Abstract
NAC proteins constitute one of the largest families of plant-specific transcription factors and play an important role in biological processes, including plant development and phytohormone homeostasis, and in responses to various environmental stresses. In this study, we isolated an NAC group A gene (named NtNAC2) from Nicotiana tabacum L. Quantitative RT-PCR (qRT-PCR) analysis indicated that NtNAC2 was significantly upregulated under drought stress, which implied NtNAC2 was important in tobacco under such conditions. Overexpression of NtNAC2 in tobacco plants exhibited enhanced drought tolerance by means of improved seedling growth. Under drought stress, organic osmoprotectants were significantly accumulated in these plants. Additionally, the activities of antioxidant defense enzymes, like superoxide dismutase (SOD) and peroxidase (POD), which could effectively scavenge accumulated reactive oxygen species (ROS), increased in NtNAC2-overexpression transgenic tobacco plants compared with wild-type plants. The net photosynthetic rate was also significantly increased in NtNAC2-overexpression transgenic lines compared with wild-type plants, and the content of malondialdehyde (MDA) and proline was lower in NtNAC2-overexpression transgenic lines than that in wild-type plants (P < 0.01). Furthermore, the expression of NtWRKY28, a drought resistance gene, was significantly increased and the δ-OAT gene was downregulated in NtNAC2-overexpression plants relative to wild-type plants. Taken together, these results indicated that NtNAC2 functions as a positive regulator of drought stress tolerance. This study provides a basis for further study of drought resistance conferred by the NtNAC2 gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth and development are often adversely affected by different stresses during growth and developmental processes, including drought, high temperature, cold, and salinity (Long et al. 2013). Among these, drought is one of the most significant abiotic stresses, limiting plant growth, development, and productivity (Thirumalaikumar et al. 2017). At the cellular level, one of the first responses of plants to drought stress is the production of reactive oxygen species (ROS), thus affecting the equilibrium of the redox state and resulting in oxidative stress. Such disruptions to redox homeostasis manifest as severe cellular damage due to peroxidation, a decline in photosynthetic efficiency, reduced cell membrane stability, and leaf wilting (Carvalho 2008; Choudhury et al. 2013; Benjamin and Nielsen 2006; Hanin et al. 2011).
In order to adapt to the stresses conferred by water deficits, plants have developed molecular mechanisms that coordinate expression of numerous genes that serve to protect them from such environmental adversities (Agarwal et al. 2006). The different expression levels of regulatory genes induced under various stresses protect cells from stresses by promoting the appropriate stress response. Typical regulatory genes encode transcription factors (TFs) and protein kinases (Nakashima 2006; Umezawa et al. 2006). Numerous studies have demonstrated that TFs play vital roles in plant gene regulation by either activating or preventing target gene expression (Puranik et al. 2012; Nakashima et al. 2012). These TFs include members of the NAC, MYB, AP2/ERF, WRKY, and bZip families (Wang et al. 2017). The NAC (NAM, ATAF, and CUC) transcription factor (NAC-TF) genes belong to a large family of TFs that encode important regulatory proteins in plants (Wang et al. 2011). The main structural features of these transcription factors include an N-terminal containing a conserved NAC domain of approximately 150 amino acid residues and a C-terminal that functions as a highly variable transcriptional regulatory region (Olsen et al. 2005). NAC TFs play vital roles in regulating plant growth and developmental processes, including abiotic stress responses (Shao et al. 2015). A few NAC genes, such as AtNAC072 (RD26), AtNAC019, and AtNAC055 from Arabidopsis and BnNAC from Brassica, have been shown to be involved in the response to various environmental stresses. Transgenic plants overexpressing three different Arabidopsis NAC genes (ANAC019, ANAC055, and ANAC072) demonstrate significantly increased drought tolerance (Tran et al. 2004). Additionally, Liu et al. (2016a, b) reported that CarNAC6 is induced by drought and functions to enhance drought tolerance in Arabidopsis. Some NAC genes, such as ATAF1 and ATAF2 from Arabidopsis and StNAC from potato, are induced by pathogen attack and wounding. Huang et al. (2015) cloned the novel NAC transcription factor gene TaNAC29 from wheat, which was shown to be upregulated by high salinity, dehydration, ABA, and H2O2 treatments. TaNAC29 enhances tolerance to high salinity and drought stress in transgenic Arabidopsis and exhibits hypersensitivity to ABA. Hifzur et al. (2016) reported that overexpression of the NAC 67 transcription factor from finger millet conferred tolerance against salinity and drought stress in rice.
