 µmol O2/mg chl/ml, PSI—for wt—98.72 ± 6.51 µmol O2/mg chl/ml and for lut2—119.11 ± 6.12 µmol O2/mg chl/ml
High-light-induced alterations in energy transfer and interaction between the pigment–protein complexes
The HL-induced changes in energy transfer and interaction within the PSII complex and between PSII and PSI were evaluated by recording and analyzing low temperature (77 K) fluorescence emission spectra of thylakoid membranes, isolated from control and illuminated for 4.5 h at room or low temperature wt and lut2 leaves. Emission spectra of thylakoid membranes were recorded at excitation with 436 nm (preferentially exciting chlorophyll a) or with 472 nm (preferentially exciting chlorophyll b). In Fig. 8, the emission spectra at excitation 436 nm of thylakoids from control and illuminated for 4.5 h with high-light leaves at room temperature of wt (Fig. 8a) and lut2 (Fig. 8b) are presented. In the emission spectra of thylakoid membranes at 77 K, three main maxima, at 685, 695, and 735 nm, are observed, emitted from the reaction center of PSII, its core antenna (CP47) (Krause and Weis 1991; Andrizhiyevskaya et al. 2005) and from the reaction center of PSI and its LHC, respectively. The spectra of thylakoids, isolated from control leaves, presented in Fig. 8 (bold lines in panel a and b), show that the relative height of the fluorescence peak at 735 nm is higher in lut2 thylakoids in comparison with those isolated from non-illuminated wt. Illumination of leaves with high-light for 4.5 h leads to a relative increase of the fluorescence peak at 735 nm, stronger expressed in lut2 leaves.
Low-temperature (77 K) fluorescence emission spectra of thylakoid membranes, isolated from non-illuminated (solid line) and photoinhibited (dashed line) at room temperature for 4.5 h leaves of wt (a) and lut2 (b). Chlorophyll fluorescence was excited at 436 nm. Spectra are normalized at 685 nm after subtraction of the baseline. Excitation and emission slits were 4 nm. Chlorophyll concentration was 15 µg chl/ml
Treatment with high-light leads to a reduction of the overall emitted fluorescence as estimated by the area under the fluorescence spectra and the degree of quenching of overall fluorescence of wt and lut2 thylakoids after excitation at 436 or 472 nm do not differ significantly (data not shown).
From the fluorescence emission spectra at excitation with 436 nm the fluorescence ratios F685/F695 (Fig. 9a) and F735/F685 (Fig. 9b) of thylakoid membranes of control and illuminated for 4.5 h at room and low temperature leaves of wt and lut2 are calculated. The ratio F685/F695 provides information about the energy interaction in the super PSII complex while F735/F685 characterizes the relative population of both photosystems, the energy distribution between them and spillover of energy from PSII to PSI. High-light treatment at both temperatures does not lead to significant alterations in the energy interaction in the supercomplex of PSII (Fig. 9a). At the same time, the ratio F735/F685, of thylakoid membranes of non-illuminated lut2 leaves is higher in comparison with non-illuminated wt leaves, 1.48 against 1.19, respectively. For both types of leaves, the ratio F735/F685 is increased in thylakoids isolated from illuminated leaves, better expressed for lut2 (Fig. 9b). For the wt leaves, the ratio F735/F685 is increased from 1.192 to 1.336 and 1.308 (with 12 and 10%) for illuminated at room and low temperature, respectively, while for lut2 illuminated leaves the increase is with 26 and 24%, from 1.48 to 1.865 and 1.830, for room and low temperature, respectively.
Alterations in fluorescence ratios F685/F695 (a) and F735/F685 (b), calculated from emission fluorescence spectra of thylakoid membranes, isolated from non-illuminated and illuminated for 4.5 h at room (22 °C) and low-temperature (8–10 °C) leaves of wt and lut2 at excitation with 436 nm. Means ± SE were calculated from three independent experiments with two parallel samples. Statistically significant changes in treated samples in comparison with thylakoid membranes of control, non-illuminated leaves, are marked by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001). Significant differences between the values of wt or lut2 at the same type of treatment are marked with plus (+P < 0.05, ++P < 0.01, +++P < 0.001)
Oxygen production reactions
To characterize the effect of high-light treatment of wt and lut2 leaves and of temperature during illumination on the functioning of oxygen evolving system in isolated thylakoid membranes from control and illuminated for 4.5 h leaves at room or low temperature, the traces of initial oxygen burst at continuous illumination without the addition of artificial electron acceptor were recorded and analyzed. The induction curve of initial oxygen burst at continuous illumination exhibits a second-order exponential decay that can be decomposed with two components with two respective amplitudes and time constants (A1 and A2, t1 and t2). The time constants of both components of the decay curve (t1 and t2) and the ratio A1/A2 characterizing the two different types of PSII centers (PSIIα—with “fast” and PSIIβ—with “slow” turnover, respectively) in oxygen evolution are presented in Table 2. It is assumed that in respect to their functioning, antenna size and location in thylakoid membrane PSII centers are two types—PSIIα and PSIIβ, situated in the grana or in the stroma-exposed thylakoids, respectively (Melis and Homann 1976) and