Abstract
The reduced content of photoreceptors, such as phytochromes, can decrease the efficiency of photosynthesis and activity of the photosystem II (PSII). For the confirmation of this hypothesis, the effect of deficiency in both phytochromes (Phy) A and B (double mutant, DM) in 7–27-day-old Arabidopsis thaliana plants on the photosynthetic activity was studied in absence and presence of UV-A radiation as a stress factor. The DM with reduced content of apoproteins of PhyA and PhyB and wild type (WT) plants with were grown in white and red light (WL and RL, respectively) of high (130 μmol quanta m−2 s−1) and low (40 μmol quanta m−2 s−1) intensity. For DM and WT grown in WL, no notable difference in the photochemical activity of PSII was observed. However, the resistance of the photosynthetic apparatus (PA) to UV-A and the rate of photosynthesis under light saturation were lower in the DM compared to those in the WT. Growth in RL, when the photoreceptors of blue light—cryptochromes—are inactive, resulted in the significant decrease of the photochemical activity of PSII in DM compared to that in WT including amounts of QB-non-reducing complexes of PSII and noticeable enhancement of thermal dissipation of absorbed light energy. In addition, marked distortion of the thylakoid membrane structure was observed for DM grown in RL. It is suggested that not only PhyA and PhyB but also cryptochromes are necessary for normal functioning of the PA and formation of the mechanisms of its resistance to UV-radiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of photoreceptors—phytochromes—in regulation of plant growth and photosynthesis under different environmental conditions is actually one of the crucial questions in photobiology and stress-physiology of plants. In particular, the change of Phy content and activity can be one of the ways to regulate the resistance of the photosynthetic apparatus (PA) to the effect of environmental stress factors (Kreslavski et al. 2013a, b). This concept is in agreement with recent studies on phytochrome mutants of Arabidopsis thaliana which are deficient in different types of phytochromes, demonstrating that deletion of phytochromes is critical for plant development (Strasser et al. 2010; Hu et al. 2013; Zhao et al. 2013). Normal seedling greening and plant development is impossible if phytochromes are absent (Strasser et al. 2010; Zhao et al. 2013). On the contrary, the excess of PhyB led to the delay of flowering and enhancement of photosynthesis (Thiele et al. 1999; Kreslavski et al. 2015). To change the phytochrome content, plant mutants deficient in various phytochrome types and transgenic Phy-superproducer plants are used. Mutants of A. thaliana and rice are frequently used in experiments, as their genome is deciphered, and there is a wide number of mutants, in particular, the A. thaliana mutant hy3, which is deficient in PhyB, and the mutant phyA-211 phyB-9, deficient in both PhyA and PhyB, simultaneously (Kreslavski et al. 2013a, 2016; Rusaczonek et al. 2015). Oxidative stress induced by the effect of UV-radiation was studied in the mutant hy2 deficient in the chromophore of all five Phy types contained in A. thaliana. The study performed in (Kreslavski et al. 2013a) focused on the influence of lack of all phytochromes on photosynthetic parameters and PA resistance, as well as the influence on transcriptional activity of key antioxidant enzymes genes, genes of some photosynthetic proteins and transcription factors of Phy signaling. It was found that Phy deficiency results in the decrease of UV-absorbing pigments and decreased carotenoid content, as well as in decrease of the activity of a number of key antioxidant enzymes, which shifts the balance of oxidants and antioxidants in the direction of oxidants. These findings are consistent with the observed reduction of PA resistance to UV-radiation and high intensity light. The decrease of the expression level of antioxidative defense and Phy signaling genes, in particular, the content of transcripts of ascorbate peroxidase and l-phenylalanine ammonia-lyase genes, can play a role in the shift of this balance. It is suggested that PhyB is a key red light (RL) sensor, whereas phytochromes C, D, and E are less important for the RL perception. The photoreceptor PhyB participates in the reactions induced by short RL treatment with relatively low intensity (Casal et al. 1998). It plays the key role in the Phy system of green plant leaves. It is involved in shading avoidance (Franklin 2008), the synthesis of photosynthetic pigments, and chloroplast development (Zhao et al. 2013), as well as in the synthesis of some photosynthetic proteins and stomatal (Boccalandro et al. 2009). Peculiarity of these reactions is the reversibility of RL-induced effects by far-red light (FRL) illumination (Kreslavski et al. 2013a, b).
PhyA is the most important FRL sensor (Hu et al. 2013). PhyA predominates in seedlings grown in the dark and quickly degrades in the light, while other phytochromes prevail in the light, where they are relatively stable. This phytochrome can be also important for stress tolerance. Thus, the study of Gururani et al. (2015) evaluated the influence of cold stress on the photosynthetic machinery of transgenic turfgrass, Zoysia japonica, expressing oat phytochrome A (PhyA) or a hyperactive mutant phytochrome A (S599A). The transgenic plants showed enhanced tolerance of PA to cold stress.
