Abstract
Key message
Pi65, a leucine-rich repeat receptor-like kinase (LRR-RLK) domain cloned from Oryza sativa japonica, is a novel rice blast disease resistance gene.
Abstract
Rice blast seriously threatens rice production worldwide. Utilizing the rice blast resistance gene to breed rice blast-resistant varieties is one of the best ways to control rice blast disease. Using a map-based cloning strategy, we cloned a novel rice blast resistance gene, Pi65, from the resistant variety GangYu129 (abbreviated GY129, Oryza sativa japonica). Overexpression of Pi65 in the susceptible variety LiaoXing1 (abbreviated LX1, Oryza sativa japonica) enhanced rice blast resistance, while knockout of Pi65 in GY129 resulted in susceptibility to rice blast disease. Pi65 encodes two transmembrane domains, with 15 LRR domains and one serine/threonine protein kinase catalytic domain, conferring resistance to isolates of Magnaporthe oryzae (abbreviated M. oryzae) collected from Northeast China. There were sixteen amino acid differences between the Pi65 resistance and susceptible alleles. Compared with the Pi65-resistant allele, the susceptible allele exhibited one LRR domain deletion. Pi65 was constitutively expressed in whole plants, and it could be induced in the early stage of M. oryzae infection. Transcriptome analysis revealed that numerous genes associated with disease resistance were specifically upregulated in GY129 24 h post inoculation (HPI); in contrast, photosynthesis and carbohydrate metabolism-related genes were particularly downregulated at 24 HPI, demonstrating that disease resistance-associated genes were activated in GY129 (carrying Pi65) after rice blast fungal infection and that cellular basal metabolism and energy metabolism were inhibited simultaneously. Our study provides genetic resources for improving rice blast resistance and enriches the study of rice blast resistance mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice blast, caused by M. oryzae, is a devastating fungal disease worldwide. The annual rice yield loss due to blast damage can be as high as 10-30% (Skamnioti and Gurr 2009). China is the largest producer of japonica rice in the world. The annual planting area of japonica rice has reached more than 10 million hectares (ha) in northern China. The demand for japonica rice relative to indica rice is increasing each year (Bian et al. 2020). However, rice blast has been a serious threat to japonica rice production in northern China for many years. It is widely accepted that breeding and cultivating disease-resistant varieties are the most economic and efficient way to control rice blast disease. Therefore, it is very important to continue to exploit blast resistance genes. In recent years, scientists have identified several new genes that mediate strong rice blast resistance without affecting rice yield and quality, such as Pigm, Ptr and bsrd1 (Li et al. 2017; Zhao et al. 2018; Zhai et al. 2019), and have developed many broad-spectrum resistant varieties; however, most of these genes are derived from indica rice. Although the substitution of genetic background can be achieved through multiple backcrosses, this is time consuming and difficult to apply in resistant breeding. Rice blast resistance breeding in northern China has lagged behind due to a lack of resistance gene resources. It is important to identify new blast resistance genes from japonica rice and to develop resistant germplasm resources in the japonica background.
The innate immune systems of plants mainly include two levels of defense. In the first level, when the fungus infects plants, the PAMP (pathogen-associated molecule pattern) stimulates the PTI (PAMP triggered immunity) of plants, the pattern recognition receptor on the surface of plant cells specifically recognizes the PAMP of pathogenic microorganisms, and pathogenic bacteria or fungi inhibit PTI through effectors; in the second level, the protein encoded by resistance (R) genes in plant cells further recognizes effectors and activates ETI (effector triggered immunity) (Ronald and Beutler 2010; Cheng et al. 2012; Liu et al. 2013). PTI is a nonspecific defense response that is characterized by a broad resistance spectrum and persistent resistance. The pathogen-related molecular patterns that stimulate PTI, such as polysaccharides, polypeptides and flagellin, are widely conserved in pathogens (or are considered not to be pathogen-specific) (Ronald and Beutler 2010; Chen and Ronald 2011; Segonzac and Zipfel 2011); however, ETI-related resistance genes are usually specific and recognize limited strains, but can mediate a strong resistance response, and most of them encode cytoplasmic proteins with nucleotide-binding site-leucine-rich repeat (NLR) domains (Liu et al. 2013).
In 2017, the rice blast resistance gene Pi65(t) was finely mapped from the japonica rice variety GY129, which is resistant to most M. oryzae isolates found in northern China (Zheng et al. 2016). The gene was located on chromosome 11, close to the Pik gene cluster, and was identified as a new gene distinct from other cloned genes. However, its structure and function remain unknown. Here, we show that Pi65 encodes a leucine rich-repeat receptor-like kinase (LRR-RLK), and we identify 16 SNPs that cause missense mutations between resistance and susceptibility alleles. The resistance function of Pi65 was further confirmed by both CRISPR/Cas9-mediated gene knockout mutation in the resistant rice variety GY129 and overexpression of Pi65 in the susceptible rice variety LX1. The spatiotemporal expression of Pi65 and the molecular mechanism of disease resistance mediated by the gene were further studied by RT-PCR and RNA sequencing. This study provides genetic resources for the molecular breeding of rice blast resistance and enriches the study of rice blast resistance mechanisms.