NAC proteins are plant-specific TFs, and the NAC family has been recently reviewed by Puranik et al. (2012). At present, a number of studies have presented bioinformatic analyses on the NAC transcription factors in tobacco. For example, Tian et al. (2009) cloned the NAC transcription factor NtNAC1 from tobacco and analyzed its physicochemical properties, advanced structure, and biological functions. Die et al. (2011)cloned NtNAC8 from tobacco, and bioinformatic analyses indicated that NtNAC8 may act as a regulatory enzyme related to intermediate metabolism and related responses in plants. At present, the research regarding NAC transcription factors is still limited to structure identification and expression analysis, with the biological role of specific NAC transcription factors still requiring functional verification.
In this study, we assessed the role of NAC transcription factors in relation to drought resistance in tobacco, and a new NAC gene, NtNAC2 (Nicotiana tabacum NAC2), was isolated and characterized. We found that NtNAC2 was induced by drought stress, and over-expression of NtNAC2 in transgenic plants conferred significantly improved drought tolerance under water-deficit conditions. Our results provide a reference for further research regarding the biological function of NtNAC2 and the signal transduction pathways influenced by this transcription factor. Our results also provide a starting point for screening and breeding new tobacco germplasm with high quality and drought tolerance with the goal of an overall improvement in crop stress resistance.
Experimental Procedures
Plant Material and Growth Conditions
Tobacco (Nicotiana tabacun ‘Xanthi’) was sown in pots containing a 3:1 mixture of nutrient soil:perlite (v/v) in a growth chamber under a 16-h light/8-h dark photoperiod, 25 °C, and 10–20% relative humidity and with photosynthetically active radiation of 200 μmol m−2 s−1. Plants were cultivated for 3–4 weeks before treatment.
Tobacco seeds were used for transformation in this study, and T4 NtNAC2-overexpressed transgenic tobacco lines were used for drought stress treatments. Tobacco seeds were surface sterilized with 75% ethanol for 30 s and H2O2 for 10 min and then rinsed four times with sterile water. Sterilized seeds were sown on nutrient agar plates containing 0.5 × MS medium at pH 5.8 and containing 30% (w/v) sucrose and 0.8% (w/v) agar. Seedlings were grown in a chamber at 22/23 °C (day/night), with photosynthetically active radiation of 200 μmol m−2 s−1, a photoperiod of 16/8 h (day/night), and 60% relative humidity. Seedlings were grown on this medium for 20 days before being transplanted into nutrient soil.
Drought Tolerance Assays
For the drought treatment, the 3-week-old T1 transgenic tobacco plants were grown with 20% polyethylene glycol (PEG) 6000 (w/v) for 7 days. The phenotype of the seedlings was recorded with photographs. A total of 50 surface-sterilized seeds from homozygous T0 transgenic lines and non-transgenic (NT) tobacco plants were germinated on MS medium at pH 5.8 and containing 30%(w/v) sucrose and 0.8% (w/v) agar and containing different mannitol concentrations (0, 150, and 300 mmol/l) at 25 °C with a 16-h light/8-h dark photoperiod. Each experiment was repeated at least three times with similar results. The seedling growth was measured by calculating the root length of ten seedlings at the end of a 2-week germination.
Total RNA Isolation
Young fresh leaves (0.1 g) were harvested, and total RNA was extracted using a HP Plant RNA Kit (R6837-02; Omega, USA). Each reverse transcription reaction was performed with total RNA (1.5 μg) using a M-MLV reverse transcriptase kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions.
Cloning of NtNAC2 and Construction of Prokaryotic and Plant Expression Vectors
The cDNA fragment of the NtNAC2 open reading frame was from Nicotiana tabacum. Using the reported tobacco NAC transcription factor, a Blastp search was performed in the GenBank database to obtain the cDNA and amino acids of tobacco homologs. Primers were designed based on the obtained cDNA sequences using the software Premier 5.0 (the primer sequences are shown in Table 1). Reverse transcription was performed to obtain a cDNA template. The PCR product was cloned into the pMD19-T vector (TaKaRa, Dalian, China) and sent for sequencing (Shanghai Ying Weijie Biotechnology Company, China). The plasmid pSH737 was digested with HindIII and XbaI, and the expression sequence of KpnI and EcoRI double digested sites was digested with KpnI and EcoRI. The expression vector pSH737-NtNAC2 was obtained using T4 DNA ligase.