Studies of photosynthesis and PSII activity in the transgenic potato plants Dara-12 and Dara-5, PhyB-superproducers, showed the enhancement of photosynthesis and increased PSII resistance to UV-radiation and high intensity light (Thiele et al. 1999; Kreslavski et al. 2015). On the contrary, reduced PA resistance to UV-radiation was found under deficiency of PhyB (Kreslavski et al. 2016). In many photomorphogenetic reactions in plants, PhyB acts together with PhyA (Casal 2000; Rusaczonek et al. 2015). Thus, it is suggested that PhyA and PhyB play regulatory role in CO2 assimilation, oxygen reactive species accumulation, and non-photochemical quenching of chlorophyll fluorescence (Rusaczonek et al. 2015). Nevertheless, the possible role of phytochromes as RL receptors in photosynthetic processes and the regulation under different environmental conditions is studied insufficiently. In particular, it would be important to use the double mutant, which is deficient in both key phytochromes (PhyA and PhyB) simultaneously, for the investigation of the role of Phy in the regulation of photosynthetic processes. In the present study, the detailed investigation of the effect of the deficiency in both apoproteins, PhyA and PhyB (double mutant, DM) on the PA activity of A. thaliana plants in comparison to wild type (WT) plants and mutants deficient in the apoprotein PhyB only (hy3), was carried out. To evaluate the role of interaction of phytochromes and receptors of blue light (cryptochromes), the plants were grown in white and red light and compared independently to exclude the activity of cryptochromes.
Materials and methods
Experiments were performed using 7–27-day-old wild type, phytochrome A and phytochrome B deficient mutants (DM) and the hy3 mutant selectively lacking the PhyB apoprotein (see Kreslavski et al. 2016) (from the European Arabidopsis Stock Centre (Nottingham, UK)) plants of Arabidopsis thaliana (ecotype Columbia-0). Plants were grown under controlled conditions (8-, 12-, or 16-h photoperiod and 16-, 12-, and 8-h dark period, respectively) at 25 °C under light and 20 °C in the dark. White fluorescent lamps (40 or 130 μmol quanta m−2 s−1) or red LEDs (656 nm, 19 nm FWHM, 40 or 130 μmol quanta m−2 s−1) were used for growing. UV-A was obtained by an ultraviolet lamp Selecta T8 18W BLB (Camelion, China) with λmax = 365 nm (I = 12 W m−2 on the leaf surface, 24 nm FWHM). For evaluation of the photochemical activity of the photosynthetic apparatus (PA), Chl fluorescence induction curves were recorded, and PAM-fluorometry was conducted.
The total amount of all plants at the end of growing was approximately 50 plants. All experiments were repeated 3–4 times (n). For each variant of our sample (WT, DM, and hy3), three healthy, developed, upper leaves with almost horizontal position of the leaf blade were chosen from three pots for fluorescence measurements. The leaves were detached, kept in the dark for 20 min until fluorescence measurements. For pigment and growth measurements, we used at least 15 leaves per each variant.
The characteristic values FV, FO, FM, and the maximum photochemical quantum yield (FV/FM); the effective photochemical quantum yield Y(II); the electron transport rate (ETR); non-photochemical quenching (NPQ); and the number of other values were identified by using PAM-fluorometer (XE-PAM, Heinz-Walz, FRG) or Junior-PAM (Walz, Germany).
The FV/FM and Y(II) were calculated as (FM − FO) / FM and (F’ M − FO) / F’ M, respectively. Here, FM and F’ M are the maximum fluorescence levels under dark- and light-adapted conditions, respectively; Fo and F’o are the minimum fluorescence in the dark-adapted state and the calculated value of the minimum fluorescence value in the light-adapted state, respectively. To measure maximal Chl fluorescence in the light-adapted state, actinic light was switched on for 10 min (usually, 190 μmol(quantum) m−2 s−1 PAR). Maximal fluorescence (FM) was measured using saturating pulses (6000 μmol(quantum) m−2 s−1 PAR, 800-ms duration).
OJIP transient, i.e., the increase of fluorescence intensity from minimal level to its maximum, was carried out by using a self-built transient fluorescence recorder as described in (Lankin et al. 2014). The intensity of saturating light was 5000 μmol quanta m−2 s−1 at λм – 470 nm. The obtained fast induction curves were evaluated by calculating the following parameters as defined below: DIO/RC FV/FO, as well as the ABS/RC ratio which denotes averaged photon flux absorbed by the Chl of the PSII antenna (ABS) per active PSII reaction center (RC); the value ABS/RC reflects the size of the PSII antenna (Kalaji et al. 2012, 2014a, b; Stirbet and Govindjee 2011). Calculations were conducted according to the following formulas:
MO—initial slope of the curve of the relative value of the variable fluorescence intensity of Chl a; expresses the rate of electron transfer at the initial stage. VJ—relative fluorescence level in phase J after 2 ms. VJ = (F2ms − FO) / (FM − FO). VI—relative fluorescence level in phase I after 30 ms. VI = (F30ms − FO) / (FM − FO). ABS—the photon flux absorbed by PSII antenna chlorophyll, as described earlier (Stirbet and Govindjee 2011; Kalaji et al. 2012, 2014a, b; Lankin et al. 2014).
