Abstract
Rice blast disease is one of the most disastrous diseases causing significant losses to the crop. In the humid weather conditions of north-eastern Himalayan region, the situation is highly devastating as the climate is very favorable to the fungus Magnaporthe oryzae. Development of resistant rice varieties is the most effective, economical, and environment-friendly way to control this disease. The study aimed to identify novel sources of resistant donor using agro-morphological and gene-based markers for their utilization in development of blast-resistant varieties with high yield potential. Phenotypic evaluation has classified the hundred landraces into resistant (13), moderately resistant (43), moderately susceptible (24), and susceptible (20). Fifty-nine genotypes were found to carry genes responsible for blast resistance, either singly or in combination. The genotype MN-62 was found to have a blast score of zero in field screening. The genetic frequencies of the major blast resistance genes ranged from 28 to 97%. The cluster analysis grouped the landraces into three major groups. Additionally, the association between blast genes was computed with blast score using a general linear model (GLM) and mixed linear model (MLM), revealing that the markers AP4007, AP56595, C1454, and RM208 were found to be associated with the trait. The landraces with six blast R-genes like Nungshang Phou, Ratkhara, Anandhi, RCPL 1–44, Ching Phou Khong Nombi, MN-31, Rona Yang, and RCPL-128 were also superior for agro-morphological traits. Similarly, the landraces MN-62, Tara Bali, Rato Bhan Joha, MN-47, RCPL 1–411, and CAU-R1 were promising regarding yield and blast resistance. The resistant lines with various combinations of blast resistance genes can be utilized for introgression of genes into the commercial varieties for durable resistance using marker-assisted backcrossing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Northeast India constituted one of the richest reservoirs for genetic resources in agri-horticultural crops. Biotic as well as abiotic stresses often led to low productivity of rice in this region (Das et al. 2011). Of the various biotic factors limiting the rice production system and productivity, blast disease continue to be an enigmatic problem in all rice ecosystems causing crop losses or yield gap especially in upland rice ecosystem in the humid rice-growing areas of India (Annegowda et al. 2021). The region is reported to be endemic to rice blast causing yield loss ranging from 40 to 46% (Ngachan et al. 2011). The evolution of new pests and diseases has caused a yield reduction in different crops that have been investigated over time (Evans 1993; Tonnang et al. 2022), and this decline is due to the devaluation of germplasm due to newly evolving strains of pathogens. More than 5% of yield loss is consistently caused by more than 70 diseases caused by various biotic factors, namely, fungi, bacteria, microbes, and nematodes (Namrata et al. 2019). Blast disease, due to its wide distribution and ability to survive in various environmental conditions, is the most detrimental biotic stress, severely affecting rice productivity. Magnaporthe oryzae, a non-obligate filamentous ascomycete, the culprit behind rice blast, is causing significant yield losses that range from 10 to 30% every year (Sakulkoo et al. 2018). Landraces, chosen by farmers, possess genes for local adaptation and thus exhibit equilibrium with the environment and pathogens and are genetically dynamic (Harlan 1975). These lines, while being less productive, are known to carry sizeable genetic variance for several biotic stresses and, thus, could be effectively utilized for rice improvement (Hanamaratti et al. 2008). The landraces from Northeast (NE) India, being very diverse, are anticipated to exhibit significant genetic variability for various biotic stresses, including the rice blast. Blast resistance has not yet been characterized systematically for NE Indian rice landraces. Due to the continual evolution of novel pathogenic variants by this genetically diverse pathogen constantly changing, many blast-resistance genes need to be introgressed. For this, the various rice landraces native to this particular region may serve as a valuable source for identifying and mining novel blast R-genes, as numerous lines show complete and/or partial blast resistance.
Through map-based cloning, 22 blast resistance genes have been isolated, including Pib, Pita, Pi54 (Pikh), Pi9, Pid2, Pi2, Piz-t, Pi36, Pi37, Pik-m, Pi5, Pid3, pi21, Pit, Pb1, Pish, and Pik (Rama Devi et al. 2015). To date, more than 125 blast resistance genes have been discovered in Japonica (45%), Indica (51%), and other genotypes (4%) (Shikari et al. 2014). In the current study, phenotyping was carried out for blast resistance vis-a-vis yielding ability of landraces. Gene profiling and phenotyping for the major blast genes have led to the conclusion that the many genotypes had a considerable source of resistance genes (Hosahatti et al. 2023). To strengthen and develop a goal-oriented breeding program on blast resistance, marker–trait association for blast resistance was assessed in 100 local germplasm. As more than 30 of the approximately 100 blast resistance genes have already been cloned and analyzed at the sequencing level (Sharma et al. 2012; Wang et al. 2022), the information could be harnessed by using gene-based markers for the selection of lines having resistant genes. Blast resistance genes like Pi9 (Wang et al. 2013) and Pi54 (Sharma et al. 2005) have a broad resistance spectrum, and others, like Pita in the US, have given immunity against the dominant racial groups in particular places. Diverse genotypes with various combinations of blast genes with a resistant reaction in the field may offer a wide scope in a breeding program to evolve multiple disease-resistant varieties combined with high yield potential. Hence, the current study sought to explore the presence of genes governing blast resistance in various germplasm of India’s north-eastern hill region using gene-based/linked microsatellite markers and assess the effect of various gene combinations on blast resistance vis-a-vis to evaluate rice germplasm for their yield potential. The identified germplasm with superior gene combinations will play a pivotal role towards development of varieties with higher yield and blast resistance.