Identification of the NtNAC2 Gene by Agrobacterium-Mediated Transformation of Tobacco and Molecular Identification
Tobacco seedlings (3 to 4 weeks old) were transformed with Agrobacterium strain EHA105 in which 35S::NtNAC2 was harbored using the tobacco leaf disc transformation method (Horsch et al. 1985). After taking the leaves of tobacco seedlings, a DNA extraction kit (Biochemical Technology Co., Ltd., Beijing, China) was used to extract DNA for PCR detection.
Quantitative RT-PCR Analyses
Total RNA was isolated from the young fresh leaves of tobacco (NT and transgenic lines) under stress, and the cDNA was synthesized according to the above procedure. Quantitative RT-PCR was carried out using an ABI 7500 real-time PCR system (Applied Biosystems, USA). Detection of qRT-PCR products was performed via staining with a QuantiNova SYBR Green PCR kit (Cat. 208054; Qiagen, Germany). The primer sequences are shown in Table 2.
Determination of Physiological Indicators
Determination of Enzyme Activity and Related Products of the Oxidation Pathway
Plants were treated with simulated drought stress (7 days), and superoxide dismutase (SOD) and peroxidase (POD) were measured with a test kit (Suzhou Keming Biotechnology Co, Ltd., China). The contents of malondialdehyde (MDA) and proline were measured as per the kit’s instructions.
Measurement of the Net Photosynthetic Rate and Transpiration Rate in Transgenic Tobacco
Using an L1-6400XT portable photosynthesis measurement system, mature leaves under the same growth conditions and at the same parts were selected, and the indexes were measured at 10:00–14:00 on sunny days. Each leaf position was measured three times.
Statistical Analysis
All data were expressed as mean ± standard error. For root length, ten seedlings were measured; for physiological parameter assays, three replicates were measured; and for qRT-PCR, three biological replicates were assessed. Microsoft Excel and GraphPad Prism 5.0 software were employed for data analysis. One-way ANOVA post hoc Duncan’s multiple range test was used for multiple variable comparisons. Any difference relative to the control at *P < 0.05 or **P < 0.01 was considered significant.
Results and Discussion
Cloning and Characterization of NtNAC2 in Tobacco
The open reading frame of NtNAC2 was obtained from tobacco (GenBank accession#: XM_016579112). Based on the sequence information, the NtNAC2 gene was predicted to be 1029 bp in length and encode 342 putative amino acids. The cloned NtNAC2 sequence was consistent with the database coding sequence.
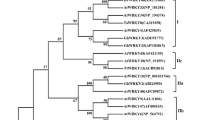
To reveal the evolutionary relationship of NtNAC2 from various plant species, we constructed a phylogenetic tree using amino acid sequences derived from the GenBank database. Blastp analysis showed that NtNAC2 shared a high degree of sequence similarity with Solanum tuberosum (XP_006364781.1) and Capsicum annuum (XP_016543940.1) during evolution (Fig. 1).
Phylogenetic tree analysis of NtNAC2 in tobacco and other species. Sequence alignment was performed using Clustal X software, and the phylogenic tree was created and visualized using MEGA 5. Protein sequences used for alignment were as follow: Capsicum annuum (XP_016543940.1), Solanum tuberosum (XP_006364781.1), Ipomoea nil (XP_019187747.1), Coffea canephora (CDP_06226.1), Vitis vinifera (XP_002283240.1), Erythranthe guttata (XP_012857403.1), Citrus clementina (XP_006430510.1), Populus trichocarpa (XP_002322608.1), Citrus sinensis (XP_006482042.1), Beta vulgaris subsp. vulgaris (XP_010673076.1), Glycine max (XP_003554106.1), Dorcoceras hygrometricum (KZV_47569.1), Glycine soja (KHN_08910.1), Spinacia oleracea (XP_021864005.1), Eucalyptus grandis (XP_010055671.1), Amborellatrichopoda (XP_006836242.2), Lupinus angustifolius (OIW_09475.1), Hevea brasiliensis (XP_021673382.1), Helianthus annuus (XP_022001413.1), Theobroma cacao (XP_007028263.1), and Cicer arietinum (XP_004493365.1)
Overexpression of NtNAC2 in Tobacco Regulates Root Growth under Drought Stress
To test whether NtNAC2 was involved in drought tolerance in tobacco, we generated eight independent NtNAC2-overexpression transgenic lines of tobacco (designated as NtNAC2 lines) (Fig. 2), and we selected three transgenic lines (5, 11, 15) with NtNAC2 transcript levels for further investigation.