In addition, the performance index for PSII PIABS (Strasser et al. 2000) was calculated according to (Živčák et al. 2008): PIABS = (FV / FM) / (MO / VJ) × (FV / FO) × (1 − VJ) / VJ and the total performance index for PA in whole PItotal according to: PItotal = PIABS × δRo / (1 − δRo), where δRo = (1 − VI) / (1 − VJ). For the calculation of QB-non-reducing centers, the method described in (Klinkovsky and Naus 1994) was used. The fluorescence intensity at the transition from the exponential fluorescence dependence to the sigmoid one “before the plateau” reflects the QA reduction in QB-non-reducing complexes and was calculated according to 1- (FM − Fpl) / FV, where Fpl is the fluorescence intensity on the plateau region.
The parameters of photosynthesis
The CO2-gas exchange of the leaves was measured by the portable system LCPro+ (ADC BioScientific Ltd., Great Britain). The dependence of the visible photosynthesis rate on the light intensity was measured in the range of from 0 to 1200 μmol photons m−2 s−1 at CO2 concentration in the air of 400 μmol mol−1 (Martirosyan et al. 2013). The light curve was approximated by the model of (Priol and Chartier 1977). The content of photosynthetic pigments was measured in μg per 1 g of fresh mass (f.m.) by measurements of the optical density of filtrated ethanol extracts using the extinction values from literature (Lichtenthaler and Wellburn 1987). UV-absorbing methanol-extracted compounds (predominantly flavonoids) were isolated from cuttings of fresh leaves by the method of (Mirecki and Teramura 1984); 15–25 discs were used, carving several discs from each leaf. The cuttings from the leaves were incubated in acidic methanol for 24 h (methanol:water:HCl, 78:20:2) under 4 °C. Then, the optical density of the solution at 327 nm was determined using the spectrophotometer M-40 (“Karl Zeiss,” Germany). From this value, the amount of flavonoids (optical density unit per leaf dry weight or 1 cm2 of its surface) was calculated.
Electron microscopy
Structural studies were carried out on leaf sections from the middle part of the leaf, fixed with 2 % glutaraldehyde in phosphate buffer with or without post fixation by 1 % osmium tetroxide. After dehydration by incubation into a series of solution with increasing alcohol and acetone concentration, the samples were embedded in Epon epoxide resin. Ultrathin sections were cut by LKB ultratome (Sweden), contrasted with uranyl acetate and lead citrate, and then investigated with an electron microscope JEM 100B (Japan) (Semenova and Romanova 2011) and photographed.
Statistics
The tables and graphs show the average values with their standard errors (SE). Three or four biological and at least nine analytical replicates were used for each experiment. The differences among the variants were analyzed by the Student t test at the 5 % significance level.
Results
White light
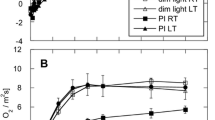
The DM plants grown at white light (WL) with light intensity of 130 μmol quanta m−2 s−1 differed in growth characteristics from WT plants independently on plant age, photoperiod, and light intensity, at which they were grown. Thus, the averaged wet weight of one leaf of 23-day-old plants grown at 8-h photoperiod was 2 ± 0.2 mg (WT) and 0.73 ± 0.07 mg (DM), whereas at 12-h photoperiod, the WT fresh weight was 8.2 ± 0.7 mg and 6.5 ± 0.6 mg for DM (Table 1). The leaves of DM and hy3 plants looked paler than those of the WT. The photosynthetic pigment content (chlorophylls and carotenoids) in DM was lower by 15–20 % in comparison to that in WT (Table 2). For the different samples, variations in the photosynthetic parameters were observed. For instance, the light intensity necessary for light saturation of photosynthesis in WT was approximately twofold higher than that in DM (Table 3). The value of photosynthesis rate at light saturation in DM was 64.5 % of WT. The light compensation point in DM was almost three times lower than that in WT, which is consistent with the significantly lower rate of CO2 evolution in the dark in DM. We also observed a significant difference in Y(II) between WT and DM, grown under the same conditions as it is shown in Table 3. The effective quantum yield Y(II) at high intensity of acting light (625 μmol quanta m−2 s−1) in WT and DM leaves was 0.29 ± 0.01 and 0.21 ± 0.017, respectively. On the other hand, no notable difference in the FV/FM ratios (Tables 1 and 4) and the shape of the OJIP transient (Fig. 1) between WT and DM grown in different photoperiods (8, 12, and 16 h) was revealed. There was also no noticeable difference in the quantum yield Y(II) at an acting light intensity of 190 μmol quanta m−2 s−1 (Table 1). We observed a tendency to an increasing ABS/RC ratio in DM compared to that in WT (Table 5) but the amount of QB-non-reducing centers of PSII was equal in WT and DM. The ratios of k p/k n, where k p is the rate constant of primary photochemical processes and k n is the rate constant of primary non-photochemical processes, and the values of thermal dissipation of absorbed light energy (DIO) per PSII RC (DIO/RC) were similar for WT and DM. There was also no significant difference between the parameters PIABS and PItotal calculated for the WT and DM samples.