related to QB-reducing and QB-non-reducing centers (Melis 1985; Neale and Melis 1991). The contribution of PSIIα and PSIIβ centers in the total amount of evolved oxygen is estimated by parameters of the two decay components of oxygen burst, “fast” and “slow,” respectively (Lazarova et al. 2014). At illumination with high-light of wt leaves, the time constants t1 and t2 increase, indicating delayed turnover of both centers, better expressed for t1, to the same extent at room and low temperature. The value for t1 of wt is increased from 0.92 ± 0.10 in the control leaves to 2.27 ± 0.05 s for photoinhibited leaves at room temperature and from 1.02 ± 0.04 s to 2.53 ± 0.28 s in illuminated at low-temperature leaves. For lut2 leaves, the same tendency of increase of time constants is observed, but the delay is stronger for room temperature illumination—t1 is increased from 1.04 ± 0.13 s in control, non-illuminated, leaves to 3.16 ± 0.73 s for illuminated ones, while for the leaves, illuminated at low temperature the increase is less expressed—from 0.99 ± 0.04 s to 2.04 ± 0.13 s. The ratio A1/A2 is also affected by treatment with high-light intensity in wt and lut2 leaves. For wt A1/A2 is decreased in illuminated at room temperature leaves with 37% (from 1.97 in control leaves to 1.25 in illuminated ones) and the process is accelerated at low temperature expressed in a decline with 50% (from 1.45 to 0.71). For lut2 leaves, the illumination-induced decline is stronger expressed—with 58 and 66% for illuminated at room and low temperature leaves, respectively.
Protein degradation
High-light-induced changes in the abundance of reaction centers proteins of PSI (PsaB) and PSII (PsbA, D1) were analyzed in thylakoid membranes, isolated from wt and lut2 leaves after illumination for 4.5 h at room and low temperature and were compared with samples from control, non-illuminated leaves. PsaB and D1 from wt and lut2 show different susceptibility to light-induced damage at room and low temperature. D1 in wt leaves is less affected at room temperature (after 4.5 h of illumination minor changes are observed while at low temperature a reduction by 11% is detected [Table 3]). In mutant leaves, D1 is reduced to the same extent—10% at both temperatures. More evident difference between wt and lut2 is observed in respect to PSI reaction center protein—PsaB. During low-temperature illumination, the content of PsaB in wt is reduced by about 35%, but for lut2 the protein is not affected. At room temperature illumination PsaB is decreased by 10–12%, to the same extent in wt and lut2.
Discussion
Higher plants absorb sun light by photosynthetic pigments, chlorophylls, and carotenoids that are covalently bound to the main photosynthetic complexes, reaction centers of PSII and PSI as well as of light-harvesting complexes. Chlorophyll a is bound to all photosynthetic pigment–protein complexes; β-carotene is connected to the proteins of reaction centers while chlorophyll b and xanthophylls comprise intrinsic part of LHC complexes (Dekker and Boekema 2005). Carotenoids are involved not only in light harvesting (Yruela et al. 1998) but perform a photoprotective function expressed in dissipation of excess absorbed light (Havaux and Niyogi 1999) and deactivation of the triplet states of chlorophyll and scavenging of light-induced singlet oxygen (Jahns and Holzwarth 2012; Krieger-Liszkay 2005; Triantaphylides and Havaux 2009). Lutein is the most abundant xanthophyll in higher plants, found in the LHC of both PSII and PSI (Morosinotto et al. 2003; Jahns and Holzwarth 2012). Its presence for the formation of stable trimeric LHCII complexes is essential (Lokstein et al. 2002). In lutein-deficient mutants lut1 and lut2 of A. thaliana, the formation of stable LHCII trimeric structure was reduced or completely abolished (Pogson et al. 1996) irrespective of replacement of lutein by the violaxanthin cycle pigments (Lokstein et al. 2002; Dall’Osto et al. 2006). Lutein is involved in the light-harvesting process as well (Siefermann-Harms 1985) but its function as an accessory pigment can be fully replaced by substituting xanthophylls (Pogson et al. 1996). The most important photoprotective function of lutein is to quench the excited states of chlorophyll in singlet and triplet state (Jahns and Holzwarth 2012; Peng et al. 2006).
The role of different carotenoid species in the photosynthetic processes and especially of lutein is intensively investigated with a special attention on its role in dissipation of excessive light energy and photoprotection in higher plants based on data about the effect of high-light illumination of whole plants of A. thaliana, wt and lutein-deficient mutants (Niyogi et al. 2001; Lokstein et al. 2002), of detached leaves (DallOsto et al. 2007) or with a focus on the role of lutein for adaptation to different light intensities (Kalituho et al. 2007). Recently, the participation of lutein in scavenging of light-induced reactive oxygen species has been supposed as the lack of lutein accelerates the photobleaching of photosynthetic pigments and degradation of light-harvesting proteins in isolated thylakoid membranes from lut2 (Dobrev et al. 2016). In the present investigation, we report on the effect of HL illumination of detached leaves of A. thaliana, wt and lutein-deficient lut2 mutant, at different temperatures, on the functioning of photosynthetic reactions with a special attention on quenching of excessive absorbed light.