The expression level of NtNAC2 in transgenic tobacco plants. The expression of NtNAC2 was analyzed via RT-PCR in NtNAC2 overexpression and WT plants grown under control conditions. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified as a control for the amount of template. WT wild type; transgenic lines: 1,2,4,5,11,12,15, and 18
The root system is the main organ of the plant that serves to absorb nutrients and moisture (Yang et al. 2016) and is the earliest part of the plant that responds to drought. Therefore, enhancing the drought resistance of roots can effectively increase the rational distribution of water to plants, reduce the damage caused by drought, and increase the plants’ survival rate under stress conditions (Tian 2016). To further understand the role of NtNAC2 in the stress response, we collected the seeds of transgenic lines for root growth experiments. Seed germination and seedling growth of T0 generation plants revealed that there was no significant difference between WT plants and the transgenic lines in the absence of mannitol treatment, though the root length of the control plants was longer than that of the transgenic plants (Fig. 3a). Under treatment with 150 mmoL L−1 mannitol, the root lengths of the three transgenic lines respectively were 23, 22, and 17% longer than those of the WT plants. Following treatment with 300 mmoL L−1 mannitol, the root lengths of the three transgenic lines were 28, 31, and 18% longer, respectively, than those of the WT plants (Fig. 3b). The root growth of the transgenic lines was significantly better than that of WT plants. This result indicated that the transgenic tobacco with the NtNAC2 gene has a more developed root system than wild type to absorb water and nutrients to compensate for the arid environment. Therefore, overexpression of NtNAC2 strengthened the drought resistance of tobacco roots.
a Root development of NT and T0 transgenic lines on MS medium containing different concentrations of mannitol. Values are means ± SE of four (root) replicates. b Control and transgenic plant roots under different concentrations of mannitol. WT wild type; transgenic lines TP-5, TP-11, and TP-15. The same is shown in Fig. 4
Overexpressing NtNAC2 in Tobacco Promotes Enhanced Drought Tolerance
Plants change in response to the external stress conferred by drought. The lack of water balance in plants leads to decreased cell water potential and turgor pressure, resulting in the occurrence of sagging and wilting in the stem and leaves. Prolonged drought duration leads to severe water loss from the plant cell protoplasts, leading to plant death (Sheng et al. 2017). In order to observe phenotypic differences under control conditions and drought, we tested the growth of WT and NtNAC2-overexpression plants under simulated drought stress. A total of 31 positive transgenic lines were obtained, and two homozygous transgenic lines (TP5 and TP15) were selected for these drought stress assays. The results indicated that there was no significant difference in the growth status or phenotype between NtNAC2-overexpression plants and WT plants under the control conditions (Fig. 4a). However, after 7 days of water stress induced by 20% PEG 6000, the leaves of WT plants were dehydrated and seriously weathered and yellowed, while the leaves of the transgenic plants appeared normal and fresh (Fig. 4b), indicating that overexpressing NtNAC2 in tobacco serves to maintain water balance in plants, helping the plant grow normally under such conditions.
Phenotypic differences between WT and transgenic tobacco seedlings under drought stress. a Photographs were taken under normal conditions. There was no significant difference in phenotypes between transgenic and control groups. Three-week-old seedlings grown at 22 °C under long days (16-h light/8-h dark). b Three-week-old seedlings watered with 20% PEG once a day. Photographs were taken 7 days after drought stress treatment. Photographs of NtNAC2-overexpression plants and WT plants grown under PEG treatment. Transgenic plants maintained normal growth; the control group suffered environmental damage that led to wilted leaves. These observations indicated increased drought tolerance in NtNAC2-overexpression plants
NtNAC2 Influences the Expression of Stress-Responsive Genes
Plants adapt to stress conditions by regulating the expression of a large number of stress-related genes. Several stress-responsive transcription factors, such as those in the myeloblastosis (MYB), WRKY, NAC, and bZIP families, are involved in plant abiotic stress responses, and some transcription factor genes have also been engineered to improve stress tolerance in model plants and crops (Li et al. 2017).
