Abstract
Aims
To unfold the full potential of plant growth-promoting rhizobacteria in crop production it is desirable to explore the mechanisms through which they promote growth. We investigated the potential mechanisms of plant growth promotion by Streptomyces rochei S32 in two crops.
Methods
The effects of S. rochei S32 on plant growth and its antagonistic activity against soil-borne pathogenic fungi were tested. The underlying molecular mechanisms were identified based on whole-genome sequencing and bioinformatics analysis. The results of genomic analysis were verified by widely targeted metabolomics and mechanism studies of plant growth promotion.
Results
S. rochei S32 significantly improved the growth of wheat and tomato. The shoot length (24.7%) and root length (25.3%) of wheat (400-fold dilution of cell-free fermentation filtrate) were increased, and the root length of tomato (200-fold dilution) was prolonged (40.9%), and the field yield was also increased. S. rochei S32 showed antagonistic activity against multiple pathogenic fungi, especially Macropoma kawatsukai. The bacterial genome contains an 8,041,158-bp chromosome and two plasmids. A total of 7486 annotated genes were classified into 31 Gene Ontology functional categories. Genomic analysis revealed the potential for the production of indole-3-acetic acid, fungal cell wall hydrolases, antibiotics (e.g., candicidin, streptothricin, borrellin, albaflavenone), and siderophores. Thirty-nine phytohormones and 2205 secondary metabolites were detected, including indole-3-acetic acid, phytosphingosine, acivicin, and corynebactin. Normal bacterial growth occurred on a nitrogen-free medium.
Conclusion
S. rochei S32 can promote plant growth directly or indirectly through nitrogen fixation and production of phytohormones, extracellular hydrolases, antibiotics, and siderophores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical fertilizers and pesticides are widely used in agricultural production to promote crop growth and control plant diseases. Inappropriate or excessive use of agrochemicals leads to the risk of environmental pollution, disturbs the community structure of beneficial soil microorganisms, and causes food safety problems (Dennis et al. 2018; Nejatzadeh-Barandozi and Gholami-Borujeni 2014; Ye et al. 2020). In contrast with agrochemicals, functional microbial inoculants have the advantages of reduced production costs and environmental impacts, with great potential in improving plant nutrient absorption, growth performance, and disease resistance (Fravel 2005; Safar et al. 2023). In particular, plant growth-promoting rhizobacteria (PGPR) provide alternatives to support the sustainable development of agriculture (Harris et al. 2021; Thilagar et al. 2016).
Streptomyces, a genus of Gram-positive actinobacteria, is ubiquitous in the soil environment and has great potential for agricultural application (Gillon et al. 2023). The filamentous morphology and sporulation ability of Streptomyces species allow them to survive in adverse environments and outcompete other species for nutrients and space (Vurukonda et al. 2018). Most known species of Streptomyces can enhance plant growth and resistance to biotic and abiotic stresses by producing various phytohormones and antibiotics (Kim et al. 2011). This is exemplified by Streptomyces rochei (Zhang et al. 2015), S. albidoflavus (Yao et al. 2021), and S. angustmyceticus (Luo et al. 2023), which play a role in plant growth promotion and disease control in different crops. Rational application of these Streptomyces spp. as PGPR necessitates an in-depth understanding of the molecular mechanisms underlying plant growth promotion.
Phytohormone biosynthesis is considered to be one of the direct mechanisms for PGPR to promote plant growth (Jamil et al. 2022). For example, Liming et al. (2018) demonstrated the potential of a Streptomyces isolate to increase plant growth by producing indole-3-acetic acid (IAA). IAA is a plant growth regulator that controls cell elongation and apical dominance (Wang et al. 2001). This phytohormone is commonly used as an indicator to study the mechanisms of plant growth promotion by PGPR. Importantly, IAA is synthesized by PGPR through different pathways—tryptophan (TRP) induction pathway and TRP-dependent pathway (Zhao et al. 2001). In Streptomyces, IAA biosynthesis is achieved mainly through the TRP dependent indole-3-acetamide pathway (Manulis et al. 1994). Other direct mechanisms of plant growth promotion by PGPR include the production of 1-aminocyclopropane-1-carboxylic acid (ACC) (Vurukonda et al. 2018) and siderophores (Zhu et al. 2023), as well as nitrogen fixation (Paungfoo-Lonhienne et al. 2014) and organophosphorus solubilization.
PGPR indirectly promote plant growth by inhibiting soil-borne pathogens. Some PGPR strains release extracellular enzymes to alleviate root and stem rot diseases caused by Fusarium oxyspora (Compant et al. 2005), and other strains produce antimicrobial metabolites to impair the growth of pathogenic fungi, bacteria, and nematodes (Fu et al. 2016; Singh et al. 2021). Streptomyces can synthesize various secondary metabolites with a wide range of biological activities, which confers substantial benefits to plant growth and disease resistance. The most prominent secondary metabolites of Streptomyces are antibiotics, such as alkaloids, macrolides, cyclopeptides, terpenoids, and polyketides (Zhang and Qian 2019). Exposure to these antibiotics can hinder the biosynthesis of cell wall components, change the permeability of cell membranes, and inhibit the activity of ribosomes, RNAs, or enzymes in harmful microorganisms (De la Cruz-Rodríguez et al. 2023; Mascher et al. 2003; Siewert and Strominger 1967; Nakamura 1959; Zhang et al. 2023).
The application of S. rochei Sr has been shown to improve plant growth and yield in a marine environment, most likely through the production of IAA and polyamine (Mathew et al. 2020). Field observations in non-marine environments indicate that S. rochei S32 also exhibits positive effects on the growth and yield of various crops, such as tomato (Solanum lycopersicum L.), watermelon (Citrullus lanatus L.), and strawberry (Fragaria × ananassa Duch.). However, whether S. rochei strains adopt the same mechanisms of plant growth promotion in various environments remains unclear. Therefore, this study verified the ability of S. rochei S32 as a PGPR strain to promote plant growth and resistance against soil-borne pathogens through petri dish experiments. The potential mechanisms of plant growth promotion and pathogen antagonism were explored based on whole-genome sequencing and biological function analysis. The results of genomic analysis were confirmed by widely targeted metabolomics and mechanism studies of plant growth promotion.
Materials and methods
Plant growth promotion test
The strain of S. rochei S32 was isolated from the soil of greenhouse watermelon with continuous cropping obstacle in Yangling (Shaanxi Province, China) and preserved in the Laboratory of Resource Microbiology, Northwest A&F University (Yangling). To verify its effect on plant growth, growth-promoting experiments in dishes and field experiments were carried out.