In the second part of the work, the effect of UV-A radiation (2 h) on mutants (DM and hy3) and WT was studied. The decrease of maximum quantum yield in DM, Phy B mutant (hy3), and WT under the action of UV-radiation on plants grown at WL was 10.0, 10.0, and 8.6 %, respectively (Table 6). The results show that the PSII resistance of A. thaliana plants grown in WL to UV-A is significantly lower in DM and hy3 as compared to WT. Thus, there is significant contribution of phytochromes in the formation of resistance to UV-radiation.
Additionally, the effect of UV-radiation on the value Pn of WT, hy3 and DM was studied. It was revealed that there is a significant difference between DM and WT in resistance of photosynthesis to UV-A (Table 6). Under UV influence, the photosynthesis rate Pn decreased by 51 % in DM, 44 % in hy3, and only 24 % in WT.
Red light
In contrast to plants grown under WL, the growing of WT plants in red light (RL) at an intensity of 130 μmol quanta m−2 s−1 led to notable differences in the induction curves of DM compared to WL, which were related to a decrease of the photochemical activity of PS II and higher thermal dissipation as characterized by the aforementioned fluorescence parameters (Fig. 1 and Table 5). In WT, the amount of QB-non-reductive centers increased from 30 % in WL to 41% in RL, but in DM, this amount increased from 31% to 58% (Table 1). Probably, the increase of QB-non-reductive centers is connected with accumulation of functionally inactive PSII complexes due to the slowdown of protein D1 synthesis (Larocca et al. 1996).
In plants grown under RL, we observed a significant increase of the ABS/RC ratio and the amount of QB-non-reducing centers of PSII (Table 1) in DM compared to that in WT (Tables 1 and 5). Values of maximum and effective quantum yields of PSII and the ratios of k p/k n in DM leaves were reliably reduced compared to WT leaves (Tables 1, 4, and 5). Bigger difference was observed for the value of thermal dissipation of absorbed light energy (DIO) per PSII RC (DIO/RC). This value increased by 60 % in DM as compared to that in WT. There was also a significant difference between parameters reflecting the PSII performance—PIABS and the performance of the photosynthetic apparatus in whole—PItotal in WT and DM. Thus, PIABS in DM was five times smaller, and PI total was seven times smaller (Table 5). In addition, the content of photosynthetic pigments at RL was lower by 30–40 % on average in DM in comparison with those in WT, and UV-absorbing pigments were less four times in DM compared to those in WT (Table 2). The photosynthetic pigment content in WT at red light was lower by 20 % on average in comparison to that in WT grown in white light. For the growth parameters, even more significant differences were observed. The plants grown in RL showed the following weight of the upper tier leaves used for fluorescent measurements: 7.5 ± 1 mg in WT and 0.8 ± 0.2 mg in DM (Table 1). The big difference between WT and DM can be explained by the lack of activity of photoreceptors such as cryptochromes in plants grown at RL. When grown at low intensity RL of 40 μmol quanta m−2 s−1, the difference between WT and DM for FV/FM ratio was even larger (Table 4).
Electron microscopy data
The foregoing data is consistent with the results of electron microscopy obtained for thylakoid membranes from WT and DM of 23-day-old A. thaliana plants grown under WL and RL at an intensity of 40 μmol quanta m−2 s−1 (Fig. 2a, b, c, d) and 130 μmol quanta m−2 s−1 (data not shown). Organization of thylakoids of the chloroplast grana in A. thaliana grown in RL of 40 μmol quanta m−2 s−1 differed from that in plants grown in white light of the same intensity. According to the data for plants grown in WL, thylakoids in grana of WT are more densely packed and have a well-defined lumen. The thickness of one thylakoid is 20 nm, from which the thickness of the double membrane is 15 nm and the lumen size is 5 nm. The electron-dense contact strip between thylakoids is well expressed (indicated by arrows). Mutants grown in WL had thylakoid membranes, which are a bit lighter than those in WT, and the electron-dense contact strip between thylakoids is also well expressed (indicated by arrows). Also, there are electron-dense granules at the luminal volume, and small swelling of thylakoids occurs. Size characteristics correspond to wild type.
Organization of thylakoids in grana chloroplasts of A. thaliana WT (a, c) and DM (b, d) plants grown in white (a, b) and red (c, d) light. Electron micrographs are presented. All conditions are described in the section “Materials and methods” (see Semenova and Romanova 2011). Arrows show the electron-dense contact strip between thylakoids. Bars on micrograph are equal to 100 nm
The lumen in grana thylakoids of WT grown in RL is decreased, and the electron-dense contact strip of the neighboring thylakoids is enhanced (indicated by arrows). The thickness of one thylakoid was 18 nm, the thickness of the double membrane was equal to 15 nm, and the lumen size was 3 nm.
The maximum difference in the thylakoid membrane organization in grana was observed in DM grown in RL. The thylakoid system in grana looked rather chaotic. Individual thylakoids in grana were poorly visible due to the lack of electron-dense contact strips. The thickness of one thylakoid was 16–17 nm; the size of lumen was not more than 2 nm.