Light-induced alterations in activity of PSII in vivo
Under conditions of high-light illumination an imbalance between the absorbed light energy and its utilization through driving primary photosynthetic reactions and energy transformation into reducing power (NADPH) and chemical energy (ATP), needed for metabolic reactions takes place. Exposure to high-light illumination leads to over-reduction of the primary electron acceptor QA and of PQ pool and increased excitation pressure of PSII that negatively affects the performance of PSII and often leads in photoinhibition (Huner et al. 1996, 1998). Exposure of higher plants to lower than optimal for their development temperature, combined either with normal or high-light intensity, leads as well to photoinhibition and/or to increase its extent. At low temperature, the rate of enzymatic reactions, including anti-oxidant systems, is decreased retarding the scavenging of stress-generated reactive oxygen species thus negatively affecting both primary photosynthetic and metabolic reactions (Allen and Ort 2001; Huner et al. 1998).
The observed effect of photoinhibitory treatment is time dependent and is better expressed for illuminated leaves of the mutant not containing lutein. The extent of light-induced inhibition is accelerated when illumination is performed at low temperature (Fig. 3a, b). The depressed photosynthetic performance of PSII as induced by HL illumination causes a reduction in the maximum rate of oxygen evolution (Fig. 2b) and inhibition of the electron transport (Figs. 2a, 5) as well as increase in the values of LCP (Fig. 2c).
The increased excitation pressure under exposure to HL that causes inhibition of the photosynthetic performance of PSII raises the question about the different mechanisms that are involved in the process of deactivation of excess absorbed light. The LHCII is involved not only in capture and transfer of light energy to the reaction center of PSII but under conditions of high-light illumination performs photoprotective function dissipating the energy, not utilized in photosynthetic processes (Derks et al. 2015). It is generally considered that the excessive absorbed light is converted to harmless heat by the process of NPQ that is triggered by build up of proton gradient (∆pH) across the thylakoid membrane induced by high-light illumination electron transport that in turn activates the functioning of the xanthophyll cycle (Demming-Adams and Adams 1992; Horton et al. 1996; Ort 2001). However, it had been supposed that only half of light energy is dissipated by NPQ (Gilmore 1997) and a part of the excessive absorbed light is dissipated by another mechanism that also takes place in the LHCII but is not ∆pH- and zeaxanthin-dependent (ΦNO) (Szyszka et al. 2007). Results presented indicate that with increase of time of illumination, irrespective of the temperature of treatment, not only the quantum efficiency of utilization of light energy by PSII (ΦPSII) is negatively affected, which is to be expected under high-light illumination that increases the excitation pressure of PSII (Fig. 3b), but the efficiency of non-photochemical quenching (NPQ and ΦNPQ) are inhibited as well (Figs. 3c, 4). The observed reduction of NPQ seems reasonable taking in mind that upon photoinhibitory treatment the inhibition of PSII activity resulted in a decrease of quantum efficiency of light conversion followed by a decrease of accumulation of H+, leading to decrease of transthylakoid proton gradient (Barenyi and Krause 1985; Laasch 1987; Tjus and Andersson 1993) which plays a decisive role in the functioning of xanthophylls cycle (Munekage et al. 2002; Demming-Adams and Adams 1992; Horton et al. 1996).
A substantial part of the excessive absorbed light is dissipated by the alternative, not dependent on the functioning of ∆pH and/or the xanthophyll cycle mechanism as indicated by the time-dependent increase of ΦNO (Figs. 3d, 4). The operation of this mechanism is more intensive in the lut2 leaves in comparison with that of wt and proceeds faster under photoinhibitory treatment at low temperature, for both types of leaves (Figs. 3d, 4). This is a clear indication that a significant part of excessive light is dissipated by an alternative mechanism that is not dependent on the light-induced ∆pH across thylakoid membrane and on operation of the xanthophyll cycle in the LHCII. Operation of this mechanism is faster in the absence of lutein and is accelerated at low temperature that can indicate to be independent on enzymatic activity.
Alterations in the quantum efficiency of PSI electron transport in leaves as affected by high-light illumination
The redox state of PSI in vivo is determined by FR illumination of leaves. In control, non-illuminated leaves of lut2 the kinetics of oxidation of P700 is slower in comparison with that in control wt leaves. In wt leaves illuminated with high-light intensity, the kinetics of photo-oxidation of P700 is slowed down, better expressed at low temperature, while for lut2 leaves photoinhibitory treatment has much less effect in achieving full oxidation of P700 (Fig. 6a, b). In addition to the linear electron transport through the electron transport chain, a cyclic electron transport around PSI is recognized and considered as a protective mechanism against photodamage of PSII (Endo et al. 1999; Munekage et al. 2002). For both types of leaves, wt and lut2, photoinhibitory treatment accelerates the cycling of electrons around PSI as determined by the decay kinetics of re-reduction of P700+ (Fig. 6; Table 1). This result is in accordance with the finding that CEF through PSI is essential for photoprotection of A. thaliana at low temperature (Munekage et al. 2004; Ivanov et al. 2012). The experimental data presented above clearly demonstrate that after photoinhibitory treatment an increase of the rate of dark reduction was observed in wt as well in lut2 leaves. Taking in mind the possible electron fluxes to P700+, this acceleration of reduction rate could be related to a stimulation of CEF (as a protective mechanisms when PSII activity is inhibited) and with abolishment of slow component of P700+ reduction related to a decrease of slowly reducing PSI units from stromal reductants (Bukhov et al. 2002).