Petri dish growth test: five treatments were used in the experiment, with five replications per treatment. Four treatment groups received the cell-free fermentation broth of S. rochei S32 (i.e., 50-, 100-, 200-, 400-fold diluted), and the control group was added with sterile water. The fermentation broth was prepared in Gauze’s synthetic medium No. 1 (GA) at 28 °C, 160 r/min for 8 d and filtered through a 0.22-μm membrane filter to remove cells. Seeds of wheat (Triticum aestivum L. cv. Xiaoyan22) and tomato (Solanum lycopersicum L. cv. Jinpeng101) were purchased from Jinpeng Seed Co., Ltd. (Xi’an, Shaanxi Province, China). After surface disinfection with sodium hypochlorite (containing 1% chlorine, 15 min), the seeds were washed with sterile water at least five times until odorless. Seeds with full grains were selected and placed uniformly on moistened filter paper in sterile petri dishes (10 seeds per dish). The seeds were cultured in a greenhouse with an average temperature of 28 °C and an average daily illumination of 12 h. Shoot length and root length were measured after 15 d of treatment with the cell-free fermentation broth (10 mL per dish).
The field experiment of wheat (Xiaoyan22) was carried out in Xixiaozhai (107°59′20′′E, 34° 14′40′′N) Village (Yangling, Shaanxi Province, China), from October 2022 to June 2023. Two treatments were set up in the experiment: S32 treatment (1.0 × 105 cfu/grain) and control without bacterial agent (Ctrl). 750 kg/ha complex fertilizer (15% N, 15% P2O5 and 15% K2O) and 150 kg/ha urea was spread at the sowing stage of wheat growth. For field experiments, experimental plots were 150 m2 (= 1.5 × 100 m) and 0.02 kg grains were sowing per 1 m2 using mechanical drilling. The two groups of wheat seeds were sown at the same time, with three replicates for each treatment. After 210 days of sowing, 12 wheat plants were randomly selected to determine the shoot length, shoot dry weight, root dry weight and panicle number. The 1000-grain weight and yield of wheat were measured during the harvest period.
The field experiment of tomato (Provence) greenhouse was carried out in Xixiaozhai (107°59′20′′E, 34°14′40′′N) Village (Yangling, Shaanxi Province, China), from October 2021 to June 2022. Two treatments were set up in the experiment: S32 treatment (1.0 × 1010 cfu/plant) and control without bacterial agent (Ctrl). The substrate seedling was transplanted when the four foliar, and the cultivation mode was one ridge and two rows. Four replicates/ridges for each treatment (28 plants per ridge). During the whole growth period, 1.5 × 104 kg/ha organic fertilizer was applied, and the total amount of N-P2O5-K2O application was 187.5–213.6-82.4 kg/ha. 12 plants were randomly selected for destructive sampling. The shoot length, root length, stem diameter, shoot dry weight and/or root dry weight were measured after 60 days and 160 days of growth. The total tomato yield of each treatment was counted.
Pathogen antagonism test
The plate standoff method was used to test the antagonistic activity of S. rochei S32 against eight plant pathogenic fungi that are commonly found in the northwest region of China (Soliman et al. 2022). Briefly, S. rochei S32 was grown on agar plates of GA medium at 28 °C for 8 d (Xu et al. 2015). The fungal strains (Table 3) were provided by the College of Life Sciences (Northwest A&F University) and cultured on potato dextrose agar (PDA) plates at 30 °C for 5 d (Okull et al. 2003). Agar plugs of S. rochei S32 and pathogenic fungi were prepared under aseptic conditions using an 8-mm punch. One plug of S. rochei S32 was placed at the center of a fresh PDA plate, with three plugs of a given fungus placed around it in a triangular shape. There were three replicates for each fungus and all plates were incubated in an incubator at 28 °C for 5 d. Colony growth was measured to determine the strength of antagonistic activity. The growth inhibition rate of pathogenic fungi was calculated as follows: inhibition rate (%) = (fungal colony radius in the control – fungal colony radius in the S. rochei S32 treatment) / fungal colony radius in the control × 100%.
Whole-genome sequencing, gene annotation, and functional analysis
The whole genome of S. rochei S32 was sequenced using a Pacbio sequel II and DNBSEQ platform at Beijing Genomics Institute (Shenzhen, China). Low-quality and short reads were filtered from the sequencing data before downstream analysis. Clean data were assembled to obtain genomic data using Canu v1.5 (https://github.com/marbl/canu/releases) and GATK v1.6–13 (http://www.broadinstitute.org/gatk/). The whole-genome sequence was used to predict coding genes by Glimmer v3.02 (http://www.cbcb.umd.edu/software/glimmer/). Then, the predicted gene sequences were annotated by comparison against available databases (e.g., CAZy, COG, GO, KEGG, antiSMASH). Comparative gene mapping was conducted using Circos v0.69–9 (Krzywinski et al. 2009).
Functional classification of annotated genes was accomplished based on the Gene Ontology (GO) database (http://geneontology.org/). Prediction of secondary metabolite biosynthesis was performed using the antiSMASH v5.2.0 database (https://antismash-db.secondarymetabolites.org/). Prediction of phytohormone and siderophore biosynthesis was carried out based on metabolic pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG) v101 database (https://www.genome.jp/kegg/). Prediction of chitinase, cellulase, β-1,3-glucanase, and other enzymes related to plant growth promotion and disease resistance was done using the Carbohydrate-active Enzymes (CAZy) database (http://www.cazy.org/).
Secondary metabolite profiling
S. rochei S32 was inoculated to the GA medium without agar and cultured at 30 °C for 8 d with 180 rpm oscillation. The culture sample was filtered through a 0.22-μm microporous filter membrane (Xiangya Instrument, Shanghai, China), and the filtrate was used for widely targeted metabolomics. First, a high-resolution time-of-flight system (Tremsin et al. 2007) was employed to scan the samples with secondary spectra and extract ion-pair information in the multiple reaction monitoring mode. Then, a specific database of the samples was constructed based on the wide-target library. A quadrupole-linear ion trap mass spectrometer (AB Sciex, Pte. Ltd., Singapore) was used to detect the compounds in the library and given a report. Statistical tools such as ANOVA were used to evaluate and process the data.
Phytohormone assay was also conducted with the culture filtrate samples. A methanol/water/formic acid solution (15:4:1, v/v/v) was used for the original extraction of phytohormones (Floková et al. 2014; Li et al. 2016). The extract was concentrated and reconstituted with an 80% methanol/water solution (4:1, v/v), and then filtered through a 0.22-μm microporous filter membrane. The filtrate was used for the detection of phytohormones and their analogues by ultra-performance liquid chromatography/tandem mass spectrometry (Q-TRAP® 6500, AB Sciex Pte. Ltd.). The peak plots of the downstream data were processed using the Analyst v1.6. software 3 (AB Sciex Pte. Ltd.). Qualitative analysis of the compounds in the samples was carried out based on the retention times and peak shapes of the standards. The relative concentration of each compound was calculated from the peak area, and its absolute concentration was calculated from the concentration of the standard.
Mechanism studies of plant growth promotion
Phosphorus and potassium solubilization. S. rochei S32 was inoculated onto agar plates of phosphorus- and potassium-solubilizing media (Yuan et al. 2015). Colony growth was observed after the inoculated plates were incubated in an incubator at 28 °C for 5–7 d. If a transparent circle (i.e., halo) was formed around the bacterial colony, it indicates that S. rochei S32 has the ability to solubilize phosphorus or potassium. The cross method was used to measure the colony diameter (d) and halo diameter (D). The D/d ratio was calculated to indicate the phosphorus- and potassium-solubilizing activity. The higher the D/d ratio, the greater the nutrient-solubilizing activity of S. rochei S32.
