Abstract
Salt stress seriously affects C and N metabolism, and secondary metabolites, further have adverse effects on the growth and yield of Glycyrrhiza uralensis. Methyl jasmonate (MeJA) plays a significant role in influencing C and N metabolism and improving the accumulation of secondary metabolites. However, the underlying mechanism of MeJA in alleviating salt stress in G. uralensis remains unknown. A pot experiment was employed to explore the responses of C and N, and secondary metabolisms to MeJA in G. uralensis seedling under salt stress. Salt significantly inhibited growth, affected activities of key enzymes involved in C and N metabolisms and decreased contents of carbohydrates and N-containing compounds, and secondary metabolites in G. uralensis, while the adverse effect was counteracted by MeJA. MeJA remarkably increased the diameter of main root and main stem, and the length of main root in the salt-stressed seedling. In addition, MeJA remarkably increased soluble sugar content and the sucrose synthase (SS) and sucrose phosphate synthase (SPS) activities. Nitrate (NO3−) and nitrite (NO2−) contents, glutamine synthetase (GS), glutamate synthase (GOGAT) and nitrate reductase (NR) activities were also higher in seedlings that were treated with MeJA. Moreover, MeJA significantly increased licochalcone A, glycyrrhizic acid, total flavonoids and total polysaccharides contents. MeJA improves the capacity of osmotic adjustment by regulating C and N metabolism, further promote the synthesis of secondary metabolites in G. uralensis seedling; it also employed different metabolites against membrane lipid peroxidation, further to improve the plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinization is one of the main threats to crop production in arid regions, and it causes severe reduced biomass and productivity (Taïbi et al. 2016). Salinization also alters various biochemical and physiological processes, thereby resulting in a metabolic imbalance in plants (Li et al. 2016). Generally, salinization negatively affected plants in three ways as follows: (1) the soil has a high concentration of salt leads to high osmotic potential in plants; (2) imbalance of ions in cells such as Na+ and Cl− leads to ion toxicity in plants; (3) excessive production of reactive oxygen species (ROS) leads to oxidative stress in plants (Salimi et al. 2016; Ahmadi et al. 2018).
Osmotic regulation is one of the biochemical changes in plants under salt stress. It promotes the protection of plant cell structure, prevents ionic toxicity, and improves water absorption through the synthesis and accumulation of a large number of compatible solutes in plants (Khan et al. 2015; Zhang et al. 2018). Many compatible solutes such as carbohydrates, amino acids and proteins which are products of carbon (C) and nitrogen (N) metabolism (Liu et al. 2014b, a). C and N metabolisms are very sensitive to environmental stress that generally decreases chlorophyll contents, depresses photosynthesis, alters expression of genes and activities of enzyme, which thus inhibit growth (Flores et al. 2004; Debouba et al. 2006; Cuellar-Ortiz et al. 2008). When plants are subjected to salt stress, the cell membrane is firstly affected and the membrane permeability is increased (Liu et al. 2015). Other significant effects of salt stress are changes in soluble sugar content and in invertase (INV) activities, sucrose synthase (SS), sucrose phosphate synthase (SPS) (Fernandes et al. 2004). Moreover, the yield of secondary metabolites is closely related to the growth conditions, and plants usually produce more secondary metabolites under moderate environmental stresses (Kleinwächter and Selmar 2013). Thus, C and N metabolism and secondary metabolites are of vital importance for stress tolerance.
Methyl jasmonate (MeJA), as an inducer or signal transduction agent, is involved in many physiological and biochemical processes of plants (Fan et al. 2016; Zaid and Mohammad 2018). MeJA also elicits beneficial effects on active components in plant. Takahashi and Hara (2014) sprayed MeJA on leaf number of plants and found that MeJA promotes the accumulation of starch by up-regulating the expression genes of starch biosynthetic in Arabidopsis thaliana. Furthermore, MeJA induces the accumulations of monoterpene and sesquiterpene by stimulating oxygenated linalool and (E)-β-farnesene or forming traumatic resin (TDs) ducts in xylem and foliage of Norway spruce (Martin et al. 2002, 2003). Zaid and Mohammad (2018) studied the interactive effect of MeJA and N in reducing the toxicity of Cd in an important medicinal and aromatic plant.