In addition, the extent of oxidation of P700 is negatively influenced with increase of time of illumination in the same manner for wt at both temperatures and for lut2 leaves, treated with high-light intensity at room temperature. At illumination at low temperature of lut2 leaves the degree of oxidation of P700 is nearly twice stronger inhibited (Fig. 6c).
high-light-induced alterations in functioning and organization of main pigment–protein complexes
Photochemical activity of PSII and PSI, activity of oxygen evolving complex, population of reaction centers of both photosystems as well as energy interaction between the main pigment–protein complexes was determined in isolated thylakoid membranes from control and photoinhibited for 4.5 h at different temperatures leaves of wt and lut2 Arabidopsis leaves. The photochemical activity of PSII and PSI was polarographically determined in the presence of artificial electron donors and acceptors thus allowing determination the activity of the reaction centers. The photochemical activity of PSII suffers comparable degree of inactivation by high-light illumination at normal and low temperature in both types of leaves (Fig. 7a) that can be due either to light-induced attack of D1 protein of PSII (Aro et al. 1993; Adams et al. 2008) and/or of the water splitting system, the donor of electrons for the reaction center of PSII. Analysis of the decay kinetics of initial oxygen burst in isolated thylakoid membranes of control and photoinhibited for 4.5 h at different temperature leaves of wt and lut2 indicates that HL illumination retards oxygen evolution by both types of PSII centers, “fast” (PSII α) and “slow” (PSII β) (Table 2), stronger expressed for the “fast” centers, situated in the grana regions. In addition, illumination at room temperature leads to a stronger delay of turnover of PSIIα (t1) in lut2 in comparison with wt, while at low temperature “fast” centers are more affected in wt. The observed decrease in the ratio A1/A2 in illuminated leaves indicates that as a result of HL treatment the relative contribution of the “slow” PSII centers in oxygen evolution is higher in comparison with that of “fast” PSII centers and this tendency is better expressed in the mutant.
The presented results concerning the alterations in photochemical activity of PSII and oxygen evolving complex are in accordance with the scheme of photoinhibition (Ohnishi et al. 2005; Hakala et al. 2005) suggesting that PSII photodamage occurs at two steps—attack of the manganese cluster of the oxygen evolving complex of PSII and inactivation of the reaction center of PSII by the absorbed light. At the same time, the photochemical activity of PSI in the presence of exogenous electron donors and acceptors is hardy affected by the high-light treatment (Fig. 7b).
High-light illumination affects as well the content of reaction center proteins of PSI (PsaB) and PSII (D1). It should be noted that PsaB content of wt is more reduced at low temperature illumination, than at room temperature. It had been reported earlier that PSI is more affected by light treatment at low than at room temperature (Terashima et al. 1994).
Exposure of detached leaves to HL illumination at room and low temperature affects as well energy interaction and transfer between the main pigment–protein complexes of photosynthetic machinery that is reflected by the emission fluorescence spectra at low (77 K) temperature. The contour of emitted fluorescence is not altered in respect to the position of the fluorescence peaks but their relative intensities are influenced. Energy interaction in the multiprotein complex of PSII (F685/F695) does not show any significant alterations as a consequence of exposure for 4.5 h to high-light at both temperatures (Fig. 9a). The relative intensity of fluorescence peak at 735 nm, emitted by the pigment–protein complex of PSI, is increased in comparison with that of F685 after high-light treatment (Figs. 8, 9b) that can be due to a number of reasons including differences in energy absorption and supply of both photosystems, energy interaction between them and/or differential energy quenching. The relative higher peak of F735 and light-induced increase in the ratio F735/F685 can be caused by a higher energy delivery to PSI either directly or via energy supply by PSII–LHCII complexes or part of them that have migrated to the stroma-exposed regions of thylakoids (spill over) where is situated the multiprotein complex of PSI and/or higher population of PSI complexes. Such type of migration of LHCII from PSII to PSI in isolated thylakoid membranes is considered to be a mechanism aiming the protection of the photosensitive PSII from excessive light (Hundal et al. 1990), thus decreasing the excitation pressure of PSII (Derks et al. 2015). Time-resolved fluorescence measurements have shown that LHCII serves a more efficient light-harvesting function when associated with PSI than with PSII in A. thaliana (Wientjes et al. 2013). The increase in ratio F735/F685 can be due as well to quenching of emitted fluorescence by PSII at 685 nm by a quencher in PSII reaction centers (Velichkova and Popova 2005; Lazarova et al. 2014; Hundal et al. 1990). In thylakoids and PSII-enriched membranes, the inactivation at the donor side of PSII most probably is accompanied by the formation of a P680+ quencher that decreases the fluorescence emitted by the PSII complexes at 685 nm (Velichkova and Popova 2005; Lazarova et al. 2014; Bruce et al. 1997; Horton and Ruban 1992). The light-induced rearrangement of photosynthetic complexes expressed in delivery of excitation energy in favor of PSI is observed not only for the thylakoid membranes of wt leaves, but as well for those of the mutant, but in lut2 thylakoids the extent of the process is nearly twice higher. It has to be mentioned here that the ratio F735/F685 of non-illuminated thylakoid membranes of lut2 leaves (1.48) is higher than in thylakoids of non-illuminated wt leaves (1.19). The higher ratio F735/F685 for lut2 thylakoids is either due to the smaller antenna of PSII in the mutant, as has been previously shown (Pogson et al. 1996; Niyogi et al. 2001) or to higher population of PSI–LHCI complexes, as in lut2 the stroma-exposed thylakoids where are situated the complexes of PSI prevail over that in wt (Andersson 1981). It has to be mentioned as well that the HL-induced alterations in the energy interaction and transfer between the main pigment–protein photosynthetic complexes are observed at excitation with 436 nm (preferentially excitation of chl a) and at excitation with 472 nm (excitation of chl b, data not shown). It can be speculated that the smaller antenna of PSII in lut2 (Pogson et al. 1996; Niyogi et al. 2001) does not influence the energy interaction in the supercomplex of PSII but the light-induced spillover in thylakoids, increasing the energy delivery towards PSI complexes is functioning more effectively in lut2 than in the wt.