Nitrogen fixation. S. rochei S32 was inoculated onto Ashby’s agar plates containing no nitrogen source (Ma et al. 2022). Colony growth was observed and recorded after incubation at 30 °C for 96 h. If colonies formed on the plates, they were transferred to fresh Ashby’s agar plates and continuously subcultured three times. If colonies still formed in the third generation, it was considered that S. rochei S32 has the ability to fix atmospheric nitrogen.
ACC deaminase production. S. rochei S32 was inoculated onto agar plates of ADF medium, in which ACC was used to replace (NH4)2SO4 in DF medium (Bai 2017). The inoculated plates were incubated at 28 °C for 7 d before the observation of colony growth. If colonies formed on the plates, they were transferred to fresh ADF agar plates and continuously subcultured three times. If colonies still formed in the third generation, it indicates that S. rochei S32 can produce ACC deaminase.
Siderophore production. The ability of S. rochei S32 to produce siderophores was determined by chrome azurol sulfonate (CAS) assay (Milagres et al. 1999). It is theorized that when the sample contains siderophores, they can chelate iron ions to form a complex; once the complex is broken, red CAS is released, forming a yellow or orange yellow halo in the solid medium. S. rochei S32 was inoculated onto agar plates of GA medium and incubated at 28 °C for 8 d. The CAS detection reagent was sterilized and cooled to 40–50 °C, with a 10-mL aliquot added to each GA agar plate. The change in the color of the plate was observed 1 h later.
Data analysis
Excel v2016 (Microsoft Corp., Redmond, USA) and SPSS v26.0 (International Business Machines Corp., New York, USA) were used to test for the significance of differences between group means. Origin v2022 (OriginLab, Massachusetts, USA) was used for graphing.
Results
Plant growth-promoting effect of S. rochei S32 on two crops
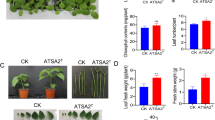
Compared with the control group, the addition of 400-fold diluted cell-free fermentation broth significantly promoted the growth of wheat plants (Fig. S1). This effect was manifested by significantly increased shoot length (24.7%) and root length (25.3%) after 15 d of treatment (Fig. 1a). The addition of cell-free fermentation broth also improved the growth of tomato plants mainly in terms of root length (Fig. S2). When treated with 100-, 200-, and 400-fold diluted cell-free fermentation broth, the root length of tomato increased by 9.7%, 40.9%, and 7.8%, respectively, compared with the control group (Fig. 1b).
Effect of S. rochei S32 on plant growth of wheat (a) and tomato (b) after 15 d of treatment. Figure shows changes in the plant height and root length of wheat (a) and root length of tomato (b) treated with 50-, 100-, 200-, and 400-fold diluted cell-free fermentation broth (S-50 to S-400). Data are the means ± standard deviation
In the wheat field experiment, the shoot length, shoot dry weight, root dry weight and panicle number increased after 210 days of growth, and the 1000-grain weight and yield increased by 11.8% and 13.2%, respectively (Table 1), compared with the control group.
In the field experiment of tomato greenhouse, the shoot length, root length and stem diameter of tomato increased significantly after 60 days of growth. After 210 days of growth, shoot length, stem diameter, shoot dry weight and root dry weight also increased. In terms of yield, after adding strain S32, the yield increased by 6.2% (Table 2).
Antagonistic activity of S. rochei S32 against soil-borne pathogens
S. rochei S32 had no or weak antagonistic effect against Sclerotinia sclerotiorum, F. graminearum, and F. oxysporum. Its inhibitory effect on Altenaria alternata, Macrophoma kawatsukai, Rhizoctonia cerealis, Colletotrichum orbiculare, and F. incarnatum was striking (Fig. 2). The highest inhibition rate was observed for M. kawatsukai (65.0%), followed by F. incarnatum (49.8%) and A. alternate (48.1%; Table 3).
Antagonistic activity of S. rochei S32 against eight pathogenic fungi grown on potato dextrose agar plates. S. rochei S32 was inoculated at the center, and a given pathogenic fungus was inoculated around it in a triangle shape. a Altenaria alternata; b Macrophoma kawatsukai; c Rhizoctonia cerealis; d Colletotrichum orbiculare; e Sclerotinia sclerotiorum; f Fusarium graminearum; g Fusarium oxysporum R1; and (h) Fusarium incarnatum
Genomic information of S. rochei S32
The whole genome of S. rochei S32 contains a linear chromosome (Fig. 3) and two plasmids. The total length of the chromosome is 8,041,158 bp with a GC ratio of 72.5%. The size and GC ratio of plasmid 1 are 171,061 bp and 69.4%, respectively. The size and GC ratio of plasmid 2 are 95,155 bp and 69.4%, respectively. The general characteristics of the bacterial genome are summarized in Table 4.
A comparative gene map of S. rochei S32 constructed using Circos. The features from outer to inner circle are genome size (circle 1), forward (circle 2) and reverse strand genes (circle 3) colored by cluster of orthologous groups, forward (circle 4) and reverse strand non-coding RNAs (circle 5), repeat (circle 6), GC (circle 7), GC-skew calculated as (nG – nC) / (nG + nC) (circle 8). The green color is skew+, which means that the content of G is greater than that of C; the purple color is skew–, which means that the content of G is less than that of C
A total of 7726 coding genes were predicted in the whole genome, and 7486 (96.8%) of them were annotated with specific functions. The number of genes annotated based on different databases was 338 (4.4%) in VFDB, 32 (0.4%) in ARDB, 433 (5.6%) in CAZy, 6133 (79.4%) in IPR, 2364 (30.6%) in Swiss-Prot, 3 (0.0%) in CARD, 7387 (95.6%) in NR, 1459 (18.9%) in T3SS, 5293 (68.5%) in COG, 4208 (54.5%) in GO, and 3866 (50.0%) in KEGG.
In the GO-based gene function classification, there were 6335, 1155, and 5054 genes involved in biological processes, cellular components, and molecular functions, respectively (Fig. 4). The three functional categories were further divided into 17, 3, and 11 subcategories, respectively. The main subcategories included “cellular processes” (1959) and “metabolic processes” (2179) in biological processes, as well as “binding” (1837) and “catalytic activity” (2495) in molecular functions.
Prediction of gene functions in plant growth promotion
Phytohormone biosynthesis
KEGG pathway enrichment analysis revealed the biosynthesis of L-TRP and indole (two precursors for IAA biosynthesis) via the TRP biosynthesis pathway in S. rochei S32. Among the common pathways of IAA biosynthesis with TRP as a substrate, the indole-3-acetamide (IAM) pathway, the indole-3-pyruvate (IPA) pathway, the tryptamine (TAM) pathway, and the indole-3-acetaldehyde oxime (IAOx) pathway were all incomplete in the TRP metabolic pathway (Figs. S3, S4). However, only the iaam gene was missing in the IAM pathway (Fig. S3), so IAA was more likely to be synthesized via the IAM pathway at the gene level.