Materials and methods
Plant materials and disease evaluation
The japonica rice variety GY129 is resistant to most of the tested M. oryzae isolates (e.g., ZA1, ZA9, ZB1, ZB13, ZC1, ZE1, ZF1 and ZG1) from Liaoning Province in China, whereas LX1 is susceptible to them (Zheng et al. 2016). In this experiment, the M. oryzae isolate QY-13 (ZA1) was selected to evaluate the disease reactions of the GY129/LX1 BC1F2 population and Pi65 knockout and overexpressed mutants. The donor variety GY129 is used in international research and breeding (Mukhina et al. 2020).
The rice plants were sown in black plastic containers (10 × 7.0 × 8.5 cm3) with a locally disinfected seedling substrate. The black plastic boxes containing the seedlings were then placed in a blue box one-third full of water (34.5 × 47 × 15 cm3). The seedlings were grown in a greenhouse at 24 to 30 °C with an 8 h dark and 16 h light cycle until the three and half leaves stages (approximately three weeks old). We spray-inoculated spore suspensions (5 × 105 spores/mL) and placed them in a blue box. The inoculated plants were covered with black plastic sheeting in darkness for 24 h at 25 to 28 °C under 100% relative humidity. After culture in the dark, the sunshade was removed, and the cells were cultured at 25-28 °C for another 5 days. The punch inoculation of detached rice leaves was performed as follows: 5 µL drops of a spore suspension were placed on three spots on each leaf with a transferpettor, and the leaves were kept in a culture dish containing 0.1% 6-benzylaminopurine (6-BA) in sterile water to provide moisture (Li et al. 2017).
Targeted mutagenesis of Pi65 in GY129 with the CRISPR/Cas9 system
Two potential candidate genes from GY129 were targeted with sgRNA spacers in the anterior segments of their exons. The highly specific sgRNA sequence (Table S1) was designed using CRISPR Design (http://crispr.mit.edu/). The CRISPR/Cas9 vector pCas9 (ZmUbi, OsU6, Hpt) plasmid (Table S2) was linearized by using BsaI(NEB) and connected to the sgRNA with T4 ligase (TIANGEN, NG201). The resulting binary vectors were introduced via electroporation into the Agrobacterium tumefaciens strain EHA105. The transformation events were selected based on hygromycin B resistance, and regenerated plants were analyzed for genome editing-induced mutations in the target gene. Chromosomal deletions were detected by PCR with primers located on both sides of each targeted gene.
Pi65 overexpression mutagenesis in LX1
The full-length cDNA of Os11g0694600 was amplified with Primer 1 (Table S1) and cloned into the T vector (pe-Blunt Simple Cloning Vector) to produce T-Pi65, which was then recombined with a pCambia1301-UbiN vector at BamHI to generate the overexpression construct pCambia1301-UbiN-OsPi65 (abbreviated as OE-Pi65) (Table S2). The construct was transformed into calli of Oryza sativa japonica LX1 mediated by Agrobacterium tumefaciens. The transgenic plants were screened with a solution containing 300 mg/mL carbenicillin and 50 mg/mL hygromycin, and the hygromycin resistance gene was detected by PCR. All transgenic plants were properly managed in an artificial climate incubator in Liaoning Province. More than 20 transgenic lines were obtained, and 3 independent T2 lines were used in this study.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from rice tissues using TRIzol. First-strand cDNA was synthesized using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, RR047A) with an Oligo (dt)18 primer according to the manufacturer’s protocol. qRT-PCR amplification was performed using TB Green® Premix Ex Taq™ II (Takara, RR820A) and a Roche LightCycler 480 System (CT, USA) following the manufacturer's instructions. qRT-PCR amplification was performed with three biological replications, and the rice Actin1 gene was used as the internal control for gene expression analysis (Table S1). The Pi65 gene-specific qRT-PCR primers are listed in Table S1.
Pi65 candidate gene screening and haplotype sequence analysis
We sequenced the candidate genes Os11g0694500, Os11g0694600, Os11g0694850 and Os11g0695000 and analyzed the sequence polymorphisms of the candidate genes to determine the target gene (Table S1). To investigate the distribution of Pi65 haplotypes in japonica rice, we tested japonica rice varieties from different areas of northern China (Table S3 and S4). Primers were used to amplify DNA sequences of candidate genes in different rice varieties using PrimeSTAR® HS (Takara, R040Q); after gel purification, DNA samples were sequenced at Tsingke Biological Technology. The DNA sequences were assembled using DNASTAR Lasergene.v7.1\SETUP\Editseq software and aligned with DNASTAR Lasergene.v7.1\SETUP \SeqMan.