Conclusions
The presented results indicate that in lut2 non-illuminated leaves the maximum rate of photosynthetic evolution (Pnmax) and efficiency of energy utilization by PSII (ΦPSII) is lower than in wt and excitation pressure on PSII (1 − qP) is nearly twice higher than in wt. The FR-induced oxidation of P700 in lut2 non-illuminated leaves is slower and intensity of CET around PSI is faster than in non-illuminated wt leaves. The high-light-induced decrease in PSII performance in respect to electron transport, efficiency of oxygen evolution, and maximum quantum efficiency of PSII is more pronounced in illuminated leaves of the mutant that do not contain lutein in comparison with the wt. The excitation pressure of PSII and effectiveness of ΦNO are stronger increased in lut2 and especially when illumination is performed at low temperature. A significant part of excessive absorbed light is deactivated by a mechanism that is not ∆pH-dependent and not related to the operation of the xanthophyll cycle in the LHCII that operates more intensive in lut2 than in wt and is accelerated at low temperature illumination. Exposure to high-light illumination delays the oxidation of P700 in wt leaves, stronger at low temperature and accelerates the CET around PSI as a photoprotective mechanism. The light-induced transfer of excitation energy from PSII to PSI (spillover) in thylakoid membranes is more intensive in lut2 mutant.
Abbreviations
- BQ:
-
1,4-Benzoquinone
- CEF:
-
Cyclic electron flow around PSI
- DCMU:
-
3-(3,4-dichlorophenyl)1,1-dimethyl urea
- DCPIP:
-
2,6-dichlorophenolindophenol
- EDTA:
-
Ethylenediamine-tetraacetic acid
- ETR:
-
Electron transport rate
- F o :
-
Minimum yield of chlorophyll fluorescence in open PSII centers
- F m :
-
Maximal chlorophyll fluorescence in dark-adapted state
- \(F^{\prime}_{{\text{m}}}\) :
-
Maximal chlorophyll fluorescence in light-adapted state
- F v :
-
Variable chlorophyll fluorescence
- ΦPSII :
-
Effective quantum yield of PSII
- ΦNPQ :
-
Quantum yield of the regulated energy dissipation of PSII
- ΦNO :
-
Quantum yield of non-regulated energy dissipation of PSII
- F v/F m :
-
Maximum photochemical efficiency of PSII in the dark-adapted state
- LCP:
-
Light compensation point
- LHCII:
-
Light-harvesting chlorophyll a/b-protein complex of PSII
- LHCI:
-
Light-harvesting chlorophyll a/b-protein complex of PSI
- MES:
-
2(N-morpholino)ethanesulfonic acid
- MV:
-
Methyl viologen
- NPQ:
-
Non-photochemical quenching
- P700:
-
Reaction center chlorophyll of PSI
- P700+ :
-
Oxidized form of PSI reaction center
- PFD:
-
Photon flux density
- PQ:
-
Plastoquinone
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- Q A, Q B :
-
Primary and secondary electron-accepting quinone in PSII
- TES:
-
N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid
- TRICINE:
-
N-tris[hydroxymethyl]methyl glycine
References
Adams WW III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B (2008) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams W, Mattoo A (eds) Photoprotection, photoinhibition, gene regulation, and environment, Springer, Dordrecht, pp 49–64
Allen JF (1995) Thylakoid protein phosphorylation, state 1- state 2 transitions, and photosystem stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant 93:196–205
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6(1):36–42
Andersson J (1981) Consequence of spatial separation of photosystem I and II in thylakoid membranes from higher plants chloroplasts. FEBS Lett 124:1–10
Andrizhiyevskaya EG, Chojnicka A, Bautista JA, Diner BA, van Grondelle R, Dekker JP (2005) Origin of the F685 and F695 fluorescence in photosystem II. Photosynth Res 84:173–180
Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Baker NR, East TM, Long SP (1983) Chilling damage to photosynthesis in young Zea mays II. Photochemical function of thylakoids in vivo. J Exp Bot 34:189–197
Barenyi B, Krause GH (1985) Inhibition of photosynthetic reactions by light. A study with isolated spinach chloroplasts. Planta 163:218–226
Bassi R, Pineau B, Dainese P, Marquardt J (1993) Carotenoid binding proteins of photosystem II. Eur J Biochem 212:297–303
Bravo LA, Saavedra-Mella FA, Vera F, Guerra A, Cavieres LA, Ivanov AG, Huner NPA, Corcuera LJ (2007) Effect of cold acclimation on the photosynthetic performance of two ecotypes of Colobanthus quitensis (Kunth) Bartl. J Exp Bot 58(13):3581–3590
Brestic M, Zivcak M, Olsovska K, Shao H-B, Kalaji HM, Allakhverdiev SI (2014) Reduced glutamine synthetase activity plays a role in control of photosynthetic responses to high light in barley leaves. Plant Physiol Biochem 81:74–83
Brestic M, Zivcak M, Kunderlikova K, Sytar O, Shao H, Kalaji H, Allakhverdiev SI (2015) Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth Res 125:151–166