Biosynthesis of fungal cell wall hydrolases
The results of CAZy enzyme activity analysis showed the presence of numerous genes encoding fungal cell wall hydrolases in the chromosome of S. rochei S32. There were 185 genes for glycoside hydrolases, 113 genes for glycosyl transferases, 30 genes for carbohydrate esterases, 132 genes for carbohydrate-binding modules, 9 genes for auxiliary activities, and 12 genes for polysaccharide lyases. Genes encoding the main enzymes that hydrolyze fungal cell walls, including chitinase, cellulase, and β-1,3-glucanase, were predicted (Fig. 5).
Secondary metabolite biosynthesis
Based on the antiSMASH database, a total of 30 biosynthetic gene clusters (BGCs) were predicted in the chromosome of S. rochei S32. Twelve BGCs had >80% similarity to known clusters with high confidence (Table 5). Among them, the products of six BGCs were identified: hopene, geosmin, albaflavenone, ectoine, isorenieratene, and 7-prenylisatin. Despite the unknown products of the remaining BGCs, they showed high similarities to those encoding borrelidin, lipopeptide, streptothricin, candicidin, and desferrioxamine B/E antibiotics. These results indicate that S. rochei S32 has the potential to produce antibiotics targeting nucleic acids, macrolides, and peptides, as well as siderophores. Other BGCs were not annotated in available databases or had low similarity to known natural BGCs, suggesting that S. rochei S32 is likely to synthesize a wide range of unknown natural products.
Phytohormone and metabolome profiles
Thirty-nine phytohormones were detected in the culture filtrate of S. rochei S32 (Fig. 6). The phytohormones were classified into seven categories: auxins, cytokinins (CKs), jasmonates (JAs), gibberellins (GAs), salicylic acid (SA), strigolactones (SLs), and abscisic acid (ABA). Additionally, 2205 secondary metabolites were detected. Among the top 200 most frequently detected metabolites (Fig. 7), “benzene and substituted derivatives” (28), “heterocyclic compounds” (25), “organic acid and its derivatives” (22), “small peptides” (19), and “sugars” (15) were relatively abundant. These metabolites included biologically active compounds, such as phytosphingosine, fosfomycin calcium, aspulvinone E, haloprogin, acivicin, nordihydrocapsiate, and corynebactin (Table S1).
Mechanisms of plant growth promotion
S. rochei S32 grew normally on the organophosphorus-solubilizing medium, inorganic phosphorus-solubilizing medium, and potassium-solubilizing medium, despite no halos (Fig. 8a). Normal bacterial growth also occurred on the nitrogen-free medium after three subcultures (Fig. 8b). These results indicate that S. rochei S32 has the ability to fix atmospheric nitrogen, but not to solubilize phosphorus or potassium. Poor colony growth was observed on the ADF medium with ACC as the sole nitrogen source (Fig. 8c), indicating no production of ACC deaminase. However, siderophores were produced on CAS plates, as indicated by the halo with a distinct orange color around colonies (Fig. 8d).
Discussion
In the present study, we verified the plant growth-promoting effect of S. rochei S32 on two different crops and its antagonistic activity against five soil-borne pathogenic fungi through petri dish experiments. We then deciphered the underlying molecular mechanisms based on whole-genome sequencing, widely targeted metabolomics, and mechanism studies of plant growth promotion. The results indicate that S. rochei S32 can promote plant growth through phytohormone biosynthesis (e.g., IAA), nitrogen fixation, and production of extracellular hydrolases, antimicrobial metabolites, and siderophores. Our findings can enable proper application of S. rochei S32 as a PGPR strain for sustainable agriculture.
Generally, when the plants were treated with the cell-free fermentation broth of S. rochei S32, their growth was inhibited with a low dilution factor. With an increase in the dilution factor, the effect of plant growth promotion was first enhanced and then diminished until it disappeared. The special pattern of wheat root growth (200-fold dilution) is likely due to the influence of external environmental factors (e.g., water). When treated with the highly diluted cell-free fermentation broth, the growth performance of wheat and tomato seedlings in petri dishes was substantially improved in terms of plant height and/or root length. In the field experiment, the growth of wheat and tomato was also improved compared with the control group. This effect can be at least partially attributed to IAA biosynthesis and secretion by S. rochei S32 (Alemneh et al. 2022; Sun et al. 2022).
Phytohormones are low-molecular-weight metabolites that play a vital role in regulating plant growth and development. Auxins, mainly represented by IAA, control cell elongation, apical dominance, and the development of main and lateral roots in plants (Sabatini et al. 1999; Wang et al. 2001). IAA also facilitates root exudation by relaxing root cell walls, and as such, improve plant growth (Pang et al. 2022). S. rochei S32 has the ability to synthesize L-TRP and indole as precursors for IAA biosynthesis, based on the results of KEGG pathway enrichment analysis. Indole biosynthesis was also predicted in the secondary metabolite gene cluster, providing strong evidence for IAA biosynthesis at the gene level. The predicted results of IAA biosynthesis were confirmed by the detection of L-TRP and IAA in the phytohormone profile of S. rochei S32.
As the primary precursor for IAA biosynthesis (Choix et al. 2018), TRP is converted into IAM through TRP monooxygenase encoded by the iaam gene. Then, IAM hydrolase encoded by the iaah gene, which is the last key enzyme for IAA biosynthesis, catalyzes the conversion of IAM to synthesize IAA (AI-Hosni et al. 2018; Wu et al. 2022). The S. rochei S32 genome contains the iaah gene (Fig. S3), but lacks the iaam gene which was first discovered in Agrobacterium tumfaciens and Pseudomonas savastanoi (Aragón et al. 2014). These findings allow us to posit that S. rochei S32 synthesizes IAA mainly through the IAM pathway, and this process is likely to involve interactions with other soil microorganisms.
In addition to auxins, S. rochei S32 was found to produce phytohormones of other categories. Among them, CKs and GAs play a role in promoting plant growth (Liu et al. 2023; Rivas et al. 2022; Yamaguchi 2008), whereas JAs, SA, SLs, and ABA mainly mediate biotic and abiotic stresses (Beigi et al. 2023; Keskin et al. 2010; Liu et al. 2023; Ma et al. 2023). S. rochei S32 produced various phytohormones without any addtions, providing a possible mechanism for plant growth promotion and disease control.
Nitrogen is essential for plant growth (Fateme et al. 2023), and nitrogen fixation is considered to be one of the direct mechanisms by which Streptomyces spp. promote crop growth (Vurukonda et al. 2018). S. rochei S32 showed an ability to fix atmospheric nitrogen, based on its normal growth on the nitrogen-free medium for at least three generations. Therefore, S. rochei S32 contributes to plant growth by converting atmospheric inorganic nitrogen into organic nitrogen that is available for plant absorption (Fowler et al. 2013). Similarly, a nitrogen-fixing strain of Burkholderia australis sp. nov. promotes plant growth of sugarcane by increasing root and shoot biomass (Paungfoo-Lonhienne et al. 2014).