Structural and comparative analysis of Pi65
The in silico structural and functional prediction of high-quality assembled sequences was performed at the following sites. Functional annotation was performed after translating the sequence into one of three reading frames. The Simple Modular Architecture Research Tool (https://smart.embl-heidelberg.de/) was used for the domain architecture analysis of GY129 and LX1, and tertiary structures were predicted using SWISS-MODEL (https://swissmodel.expasy.org/).
The peptide sequences encoded by Pi65 were subjected to search strings in the nonredundant protein Rice Information GateWay (RIGW) database (https://rice.hzau.edu.cn/rice_rs3/), and the top 24 high-similarity homologous genes were downloaded for further analyses. Hmmer software was used to identify the LRR and kinase domain of each LRR-RLK gene. Clustal X was used for multisequence alignments, and the phylogenetic tree was constructed using a neighbor-joining method in MEGA software (Saitou and Nei 1987; Kumar et al. 2018).
Transcriptome sequencing of wild-type GY129 and its Pi65 knockout mutants
To study the biological processes mediated by Pi65, we analyzed the Pi65-associated gene expression pattern. Total RNA was extracted from young seedlings of GY129 (carrying the Pi65 gene) and the Pi65 knockout mutant KO-B 6 (abbreviated KOPi65) with three biological replicates using TRIzol reagent according to the manufacturer’s instructions. cDNA library preparation and sequencing reactions were conducted at the Biomarker Technology Company (Beijing, China). RNA-sequencing (RNA-Seq) analysis was performed following Zheng et al. (2013). Gene expression levels were measured in the RNA-Seq analyses as the numbers of reads normalized via the reads per kilobase of transcript per million mapped reads (RPKM) method. EdgeR software was used to identify differentially expressed genes (DEGs) in pairwise comparisons, and the results of all statistical tests were corrected for multiple tests according to the Benjamini-Hochberg false discovery rate (FDR < 0.05). Genes were considered to be significantly differentially expressed if the adjusted P-value was < 0.05 and log2FC ≥ 2 and log2FC ≤ -2 between two libraries.
Results
Molecular cloning and functional analysis of Pi65
In our previous work, Pi65(t) was localized in a 430 kb region between InDel-1 and SNP-4 on the short arm of chromosome 11 (Zheng et al. 2016). Within the interval, we found that four genes, Os11g0694500, Os11g0694600, Os11g0694850 and Os11g0695000, all contain typical LRR domains. Sequence analysis results showed that for Os11g0694500 and Os11g0695000, there were no sequence differences between the resistant parent GY129 and the susceptible parent LX1. However, in the other two candidate genes, Os11g0694600 and Os11g0694850, sequence polymorphisms were present between the resistant and susceptible parents. Therefore, Os11g0694600-R and Os11g0694850-R were used for further functional analysis.
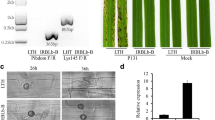
Using a CRISPR/Cas9 gene mutation strategy, we designed two gene-specific sgRNAs to target Os11g0694600-R and Os11g0694850-R (Fig. 1a and S1a). We obtained a total of 12 and 4 independent T0 CRISPR knockout transgenic lines for Os11g0694600-R and Os11g0694850-R, respectively. Six editing types of Os11g0694600-R (KO-B 1-6) (Fig. 1b) and two editing types of Os11g0694850-R (KO-C 1-2) were obtained (Fig. S1). Homozygous mutants for these two genes in generation T2 were selected for further rice blast resistance evaluation. The rice blast inoculation results showed that in the GY129 background, when Os11g0694850-R was knocked out, the mutants were still resistant to the M. oryzae isolate of QY-13 (Fig. 1b). However, when Os11g0694600-R was knocked out, the mutants became susceptible to QY-13. A comparison of the lesion areas of GY129 and KOPi65 after inoculation showed that the lesion areas of KOPi65 were significantly larger than those of GY129 (Fig. 1b). To further determine the function of Os11g0694600-R, we generated an Os11g0694600-R overexpression vector and transformed it into the susceptible rice variety LX1. The rice blast inoculation results indicated that overexpression of Pi65 in LX1 could enhance its resistance to the M. oryzae isolate QY-13 (Fig. 1c). Although R gene overexpression often leads to nonspecific resistance, a combination of wild-type, knockout mutant, and overexpressed lines suggests that these phenotypic changes are indeed due to Pi65, demonstrated that Os11g0694600-R is the bona-fide rice blast resistance gene of Pi65.