Materials and Methods
Experimental Field and Climatic Description
The study was conducted at Plant Breeding Farm, ICAR-RCNEH Umiam, Meghalaya, situated at a latitude of 25° 41′ 8.13″ north and longitude of 91° 54′ 59.082″ east and an altitude of 965 m above mean sea level. The climatic condition of the region as a whole is sub-tropical humid, having warm summer and cold winter. The mean temperature (19.19 °C), average rainfall (383.57 mm), and relative humidity (85.29%) during the entire crop growth period, i.e., June to December 2021 and 2022, were observed. The average number of rainy days was 15.43 days per month, whereas the average sunshine hours were 5.75 during the entire crop growth period. The soils of the experimental field were acidic, with a pH of 5 to 5.3 and very little availability of nutrients (Supplementary Table 1).
Plant Materials and Experimental Design
A set of 100 landraces, collected from the north-eastern states of India, along with five checks (Shahsarang, MTU-1010, Swarna, Shahbhagi Dhan, and Sakurd Dhan) were used for the present study. Blast susceptible lines were also sown at regular intervals as a source of inoculum for blast disease. The landraces were planted in an augmented design with 4 blocks and 5 checks in upland field conditions during kharif 2021 and 2022 to ensure a sufficient degree of freedom. The landraces were screened morphologically for leaf blast using the “leaf blast score scale” from 0 to 9 (Singh et al. 2013).
Culture Preparation and Inoculation
The blast fungus was cultured under sterile lab conditions for artificial inoculation. Spores were harvested by flooding the plates with sterile distilled water and scrapping the growth with a spatula. The spore suspension was adjusted to the desired concentration of 1 × 105 spores/ml with the help of a hemocytometer, and a drop of a surfactant (Tween 20) was added to ensure the uniform dispersal of spores. The suspension was used for field inoculations. Thirty-day-old seedlings were inoculated by spraying the inoculum (1 × 105 spores/ml) on the foliage using a Knapsack power sprayer in the evening.
Field Screening
The landraces were screened morphologically for blast lesions in upland conditions. The screening for the blast was performed at an interval of 10–15 days, starting from seedling till the maturity stage. Scoring for leaf blast disease was done under natural field conditions (upland) from 0 to 9 using the leaf blast score scale. The range from 0 to 3 was considered resistant, and 7–9 fell under susceptible type, while 4–6 varied from moderately resistant to moderately susceptible (IRRI 2013; Singh et al. 2013). Susceptible genotypes, namely, Manipur, Pnah long, Rukezie, and Ranga bora, identified in previous studies, were planted at successive intervals for evaluating the genotypes and spreading the inoculum. Further, the percent disease index was calculated from the scoring data. Percent disease index was calculated based on scores as follows (Villareal and Lantican 1965)
Agro-morphological Characterization of Landraces
The landraces were grown in the augmented block with a spacing of 20 cm between each row and 15 cm between plants. Four rows for each landrace were sown, and the data were recorded for five randomly selected plants on various traits like days to 50% flowering, days to 80% maturity, plant height (cm), number of effective tillers per plant, panicle length (cm), total number of spikelets per panicle, number of filled grains per panicle, number of chaffy grains per panicle, spikelet fertility percentage (%), biological yield (g), test weight (g), harvest index (%), yield per plant (g), and yield per ha (kg/ha) (Fig. 2).
Genomic DNA Isolation
The leaves were collected from plants of each genotype from the field at a very early stage of their growth (30–35 days after planting). The leaves were collected in aluminum foils, immediately kept in the icebox, and stored at − 20 °C until further use. Plant DNA was extracted using the CTAB (cetyltrimethylammonium bromide) method (Doyle and Doyle 1987). DNA sample concentration was determined using a Nanodrop 2000 (Thermo Fisher Scientific) followed by 0.8% ethidium bromide-stained agarose gel electrophoresis.
Gene Profiling
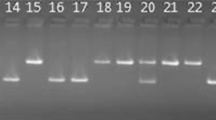
PCR analysis was done for landraces and checks to identify the presence of seven major known blast resistance genes, namely, Pi54, Pib, Pi2, Pi5, Pita2, Pi1, and Pi9, using previously reported candidate/gene-based markers associated with these major blast R-genes (Supplementary Table 2). These genes were located on chromosomes 2 (Pib), 6 (Pi2), 9 (Pi5 (t)), 11 (Pi54, Pi1), and 12 (Pita2). Ten markers linked to these genes, namely, AP4007, AP56595, C1454, Pi54MAS, PIBDOM, RM208, RM224, YL153/154, YL155/87, and MSM1, were used for studying the marker–trait association. Scoring was done based on the resolution of bands in 3.5% agarose gel. The PCR products were analyzed in gel electrophoresis using 100 bp DNA ladder, and the gels containing DNA bands were visualized in the Bio-Rad Gel Documentation System using Quantity One software. The presence of these resistance genes was detected among the landraces and checks studied by comparing the marker allele data with already reported allele size for resistant reaction.
Statistical Analysis
Morphological Traits
The data was recorded for 100 genotypes and five checks for agro-morphological traits for 2 years during kharif 2021 and 2022. The data was averaged and analyzed as per the design for working out different genetic variability parameters, ANOVA (augmentedRCBD package) and correlation (corr package) using R 4.2.1 software.