When growing the DM plants both in white and red light with higher intensity (130 μmol quanta m−2 s−1), the noticeable difference in the thylakoid membrane structure in WT and DM was not revealed.
Discussion
It is known that various types of Phy are involved in mechanisms of adaptation to environmental stress factors (Carvalho et al. 2011). Thus, PhyB can participate in plant adaptation to high and low temperatures, in protection of plants against high intensity light and UV-radiation (Thiele et al. 1999; Konstantinova et al. 2004; Foreman et al. 2011; Carvalho et al. 2011; Kreslavski et al. 2013a, b; 2016). UV-radiation damages various target molecules and systems of the PA, especially PSII, and most of all such components as QA, QB, PQ, and the D1 protein (Kolli et al. 1998; Babu et al. 1999). On the other hand, at moderate doses UV-radiation activates different protection systems. Thus, the activity or biosynthesis of antioxidant enzymes and accumulation of low molecular weight antioxidants and UV-absorbing photoprotective compounds increase with time (Strid et al. 1994; Häder et al. 2003; Solovchenko and Merzlyak 2008; Schmitt et al. 2014; Schmitt et al. 2015). The visible light of low intensity, primarily of the blue region of the spectrum (Han et al. 2001; Häder et al. 2003) and also red light (Joshi et al. 1991; Biswal et al. 2003) can play an important role in photoprotection of the PA against UV-radiation and/or photoreactivation processes.
There is little information about the Phy regulation of photosynthetic processes. Thus, no significant differences were found between non-transformed control and transformed potato plants (Solanum tuberosum L.), PhyB-superproducers Dara-5 and Dara-12, regarding the photosynthesis rate (Pn) and fluorescence parameters (FV/FM, Y(II), qN, and qP), whereas the transgenic plants had higher content of photosynthetic pigments per 1 cm2 of leaf area. The deficiency of PhyB had little effect on PSII activity, but it resulted in the decrease of the content of photosynthetic pigments (Kreslavski et al. 2016). The work of Rusaczonek et al. 2015 did not reveal the significant difference between WT and A. thaliana mutants deficient in both PhyA and PhyB in such parameters of the PA photochemical activity as FV/FM, Y(II), qN, and qP, but they found the decreased content of chlorophylls and carotenoids in mutants deficient in PhyB and the same content of these pigments in mutants deficient in both PhyA and PhyB.
It has been shown that the active form of PhyB is involved in the formation of the mechanism of PSII resistance to UV-radiation in A. thaliana and lettuce (Kreslavski et al. 2013a, b). The content of the active form of Phy was increased by pre-irradiation of the plants with RL, which resulted in the increase of the active form of PhyB. The short pre-irradiation with RL (10 min, 1–2 W m−2) in UV-irradiated plants (10 W m−2, 2 h) resulted in partial removing of the inhibitory effect of UV-radiation on the PA activity, in particular on PSII. In addition, the effect of such a protective effect of RL pre-irradiation found in WT, in its turn, was eliminated by a second pre-irradiation with FRL (RL-FRL). Such effects of RL/FRL reversibility are typical for PhyB-controlled processes and consistent with its involvement in this process (Casal et al. 1998). This indicates the important role of PhyB in the PSII resistance to UV-radiation. On the other hand, the interaction of PhyA and PhyB is observed in many photomorphogenetic processes (Casal 2000; Rusaczonek et al. 2015). Therefore, it was important to compare the influence of UV-radiation on the photosynthetic activity of mutants deficient in both PhyA and PhyB with the hy3 mutant and WT. The mutant that lacks apoprotein PhyB (hy3) is sufficiently studied genetically (Somers et al. 1991). It is indicated that hy3 has 20–30-fold lower amount of PhyB and 20–30-fold deficiency of PhyB transcripts compared to WT. In addition, the levels of proteins and transcripts for PhyC and PhyA were similar to those in WT. The DM mutant is less studied. The investigation of morphometric characteristics of this mutant revealed that there are no noticeable differences in fresh biomass between WT and DM leaves at WL, while it was obvious when growing plants in RL. This difference appears to be the consequence of higher photosynthetic activity in WT plants and respiration rate in comparison to DM (Table 3).
WT plants have all photoreceptors, while DM plants are deficient in PhyA and PhyB. However, there is no difference in the photochemical activity of PSII between WT and DM plants grown under white light when they were exposed to moderate acting light (190 μmol quanta m−2 s−1). These differences become evident under strong acting light. Here, both secondary dark processes of CO2 fixation and primary light processes are different in WT and DM. For instance, DM shows decreased photosynthesis under light saturation as compared to WT (Table 3), and the quantum yield Y(II) at high acting light intensity was also smaller in DM. On the other hand, we do not see a significant difference in activity of the PA between the WT and the hy3 mutant. Likely, reciprocal actions between PhyA and PhyB are responsible for the decrease of photosynthesis efficiency of the PA of the PhyAB mutant compared to the WT.