CRISPR/Cas9-mediated mutation of two candidate genes in GY129 and disease reactions of Pi65-overexpressing lines of LX1. a The candidate gene Os11g0694600 was knocked out with specific gRNAs. WT, wild-type Os11g0694600 in GY129, and KO-B 1-6, edited types of Os11g0694600. b Disease reactions in wild-type GY129 and CRISPR-edited mutant leaves after spray-inoculation with the QY-13 isolate. KO-B and KO-C are Os11g0694600 and Os11g0694850, respectively; lesion lengths were determined on inoculated leaves at 7 days post-inoculation (Student’s t-test; *P < 0.05, ***P < 0.001, ****P < 0.0001). c Blast reactions in OE-Pi65 and LX1 plants. Leaves of 4-week-old plants were punch-inoculated; lesion lengths were determined on inoculated leaves at 7 days post-inoculation (Student’s t-test; *P < 0.05)
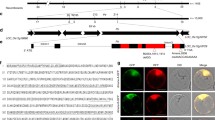
Sequence structure of Pi65
Pi65 contains 2 introns with lengths of 2,923 bp and 386 bp. The full-length cDNA of Pi65 has a 3,309 bp open reading frame (ORF), encoding 1,102 amino acids (aa). The Pi65 protein has 15 LRR domains and one serine/threonine protein kinase domain (Fig. 2a and b). The structural annotation results showed that the Pi65 R allele (Os11g0694600-R in GY129) had one more LRR domain than the Pi65 S allele (Os11g0694600-S in LX1) at aa sites 543 to 569. This LRR domain difference was due to the nonsynonymous changes caused by the 16 SNPs between Os11g0694600-R and Os11g0694600-S (Fig. 2b). To further detect the potential structural differences between Os11g0694600-R and Os11g0694600-S, we performed protein structure prediction, and the results revealed significant structural variation in the 420-580 aa regions between the S-allele and R-allele of the Pi65 gene (Fig. 2c), implying that the R-allele in this region is essential for the rice blast resistance function of the gene.
Structure of Pi65 and its deduced amino acid sequence. a LRRs and serine/threonine protein kinases are shown in the CDS. b Deduced peptide sequence encoded by Pi65. The bold and enlarged amino acid sequences are specifically present in GY129 and not in LX1. c Red arrows indicate the tertiary structural differences in Pi65 between GY129 and LX1 (colour figure online)
Pi65 phylogeny
The top 24 genes with high homology with Pi65 were found in the RIGW gene bank for the construction of the evolutionary tree (Fig. S1b). These 25 genes were divided into four groups according to their kinships. Group I contained Pi65 and another two genes, LOC_Os11g47210 and LOC_Os11g46980, and LOC_Os11g47210 is the previously cloned Bacterial Blight resistance gene Xa26 (Yang et al. 2003). LOC_Os11g46980 is involved in the response to high temperature, and its LRR domain is important for the perception of elevated temperature (Zhang et al. 2011). It is interesting that the closest gene to Pi65 is Xa26. Further study of these two genes will help us to better understand their resistance differentiation.
Distribution of Pi65 and its alleles in different rice varieties
A previous study indicated that temperate japonica is the most rice blast disease-susceptible rice subpopulation (Kang et al. 2016). The analysis of Pi65 alleles and their distribution will help breeders make better use of this rice blast resistance gene. We collected 38 japonica rice varieties from 7 regions of China, including Beijing, Xinjiang, Ningxia, Henan, Heilongjiang, Jilin and Liaoning (Fig. S2, Table S3). Through sequence analysis, we identified three haplotypes of Pi65, Hap1 (GY129, etc.), Hap2 (LX1, etc.) and Hap3 (JingDao2, abbreviated JD2, etc., Oryzasativa japonica) (Table S4). Hap1 and Hap3 were functional haplotypes (resistant) according to the spray inoculation results. Hap1 was present in 15 varieties that came from Liaoning, Heilongjiang, Henan and Ningxia. Compared with Hap1, Hap3 had a G/A SNP at position 3264, leading to a missense mutation. In addition, 16 SNPs led to missense mutation differences between Hap2 and Hap1, and most rice cultivars carrying Hap2 were susceptible to QY-13, indicating that the SNPs in Hap2 abolished the rice blast resistance function of Pi65.
Spatiotemporal expression profile of Pi65
To investigate Pi65 expression patterns, we detected the expression of Pi65 in the GY129 seedling (root, leaf and stem) and booting (leaf, rachilla, panicle, stem and sheath) stages. The strongest expression was found in the booting stage (16 weeks) in leaves, and there was relatively weak expression in the seedling stage (4 weeks) in roots, young leaves and young sheaths, indicating that Pi65 is constitutively expressed at different developmental stages and in different tested organs (Fig. 3a). Furthermore, to determine whether the expression of Pi65 in GY129 could be induced during M. oryzae infection, we performed the rice blast inoculation and conducted quantitative (q) RT-PCR analysis at six time points (0, 12, 24, 48, 72 and 96 HPI) (Fig. 3b) using the three- and half-leaf stage seedlings of GY129 (resistant variety) and LX1 (susceptible variety) as the materials. During the early stage (12 HPI) after M. oryzae inoculation, the expression level of Pi65 was significantly increased in GY129 compared to the control (mock treatment), reaching a peak at 72 HPI. However, in LX1, pi65 (Pi65’s susceptible allele) decreased in early stages (both 12 and 24 HPI) and then increased at 48 HPI. In brief, the expression level of Pi65 in GY129 was higher than that in LX1 before and after inoculation, except at 48 HPI. In conclusion, Pi65 is constitutively expressed, and its expression could be quickly induced in resistant rice varieties but could not be induced in the early stage in susceptible rice varieties.