Marker–Trait Association Using SSR Markers
The association tests between the marker and the traits in the population were run based on two models, general linear model (GLM) and mixed linear model (MLM), using TASSEL 5.0 software. The MLM was performed using the Kinship file generated from genotypic data and the Q matrix generated by running population structure at optimized K value to minimize the false positive associations (Yu et al. 2006; Gupta et al. 2014). The significant marker–trait association was determined by P < 0.05 and the magnitude of the QTL effects by marker R2.
Results
Agro-morphological Characterization
Identification of candidate germplasm based on yield potential vis-a-vis resistance to blast disease is of paramount importance. Their deployment as valuable donors in the breeding of blast-resistant rice varieties and new sources for novel blast-resistant genes can augment the productivity of rice in the NEH region of India. The selected genotypes and five checks (Shahsarang, MTU-1010, Swarna, Shahbhagi Dhan, and Sakurd Dhan) were characterized for various yield attributing traits and the genetic variability parameter, viz., mean, standard error, minimum, maximum, coefficient of variation, phenotypic coefficient of variation (PCV), genotypic coefficient of variation (GCV), heritability (broad sense), and genetic advance as percent of mean for all the 14 characters studied, are furnished in Table 1. This study helps us to understand whether the nature of variability in the genotype is genetic or environmental and helps to determine the heritable nature of those traits. The yield per plant varied significantly from 0.27 to 17.18 g, with a mean of 4.78 g (Fig. 1). The lowest yield per plant was recorded for the genotype Kunta Mah (0.27 g), followed by Machang Kaoyeing (0.47 g) and Shamphai (0.47 g), and the highest yield per plant was recorded for the genotype CAU R1 (17.18 g) followed by Shahsarang (16.3 g) and Phehsa (15.09 g). The values for genotypic (55.86) and phenotypic (72.24) coefficient of variation were observed to be high for this trait coupled with moderate heritability estimates of 59.81% and high genetic advance as a percent of the mean (89.13). As far as spikelet fertility percentage is concerned, the low genotypic (8.81) and high phenotypic (31.50) coefficient of variation was observed for this trait coupled with low heritability estimates of 7.82% and low genetic advance as percent of mean (5.06). Traits, namely, plant height (24.43, 25.02), total number of spikelets per panicle (32.69, 36.07), number of filled grains per panicle (31.07, 42.20), test weight (29.10, 31.02), and harvest index (31.05, 48.55), have shown higher value for the genotypic and phenotypic coefficient of variation. Likewise, panicle length (80.76), total number of spikelets per panicle (82.13), and test weight (88.01) have high heritability values > 80%. As far as genetic advance over mean is concerned, plant height (49.21), panicle length (21.18), total number of spikelets per panicle (61.11), number of filled grains per panicle (47.20), test weight (56.32), and harvest index (40.97) were found to have higher values. It can be inferred that these traits were having a low environmental impact on the expression of these traits, and selection would be fairly helpful for plant breeders to make effective and easy selection.
A violin and box plot for agro-morphological traits in rice germplasm. DFF = days to 50% flowering, PH = plant height (cm), NET = number of effective tillers per plant, PL = panicle length (cm), TSP = total number of spikelets per panicle, FGP = number of filled grains per panicle, YPP = yield per plant (g), TW = test weight (g), and HI = harvest index (%)
Analysis for Variance for Agronomic Traits
Analysis of variance was done using augmentedRCBD package in R 4.2.1. The data recorded for the yield and yield attributing traits was subjected to analysis of variance for augmented randomized complete block design to test the significant difference among the genotypes for all the traits. Significance at P < 0.05 and P < 0.01 was considered for assessing the significance of variability. ANOVA for all traits studied revealed that there was a presence of significant and considerable variation among landraces except for the traits like the number of effective tillers and spikelet fertility (Table 2).
Association Among Agro-morphological Traits
Pearson correlation was calculated among different traits to assess the nature of association among the yield and its component traits. This would give predictive information for selecting elite genotypes from diverse genetic populations to enhance overall productivity. The correlation estimates are presented in Table 3. Correlation analysis revealed that the yield per plant was significantly correlated with various yield-related traits. Positive and highly significant correlations with the harvest index (0.748) followed by number of filled grain per panicle (0.729), biological yield (0.679), total number of spikelets per panicle (0.488), panicle length (0.413), test weight (0.409), and the number of effective tillers per plant (0.343) were observed. Other traits, such as plant height and number of chaffy grain per panicle, showed a non-significant correlation whereas plant height was positively correlated (0.093).
Leaf Blast Phenotyping
Scoring was done on a scale of 0–9 (Fig. 2). Readings for leaf/neck blast were taken at five different times during the crop growth period. The first reading was taken 30 days after sowing, followed by 45, 60, 75, and 90 days after sowing. The average score of the five readings was taken for the analysis. Several genotypes showed a susceptible reaction in the early stage but gradually overcame the pathogen attack and showed a moderately resistant response, while several others were not attacked by the pathogen in the early stage but later developed the disease symptoms and showed a susceptible reaction. A 0–9 leaf blast score scale standardized by IRRI was used to classify the landraces based on the level of resistance. The mean score of resistant genotypes ranged from 0 to 3, while for susceptible, it ranged from 7 to 9. It was evident that out of 100 genotypes screened, only one landrace showed high resistance with a disease score of 0, whereas 10 (resistant) with a disease score of 1, 48 (moderately resistant) with a disease score of 2–3, 28 (moderately susceptible) with disease score of 4–6, 11 (susceptible) with disease score of 7, and 4 (highly susceptible) with disease score of 8–9 were observed. Among the checks, MTU-1010 was resistant with a disease score of 1, while Shahsarang, Swarna, Shahbhagi Dhan, and Sakurd Dhan were moderately resistant with a disease score of 2–3 (Table 4). The results obtained from the disease score and disease index showed that most of the genotypes were moderately resistant (Table 5, Fig. 3).