To investigate the expression pattern of NtNAC2 and related genes under abiotic stresses, we examined the transcript accumulation of NtNAC2 and some typical stress response genes, including δ-OAT and NtWRKY28, via qRT-PCR. The expression of NtNAC2 in NtNAC2-overexpressed transgenic tobacco lines (especially in TP-5) was 7.0 times higher than that in WT plants when exposed to PEG (Fig. 5a), and the expression of δ-OAT in NtNAC2-overexpressed transgenic tobacco was 0.29 times and 0.83 times lower than that in WT plants under these conditions (Fig. 5b). The expression of NtWRKY28 was significantly increased in transgenic tobacco (in TP-5), reaching up to 3.3 times and 2.3 times higher than that in WT plants (Fig. 5c). These results indicated that overexpression of NtNAC2 in tobacco could significantly induce the expression of NtWRKY28 and decrease the expression of δ-OAT, suggesting that NtNAC2 may be involved in the process of osmotic adjustment during drought stress and might function to improve drought tolerance in tobacco.
Expression patterns of NtNAC2 and some typical stress response genes, δ-OAT and NtWRKY28, in transgenic tobacco lines under drought stress, as assessed by quantitative RT-PCR. a Overexpression of NtNAC2 in transgenic tobacco lines (especially in TP-5) was dramatically enhanced. b The expression of δ-OAT was decreased. c Overexpression of NtNAC2 in tobacco plants could significantly induce the expression of NtWRKY28. Total RNA was isolated from tobacco leaves at the indicated treatment time points. PEG polyethylene glycol 6000 treatment. Data are mean ± SD calculated from three replicates
Effects of NtNAC2 Overexpressed in Tobacco on the Activities of Antioxidant Enzymes and Osmoprotectants
Reactive oxygen species (ROS) homeostasis is a key concern for plants, and plants have evolved an active antioxidant defense, including SOD, POD, and the synthesis and accumulation of osmoprotectants such as proline (Ashraf and Foolad 2007; Molinari et al. 2007), to remove excessive ROS. SOD converts superoxide radicals (O2−) into hydrogen peroxide (H2O2), and POD converts H2O2 to water on various substrates, such as electron donors. MDA is the final product of plant cell membrane lipid peroxidation and is one important marker of membrane system injury (Sun et al. 2010). Wang et al. (2009) found that the increase of alfalfa membrane permeability was significantly and positively correlated with the accumulation of MDA, while MDA accumulation was negatively correlated with the amount of growth.
The activation of antioxidant systems is often related to transcription factors. To investigate the role of NtNAC2 in antioxidant defense under stress conditions, the content of MDA and proline and the activities of the antioxidant enzymes SOD and POD were analyzed in NtNAC2-overexpression tobacco plants exposed to water stress. Under 20% PEG 6000 treatment, the SOD activities of the transgenic plants were 124.9, 130.3, and 105.6 U g−1 (FW), respectively, and the average SOD activity of WT plants was only 65.73 U g−1 (FW), indicating that the SOD activity of transgenic plants was 82.99% higher than that of the control (Fig. 6a). The POD activities of transgenic plants were 393.9, 445.1, and 415.3 U g−1 (FW), respectively, and that of WT plants was 312.4 U g−1(FW), indicating that the POD activity of transgenic plants was 33.81% higher than that of WT plants (Fig. 6b). The MDA content in WT plants was 8.6 μmol g−1 on average, and in the transgenic plants, it was 4.1, 2.3, and 3.2 μmol g−1, respectively. As such, the MDA content in the transgenic plants was 57.1% lower than that in the control (Fig. 6c) (FW). The proline content of WT plants was 256.2 μg g−1, and in the transgenic plants, it was 35.1, 76.1, and 74.6 μg g−1 (FW), respectively, indicating that the proline content was 75.8% lower than that in WT plants (Fig. 6d). Proline is an important osmotic regulator in plants, and increasing proline content under drought stress helps maintain osmotic regulation, enhances cell membrane system stability, and scavenges ROS. When the proline content is low, this indicates that plants are not subject to minor drought stress and drought has less of an impact on the plant. The membrane system defense enzymes SOD and POD were overexpressed in the transgenic tobacco, serving to resist stress and senescence under drought conditions. The MDA content was lower in the transgenic tobacco than WT, indicating that the membrane system of the transgenic tobacco was intact, indicating that the cell membrane of wild-type tobacco was seriously damaged. Together, the above results indicate that the transgenic tobacco plants were more resistant to drought than the WT plants.