It is suggested that the red component of the spectrum in the visible region transforms Phy into its active form, which maintains the level of chlorophyll and other pigments as well as the structure and activity of chloroplasts during leaf senescence (Joshi et al. 1991; Lingakumar and Kulandaivelu 1993; Biswal et al. 2003). Indeed, in DM, that has a low level of active phytochrome form, while growing in RL with low light intensity, the thylakoid membrane structure is disrupted (Fig. 2d). It is likely connected with both decreased level of PhyA and PhyB, and inactive blue light photoreceptors. The reason of the disruption can be the reduced content of the chlorophyll and carotenoid pigments in the mutant grown under low intensity RL conditions (data not shown) compared to that in WT. Such small pigment content could explain disruption of the thylakoid membrane structure. It is assumed that the contact strip has a protein nature, according to the data of Semenova et al. (1977). Therefore, another reason of the randomized picture observed in the Fig. 2d can be the deficit of membrane proteins participating in stacking and formation of electron-dense contact strip between thylakoids due to deficit of photoreceptors.
On the contrary to that, DM grown in WL has a more expressed contact strip between thylakoids than DT. In this case, the synthesis of membrane proteins, especially antenna pigment-protein complexes of LHC2, is likely enhanced. It is suggested that there exists a link between the size of PSII light-harvesting antenna and the synthesis and content of antenna proteins (Kouril et al. 2013; Borisova-Mubarakshina et al. 2014). For example, such connection was observed when increasing the illumination intensity of A. thaliana, which leads to a decrease in content of almost all proteins of extrinsic antenna pigment-protein complexes of LHC2 and of the PSII antenna size (Kouril et al. 2013). Following this hypothesis, the mutant could demonstrate the increased size of PSII light-harvesting antenna. Indeed, according to our data, there is a tendency to increasing of ABS/RC ratio that reflects the size of antenna complexes of PSII (Stirbet and Govindjee 2011). However, for final decision, the added study is necessary.
Thus, the deficiency of PhyA and PhyB does not affect much the photochemical activity of PSII under conditions, when the blue light photoreceptors (cryptochromes) are active. However, the growing of the plants under RL, when cryptochromes are inactive, leads to some decrease of the photochemical activity of the PSII in WT and a significant decrease in DM. This is consistent with the fact of the decrease of the performance indexes IDO and IDtotal as well as FV/FM in DM compared to that in WT grown in RL, and the increasing of the rate of thermal dissipation of absorbed energy.
We suggest that one of the reasons of decreasing PSII activity characterized by parameters such as photochemical maximum, effective quantum yields, and performance index PIABS (Tables 1, 4, and 5) as well as activity of PA in whole characterized by PItotal in plants grown under RL is explained by the increase of the amount of PSII active QB—non-reducing centers and/or the enhancement of thermal dissipation processes of energy absorbed in PSII.
Reduced resistance of PA of DM to UV-A indicated in Table 6 can be due to a decreased amount of carotenoids and UV-absorbing pigments as found for DM as compared to that in WT samples.
Conclusion
We demonstrated a strong decrease in the PA activity in DM plants grown under RL as compared to that in WT under same conditions, and the large quantity of this effect does not depend on the photoperiod and plant age. Consequently, for normal functioning of the PA, both cryptochromes and two key phytochromes—PhyA and PhyB are required. The notable decrease of the content of photosynthetic pigments in the DM when growing in RL results in changes of the thylakoid membrane structure and a consequent decrease of the photochemical activity. Markedly, these tendencies with a full disruption of the thylakoid membrane appear when plants are grown in RL with low intensity.
According to the obtained data, it is suggested that for functioning of PA under normal conditions the content of PhyA and PhyB is not crucial, whereas under the oxidative stress induced by UV-radiation or high intensity light, the presence of PhyA and PhyB can be crucial for the PA resistance, especially for PA of plants grown under RL when blue-light photoreceptors are inactive.