Genetic Profiling with Gene-Based Marker
PIBDOM, YL153/154, and YL155/87 are dominant markers and scored for the presence or absence of allele. In contrast, the other seven markers are co-dominant markers and scored according to the already reported allele size for resistant reaction. The list of markers, along with their association with blast genes and resistant allele information, is provided in Table 6. All the genotypes, along with checks, were screened for the presence of a resistance gene with these ten-blast gene-based markers, and it was found that they had at least one blast resistance gene. Gel electrophoresis pattern of genotypes for blast gene based markers is represented in Fig. 4. The gene profiling of landraces revealed that the Pib gene was found in all the landraces studied, followed by Pita2 gene in 72 (72%) landraces, while the Pi54 and Pi1 genes were present in 28 (28%) and 29 (29%) landraces, respectively. Out of the total, six lines, namely, MN-36, Ratkhara, Ching Phou Khong Nembi, MN-68, RCPL 1–110, and RCPL 1–44, showed amplification for the maximum number of gene-based markers, i.e., 7 out of 10 gene-based markers followed by 13 landraces, namely, MN-56, Makhara, Naga Phou, Sijali, Nungshang Phou, Anandhi, RCPL 1–103, MN-31, Chamra Phou, Rona Yang, RCPL 1–127, KMP-34, and RCPL-128 with amplification for six gene-based markers. The genotypes Niphuthokpi, Ramphiatama, and Shahsarang showed amplification for only one gene-based marker (Table 7).
The genotypes with three R-genes showed a low blast score and percent disease index compared to those with two R-genes and a single R-gene. The landraces with Pi54 gene in all three/four/five gene combinations were found to be more effective than the other combinations (Table 8).
Similarly, the genotypes with four R-genes showed a low blast score and percent disease index compared to those with three R-genes. The genotypes such as Naga Phou, Ching Phou Khong Nembi, Nungshung Phou, and Ratkhara were found to be more frequent in the four R-gene combinations (Table 9).
One hundred landraces were used to find the association between the marker and trait studied using the general linear model (GLM) and mixed linear model (MLM) through TASSEL 5.0 software based on the P value for each marker. Four marker–trait associations with a significant P value of less than 0.05 were observed. In the general linear model (GLM), one marker AP56595 present on chromosome 6 showed an association with leaf blast with a significant P value of 0.03, and at this location, blast gene Pi2 was reported. Another marker, C1454 present on chromosome 9 gene, showed an association with leaf blast with a significant P value of 0.025, and at this location, blast gene Pi5 was reported. In the mixed linear model (MLM), one marker, namely, AP4007, present on chromosome 6 showed an association with leaf blast with a significant P value of 0.000372, and at this location, blast gene Pi2 was reported. RM208 linked to the Pi2 gene was also associated with the blast resistance in the current set of genotypes (Table 10.). Allelic variation among rice genotypes based on gene-based markers is shown in Fig. 4.
Clustering of Genotypes Based on Blast Gene-Based/Linked Markers
The rice genotypes were further grouped into three major clusters based on blast gene-based/linked markers (Fig. 5). Cluster I had the maximum number of genotypes (54 genotypes), representing 52% of the population, followed by cluster III (28 genotypes), which represents 27% of the total population and cluster II with 23 genotypes representing only 22% of the total population. The majority of genotypes in cluster III were found to possess five resistant genes with Pita2 and Pib as common genes, whereas cluster II had genotypes with four genes with Pi2 as a common gene.
Discussion
The ability of Magnaporthe species to evolve into new pathotypes within a short period has resulted into considerable variability in the M. oryzae population of India (Prasad et al. 2011). Thus, the biggest challenge for rice breeders is the breakdown of resistance in existing rice varieties over the years. Although several control measures of blast disease are employed at the field level, the most efficient and cost-effective method for managing blast disease has been the development of resistant cultivars. However, the distribution of major blast-resistant genes has yet to be extensively investigated in north-eastern landraces. In the study, a set of 100 genotypes were evaluated, and it was observed that the majority of the genotypes were moderately resistant at the field level, with a blast score of 2 to 3 and a percent disease index of 20–40% (Fig. 2). The same result has also been observed by ManojKumar et al. (2020) after a screening of 82 genotypes, with a score of 2 to 3 and moderate resistance reaction. In our study, genotypes that showed resistance had four R-genes, as observed by Khan et al. (2014). The gene profiling for seven major blast resistance genes (Pi1, Pi2, Pi5, Pi54, Pib, Pi9, and Pita2) with gene-based markers identified landraces possessing different R-genes singly or in combination with other R-genes. The results were found to be similar to the findings of Umakanth et al. (2017). Screening of landraces with candidate/gene-based markers has revealed that they had one to several blast resistance genes, which indicates the richness of diversity for genes associated with blast resistance in the NEH region of India. Similar results were found by Mahendra et al. (2012) by characterizing various accessions of Manipur.