The SOD and POD activity and MDA and proline (PRO) content of transgenic and wild-type tobacco plants under 20% PEG 6000 treatment. Analysis of SOD (a) and POD (b) activities in WT and transgenic lines (TP-5, TP-11, and TP-15) under drought conditions. Twenty-day-old tobacco plants were deprived of water for 7 days, and leaves were then collected to detect SOD and POD activities. SOD activity and POD activity in the transgenic lines were both significantly higher than those in the control. c Content of the MDA in transgenic lines and WT tobacco under drought stress for 7 days. d Content of free proline in transgenic lines and WT tobacco under drought stress for 7 days. The contents of MDA and proline in the transgenic plants were significantly lower than in the control plants, indicating that the transgenic plants received less drought stress. Data are mean ± SD calculated from three replicates. Asterisks indicate a significant difference between the WT and the two transgenic lines (*P < 0.05, **P < 0.01). Three biological replicates were performed, which produced similar results
Overexpression of NtNAC2 in Tobacco Enhances the Net Photosynthetic Rate
Photosynthesis is an important metabolic process for crop growth and is a major source of material and energy for plant growth and development. Improvements in plant water use and efficiency under drought stress is an important approach for enhancing plant drought tolerance. Previous studies have suggested that improving water use efficiency in plants can be achieved by increasing the net photosynthetic rate and by inducing stomatal closure to reduce stomatal conductance. Under drought stress, stomatal opening is inhibited and water dissipation is reduced, so the final plant water retention capacity is increased, resulting in an increased tolerance of plants to drought (Guo et al. 2014). In this current study, the results of net photosynthetic rate analysis showed that this rate was higher in the transgenic plants compared to that in WT plants, with the net photosynthetic rate of the transgenic plants substantially higher than that of WT over the whole day (Fig. 7a). These results indicated that the expression of NtNAC2 improved the photosynthetic characteristics in tobacco and helped maintain strong photosynthesis under drought conditions, serving to maintain plant growth and development. The level of CO2 was 16.27% lower in the transgenic tobacco plants compared to WT (Fig. 7c), but the net photosynthetic rate was 107.45% higher in the transgenic tobacco plants compared to WT, which indicated that the transgenic tobacco plants had a better ability to maintain water than the WT plants.
Conclusions
NAC TFs act as pivotal transcriptional regulators in the adaption of plants to environmental stresses. NAC genes have not been found in yeast, but they have been identified in dicotyledons and monocotyledons (Liu et al. 2018). A variety of information has been learned regarding NAC TFs since their discovery; however, research in this area is still in its infancy (Shao et al. 2015). Based on a review of the literature, few tobacco NAC genes have been verified to function in abiotic stress tolerance. To further study the function of the NAC TFs in tobacco, we isolated a NAC gene, named NtNAC2, from Nicotiana tabacum (K326) and demonstrated that NtNAC2 actively regulates tolerance to drought stresses in transgenic tobacco plants.
Han et al. (2014) cloned the NtNAC2 gene and analyzed its structure and tissue-specific expression. Construction of a phylogenetic tree suggested that all known NAC proteins involved in abiotic stress tolerance are closely related. The NtNAC2 protein was extremely similar to the NAC proteins RD26, ANAC055, and ANAC019, which all belonged to the RD26 sub-group. The RD26 gene was studied in Arabidopsis thaliana and reported to be induced by drought, and members of this sub-group are highly expressed when plants face conditions of biotic or abiotic stress (Fujita et al. 2004).