Expression of Pi65 in different organs and at different time points in both compatible and incompatible interactions. a Constitutive expression of Pi65 in different organs of rice at the seedling stage. Constitutive expression of Pi65 in different organs of rice at the booting stage. b Profiles of Pi65 expression in GY129 at different time points (0, 12, 24, 48, 72 and 96 HPI) after inoculation detected by qRT-PCR using the relative 2-△△CT method with Actin1 as an internal control. Data represent means with error bars showing ± s.d. (n = 3) (Student’s t-test; *P < 0.05, **P < 0.01, ***P < 0.001)
Transcriptome analysis of the expression pattern of Pi65-associated genes
To further investigate the regulatory mechanism mediated by Pi65, transcriptome sequencing was performed on samples from GY129 and KOPi65 mutant plants. Only those DEGs found in three independent biological replicates were selected for further analyses. A total of 2,709 DEGs (619 upregulated genes and 2,090 downregulated genes) and 2,221 DEGs (299 upregulated genes and 1,922 downregulated genes) were detected in GY129 and the KOPi65 mutant, respectively (Fig. 4a). Among all DEGs, 128 upregulated DEGs and 1,399 downregulated DEGs were shared between GY129 and the KOPi65 mutant. In addition, 489 genes and 690 genes were specifically upregulated and downregulated, respectively, in GY129.
DEG analysis of GY129 and KOPi65. a Venn diagram analysis of upregulated and downregulated genes in GY129 and KOPi65 at 24 HPI. b GO enrichment analysis for all DEGs in GY129 and KOPi65. The X-axis represents the -log10 (Pvalue), and the left side of the Y-axis represents GO item types. c KEGG enrichment analysis of all DEGs in GY129 and KOPi65. The X-axis represents the Rich Factor, and the left side of the Y-axis represents KEGG pathways
Gene Ontology (GO) analysis showed that these DEGs were mainly enriched in the categories of “single-organism process” (GO:0,044,699), “response to stimulus” (GO:0,050,896), “response to chemical” (GO:0,042,221), “response to oxygen-containing compound” (GO:1,901,700) and “biological regulation” (GO:0,065,007) (Fig. 4b). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that the pathways that were most highly enriched among all DEGs in GY129 and KOPi65 were associated with metabolic pathways, biosynthesis of secondary metabolites, phenylpropanoid biosynthesis, cutin, suberin and wax biosynthesis and fatty acid metabolites (Fig. 4c). The results indicated that although both GY129 and KOPi65 mutants presented resistance responses after inoculation with M. oryzae, the resistance responses in GY129 were much stronger than those in KOPi65.
Additionally, 489 DEGs that were specifically upregulated in GY129 exhibited enrichment in several GO terms associated with disease defense, such as “response to oxygen-containing compound” (GO:1,901,700),“response to chemical”(GO:0,042,221),“response to stimulus” (GO:0,050,896),“defense response” (GO:0,006,952), “response to stress” (GO:0,006,950) and “response to fungus” (GO:0,009,620), including 10 disease resistance proteins (LOC_Os11g12340, etc.), 6 E3 ubiquitin-protein ligases, 6 G-type lectin S-receptor-like serine/threonine-protein kinases, 15 transcription factors (LOC_Os04g43680, LOC_Os11g02520, LOC_Os11g45740, etc.), 6 L-type lectin-domain containing receptor kinases, 2 pentatricopeptide repeat-containing proteins, 13 probable LRR receptor-like serine/threonine-protein kinases, 5 probable protein phosphatase 2C proteins, 1 probable serine/threonine-protein kinase, 4 putative disease resistance proteins, 1 receptor kinase-like protein, 1 serine/threonine-protein phosphatase, and 23 wall-associated receptor kinases (LOC_Os02g42150, LOC_Os09g38850, etc.). Furthermore, some of these DEGs that were specifically upregulated in GY129 were downregulated in the KOPi65 mutant, such as Bowman-Birk serine protease inhibitor (LOC_Os01g03330) and PPR repeat family (LOC_Os07g41810) genes (Fig. 5a and Tables S5). OsRSR1 (LOC_Os11g12340) and OsRLCK5 can enhance the resistance of rice to sheath blight by regulating ROS balance through the ascorbate-glutathione circulation system (Wang et al. 2021). LOC_Os04g43680, LOC_Os11g02520 and LOC_Os11g45740 are MYB and WRKY transcription factors, respectively, which are involved in rice blast resistance (Wang et al. 2007; Cao et al. 2015; Kishi-Kaboshi et al. 2018). LOC_Os02g42150 and LOC_Os09g38850 are wall-associated receptor kinase genes, both of which are positive regulatory factors of rice resistance to M. oryzae infection (Delteil et al. 2016).