As far as distribution of blast genes is concerned, forty landraces (40%) with Pi2, twenty-four landraces (24%) with Pi54 gene, twenty-nine landraces (29%) with Pi1 gene, thirty-nine landraces (39%) with Pi9 gene, and seventy-six genotypes (76%) with Pita2 gene were identified. Yadav et al. (2017) found similar results by screening 80 natural rice varieties (NRVs), where Pi2 gene was present in all the NRVs, Pi54 gene in 56 NRVs (70%), Pi1 gene in 20 (25%), Pi9 gene in 15 (18.75%), and Pita2 gene was detected in 26 NRVs (32.50%). In another study, 60 landraces harbored Pi2 gene with a genetic frequency of 72.28%, whereas in 39 landraces, Pi1 gene was detected with a genetic frequency of 46.98% (Ingole et al. 2014). Pi54 gene, positioned on chromosome 11, was originally isolated from the indica variety Tetep (Sharma et al. 2005). In a study by Singh et al. (2015), Pi54 gene was detected in 52 accessions out of 192 rice germplasm accessions. A similar study by Shikari et al. (2013) showed 27% genetic frequency of Pita 2 gene in indica rice varieties.
In the current set of landraces, the blast-resistant gene Pib was the most abundant blast-resistant gene found to be widely distributed in all the landraces followed by Pita2 gene (76%), Pi2 gene (40%), Pi5 (52%), Pi1 (29%), and Pi54 gene (24%). The high frequency of Pib gene detected in the present study is consistent with Anupam et al. (2017) on screening 74 rice germplasm lines including landraces from Tripura for blast-linked markers. Similar results were found by Singh et al. (2015) by screening 192 rice accessions where the dominant gene-based marker YL155/YL87 tightly linked to the resistant gene Pita2 was present in 11 (34%) out of the 32 accessions with positive bands of 1042 bp for this gene. The result agreed with the current study in which 72 of the 100 landraces showed amplification for the marker YL155/YL87 linked to Pita2 gene. The marker AP56595 has been found to share 279 bp resistance allele sizes for the germplasm carrying the Pi9 gene with frequencies of 39%. The results were in accordance with Shikhari et al. (2014) by screening the genotypes for Pi9 gene. The marker Pi54MAS has been found to share a fragment size of 216 bp for the resistant allele in the germplasm carrying the Pi54 gene with a frequency of 24%. Similar results were found by Manojkumar et al. (2020) where ten released varieties and advanced breeding materials and 13 traditional rice varieties were found positive for Pi54MAS marker with a fragment size of 216 bp for the resistant allele. The Pib gene located on chromosome 2 appeared omnipresent and was detected in all the genotypes studied. Mahendar et al. (2012) and Yadav et al. (2017) also found similar results concluding that Pib gene was present in all the genotypes studied. The SSR marker RM208 shared a fragment size of 170 bp in 26 genotypes carrying Pib gene while the dominant marker PIBDOM showed positive bands at 365 bp in 97 genotypes carrying Pib gene. The result was also similar to the findings of Roychowdhury et al. (2012) and Singh et al. (2015) wherein 75 out of 192 accessions show a positive allele at 170 bp for the marker RM208 linked to the Pib gene. As RM208 is a linked marker, segregation between the marker and gene might be responsible for identifying a lesser number of genotypes, whereas PIBDOM is more accurate in identifying genotypes with blast-resistant alleles. The marker RM224 showed amplification for resistant allele size of 140 bp in the germplasm carrying the Pi1 gene with a frequency of 29% in the current study. In another similar study conducted by Dubey et al. (2014) and Singh et al. (2015), 14 out of 100 genotypes and 85 out of 192 accessions showed amplification at 140 bp for the marker RM224 linked to the Pi1 gene. The marker AP4007 and MSM1 shared a fragment size of 186 bp and 190 bp, respectively, for the resistant allele in the germplasm carrying the Pi2 gene with a frequency of 9% and 31%. Similar results were found by Yadav et al. (2017). Gene profiling was carried out by Azameti et al. (2020) using known markers for the Pi1, Pi2, Pi9, Pi54, and Pitp genes, and it was discovered that 13.6% of the 103 accessions tested contained at least one of the genes profiled. Similarly, the majority of accessions in the present study contained the resistant gene Pib.
The genotypes MN-62, MTU-1010, and Swarna had two R-genes, and Sahsarang had a single R-gene but showed high resistance with low blast score and percent disease index. This indicates that the resistance in these genotypes was governed by other/novel blast resistance genes not included in the study. The genotypes such as KD-62, K-2, K-20, Kong Youh, Machang Kaoyeing, Daram Phou, Tampha Phou, China-1, RCPL 1–111, RCM-10, and RCM-11 had three R-genes and the genotypes such as Dharmali-2 and WR-3–2-1 had four R-genes but were susceptible with high percent disease index, which means that the combination of resistant genes (name of genes) present in those genotypes was less efficient against blast pathogen or prone to some new races of pathogen evolved. Similar results were also found by Manojkumar et al. (2020).