Bruce D, Samson G, Carpenter C (1997) The origins of non-photochemical quenching of chlorophyll in photosynthesis. Direct quenching by P680(+) in photosystem II enriched membranes at low pH. Biochemistry 36:749–755
Bukhov N, Egorova E, Carpentier R (2002) Electron flow to photosystem I from stromal reductants in vivo: the size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 215:812–820
Dall’Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. MBC Plant Biol 6:32
Dall’Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R (2007) Different roles of α- and β-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem 282:35056–35068
Dall’Osto L, Ünlü C, Cazzaniga S, van Amerongen H (2014) Disturbed excitation energy transfer in Arabidopsis thaliana mutants lacking antenna complexes of photosystem II. Biochim Biophys Acta 1837:1981–1988
Dekker JP, Boekema EJ (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta 1706:12–39
Delrieu MJ (1998) Regulation of thermal dissipation of absorbed excitation energy and violaxanthin deepoxidation in the thylakoids of Lactuca sativa. Photoprotective mechanism of a population of photosystem II centers. Biochim Biophys Acta 1363:157–173
Demming-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Derks A, Schaven K, Bruce D (2015) Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim Biophys Acta 1847:468–485
Dobrev K, Stanoeva D, Velitchkova M, Popova AV (2016) The lack of lutein accelerates the extent of light-induced bleaching of photosynthetic pigments in thylakoid membranes of Arabidopsis thaliana. Photochem Photobiol 92:436–445
Endo T, Shikanai T, Takabayashi A, Asada K, Sato F (1999) The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett 457:5–8
Fan D-Y, Hope AB, Jia H, Chow WS (2008) Separation of light-induced linear, cyclic and stroma-sourced electron fluxes to P700+ in cucumber leaf discs after pre-illumination at low temperature. Plant Cell Physiol 49:901–911
Frank HA, Cogdell RJ (1996) Carotenoids in photosynthesis. Photochem Photobiol 63:257–264
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Giersch C, Krause GH (1991) A simple model relating photoinhibitory fluorescence quenching in chloroplasts to a population of altered photosystem II reaction centers. Photosynth Res 30:115–121
Gilmore AM (1997) Mechanistic aspects of xanthophylls cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant 99:197–209
Gray GR, Savitch LV, Ivanov AG, Hüner NPA (1996) Photosystem II excitation pressure and development of resistance to photoinhibition. II. Adjustment of photosynthetic capacity in winter wheat and winter rye. Plant Physiol 110:61–71
Groce R, Weiss S, Bassi R (1999) Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J Biol Chem 274:29613–29623
Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II. Biochim Biophys Acta 1706:68–80
Haldrup A, Jensen PE, Lunde C, Scheller HV (2001) Balance of power: a view of the mechanism of photosynthetic state transitions. Trends Plant Sci 6:301–305
Havaux M, Niyogi K (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96:8762–8767
Havaux M, DallÓsto L, Cuine S, Giuliano G, Bassi R (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279(14):13878–13888
Hendrickson L, Furbank RT, Show WS (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82:73–81
Horton P, Ruban A (1992) Regulation of photosystem II. Photosynth Res 34:375–385
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Huang H-Y, Zhang Q, Zhao LP, Feng J-N, Peng C-L (2010) Does lutein plays a key role in the protection of photosynthetic apparatus in Arabidopsis under severe oxidative stress? Pak J Bot 42:2765–2774
Huang W, Yang YJ, Zhang SB (2017) Specific roles of cyclic electron flow around photosystem I in photosynthetic regulation in immature and mature leave. J Plant Physiol 209:76–83
Hundal T, Virgin I, Stryng S, Andersson B (1990) Changes of the organization of photosystem II following light-induced D1-protein degradation. Biochim Biophys Acta 1017:235–241
Huner NPA, Maxwell DP, Gray GR, Savich LV, Krol M, Ivanov AG, Falk S (1996) Sensing environmental change: PSII excitation pressure and redox signaling. Physiol Plant 98:358–364
Huner NPA, Oquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3:224–230
Ilioaia C, Johnson MP, Liao P-N, Pascal AA, van Grondelle R, Walla PJ, Ruban AV, Robert B (2011) Photoprotection in plants involves a change in lutein1 binding domain in the major light-harvesting complex of photosystem II. J Biol Chem 286:27247–27254
Ivanov AG, Morgan R, Gray GR, Velitchkova MY, Huner NPA (1998) Temperature/light dependent development of selective resistance of photoinhibition of photosystem I. FEBS Lett 430:288–292