Abbreviations
- Chl:
-
Chlorophyll
- Phy:
-
Phytochrome
- RL:
-
Red light
- FRL:
-
Far-red light
- PSII:
-
Photosystem II
- PA:
-
Photosynthetic apparatus
- WT:
-
Wild type
- DM:
-
phytochrome A, phytochrome B mutant
References
Babu TS, Jansen MAK, Greenberg BM, Gaba V, Malkin S, Mattoo AK, Edelman M (1999) Amplified degradation of photosystem II D1 and D2 proteins under a mixture of photosynthetically active radiation and UV-B radiation: dependence on redox status of photosystem II. Photochem Photobiol 69:553–559
Biswal UC, Biswal B, Raval MK (2003) Chloroplast biogenesis. From propastid to gerontoplast. Kluwer Academic Publishers, Dordrecht
Boccalandro HE, Rugnone ML, Moreno JE, Ploschuk EL, Serna L, Yanovsky MJ, Casal JJ (2009) Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol 150:1083–1092
Borisova-Mubarakshina MM, Vetoshkina DV, Rudenko NN, Shirshikova GN, Fedorchuk TP, Naydov IA, Ivanov BN (2014) The size of the light-harvesting antenna of higher plant photosystem II Is regulated by illumination intensity through transcription of antenna protein genes. Biochem Mosc 79:520–523
Carvalho RF, Campos ML, Azevedo RA (2011) The role of phytochrome in stress tolerance. J Integr Plant Biol 53:920–929
Casal JJ (2000) Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 71:1–11
Casal JJ, Sánchez RA, Botto JF (1998) Modes of action of phytochromes. J Exp Bot 49:127–138
Foreman J, Johansson H, Hornitschek P, Josse EM, Fankhauser C, Halliday KJ (2011) Light receptor action is critical for maintaining plant biomass at warm ambient temperatures. Plant J 65:441–452
Franklin KA (2008) Shade avoidance. New Phytol 179:901–1201
Gururani MA, Venkatesh J, Ganesan M, Strasser RJ, Han Y, Kim JI, Lee HY, Song PS (2015) In vivo assessment of cold tolerance through chlorophyll-a fluorescence in transgenic zoysiagrass expressing mutant phytochrome A. PLoS One 10(5):e0127200
Häder DP, Kumar HD, Smith RC, Worrest RC (2003) Aquatic ecosystems: effects of solar ultraviolet radiation and interactions with other climatic change factors. Photochem Photobiol Sci 2:39–50
Han T, Sinha RP, Häder DP (2001) UV-A/blue light-induced reactivation of photosynthesis in UV-B irradiated cyanobacterium, Anabaena sp. J Plant Physiol 158:1403–1413
Hu W, Franklin KA, Sharrock RA, Jones MA, Harmer SL, Lagarias JC (2013) Unanticipated regulatory roles for Arabidopsis phytochromes revealed by null mutant analysis. Proc Natl Acad Sci U S A 110:1542–1547
Joshi PN, Biswal B, Biswal VC (1991) Effect of UV-A on aging of wheat leaves and role of phytochrome. Environ Exp Bot 31:267–276
Kalaji HM, Golstev V, Bosa K, Allakhverdiev SI, Strasser RJ, Govindjee (2012) Experimental in vivo measurements of light emission in plants: a perspective dedicated to David Walker. Photosynth Res 114:69–96
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI, Brestic M, Bussotti F, Calatayud A, Dąbrowski P, Elsheery NI, Ferroni L, Guidi L, Hogewoning SW, Jajoo A, Misra AN, Nebauer SG, Pancaldi S, Penella C, DorothyBelle P, Pollastrini M, Romanowska-Duda ZB, Rutkowska B, Serodio J, Suresh K, Szulc W, Tambussi E, Yanniccari M, Zivcak M (2014a) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122:121–158
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska I, Cetner MD, Goltsev V, Ladle RJ, Dąbrowski P, Ahmad P (2014b) The use of chlorophyll fluorescence kinetics analysis to study the performance of photosynthetic machinery in plants. In: Ahmad P (ed.) Emerging Technologies and Management of Crop Stress Tolerance (Elsevier), Vol. II, 347 – 384
Klinkovsky T, Naus J (1994) Sensitivity of the relative Fpl level of chlorophyll fluorescence induction in leaves to the heat stress. Photosynth Res 39:201–204
Kolli BK, Tiwari S, Mohanty P (1998) Ultraviolet-B induced damage to photosystem II in intact filaments of Spirulina platensis. Z Naturforsch 53:369–377
Konstantinova TN, Aksenova NP, Gukasyan IA, Golyanovskaya SA, Romanov GA (2004) An Improved tolerance of PHYB_transgenic potato plants to the middle_wave ultraviolet irradiation. Dokl Biol Sci 395:130–132
Kouril R, Wientjes E, Bultema JB, Croce R, Boekema E (2013) High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta 1827:411–419
Kreslavski VD, Shirshikova GN, Lyubimov VY, Shmarev AN, Boutanaev AM, Kosobryukhov AA, Schmitt FJ, Friedrich T, Allakhverdiev SI (2013a) Effect of preillumination with red light on photosynthetic parameters and oxidant-/antioxidant balance in Arabidopsis thaliana in response to UV-A. J Photochem Photobiol B Biol 127:229–236
Kreslavski VD, Lyubimov VY, Shirshikova GN, Shmarev AN, Kosobryukhov AA, Schmitt FJ, Friedrich T, Allakhverdiev SI (2013b) Preillumination of lettuce seedlings with red light enhances the resistance of photosynthetic apparatus to UV-A. J Photochem Photobiol B Biol 122:1–6