The genotypes with six blast resistance genes, such as Ching Phou Khong Nembi, and five blast resistance genes, such as Naga Phou, Ratkhara, and RCPL 1–44, showed more resistance as compared to the genotypes having less than five blast resistance genes. Many studies have demonstrated that resistance is significantly correlated with the number of R-genes, meaning that the more R-genes present in the accessions, the higher their resistance to M. oryzae (Wu et al. 2015; Li et al. 2020).
Conversely, the rare presence or absence of Pi9 from the germplasm lines that originated from Northeast India was reported earlier by Mahender et al. (2012) and Anupam et al.(2017) considering narrow geographical or set of germplasm lines. However, the present study has detected Pi9 in about 43 genotypes (Table 7). The detection of broad spectrum blast-resistant genes suggested the presence of novel resistant lines included in the present study.
Association analysis through TASSEL 5.0 software revealed genetic relatedness of blast scores with gene-based markers using GLM and MLM. This analysis identified four markers associated with blast resistance with a significant P value of less than 0.05 in the current set of genotypes. Based on the general linear model (GLM), two markers, namely, AP56595 linked to Pi9 and C1454 linked to Pi5 gene, had a P value of 0.0312 and 0.0255, respectively. Through a mixed linear model (MLM), two markers, namely, AP4007 linked to Pi2 gene and RM208 linked to Pib gene, had a P value of 0.00037 and < 0.00001, respectively. This result indicates that these markers were responsible for resistance to blast disease. Out of 10 candidate/gene-based markers used, only four markers linked to the four genes showed association, which means that these four genes, namely, Pi2, Pi9, Pib, and Pi5, were more effective towards blast resistance in the current set of genotypes studied.
Umakanth et al. (2017) also found a similar result by identifying 20 marker associations for blast disease and other agronomic traits based on the general linear model (GLM). Seventeen marker–trait associations were found based on the mixed linear model (MLM). Zarbafi et al. (2020) evaluated the variety of the 121 distinct rice genotypes using 42 SSR markers linked to blast resistance loci across the rice genome and discovered that 30 and 29 markers showed a significant linkage with leaf blast features in GLM and MLM, respectively. Yadav et al. (2019) performed an association study using the GLM and found that out of 36 primers used, two markers linked to 2 blast R-genes, Pi56(t) and Pi21, were significantly related to resistance reaction. With a significant P value of less than 0.05, Azameti et al. (2020) performed an association analysis utilizing the GLM and found that ten markers were related to the blast resistance with marker R2 ranging between 8 and 16% and two were related to the blast resistance via MLM with marker R2 ranging between 9 and 10%.
Genotypes like Sijali, Ramphiatama, Naga Phou, MN-3, Makhara, and KMP-34 were found to be resistant to disease in field conditions. The genotypes with five blast R-genes, such as Nungshang Phou, Ratkhara, Anandhi, RCPL 1–44, Ching Phou Khong Nombi, MN-31, Rona Yang, and RCPL-128, were found to be superior in field conditions as well. Similarly, the genotypes MN-62, Tara Bali, Rato Bhan Joha, MN-47, RCPL 1–411, and CAU-R1 were promising regarding yield and blast resistance. Among checks, MTU-1010 was resistant with a disease score of 1, while Shahsarang, Swarna, Shahbhagi Dhan, and Sakurd Dhan were moderately resistant with disease scores of 2–3. Through the general linear model (GLM) and mixed linear model (MLM), markers AP4007, AP56595, C1454, and RM208 were found to be associated with the trait.
As, Northeast India being endemic to rice blast, there is a mixture of races of M. oryzae, presence of a mere single R-gene is not enough for durable blast resistance over the long run. Hence, future resistance breeding programs should exploit the modern biotechnology tools and conventional breeding approaches in developing durable blast resistance varieties harboring multiple R-genes (Annegowda et al. 2021). The identified lines with four and five blast resistance genes and higher yield potential can be deployed in breeding program to develop lines with much longer resistance against the fungus.
Conclusion
The competition between hosts and pathogens is a persistent occurrence in evolution. The goal of collecting, analyzing, and characterizing unknown northeast landraces is to find donors with various blast resistance genes that can be used right away in breeding initiatives. The six distinct genotypes with resistance to leaf blast (Sijali, Ramphiatama, Naga Phou, MN-3, Makhara, and KMP-34) can be studied to find novel genes for enlarging the gene pool to combat disease competition. Four gene-based markers were identified by analyzing marker–trait association, and these markers have the potential for validation in larger populations because they are very much associated with blast resistance. The findings of the present study would be useful in the development of durable blast-resistant varieties and preserving precious genetics resources of Northeast Indian rice germplasm.
Availability of Data and Materials
The data set and materials may be assessed as per the request to the corresponding author.