GO enrichment analysis of GY129 of DEGs. a GO enrichment analysis of specifically up-DEGs in GY129, where the X-axis represents the Rich Factor, and the left side of the Y-axis represents GO term types. b GO enrichment analysis of specifically downregulated DEGs with GY129. The X-axis represents the Rich Factor, and the left side of the Y-axis represents GO term types
The specifically downregulated DEGs in GY129 were mainly involved in the “chloroplast”, “photosynthesis” and “single-organism biosynthetic process” pathways, and most of these pathways were associated with amino acid metabolism (three pathways), energy metabolism (one pathway), carbohydrate metabolism (four pathways) and transport and catabolism (two pathways) (Fig. 5b). These results indicated that, relative to KOPi65, GY129 specifically presented decreases in photosynthesis, carbohydrate metabolism and amino acid metabolism after infection by M. oryzae, which may be closely related to plant resistance to M. oryzae (Table S6).
Discussion
In previous work, Pi65 was finely mapped to the interval between SNP-2 and SNP-8 located in the region from 30.42 to 30.85 Mb on chromosome 11. In this study, we cloned Pi65 and confirmed that, unlike most NBS-LRR blast resistance genes, it contained a typical kinase domain encoding a leucine-rich receptor protein kinase. Phylogeny studies showed that Pi65 was closest to Xa26. Similarly, the differences between Xa26 and its alleles are mainly within the xxLxLxx as well (Li et al. 2012). Protein kinases are enzymes with catalytic subunits that transfer the primary (terminal) phosphoric acid of nucleoside triphosphate (usually ATP) to one or more amino acid residues in the protein substrate side chain, resulting in conformational changes that affect protein function (Hanks et al. 1988). The variable amino acids in the motif of LRR determine the specificity of its binding with the interacting protein (Kobe and Eisenhofer 1995). In the tertiary structure, the LRR domain forms an α/β helix, which is located on the surface of the spatial structure of the protein and is involved in the interaction between proteins. This mechanism of action is the basis of the cellular molecular recognition process (Shiu and Bleecker 2001). In this study, we found that Pi65-Hap1 (in GY129) has one more LRR domain than Pi65-Hap2 (in LX1) from amino acids 543-569; meanwhile, the expression level of Pi65 in GY129 was higher than that of its allele in LX1. Therefore, the specific structure of Pi65 and the higher level of gene expression may represent the key mechanism that enforces Pi65 resistance to rice blast.
To further clarify the molecular mechanism of Pi65-mediated blast resistance, we performed transcriptome sequencing to investigate gene expression profiles during the compatible and incompatible interactions of GY129 and the KOPi65 mutant with M. oryzae isolates. A total of 1,530 DEGs were common to the two lines. Among these DEGs, 128 involved in the biosynthesis of secondary metabolites, fatty acid metabolites and phenylpropanoid biosynthesis were upregulated in both GY129 and the KOPi65 mutant after inoculation. The results showed that M. oryzae infection influences many of the same physiological processes in GY129 and the KOPi65 mutant.
In addition to the shared DEGs, we found significant differences between GY129 and the KOPi65 mutant in response to rice blast pathogen infection in genes such as those involved in photosynthesis, carbohydrate metabolism and energy production. Several earlier studies have shown that the allocation of resources toward a defense response occurs at the expense of plant fitness (growth and yield), suggesting that defense-related products are autotoxic or that resistance is energetically costly (Bolton 2009). Reducing the photosynthetic rate to allocate resources to defense against pathogens at the expense of photosynthesis has been suggested to be an effective defense mechanism in early infection stages (Hanssen et al. 2011). Comparative phosphoproteomic analysis revealed that a number of photosynthesis-related phosphoproteins were downregulated in both compatible and incompatible interactions between rice and M. oryzae (Li et al. 2015). Similarly, Hanssen et al. (2011) showed that a number of photosynthesis-related genes were downregulated in tomato plants infected with Pepino mosaic virus during early stages of infection. However, in the present study, 112 DEGs associated with plant cell-based metabolism were specifically identified in GY129; these genes were associated with plant cell-based metabolism, including carbohydrate metabolism, lipid metabolism, amino acid metabolism, biosynthesis of other secondary metabolites, energy metabolism, nucleotide metabolism, metabolism of cofactors and vitamins and metabolism of terpenoids and polyketides. In contrast, these DEGs were not found in KOPi65 mutant plants, so we suspect that Pi65 plays an important role in reducing photosynthesis and cellular energy metabolism, which may be important for starving the pathogen and thus limiting its reproduction and expansion.
The analysis of the top 20 GO entries showed that the DEGs that were specifically upregulated in GY129 were mainly involved in the “defense response”, “response to biotic stimulus”, “regulation of response to stress”, “response to other organism”, “response to external biotic stimulus”, “response to salicylic acid” and “response to fungus” categories. Genes related to disease defense accounted for the majority of the DEGs, indicating that many genes related to disease defense were activated in GY129 (with Pi65) 24 HPI.