Infection with soil-borne plant pathogens seriously affects crop health and yield (Lamichhane et al. 2023). Under the experimental conditions, inoculation with S. rochei S32 hindered the growth of most of the pathogenic fungi tested. This inhibitory effect varied with fungal species and was possibly due to the production of diverse secondary metabolites with biological activities. However, it may also be due to the competition between microorganisms for growth space or different growth rates. Based on the CAZy database, S. rochei S32 was predicted to produce chitinase, cellulase, and β-1,3-glucanase. These hydrolases can degrade fungal cell walls and curb hyphal growth (Luo et al. 2023; Woith et al. 2021). Additionally, 12 BGCs with high similarity to known gene clusters that encode antimicrobial substances were predicted in the whole genome of S. rochei S32. Antimicrobial substances (e.g., antibiotics) produced by bacteria can inhibit the growth of pathogens (EI-Sharkawy and Abdelrazik 2022).
Among the possible products of the BGCs predicted in the S. rochei S32 genome, candicidin is a polyene antifungal agent that binds to ergosterol and primarily affects cell membrane permeability and integrity (Barke et al. 2010). Thus, candicidin can serve as a target to screening for biocontrol microorganisms (Yao et al. 2021). Lipopeptide and borrelidin are both antimicrobial peptides synthesized by non-ribosomal peptide synthetase. Lipopeptide is a biosurfactant that exerts a broad-spectrum antimicrobial effect by disrupting the integrity of cell outer and inner membranes (Dasgupta et al. 2023; Zhang et al. 2023). Borrelidin, an inhibitor of threonine transfer RNA synthase (Li et al. 2014), inhibits protein synthesis in bacteria, fungi, and viruses (Shirokikh et al. 2023). Streptothricin also inhibits protein synthesis in bacteria and provides an effective bactericidal antibiotic against Gram-negative bacteria (Morgan et al. 2023). 7-Prenylisatin is a derivative of indole, which has prominent bactericidal activity against Bacillus subtilis (Wu et al. 2015). Albaflavenone is a tricyclic sesquiterpene antibiotic with antibacterial effects and a similar aroma to geosmin (Gürtler et al. 1994). Geosmin is a specific characteristic product of Streptomyces culture (Becher et al. 2020), with no clear antibacterial effects. However, geosmin can interact with neighboring microorganisms and drive bacterial response or adaptation to environmental changes, affecting interspecific competition and cooperation (Audrain et al. 2015). All these secondary metabolites have been reported to have antibacterial activity, which can enable S. rochei S32 to benefit plant growth by antagonizing pathogenic microorganisms.
Unlike the antimicrobial metabolites, desferrioxamine specifically chelates iron in the environment (Mahajan et al. 2021). Desferrioxamine-producing microorganisms mainly compete with harmful microorganisms for the iron element. This competition would hinder the growth of harmful microorganisms and slow down pathogenic infection, thereby promoting plant growth (Jarmusch et al. 2021). Siderophore production is also one of the mechanisms that allow S. rochei S32 to promote plant growth. In addition to iron, siderophores chelate other metal cations, reducing their free concentration and toxicity (Schalk et al. 2011). Compared with common metal chelating agents, siderophores are biodegradable and pollution-free in the soil environment (Dimkpa et al. 2009). Among the metabolites of S. rochei S32, we detected the presence of corynebactin, a cyclic catecholate siderophore (Budzikiewicz et al. 2014). Corynebactin has a unique structure with methylation of trilactone ring and glycine spacer, which is more stable than enterobactin and mediates iron transport (Raymond et al. 2003). Despite abundant predictable BGCs at the gene level, only a portion of them can be actually expressed to produce detectable levels of secondary metabolites, depending on the culture conditions and substrates (Nofiani et al. 2023).
Furthermore, we found that the metabolites produced by S. rochei S32 included benzene and its derivatives, heterocyclic compounds, organic acids and their derivatives, small peptide and diverse antibiotics (Table S1). The biosynthesis of aromatic volatiles can activate plant growth, defense responses, and abiotic stress resistance (Mares-Rodriguez et al. 2023). Organic acids and their derivatives can penetrate the cell membrane of bacteria and acidify their cytoplasm to exhibit outstanding antibacterial effects (Kovanda et al. 2019). Small peptides can be recognized by plants to regulate immune responses to pathogens (Lyapina et al. 2021). All these metabolites are expected to confer the ability of S. rochei S32 to enhance plant growth and resist pathogens.
In summary, when S. rochei S32 is applied to crops, it contributes to plant growth and resistance to pathogenic fungi through the production of phytohormones, hydrolases, bioactive metabolites (including antibiotics), and siderophores, as well as nitrogen fixation. This is despite the fact that the relative contributions of various mechanisms cannot be quantified. Based on the functional analysis and verification of genomic analysis results, this study provides novel insights into the known and potential mechanisms of how S. rochei S32 promotes plant growth and antagonizes soil-borne pathogens. Our findings offer guidance for the application of S. rochei S32 as a PGPR strain in agriculture and further exploration of known and unknown secondary metabolites produced by this bacterial strain. While this study has only looked at the possible effects and mechanisms of S. rochei S32 on crop plants, further research is needed to ascertain plant responses to the bacterial strain and its possible interactions with other microorganisms in the soil environment.
Data availability
The data sets generated during the current study are available in the NCBI repository, under accession numbers: CP133098, CP133099 and CP133100, for genome, plasmid 1 and plasmid 2, respectively.