References
Annegowda DC, Prasannakumar MK, Mahesh HB, Siddabasappa CB, Devanna P, Banakar SN, Manojkumar HB, Prasad SR (2021) Rice blast disease in India: present status and future challenges. Integrative Advances in Rice Research 21:157–197
Anupam A, Imam J, Quatadah SM, Siddaiah A, Das SP, Variar M, Mandal NP (2017) Genetic diversity analysis of rice germplasm in Tripura State of Northeast India using drought and blast linked markers. Rice Sci 24(1):10–20
Arunakanthi B, Prasad MS, Madhanmohan K, Balachandran SM, Madhav MS, Reddy CS, Viraktamath BC (2008) Introgression of major blast resistance genes Pi-1, Pi-2 and Pi-kh in indica rice cultivars Samba Mahsuri and Swarna. J Mycol Plant Pathol 38(3):625–630
Azameti MK, Vishalakshi B, Umakanth B, Balram M, Srinivas Prasad M, Madha MS (2020) Molecular characterization of popular rice (Oryza sativa L.) varieties of India and association analysis for blast resistance. Genet Resour Crop Evol 67(8):2225–2236
Das A, Kesari V, Satyanarayana VM, Parida A, Rangan L (2011) Genetic relationship of Curcuma species from Northeast India using PCR-based markers. Mol Biotechnol 49:65–76
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Dubey AK, Pandian RTP, Rajashekara H, Khanna A, Ellur RK, Sharma P, Singh UD (2014) Molecular validation for blast and sheath blight resistance in improved rice genotypes and landraces. Indian Phytopath 67(3):216–221
Evans LT (1993) Crop evolution, adaptation and yield. Cambridge University Press, Cambridge, UK, p 500
Fjellstrom R, McClung AM, Shank AR (2006) SSR markers closely linked to the Pi-z locus are useful for selection of blast resistance in a broad array of rice germplasm. Mol Breeding 17(2):149–157
Gupta PK, Kulwal PL, Jaiswal V (2014) Association mapping in crop plants: opportunities and challenges. Adv Genet 85:109–147
Hanamaratti NG, Prashanthi SK, Salimath PM, Hanchinal RR, Mohankumar HD, Parameshwarappa KG et al (2008) Traditional landraces of rice in Karnataka: reservoirs of valuable traits. Curr Sci 94:242–247
Harlan JR (1975) Crops and man, 2nd edn. American Society of Agronomy and Crop Science Society of America Inc., Madison
Hittalmani S, Parco A, Mew TV, Zeigler RS, Huang N (2000) Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theor Appl Genet 100(7):1121–1128
Hosahatti BJ, Koti R, Devappa PS, Ngangkham VH, Devanna U, Yadav P, Mishra MK, Aditya KK, Boraiah JP, Gaber PK, Hossain AA (2023) Phenotypic and genotypic screening of fifty-two rice (Oryza sativa L.) genotypes for desirable cultivars against blast disease. PLoS One 18(3):e0280762. https://doi.org/10.1371/journal.pone.0280762
Hossain M, Ali MA, Hossain MD (2018) Mia MA (2018) Detection of blast resistant gene in rice by host-pathogen interaction and DNA-marker. Universal Journal of Agricultural Research 6(1):23–30
Imam J, Alam S, Mandal NP, Variar M, Shukla P (2014) Molecular screening for identification of blast resistance genes in North East and Eastern Indian rice germplasm (Oryza sativa L.) with PCR based makers. Euphytica 196(2):199–211
Ingole KD, Prashanthi SK, Krishnaraj PU (2014) Mining for major blast resistance genes in rice landraces of Karnataka. Indian J Genet Plant Breed 74:378–383
IRRI (2013) Standardization evaluation system for rice. International Rice Research Institute, P.O. Box 933, 1099 Manila,Philippines 5: 18
Jeon JS, Chen D, Yi GH, Wang GL, Ronald PC (2003) Genetic and physical mapping of Pi5 (t), a locus associated with broad-spectrum resistance to rice blast. Mol Genet Genomics 269:280–289
Khan MAI, Sen PP, Bhuiyan R, Kabir E, Chowdhury AK, Fukuta Y, Latif MA (2014) Phenotypic screening and molecular analysis of blast resistance in fragrant rice for marker-assisted selection. CR Biol 337(5):318–324
Li W, Deng Y, Ning Y, He Z, Wang GL (2020) Exploiting broad-spectrum disease resistance in crops: from molecular dissection to breeding. Annu Rev Plant Biol 71:575–603
Mahendar A, Swain DM, Subudhi HN, Rao GJN (2012) Molecular analysis of native Manipur rice accessions for resistance against blast. Afr J Biotechnol 11(6):1321–1329
Manojkumar HB, Deepak CA, Harinikumar KM, Chethana RMPB (2020) Molecular profiling of blast resistance genes and evaluation of leaf and neck blast disease reaction in rice. J Genet 99(1):1–10
Namrata S, Verma RP, Bisen RP, Singh P, Teli B (2019) Inheritance of blast disease resistance in the cross HUR 3022 X TETEP of rice (Oryza sativa L.). J Exp Biol Agric Sci 7(6):529–535
Ngachan SV, Mohanty AK, Pattanayak A (2011) Status paper on rice in North East India. ICAR Research Complex for NEH Region, Rice Knowledge Management Portal (RKMP) Directorate of Rice Research
Prasad MS, Madhav MS, Laha GS, Lakshmi DL, Krishnaveni D, Mangrauthia SK, Balachandran SM, Sundaram RM, Arunakanthi B, Mohan KM, Madhavi KR, Kumar V, Virakthamat BC (2011) Technical Bulletin No 57. Directorate of Rice Research, ICAR, Rajendranagar, Hyderabad, India. 52