In summary, the rice blast resistance gene Pi65 was identified from japonica rice variety GY129, and its disease resistance function was confirmed. Pi65 encodes a leucine-rich receptor-like protein kinase. The susceptibility allele of Pi65 has one fewer LRR domain, and the tertiary structure of the encoded protein is significantly different, which may be the key factor whereby Pi65 confers resistance to rice blast. Transcriptome sequencing results showed that 24 h after rice blast fungus inoculation, a large number of genes associated with disease resistance were upregulated specifically in GY129, and photosynthesis- and carbohydrate metabolism-related genes were simultaneously significantly downregulated, showing that disease resistance genes were activated after rice blast fungus infection. At the same time, there was a significant reduction in the basal metabolism of cells, and the combination of these factors endowed GY129 with resistance to rice blast. Our study provides genetic resources for the improvement of rice blast resistance in japonica rice and enriches the study of rice blast resistance mechanisms.
References
Bian JL, Ren GL, Han C, Xu FF, Qiu S, Tang JH, Zhang HC, Wei HY, Gao H (2020) Comparative analysis on grain quality and yield of different panicle weight indica-japonica hybrid rice (Oryza sativa L.) cultivars. J Integr Agric 19:999-1009. https://doi.org/10.1016/S2095-3119(19)62798-X
Bolton MD (2009) Primary metabolism and plant defense-fuel for the fire. Mol Plant Microbe Interact 22:487-497. https://doi.org/10.1094/mpmi-22-5-0487
Cao WL, Chu RZ, Zhang Y, Luo J, Su YY, Xie LJ, Zhang HS, Wang JF, Bao YM (2015) OsJAMyb, a R2R3-type MYB transcription factor, enhanced blast resistance in transgenic rice. Physiol Mol Plant Pathol 92:154-160. https://doi.org/10.1016/j.pmpp.2015.04.008
Chen XW, Ronald PC (2011) Innate immunity in rice. Trends Plant Sci 16:451-459. https://doi.org/10.1016/j.tplants.2011.04.003
Cheng X, Tian CJ, Li AN, Qiu JL (2012) Advances on molecular mechanisms of plant-pathogen interactions. Hereditas 34:134-144. https://doi.org/10.3724/sp.j.1005.2012.00134
Delteil A, Gobbato E, Cayrol B, Estevan J, Michel-Romiti C, Dievart A, Kroj T, Morel JB (2016) Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol 16:17. https://doi.org/10.1186/s12870-016-0711-x
Hanks SK, Quinn A, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. https://doi.org/10.1126/science.3291115
Hanssen IM, Esse HPV, Ballester AR, Hogewoning S, Thomma B (2011) Differential tomato transcriptomic responses induced by pepino mosaic virus isolates with differential aggressiveness. Plant Physiol 156:301-318. https://doi.org/10.1104/pp.111.173906
Kang HX, Wang Y, Peng SS, Zhang YL, Xiao YH, Wang D, Qu SH, Li ZQ, Yan SY, Wang ZL, Liu WD, Ning YS, Korniliev P, Leung H, Mezey J, McCouch SR, Wang GL (2016) Dissection of the genetic architecture of rice resistance to the blast fungus Magnaporthe oryzae. Mol Plant Pathol 17:959-972. https://doi.org/10.1111/mpp.12340
Kishi-Kaboshi M, Seo S, Takahashi A, Hirochika H (2018) The MAMP-responsive MYB transcription factors MYB30, MYB55 and MYB110 activate the HCAA synthesis pathway and enhance immunity in rice. Plant Cell Physiol 59:903-915. https://doi.org/10.1093/pcp/pcy062
Kobe B, Eisenhofer D (1995) A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 374:183-186. https://doi.org/10.1038/374183a0
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547-1549. https://doi.org/10.1093/molbev/msy096
Li HJ, Li XH, Xiao JH, Wing RA, Wang SP (2012) Ortholog alleles at Xa3/Xa26 locus confer conserved race-specific resistance against Xanthomonas oryzae in rice. Mol Plant 5:281-290. https://doi.org/10.1093/mp/ssr079
Li WT, Zhu ZW, Chern MS, Yin JJ, Yang C, Li R et al (2017) A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 170:114-126. https://doi.org/10.1016/j.cell.2017.06.008
Li YF, Ye ZJ, Nie YF, Zhang J, Wang GL, Wang ZZ (2015) Comparative phosphoproteome analysis of Magnaporthe oryzae-responsive proteins in susceptible and resistant rice cultivars. J Proteomics 115:66-80. https://doi.org/10.1016/j.jprot.2014.12.007
Liu WD, Liu JL, Ning YS, Ding B, Wang XL, Wang ZL, Wang GL (2013) Recent progress in understanding PAMP-and effector-triggered immunity against the rice blast fungus Magnaporthe oryzae. Mol Plant 6:605-620. https://doi.org/10.1093/mp/sst015