In this study, we selected three transgenic lines (5, 11, 15) for root growth experiments under drought stress and drought resistance in T1 tobacco plants. We observed that the transgenic plants had stronger drought tolerance, which could be explained by various indicators. Under different levels of drought stress (150 and 300 mmoL L−1 mannitol), the roots of the transgenic plants were longer. This result is consistent with the results of Liu et al. (2016a). The roots of drought-resistant plants are longer than those of the control under drought stress. Our test results also show that the net photosynthetic rate and stomatal conductance of the transgenic plants were larger than those of WT. In addition, the increased drought resistance of the transgenic plants could be explained by the increased expression of drought-resistant genes. Both the NAC and WRKY transcription factors play important roles to improve abiotic and biotic stress in transgenic plants (Erpen et al. 2018). Our results show that both NtNAC2 and NtWRKY28 expression are upregulated in transgenic tobacco, indicating that they played the active roles under drought stress. NtNAC2-overexpression tobacco plants showed higher activity levels of SOD and POD under drought stress conditions, and SOD and POD are beneficial to the maintenance of ROS levels during drought, and the content of MDA was lower in these transgenic plants than that in WT. Antioxidant enzyme activity results are consistent with Ning et al. (2017), which indicated that overexpression of NtNAC2 gene enhances the tobacco stronger drought tolerance. The above observations indicate that NtNAC2 may be involved in the process of osmotic adjustment in tobacco plants during drought stress and likely functions to improve plant drought tolerance.
Results of this study indicate that NtNAC2 may serve as a novel target for engineering drought tolerance in crop plants and highlights important candidate genes involved in the stress response of crops. Additionally, by using genetic engineering methods, this study has opened up a possibility toward improving the drought resistance of tobacco and has provided a molecular basis for improving the drought resistant of other crops.
References
Agarwal PK, Agarwal P, Reddy MK, Sopory S (2006) Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep 25(12):1263–1274
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant biotic stress resistance. Environ Exp Bot 59(2):206–216
Benjamin JG, Nielsen DC (2006) Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crop Res 97(2):248–253
Carvalho MHC (2008) Drought stress and reactive oxygen species. Plant Signal Behav 3:156–165
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8(4)
Die HU, Liu H, Chen DM, Tian Y (2011) Cloning and sequence analysis of the NAC transcription factor NtNAC8 from tobacco. Journal of Huaihua University 30(05):33–37
Erpen L, Devi HS, Grosser JW, Dutt M(2018) Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tissue Organ Cult 132(1):1–25
Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M,Tran LS, Yamaguchi-Shinozaki K, Shinozaki K(2004) A dehydration-induced nac protein, RD26, is involved in a novel aba-dependent stresssignaling pathway. Plant Journal 39(6):863–876
Guo GH, Liu HY, Li GH, Liu M, Li Y, Wang SH, Liu ZH, Tang S, Ding YF (2014) Analysis of physiological characteristics about ABA alleviating rice booting stage drought stress. Chinese Journal of Agricultural Sciences 47(22):4380–4391
Han QQ, Qiao P, Song YZ, Zhang JY (2014) Structural analysis and tissue-specific expression patterns of a novel salt-inducible NAC transcription factor gene from Nicotiana tabacum cv. Xanthi. Journal of Pomology & Horticultural Science 89(6):700–706
Hanin M, Brini F, Ebel C, Toda Y, Takeda S, Masmoudi K (2011) Plant dehydrins and stress tolerance: versatile proteins for complex mechanisms. Plant Signal Behav 6(10):1503–1509
Hifzur R, Valarmathi R, Jagedeeshselvam N, Sudhakar D, Raveendran M (2016) Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol 1(1):35
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Huang QJ, Wang Y, Li B, Chang JL, Chen MJ, Li KX, Yang GX, He GY (2015) TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol 15(1):268
Li Y, Chen Q, Nan H, Li X, Lu S, Zhao X, Liu B, Guo C, Kong F, Cao D (2017) Overexpression of GmFDL19 enhances tolerance to drought and salt stresses in soybean. PLoS One 12(6):e0179554