Rama Devi SJS, Singh K, Umakanth B, Vishalakshi B, Renuka P, Vijay Sudhakar K, Prasad MS, Viraktamath BC, Ravindra Babu V, Madhav MS (2015) Development and identification of novel rice blast resistant sources and their characterization using molecular markers. Rice Science 22(6):300–308
Ramkumar G, Srinivasarao K, Mohan KM, Sudarshan I, Sivaranjani AKP, Gopalakrishna K, Neeraja CN, Balachandran SM, Sundaram RM, Prasad MS, Shobha Rani N, Rama Prasad AM, Viraktamath BC, Madhav MS (2011) Development and validation of functional marker targeting an InDel in the major rice blast disease resistance gene Pi54 (Pikh). Mol Breeding 27(1):129–135
Roychowdhury M, Jia Y, Jia MH, Fjellstrom R, Cartwright RD (2012) Identification of the rice blast resistance gene Pib in the national small grains collection. Phytopathology 102(7):700–706
Sakulkoo W, Oses-Ruiz M, Garcia EO, Soanes DM, Littlejohn GR, Hacker C, Correia A, Valent B, Talbot NJ (2018) A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science 359(6382):1399–1403. https://doi.org/10.1126/science.aaq0892
Sharma TR, Madhav MS, Singh BK, Shanker P, Jana TK, Dalal V, Pandit A, Singh A, Gaikwad K, Upreti HC, Singh NK (2005) High resolution mapping, cloning and molecular characterization of the Pi-kh gene of rice, which confers resistance to Magnaporthe grisea. Mol Genet Genomics 274:569–578
Sharma TR, Rai AK, Gupta SK, Vijayan J, Devanna BN, Ray S (2012) Rice blast management through host-plant resistance: retrospect and prospects. Agric Res 1:37–52
Shikari AB, Rajashekara H, Khanna A, Krishnan SG, Rathour R, Singh UD, Singh AK (2014) Identification and validation of rice blast resistance genes in Indian rice germplasm. Indian J Genet 74(3):286–299
Shikari AB, Khanna A, Krishnan SG, Singh UD, Rathour R, Tonapi V, Singh AK (2013) Molecular analysis and phenotypic validation of blast resistance genes Pita and Pita2 in landraces of rice (Oryza sativa L.). Indian J. Genet 73(2):131–141
Singh VK, Singh A, Singh SP, Ellur RK, Singh D, Gopala Krishnan S, Singh AK (2013) Marker-assisted simultaneous but stepwise backcross breeding for pyramiding blast resistance genes Piz5 and Pi54 into an elite Basmati rice restorer line ‘PRR 78.’ Plant Breeding 132(5):486–495
Singh AK, Singh PK, Arya M, Singh NK, Singh US (2015) Molecular screening of blast resistance genes in rice using SSR markers. Plant Pathol J 31(1):12
Tonnang HE, Sokame BM, Abdel-Rahman EM, Dubois T (2022) Measuring and modelling crop yield losses due to invasive insect pests under climate change. Curr. Opin. Insert Sci 100873. https://doi.org/10.1016/j.cois.2022.100873
Umakanth B, Vishalakshi B, Sathish Kumar P, Rama Devi SJS, Bhadana VP, Senguttuvel P, Madhav MS (2017) Diverse rice landraces of North-East India enables the identification of novel genetic resources for Magnaporthe resistance. Front Plant Sci 8:1500
Villareal RL, Lantican RM (1965) The cytoplasmic inheritance of susceptibility to Helminthosporium leaf spot in corn
Wang JC, Jia Y, Wen JW, Liu WP, Liu XM, Li L, Jiang ZY, Zhang JH, Guo XL, Ren JP (2013) Identification of rice blast resistance genes using international monogenic differentials. Crop Prot 45:109–116
Wang L, Ma Z, Kang H, Gu S, Mukhina Z, Wang C et al (2022) Cloning and functional analysis of the novel rice blast resistance gene Pi65 in japonica rice. Theor Appl Genet 135:173–183. https://doi.org/10.1007/s00122-021-03957-1
Wu Y, Xiao N, Yu L, Pan C, Li Y, Zhang X, Li A (2015) Combination patterns of major R genes determine the level of resistance to the M. oryzae in rice (Oryza sativa L.). PLoS One 10(6):e0126130
Yadav MK, Ngangkham U, Shubudhi HN, Bag MK, Adak T, Munda S, Jena M (2017) Use of molecular markers in identification and characterization of resistance to rice blast in India. PLoS ONE 12(4):e0176236
Yadav MK, Aravindan S, Ngangkham U, Prabhukarthikeyan SR, Keerthana U, Raghu S, Rath PC (2019) Candidate screening of blast resistance donors for rice breeding. J Genet 98(3):1–13
Yu J, Pressoir G, Briggs WH, Vroh Bi I, Yamasaki M, Doebley JF, Buckler ES (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38(2):203–208
Zarbafi SS, Rabiei B, Ebadi AA, Ham JH (2020) Association mapping of traits related to leaf blast disease in rice (Oryza sativa L.). Australas Plant Pathol 49(1):31–43
Funding
The research work was supported by Director, ICAR RC NEH Umiam, Meghalaya.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by RR, AK, PWS, and BC. Supervision and data analysis were performed by MR, LT, AK, SPD, SDK, and VKM. The editing of the manuscript was done by VKM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
NA.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Designation: Senior Scientist (Plant Breeding), ICAR Research Complex for NEH Region, Umiam, Meghalaya, India
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raj, R., Kumar, A., C, B. et al. Revealing the Novel Genetic Resources for Blast Resistance in Diverse Rice Landraces of North-Eastern Hills of Himalayas. Plant Mol Biol Rep (2024). https://doi.org/10.1007/s11105-024-01463-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11105-024-01463-4