Mukhina ZM, Savenko EG, Korotenko TL, Suprun II, Zheng WJ, Ma ZB, Wang CH (2020) Development of rice pre-breeding resources with blast resistance. E3S Web of Conferences 224:04020. https://doi.org/10.1051/e3sconf/202022404020
Ronald PC, Beutler B (2010) Plant and animal sensors of conserved microbial signatures. Science 330:1061-1064. https://doi.org/10.1126/science.1189468
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406-425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Segonzac C, Zipfel C (2011) Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol 14:54-61. https://doi.org/10.1016/j.mib.2010.12.005
Shiu SH, Bleecker AB (2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 2001:re22. https://doi.org/10.1126/stke.2001.113.re22
Skamnioti P, Gurr SJ (2009) Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol 27:141-150. https://doi.org/10.1016/j.tibtech.2008.12.002
Wang AJ, Shu XY, Jing X, Jiao CZ, Chen L, Zhang JF, Ma L, Jiang YQ, Yamamoto N, Li SC, Deng QM, Wang SQ, Zhu J, Liang YY, Zou T, Liu HN, Wang LX, Huang YB, Li P, Zheng AP (2021) Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome-wide association study. Plant Biotechnol J 19:1553-1566. https://doi.org/10.1111/pbi.13569
Wang HH, Hao JJ, Chen XJ, Hao ZN, Wang X, Lou YG, Peng YL, Guo ZJ (2007) Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol Biol 65:799-815. https://doi.org/10.1007/s11103-007-9244-x
Yang Z, Sun X, Wang S, Zhang Q (2003) Genetic and physical mapping of a new gene for bacterial blight resistance in rice. Theor Appl Genet 106:1467-1472. https://doi.org/10.1007/s00122-003-1205-4
Zhai KR, Deng YW, Liang D, Tang J, Liu J, Yan BX, Yin X, Lin H, Chen FD, Yang DY, Xie Z, Liu JY, Li Q, Zhang L, He ZH (2019) RRM transcription factors interact with NLRs and regulate broad-spectrum blast resistance in rice. Mol Cell 74:996-1009. https://doi.org/10.1016/j.molcel.2019.03.013
Zhang HT, Cao YL, Zhao J, Li XH, Xiao JH, Wang SP (2011) A pair of orthologs of a leucine-rich repeat receptor kinase-like disease resistance gene family regulates rice response to raised temperature. BMC Plant Biol 11:160. https://doi.org/10.1186/1471-2229-11-160
Zhao HJ, Wang XY, Jia YL, Minkenberg B, Wheatley M, Fan JB, Jia MH, Famoso A, Edwards JD, Wamishe Y, Valent B, Wang GL, Yang YN (2018) The rice blast resistance gene Ptr encodes an atypical protein required for broad-spectrum disease resistance. Nat Commun 9:2039. https://doi.org/10.1038/s41467-018-04369-4
Zheng WJ, Ma L, Zhao JM, Li ZQ, Sun FY, Lu XC (2013) Comparative transcriptome analysis of two rice varieties in response to rice stripe virus and small brown planthoppers during early interaction. PLoS One 8:e82126. https://doi.org/10.1371/journal.pone.0082126
Zheng WJ, Wang Y, Wang LL, Ma ZB, Zhao JM, Wang P, Zhang LX, Liu ZH, Lu XC (2016) Genetic mapping and molecular marker development for Pi65(t), a novel broad-spectrum resistance gene to rice blast using next-generation sequencing. Theor Appl Genet 129:1035-1044. https://doi.org/10.1007/s00122-016-2681-7
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31772107), the Liao Ning Revitalization Talents Program (No. XLYC1808003) and Liaoning Key Agricultural Program (2019JH1/10200001-2). I would like to thank Dr. Zhenxing Zhu of Liaoning Academy of Agricultural Sciences for his selfless help throughout the process of research implementation and article modification.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31772107), the Liao Ning Revitalization Talents Program (No. XLYC1808003) and Liaoning Key Agricultural Program (2019JH1/10200001-2).
Author information
Authors and Affiliations
Contributions
LW, WZ, DM, and ZM conceptualized and designed the experiments; MZ, SG, and CW prepared the materials. LW conducted the experiment; LW, ZM, MZ, SG, ZM and CW acquired data; LW, ZM, HW, YB and GS analyzed and interpreted the data; LW drafted the manuscript; and LW, ZM, MZ, SG, ZM, CW, HW, YB, GS, DM and WZ read, revised and approved the manuscript. All authors edited, reviewed, and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Data availability
The data sets generated and analyzed in this study are available upon reasonable request from the corresponding authors.
Additional information
Communicated by Beat Keller.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Ma, Z., Kang, H. et al. Cloning and functional analysis of the novel rice blast resistance gene Pi65 in japonica rice. Theor Appl Genet 135, 173–183 (2022). https://doi.org/10.1007/s00122-021-03957-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-03957-1