Ivanov AG, Sane P, Hurry V, Krol M, Sveshnikov D, Huner NPA, Öquist G (2003) Low-temperature modulation of the redox properties of the acceptor side of photosystem II: photoprotection through reaction centre quenching of excess energy. Physiol Plant 119:376–383
Ivanov AG, Sane PV, Hurry V, Öquist G, Huner NPA (2008) Photosystem II reaction centre quenching: mechanisms and physiological role. Photosynth Res 98:565–574
Ivanov AG, Rosso D, Savitch LV, Stachula P, Rosembert M, Oquist G, Hurry V, Huner NPA (2012) Implications of alternative electron sinks in increased resistance of PSII and PSI photochemistry to high light stress in cold-acclimated Arabidopsis thaliana. Photosynth Res 113:191–206
Jahns P, Holzwarth AR (2012) The role of xanthophylls cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Johnson GN (2011) Physiology of PSI cyclic electron transport in higher plants. Biochem Biophys Acta 1807:384–389
Kalituho L, Rech J, Jahns P (2007) The roles of specific xanthophylls in light utilization. Planta 225:423–439
Klughammer C, Schreiber U (1991) Analysis of light-induced absorbency changes in the near-infrared spectral region. 1. Characterization of various components in isolated chloroplasts. Z Naturforsch C 46:233–244
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basis. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56:337–346
Laasch H (1987) Non-photochemical quenching of chlorophyll a fluorescence in isolated chloroplasts under conditions of stressed photosynthesis. Planta 171:220–225
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laisk A, Talts E, Oja V, Eichelmann H, Peterson RB (2010) Fast cyclic electron transport around photosystem I in leaves under far-red light: a proton-uncoupled pathway? Photosynth Res 103:79–95
Lazarova D, Stanoeva D, Popova A, Vasilev D, Velitchkova M (2014) UV-B induced alteration of oxygen evolving reactions in pea thylakoid membranes as affected by scavengers of reactive oxygen species. Biol Plant 58:319–327
Lee HY, Hong YN, Chow WS (2001) Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in Capsicum annuum L. leaves. Planta 212:332–342
Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, Bassi R, Fleming GR, Keasling JD, Niyogi KK (2009) Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 21:1798–1812
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lokstein H, Tian L, Polle J, DellaPenna D (2002) Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim Biophys Acta 1553:309–319
Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45:633–642
Matsubara S, Chow W-S (2004) Populations of photoinactivated photosystem II reaction centers characterized by chlorophyll a fluorescence lifetime in vivo. Proc Natl Acad Sci USA 101:18234–18239
Maxwell PC, Biggins J (1976) Role of cyclic electron transport in photosynthesis as measured by turnover of P700 in vivo. Biochemistry 15:3975–3981
Melis A (1985) Functional properties of photosystem IIβ in spinach chloroplasts. Biochim Biophys Acta 808:334–342
Melis A, Homann PH (1976) Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol 23:343–350
Millaleo R, Reyes-Diaz M, Alberdi M, Ivanov AG, Krol M, Hunner NPA (2013) Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J Ex Bot 64(1):343–354
Miyake C (2010) Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963
Miyake C, Horiguchi S, Makino A, Shinzaki Y, Yamamoto H, Tomizawa K (2005) Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of Chl fluorescence in tobacco leaves. Plant Cell Physiol 46:1819–1830
Morosinotto T, Caffari S, DallOsto L, Bassi R (2003) Mechanistic aspects of the xanthophylls dynamics of higher plant thylakoids. Physiol Plant 119:347–354
Moskalenko AA, Karapetyan NV (1996) Structural role of carotenoids in photosynthetic membranes. Z Naturforsch 51c:763–771
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371
Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429:579–582
Neale PJ, Melis A (1991) Dynamics of photosystem II heterogeneity during photoinhibition: depletion of PSIIβ from non-appressed thylakoids during strong irradiance exposure of Chlamydomonas reinhardtii. Biochim Biophys Acta 1056:195–203
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Rev Plant Physiol Plant Mol Biol 50:333–359
Niyogi KK, Bjorkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94:14162–14167
Niyogi KK, Shih C, Chow WS, Pogson BJ, DellaPenna D, Bjoerkman O (2001) Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth Res 67:139–145
Öguist G, Huner NPA (2003) Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol 54:329–355
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44:8494–8499
Ort DR (2001) When there is too much light. Plant Physiol 125:29–32
Peng CL, Gilmore AM (2003) Contrasting changes of photosystem efficiency in Arabidopsis xanthophyll mutants at room or low temperature under high irradiance stress. Photosynthetica 41(2):233–239