Abbreviations
- PGPR:
-
Plant growth-promoting rhizobacteria
- IAA:
-
Indole-3-acetic acid
- TRP:
-
Tryptophan
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- PDA :
-
Potato dextrose agar
- GA:
-
Gauze’s synthetic medium No. 1
- CAS:
-
Chrome azurol sulfonate
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- CAZy:
-
Carbohydrate-active enzymes
- IPA:
-
Indole-3-pyruvate
- IAM:
-
Indole-3-acetamide
- TAM:
-
Tryptamine
- BGCs :
-
Biosynthetic gene clusters
- CKs :
-
Cytokinins
- JAs:
-
Jasmonates
- GAs :
-
Gibberellins
- SA:
-
Salicylic acid
- SLs:
-
Strigolactones
- ABA :
-
Abscisic acid
References
AI-Hosni K, Shahzad R, Khan LA, Imran MQ, Harrasi AA, Rawahi AA, Asaf S, Kang S, Yun B, In-Jung LI (2018) Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J Plant Interact 13:112–118. https://doi.org/10.6084/m9.figshare.5895955.v1
Aragón IM, Pérez-Martínez I, Moreno-Pérez A, Cerezo M, Ramos C (2014) New insights into the role of indole-3-acetic acid in the virulence of pseudomonas savastanoi pv. Savastanoi. FEMS Microbiol Lett 356:184–192. https://doi.org/10.1111/1574-6968.12413
Alemneh AA, Zhou Y, Ryder MH, Denton MD (2022) Soil environment influences plant growth-promotion traits of isolated rhizobacteria. Pedobiologia (Jena) 90:150785 http://purl.org/au-research/grants/arc/IH140100013
Audrain B, Farag MA, Ryu CM, Ghigo JM (2015) Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol Rev 39:222–233. https://doi.org/10.1093/femsre/fuu013
Bai JL (2017) Study on from northern the diversity of rhizosphere actinomycetes the typical halophytes in coastal zone of Jiangsu and their plant growth-promoting effect under salt stress. Master dissertation, Jiangsu Normal University (in Chinese)
Barke J, Seipke RF, Grüschow S, Heavens D, Drou N, Bibb MJ, Goss RJ, Yu DW, Hutchings MI (2010) A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol 8:109. https://doi.org/10.1186/1741-7007-8-109
Becher PG, Verschut V, Bibb MJ, Bush MJ, Molnár BP, Barane E, Al-Bassam MM, Chandra G, Song L, Challis GL, Buttner MJ, Flärdh K (2020) Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat Microbiol 5:821–829. https://doi.org/10.1038/s41564-020-0697-x
Beigi A, Ghooshchi F, Moghaddam HR, Nasiri M, Kasraie P (2023) Effect of methyl jasmonate and potassium and their combined action on drought tolerance of fodder corn. Russ J Plant Physiol 70:102. https://doi.org/10.1134/S1021443723600733
Budzikiewicz H, Bössenkamp A, Taraz K, Pandey A, Meyer J-M (2014) Corynebactin, a cyclic catecholate siderophore from: Corynebacterium glutamicum ATCC 14067 (Brevibacterium sp. DSM 20411). Z Naturforsch C Biosci 52:551–554. https://doi.org/10.1515/znc-1997-7-820
Choix FJ, López-Cisneros CG, Méndez-Acosta HO (2018) Azospirillum brasilense increases CO2 fixation on microalgae Scenedesmus obliquus, Chlorella vulgaris, and Chlamydomonas reinhardtii cultured on high CO2 concentrations. Microb Ecol 76:430–442. https://doi.org/10.1007/s00248-017-1139-z
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959. https://doi.org/10.1128/AEM.71.9.4951-4959.2005
Dasgupta A, Saha S, Ganguli P, Das I, De D, Chaudhuri S (2023) Characterization of pumilacidin, a lipopeptide biosurfactant produced from Bacillus pumilus NITDID1 and its prospect in bioremediation of hazardous pollutants. Arch Microbiol 205:274. https://doi.org/10.1007/s00203-023-03619-4
De la Cruz-Rodríguez Y, Adrián-López J, Martínez-López J, Neri-Márquez BI, García-Pineda E, Alvarado-Gutiérrez A, Fraire-Velázquez S (2023) Biosynthetic gene clusters in sequenced genomes of four contrasting rhizobacteria in phytopathogen inhibition and interaction with Capsicum annuum roots. Microbiology spectrum 11:e0307222. https://doi.org/10.1128/spectrum.03072-22
Dennis PG, Kukulies T, Forstner C, Orton TG, Pattison AB (2018) The effects of glyphosate, glufosinate, paraquat and paraquat-diquat on soil microbial activity and bacterial, archaeal and nematode diversity. Sci Rep 8:2119. https://doi.org/10.1038/s41598-018-20589-6
Dimkpa CO, Merten D, Svatos A, Büchel G, Kothe E (2009) Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107:1687–1696. https://doi.org/10.1111/j.1365-2672.2009.04355.x
EI-Sharkawy EE, Abdelrazik E (2022) Biocontrol of fusarium root rot in squash using mycorrhizal fungi and antagonistic microorganisms. Egypt J Biol Pest Control 32:1–11. https://doi.org/10.1186/s41938-022-00513-x
Fateme R, Morteza S, Mahdieh E (2023) Improved drought tolerance in Festuca ovina L. using plant growth promoting bacteria. J Arid Land 6:740–755. https://doi.org/10.1007/s40333-023-0015-6
Floková K, Tarkowská D, Miersch O, Strnad M, Wasternack C, Novák O (2014) UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 105:147–157. https://doi.org/10.1016/j.phytochem.2014.05.015
Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M (2013) The global nitrogen cycle in the twenty-first century. Philos Trans R Soc Lond Ser B Biol Sci 368:20130164. https://doi.org/10.1098/rstb.2013.0164
Fravel DR (2005) Commercialization and implementation of biocontrol. Annu Rev Phytopathol 43:337–359. https://doi.org/10.1146/annurev.phyto.43.032904.092924
Fu SF, Sun PF, Lu HY, Wei JY, Xiao HS, Fang WT, Cheng BY, Chou JY (2016) Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata lab. Fungal Biol 120:433–448. https://doi.org/10.1016/j.funbio.2015.12.006
Gillon A, Abdelrahman O, Abou-Mansour E, L'Haridon F, Falquet L, Allard PM, Weisskopf L (2023) Comparative genomic and metabolomic study of three Streptomyces sp. differing in biological activity. MicrobiologyOpen 12:e1389. https://doi.org/10.1002/mbo3.1389
Gürtler H, Pedersen R, Anthoni U, Christophersen C, Nielsen PH, Wellington EM, Pedersen C, Bock K (1994) Albaflavenone, a sesquiterpene ketone with a zizaene skeleton produced by a streptomycete with a new rope morphology. J Antibiot 47:434–439. https://doi.org/10.7164/antibiotics.47.434
Harris BA, Bauske EM, Pennisi SV (2021) Cultural practices and microbial inoculants have variable impact on a bedding plant (Lantana camara L.) performance in the landscape. Sci Hortic 282:110059. https://doi.org/10.1016/j.scienta.2021.110059
Jamil F, Mukhtar H, Fouillaud M, Dufossé L (2022) Rhizosphere signaling: insights into plant-rhizomicrobiome interactions for sustainable agronomy. Microorganisms 10:899. https://doi.org/10.3390/microorganisms10050899
Jarmusch SA, Lagos-Susaeta D, Diab E, Salazar O, Asenjo JA, Ebel R, Jaspars M (2021) Iron-meditated fungal starvation by lupine rhizosphere-associated and extremotolerant Streptomyces sp. S29 desferrioxamine production. Mol Omics 17:95–107. https://doi.org/10.1039/d0mo00084a