Liu Y, Yao XZ, Lu LT, Lei YT, Dai TT, Zhao DG (2016a) Cloning of Sb SKIP gene from sorghum (Sorghum bicolor) and analysis of drought-resistant function in tobacco (Nicotiana tabacum). J Agric Biotechnol 24(10):1500–1511
Liu YM, Yu XW, Liu SS, Peng H, Mijiti A, Wang Z, Zhang H, Ma H (2016b) A chickpea NAC-type transcription factor, CarNAC6, confers enhanced dehydration tolerance in Arabidopsis. Plant Mol Biol Report 35(1):1–14
Liu H, Zhou Y, Li H, Wang T, Zhang J, Ouyang B (2018) Molecular and functional characterization of shnac1, an NAC transcription factor from solanum habrochaites. Plant Sci 271:9–19
Long L, Gao W, Xu L, Liu M, Luo X, He X, Yang X, Zhang X, Zhu L (2013) GbMPK3, a mitogen-activated protein kinase from cotton, enhances drought and oxidative stress tolerance in tobacco. Plant Cell Tissue Organ Cult 116(2):153–162
Molinari HBC, Marur CJ, Daros E, Campos MKF, Carvalho JFRP, Bespalhok-Filho JC, Pereira LFP (2007) Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant 130(2):218–229
Nakashima K (2006) Regulons involved in osmotic stress-responsive and cold stress-responsive gene expression in plants. Physiol Plant 126(1):62–71
Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819(2):97–103
Ning W, Zhai H, Yu J, Liang S, Yang X, Xing X (2017) Overexpression of glycine soja WRKY20, enhances drought tolerance and improves plant yields under drought stress in transgenic soybean. Mol Breed 37(2):19
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10(2):79–87
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17(6):369–381
Shao H, Wang H, Tang X (2015) NAC transcription factors in plant multiple abiotic stress responses: progress and prospects. Front Plant Sci 6:902
Sheng SY, Wu YX, Zheng YS (2017) Review on drought response in plants from phenotype to molecular. Curr Biotechnol 7(3):169–176
Sun CH, Du W, Cheng XL, Xu XN, Zhang YH, Sun D, Shi JJ (2010) The effects of drought stress on the activity of acid phosphatase and its protective enzymes in pigweed leaves. Afr J Biotechnol 9(6):825–833
Thirumalaikumar VP, Devkar V, Mehterov N, Ali S, Ozgur R, and Turkan I et al. (2017) Nac transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnol J 16(2):354–366
Tian Y (2016) The responding mechanism of physiology and proteomics in Prunus mira root under drought. Northeast Forestry University, Haerbin Shi
Tian Y, Fang J, Xiang-Yang LU (2009) Cloning and bioinformatics analysis of nac transcription factor ntnac1 in tobacco. Acta Tabacaria Sinica 15(5):67–72
Tran L-SP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Isolation and functional analysis of Arabidopsis stress-inducible zinc finger homeodomain transcription factor ZFHD1: role of the ZFHD1 and NAC transcription factors that bind to a drought-responsive ciselement in the early responsive to dehy-dration stress 1 promoter. Plant Cell Physiol Suppl 16:2481–2498
Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol 17(2):113–122
Wang WB, Kim YH, Deng XP, Kwak SS, Wang ZH, Zhao ZP (2009) Physiological and biological responses of alfalfa shoots and roots to salt stress. J Northwest A & F Univ 37(5):217–223
Wang H, Zhao Q, Chen F, Wang M, Dixon RA (2011) NAC domain function and transcriptional control of a secondary cell wall master switch. Plant J 68(6):1104–1114
Wang Y, Wang Q, Liu ML, Bo C, Wang X, Ma Q (2017) Overexpression of a maize MYB48 gene confers drought tolerance in transgenic arabidopsis plants. Journal of Plant Biology 60(6):612–621
Yang ZQ, Qiu YX, Liu CX, Chen YQ, Tan W (2016) The effects of soil moisture stress on the growth of root and above-ground parts of greenhouse tomato crops. Chinese Journal of Ecology 36(3):748–757
Acknowledgments
This work was funded by the National Natural Science Foundation (No. 31160149) and by the Major Projects of National New Varieties of Genetically Modified Organisms.
(No. 2014ZX08010-003-2016ZX08010-003). The authors thank Litang Lu for the technical assistance.
Author Contribution Statement
XX and YX designed and conducted the experiments. XX wrote the manuscript. LL contributed by helping with some experiments presented in the manuscript. LL helped to edit the manuscript. LL and ZD supervised the studies and revised the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, X., Yao, X., Lu, L. et al. Overexpression of the Transcription Factor NtNAC2 Confers Drought Tolerance in Tobacco. Plant Mol Biol Rep 36, 543–552 (2018). https://doi.org/10.1007/s11105-018-1096-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-018-1096-9