Peng CL, Lin Z-F, Su Y-Z, Lin G-Z, Dou H-Y, Zhao C-X (2006) The antioxidative function of lutein: electron spin resonance studies and chemical detection. Funct Plant Biol 33:839–846
Peter GF, Thornber JP (1991) Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J Biol Chem 266:16745–16754
Peterman ELG, Dukker FM, van Grondelle R, von Amerongen H (1995) Chlorophyll a and carotenoid triplet states in light-harvesting complex II of higher plants. Biophys J 69:2670–2678
Plumley FG, Schmidt DW (1987) Reconstitution of chlorophyll a/b light-harvesting complexes: xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci USA 84:146–150
Pogson B, McDonald KA, Truong M, Britton G, DellaPenna D (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8:1627–1639
Popova AV, Velitchkova M, Zeinalov Y (2007) Effect of membrane fluidity on photosynthetic oxygen production reactions. Z Naturforsch 62c:253–260
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35:15–44
Ravenel J, Peltier G, Havaux M (1994) The cyclic electron pathways around photosystem I in Chlamydomonas reinhardtii as determined in vivo by photoacoustic measurements of energy storage. Planta 193:251–259
Rochaix J-D (2004) Genetics of the biogenesis and dynamics of the photosynthetic machinery in eukaryotes. Plant Cell 16:1650–1660
Sane PV, Ivanov AG, Hurry V, Huner NPA, Öquist G (2003) Changes in the redox potential of primary and secondary electron accepting quinones in photosystem II confer increased resistance to photoinhibition in low-temperature-acclimated Arabidopsis. Plant Physiol 132:2144–2151
Savitch LV, Ivanov AG, Gudynaite-Savitch L, Huner NPA, Simmonds J (2011) Cold stress effects on PSI photochemistry in Zea mays: differential increase of FQR-dependent cyclic electron flow and functional implications. Plant Cell Physiol 52:1042–1054
Siefermann-Harms D (1985) Carotenoids in photosynthesis. I. Location in photosynthetic membranes and light-harvesting function. Biochim Biophys Acta 811:325–335
Sonoike K (1998) Various aspects of inhibition of photosynthesis under light/chilling stress: “photoinhibition at chilling temperatures” versus “chilling damage in the light”. J Plant Res 111:121–129
Sonoike K, Kamo M, Hihara Y, Hiyama T, Enami I (1997) The mechanism of the degradation of PsaB gene product, one of the photosynthetic reaction center subunits of photosystem I, upon photoinhibition. Photosynth Res 53:55–63
Szyszka B, Ivanov AG, Huner NPA (2007) Psychrophily is associated with differential energy partitioning, photosystem stoichiometry and polypeptide phosphorylation in Chlamidomonas taudensis. Biochim Biophys Acta 1757:789–800
Terashima I, Funayama S, Sonoike K (1994) The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta 193(2):300–306
Tjus SE, Andersson B (1993) Loss of the trans-thylakoid proton gradient is an early event during photoinhibitory illumination of chloroplast preparations. Biochim Biophys Acta 1183:315–322
Triantaphylides C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14:219–228
van Kooten O, Snell JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Velichkova M, Popova A (2005) High light-induced changes of 77K fluorescence spectral characteristics of thylakoid membranes with modified fluidity. Bioelectrochemistry 67:81–90
Ware MA, Dall’Osto L, Ruban AV (2016) An in vivo quantitative comparison of photoprotection in Arabidopsis xanthophyll mutants. Front Plant Sci 7:841
Wei H, Yang Y-J, Zhang S-B (2017) Specific roles of cyclic electron flow around photosystem I in photosynthetic regulation in immature and mature leaves. J Plant Physiol 209:76–83
Wientjes E, van Amerongen H, Croce R (2013) LHCII is an antenna for both photosystems after long-term acclimation. Biochim Biophys Acta 1827:420–426
Yamori W, Makino A, Shikanai T (2016) A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci Rep 6:20147
Yruela I, Tomás R, Sanjuán ML, Torrado E, Aured M, Picorel R (1998) The configuration of β-carotene in the photosystem II reaction center. Photochem Photobiol 68:729–737
Zeinalov Y (2002) An equipment for investigations of photosynthetic oxygen production reactions. Bulg J Plant Physiol 28:57–67
Acknowledgements
This work was partially supported by Bulgarian-Swiss Research Program, Project IZEBZO-143169/1. The seeds of the wt and mutant lut2 of A. thaliana were a generous gift from Prof. R. Bassi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popova, A.V., Dobrev, K., Velitchkova, M. et al. Differential temperature effects on dissipation of excess light energy and energy partitioning in lut2 mutant of Arabidopsis thaliana under photoinhibitory conditions. Photosynth Res 139, 367–385 (2019). https://doi.org/10.1007/s11120-018-0511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0511-2