Keskin BC, Sarikaya AT, Yuksel B, Memon AR (2010) Abscisic acid regulated gene expression in bread wheat (Triticum aestivum L.). Aust J Crop Sci 4:617–625. https://doi.org/10.1016/j.jhazmat.2023.130947
Kim YC, Leveau J, McSpadden Gardener BB, Pierson EA, Pierson LS 3rd, Ryu CM (2011) The multifactorial basis for plant health promotion by plant-associated bacteria. Appl Environ Microbiol 77:1548–1555. https://doi.org/10.1128/AEM.01867-10
Kovanda L, Li XD, Zhang W, Wei XH, Luo J, Wu XX, Atwill ER, Sygall R, Lum J, Liu YH (2019) 169 susceptibility of several species of gram-negative and gram-positive bacteria to organic acids and their derivatives. J Anim Sci 97:2. https://doi.org/10.1093/jas/skz122.173
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645. https://doi.org/10.1101/gr.092759.109
Lamichhane JR, Barbetti MJ, Chilvers MI, Pandey AK, Steinberg C (2023) Exploiting root exudates to manage soil-borne disease complexes in a changing climate. Trends Microbiol S0966-842X(23)00223–8. https://doi.org/10.1016/j.tim.2023.07.011
Li M, Zhang J, Liu C, Fang B, Wang X, Xiang W (2014) Identification of borrelidin binding site on threonyl-tRNA synthetase. Biochem Biophys Res Commun 451:485–490. https://doi.org/10.1016/j.bbrc.2014.07.100
Li Y, Zhou C, Yan X, Zhang J, Xu J (2016) Simultaneous analysis of ten phytohormones in Sargassum horneri by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J Sep Sci 39:1804–1813. https://doi.org/10.1002/jssc.201501239
Liming S, Thinn TN, Beibei G, Wenjun Z, Binghua L, Hailan C, Kecheng Z (2018) Antifungal and plant growth-promoting activities of Streptomyces roseoflavus strain NKZ-259. Biol Control 125:57–64. https://doi.org/10.1016/j.biocontrol.2018.06.012
Liu X, Yu Y, Yao W, Yin Z, Wang Y, Huang Z, Zhou JQ, Liu J, Lu X, Wang F, Zhang G, Chen G, Xiao Y, Deng H, Tang W (2023) CRISPR/Cas9-mediated simultaneous mutation of three salicylic acid 5-hydroxylase (OsS5H) genes confers broad-spectrum disease resistance in rice. Plant Biotechnol J 21:1873–1886. https://doi.org/10.1111/pbi.14099
Luo X, Qu J, Ren M (2023) Complete genome sequence data of novel Streptomyces angustmyceticus strain CQUSa03, a potential biological control agent for potato oomycete and fungal diseases. Plant Dis 107:1609–1612. https://doi.org/10.1094/PDIS-08-22-1927-A
Lyapina I, Filippova A, Kovalchuk S, Ziganshin R, Mamaeva A, Lazarev V, Latsis I, Mikhalchik E, Panasenko O, Ivanov O, Ivanov V, Fesenko I (2021) Possible role of small secreted peptides (SSPs) in immune signaling in bryophytes. Plant Mol Biol 106:123–143. https://doi.org/10.1007/s11103-021-01133-z
Ma Q, Kong D, Zhang Q, Li M, Han X, Che J, Zhou Y, Zhang W, Jiang X, Ruan Z (2022) Microbacterium sulfonylureivorans sp. nov., isolated from sulfonylurea herbicides degrading consortium. Arch Microbiol 204:136. https://doi.org/10.1007/s00203-021-02750-4
Ma X, Yu X, Cui GC, Guo ZG, Lang DY, Zhang XH (2023) Methyl jasmonate mitigates osmotic stress by regulating carbon and nitrogen metabolism of Glycyrrhiza uralensis seedlings subjected to salt stress. Acta Physiol Plant 45:96. https://doi.org/10.1007/s11738-023-03574-z
Mahajan SG, Nandre VS, Kodam KM, Kulkarni MV (2021) Desferrioxamine E produced by an indigenous salt tolerant pseudomonas stutzeri stimulates iron uptake of Triticum aestivum L. Biocatal Agric Biotechnol 35:102057. https://doi.org/10.1016/j.bcab.2021.102057
Manulis S, Shafrir H, Epstein E, Lichter A, Barash I (1994) Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology (Reading) 140:1045–1050. https://doi.org/10.1099/13500872-140-5-1045
Mares-Rodriguez FJ, Aréchiga-Carvajal ET, Ruiz-Herrera ŦJ, Moreno-Jiménez MR, González-Herrera SM, León-Ramírez CG, Rutiaga-Quiñones OM (2023) A new bacterial endosymbiotic relationship in Kluyveromyces marxianus isolated from the mezcal fermentation process. Process Biochem 131:133–143. https://doi.org/10.1016/j.procbio.2023.06.008
Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD (2003) Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol 50:1591–1604. https://doi.org/10.1046/j.1365-2958.2003.03786.x
Mathew BT, Torky Y, Amin A, Mourad AI, Ayyash MM, El-Keblawy A, Hilal-Alnaqbi A, AbuQamar SF, El-Tarabily KA (2020) Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front Microbiol 11:552. https://doi.org/10.3389/fmicb.2020.00552
Milagres MA, Machuca A, Napoleão D (1999) Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J Microbiol Methods 37:1–6. https://doi.org/10.1016/s0167-7012(99)00028-7
Morgan CE, Kang YS, Green AB, Smith KP, Dowgiallo MG, Miller BC, Chiaraviglio L, Truelson KA, Zulauf KE, Rodriguez S, Kang AD, Manetsch R, Yu EW, Kirby JE (2023) Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome. PLoS Biol 21:e3002091. https://doi.org/10.1371/journal.pbio.3002091
Nakamura T (1959) Inhibition of several antibiotics on d-amino acid oxidase. J Vitaminol (Kyoto) 5:277–286. https://doi.org/10.5925/jnsv1954.5.277
Nejatzadeh-Barandozi F, Gholami-Borujeni F (2014) Application of different fertilizers on morphological traits of dill (Anethum graveolens L.). Org Med Chem Lett 4:4. https://doi.org/10.1186/s13588-014-0004-z
Nofiani R, Rudiyansyah, Ardiningsih P, Rizky, Zahra STA, Sukito A, Weisberg AJ, Chang JH, Mahmud T (2023) Genome features and secondary metabolite potential of the marine symbiont Streptomyces sp. RS2. Arch Microbiol 205:244. https://doi.org/10.1007/s00203-023-03556-2
Okull DO, Beelman RB, Gourama H (2003) Antifungal activity of 10-oxo-trans-8-decenoic acid and 1-octen-3-ol against Penicillium expansum in potato dextrose agar medium. J Food Prot 66:1503–1505. https://doi.org/10.4315/0362-028x-66.8.1503
Pang F, Solanki MK, Wang Z (2022) Streptomyces can be an excellent plant growth manager. World J Microbiol Biotechnol 38:193. https://doi.org/10.1007/s11274-022-03380-8
Paungfoo-Lonhienne C, Lonhienne TG, Yeoh YK, Webb RI, Lakshmanan P, Chan CX, Lim PE, Ragan MA, Schmidt S, Hugenholtz P (2014) A new species of Burkholderia isolated from sugarcane roots promotes plant growth. Microb Biotechnol 7:142–154. https://doi.org/10.1111/1751-7915.12105
Raymond KN, Dertz EA, Kim SS (2003) Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci USA 100:3584–3588. https://doi.org/10.1073/pnas.0630018100
Rivas MÁ, Friero I, Alarcón MV, Salguero J (2022) Auxin-cytokinin balance shapes maize root architecture by controlling primary root elongation and lateral root development. Front Plant Sci 13:836592. https://doi.org/10.3389/fpls.2022.836592
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472. https://doi.org/10.1016/s0092-8674(00)81535-4