Kreslavski VD, Kosobryukhov AA, Shmarev AN, Aksenova NP, Konstantinova TN, Golyanovskaya SA, Romanov GA (2015) Introduction of the Arabidopsis PHYB gene increases resistance of photosynthetic apparatus in transgenic Solanum tuberosum plants to UV-B radiation. Russ J Plant Physiol 62:204–209
Kreslavski VD, Schmitt FJ, Keuer C, Friedrich T, Shirshikova GN, Zharmukhamedov SK, Kosobryukhov AA, Allakhverdiev SI (2016) Response of the photosynthetic apparatus to UV-A and red light in the phytochrome B deficient Arabidopsis thaliana L. hy3 mutant. Photosynthetica 54(3):321–330
Lankin AV, Kreslavski VD, Khudyakova AY, Zharmukhamedov SK, Allakhverdiev SI (2014) Effect of naphthalene on photosystem 2 photochemical activity of pea plants. Biochem Mosc 79:1216–1225
Larocca N, Barbato R, Casadoro G, Rascio N (1996) Eearly degradation of photosynthetic membranes in carob and sunflower cotyledons. Physiologia Plant 96:513–518
Lichtenthaler HK, Wellburn AR (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lingakumar K, Kulandaivelu G (1993) Regulatory role of phytochrome on ultraviolet-B (280–315 nm) induced changes in growth and photosynthetic activities of Vigna sinensis L. Photosynthetica 29:341–351
Martirosyan YT, Polyakova MN, Dilovarova TA, Kosobryukhov AA (2013) Photosynthesis and productivity of potato plants in the conditions of different spectral irradiation. Agric Biol №1:107–112
Mirecki RM, Teramura AH (1984) Effect of ultraviolet B irradiance on soybean. V. The dependence of plant sensitivity on photosynthesis flux density during and after leaf expansion. Plant Physiol 74:475–480
Priol JL, Chartier P (1977) Partitioning of transfer and carboxilation components of intracellular resistance to photosynthetic CO2 fixation: a critical analysis of the methods used. Ann Bot 41:789–800
Rusaczonek A, Czarnocka W, Kacprzak S, Witoń D, Ślesak I, Szechyńska-Hebda M, Gawroński P, Karpiński S (2015) Role of phytochromes A and B in the regulation of cell death and acclimatory responses to UV stress in Arabidopsis thaliana. J Exp Bot 66:6679–6695
Schmitt F-J, Renger G, Friedrich T, Kreslavski VD, Zharmukhamedov SK, Los DA, Kuznetsov VV, Allakhverdiev SI (2014) Reevaluation of reactive oxygen species: monitoring, generation and role in stress signaling of phototrophic organisms. Biochim Biophys Acta 1837:835–848
Schmitt F-J, Kreslavski VD, Zharmukhamedov SK, Friedrich T, Renger G, Los DA, Kuznetsov VV, Allakhverdiev SI (2015) The multiple roles of various reactive oxygen species (ROS) in photosynthetic organisms. In: Allakhverdiev S.I. (ed.) Photosynthesis: New Approaches to the Molecular, Cellular, and Organismal Levels (Scrivener Publishing LLC), Willey, 4–82
Semenova GA, Romanova AK (2011) Crystals in sugar beet (Beta vulgaris L.) leaves. Cell and Tissue Biol 5:74–80
Semenova GA, Ladygin VG, Tageeva SV (1977) Ultrastructural organization of membrane system of Chlamydomonas reinhardtii mutants chloroplasts with inactive photosystems. Russian J Plant Physiol 24:18–22
Solovchenko AE, Merzlyak MN (2008) Screening of visible and UV radiation as a photoprotective mechanism in plants. Russian J Plant Physiol 55:719–737
Somers DE, Sharrock RA, James M, Teppermaq JM, Quail PH (1991) The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell 3:1263–1274
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B Biol 104:36–57
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor and Francis, London, pp 445–483
Strasser B, Sánchez-Lamas M, Yanovsky MJ, Casal JJ, Cerdán PD (2010) Arabidopsis thaliana life without phytochromes. Proc Natl Acad Sci U S A 107:4776–4781
Strid AW, Chow S, Anderson JM (1994) UV-B damage and protection at the molecular level in plants. Photosynth Res 39:475–489
Thiele A, Herold M, Lenk I, Quail PH, Gatz C (1999) Heterologous expression of Arabidopsis phytochrome B in transgenic potato influences photosynthetic performance and tuber development. Plant Physiol 120:73–81
Zhao J, Zhou JJ, Wang YY, Gu JW, Xie XZ (2013) Positive regulation of phytochrome B on chlorophyll biosynthesis and chloroplast development in rice. Rice Sci 20:243–248
Živčák M, Brestič M, Olšovská K, Slamka P (2008) Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ 54:133–139
Acknowledgments
This work was supported by grants from the Russian Foundation for Basic Research (Nos: 15-04-01199 and 14-04-01549), and by the Molecular and Cell Biology Programs from the Russian Academy of Sciences. The authors acknowledge support by the German Research Foundation DFG (cluster of excellence “Unifying Concepts in Catalysis”) and the COST MP1205 framework.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Jaideep Mathur
Rights and permissions
About this article
Cite this article
Kreslavski, V.D., Kosobryukhov, A.A., Schmitt, FJ. et al. Photochemical activity and the structure of chloroplasts in Arabidopsis thaliana L. mutants deficient in phytochrome A and B. Protoplasma 254, 1283–1293 (2017). https://doi.org/10.1007/s00709-016-1020-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1020-9