Safar N, Sohrab M, Kazem G, Yaghoub R, Weria W (2023) PGPR and vermicompost with reduced chemical fertilizer enhances biodiesel production, nutrient uptake and improve oil composition of rapeseed grown under water deficit stress. S Afr J Bot 159:17–25. https://doi.org/10.1016/j.sajb.2023.06.001
Schalk IJ, Hannauer M, Braud A (2011) New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol 13:2844–2854. https://doi.org/10.1111/j.1462-2920.2011.02556.x
Shirokikh IG, Osterman IA, Lukianov DA, Marina VI, Biryukov MV, Belozerova OA, Guglya EB, Shirokikh AA, Nazarova YI, Bokov NA, Zakalyukina YV (2023) Biocontrol potential of novel borrelidin-producing Streptomyces rochei 3IZ-6 isolated from soil. Eurasian Soil Sci 56:619–627. https://doi.org/10.1134/S1064229323600161
Siewert G, Strominger JL (1967) Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci USA 57:767–773. https://doi.org/10.1073/pnas.57.3.767
Singh P, Singh RK, Guo DJ, Sharma A, Singh RN, Li DP, Malviya MK, Song XP, Lakshmanan P, Yang LT, Li YR (2021) Whole genome analysis of sugarcane root-associated endophyte Pseudomonas aeruginosa B18-a plant growth-promoting bacterium with antagonistic potential against Sporisorium scitamineum. Front Microbiol 12:628376. https://doi.org/10.3389/fmicb.2021.628376
Soliman SA, Khaleil MM, Metwally RA (2022) Evaluation of the antifungal activity of bacillus amyloliquefaciens and B. Velezensis and characterization of the bioactive secondary metabolites produced against plant pathogenic fungi. Biology (Basel) 11:1390. https://doi.org/10.3390/biology11101390
Sun H, Zhang J, Liu W, Wenhui E, Wang X, Li H, Cui Y, Zhao D, Liu K, Du B, Ding Y, Wang C (2022) Identification and combinatorial engineering of indole-3-acetic acid synthetic pathways in Paenibacillus polymyxa. Biotechnol Biofuels Bioprod 15:81. https://doi.org/10.1186/s13068-022-02181-3
Thilagar G, Bagyaraj DJ, Rao MS (2016) Selected microbial consortia developed for chilly reduces application of chemical fertilizers by 50% under field conditions. Sci Hortic 198:27–35. https://doi.org/10.1016/j.scienta.2015.11.021
Tremsin AS, Lebedev GV, Siegmund OHW, Vallerga JV, McPhate JB, Hussain Z (2007) High-resolution detection system for time-of-flight electron spectrometry. Nucl Inst Methods Phys Res A: Accelerators, Spectrometers, Detectors and Associated Equipment 582:172–174. https://doi.org/10.1016/j.nima.2007.08.101
Vurukonda SSKP, Giovanardi D, Stefani E (2018) Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int J Mol Sci 19:952. https://doi.org/10.3390/ijms19040952
Wang Y, Mopper S, Hasenstein KH (2001) Effects of salinity on endogenous ABA, IAA, JA, and SA in iris hexagona. J Chem Ecol 27:327–342. https://doi.org/10.1023/a:1005632506230
Woith E, Guerriero G, Hausman JF, Renaut J, Leclercq CC, Weise C, Legay S, Weng A, Melzig MF (2021) Plant extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int J Mol Sci 22:3719. https://doi.org/10.3390/ijms22073719
Wu C, Du C, Gubbens J, Choi YH, van Wezel GP (2015) Metabolomics-driven discovery of a prenylated isatin antibiotic produced by Streptomyces species MBT28. J Nat Prod 78:2355–2363. https://doi.org/10.1021/acs.jnatprod.5b00276
Wu X, Kong L, Pan J, Feng Y, Liu S (2022) Metagenomic approaches to explore the quorum sensing-mediated interactions between algae and bacteria in sequence membrane photo-bioreactors. Front Bioeng Biotechnol 10:851376. https://doi.org/10.3389/fbioe.2022.851376
Xu B, Chen W, Wu ZM, Long Y, Li KT (2015) A novel and effective Streptomyces sp. N2 against various phytopathogenic fungi. Appl Biochem Biotechnol 177:1338–1347. https://doi.org/10.1007/s12010-015-1818-5
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251. https://doi.org/10.1146/annurev.arplant.59.032607.092804
Yao X, Zhang Z, Huang J, Wei S, Sun X, Chen Y, Liu H, Li S (2021) Candicidin isomer production is essential for biocontrol of cucumber rhizoctonia rot by Streptomyces albidoflavus W68. Appl Environ Microbiol 87:e03078–e03020. https://doi.org/10.1128/AEM.03078-20
Ye L, Zhao X, Bao E, Li J, Zou Z, Cao K (2020) Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci Rep 10:177. https://doi.org/10.1038/s41598-019-56954-2
Yuan ZS, Liu F, Zhang GF (2015) Characteristics and biodiversity of endophytic phosphorus- and potassium-solubilizing bacteria in Moso bamboo (Phyllostachys edulis). Acta Biol Hung 66:449–459. https://doi.org/10.1556/018.66.2015.4.9
Zhang QM, Yong DJ, Zhang Y, Shi XP, Li BH, Li GF, Liang WX, Wang CX (2015) Streptomyces rochei A-1 induces resistance and defense-related responses against Botryosphaeria dothidea in apple fruit during storage. Postharvest Biol Technol 115:30–37. https://doi.org/10.1016/j.postharvbio.2015.12.013
Zhang Q, Qian SY (2019) Progress in research on alkaloids and pharmacological activities from Streptomyce. Nat Prod Res Dev 31:1461–1473. https://doi.org/10.16333/j.1001-6880.2019.8.023 (in Chinese)
Zhang Z, Zhou Y, Zhang H, Du X, Cao Z, Wu Y, Liu C, Sun Y (2023) Antibacterial activity and mechanisms of TroHepc2-22, a derived peptide of hepcidin2 from golden pompano (Trachinotus ovatus). Int J Mol Sci 24:9251. https://doi.org/10.3390/ijms24119251
Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291:306–309. https://doi.org/10.1126/science.291.5502.306
Zhu HX, Hu LF, Hu HY, Zhou F, Wu LL, Wang SW, Rozhkova T, Li CW (2023) Identification of a novel Streptomyces sp. strain HU2014 showing growth promotion and biocontrol effect against Rhizoctonia spp. in wheat. Plant Dis 107:1139–1150. https://doi.org/10.1094/PDIS-06-22-1493-RE
Acknowledgments
We thank Chaofeng Lin of eWin Editing in Qingdao, China, for improving the English.
Funding
This work was reported by the National Key Research and Development Program of China (2021YFD17002) and the earmarked fund for China Agriculture Research System-Green manure (CARS-22).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. WM wrote the manuscript and completed data collection and analysis. JMF, NXB, WCC, YXC and LYT performed material preparation and participated in some experiments. WM and WXM reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Stavros D. Veresoglou.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 658 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, M., Jiao, M., Nie, X. et al. Genomic and metabolomic profiling reveal Streptomyces rochei S32 contributes to plant growth by nitrogen fixation and production of bioactive substances. Plant Soil 501, 343–360 (2024). https://doi.org/10.1007/s11104-024-06667-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-024-06667-x