Glycyrrhiza uralensis Fisch. (G. uralensis) is one of the most widely used herbal medicine and food additives (Egamberdieva et al. 2017). Generally, wild G. uralensis resource grows in arid and salinization areas and therefore has the ability to adapt to drought and salt conditions. Nevertheless, studies and practices proved that the salt tolerance ability of cultivated G. uralensis is lower than wild plants (Zhang et al. 2018). Actually, soil salinization and water resource shortage are universal in regions of G. uralensis cultivation. Thus, improving the salt and drought tolerance of cultivated G. uralensis has become an important goal for sustainable development. However, there is limited information on MeJA’s response to C and N metabolism and secondary metabolites to abiotic stresses including salt stress, and thus in light of the important role of MeJA in plant stress response, this research was conducted to study the effects of MeJA on the growth parameters, key metabolites and key enzyme activities related to C and N metabolism, and the contents of bioactive components in G. uralensis under salt stress.
Materials and methods
Plant materials and growth conditions
The seeds of G. uralensis, we utilized were provided by Ningxia Academy of Agriculture and Forestry Sciences, China, Yinchuan, Ningxia Province, China, and it is identified as G. uralensis by Ming Li Professor.
The seeds of G. uralensis were steeped with 85% H2SO4 for 45 min to break the seed coat, then surface sterilized with 0.1% H2O2 for 10 min, cleaned 3 times using distilled water and imbibed in distilled water for 8 h at 25 °C. Eighty seeds of uniform size that were pre-treated were planted in plastic boxes (6 × 12 × 12 cm) filled with 1400 g autoclaved sand medium that were pre-irrigated. The treatments include control group (CK), with 370 mL of distilled water; salt stress group (S), with 370 mL of distilled water containing 75 mM NaCl; salt stress combined with methyl jasmonate group (S + MeJA), with 370 mL of distilled water containing 75 mM NaCl and 30 μM MeJA. Seedling growth conditions were maintained at 12 h/12 h, 28 °C/20 °C (day/night).
A weighing method (every afternoon weighing and watering) was used to control saturation moisture content 65–75% for all treatments. Three replications per treatment were used, and all pots were randomly arranged and periodically rotated to minimize the effects of environmental heterogeneity. Whole G. uralensis seedlings from all treatments were collected in the morning at 45 day after treatment for determination of all parameters.
Determination of carbohydrates
Soluble sugar and sucrose were determined according to the method of Loutfy et al. (2012). Specifically, 0.2 g of fresh seedlings were extracted with 10 mL of distilled water at 100 °C for 30 min, then the homogenate was centrifuged at 3000 g for 10 min, and 0.5 mL of supernatant added 5 mL of ketone concentrated sulfuric acid reagent at 100 °C for 1 min.
Finally, the absorbance was measured at 630 nm. In addition, a standard curve was plotted with 0–100 mg of glucose, and standard curves as Y = 0.00847X−0.0059. For sucrose, 0.2 g fresh seedlings were extracted with 4 mL distilled water, then homogenate was centrifuged at 3000 g for 10 min and supernatant (1 mL) contained 2 M of NaOH (1 mL), then kept in boiling water bath for 10 min, stop the reaction by adding 1 mL of 0.1% (w/v) resorcinol, and 10 M of HCl, stop the reaction by keeping in boiling water bath for 8 min. Finally, the absorbance was measured at 500 nm. In addition, the standard curve was plotted with 0–100 mg of glucose, and standard curves as Y = 0.00847X−0.0059 for soluble sugar and Y = 0.019X + 0.0009 for sucrose.
Determination of N forms
To determine NO3−, NH4+ and NO2−, 0.2 g fresh seedlings were homogenized with deionized water, centrifuged at 4 °C and 8300 g for 10 min. The NO3− and NH4+ in the supernatant were quantified photometrically at 410 and 630 nm, respectively, and KNO3 and (NH4)2SO4 were used as standard, respectively (Zhang et al. 2017). The NO2− in the supernatant was quantified photometrically at 520 nm.
C and N metabolism-related enzyme extraction and assays
Invertase (INV) activity was determined as described by Yang et al. (2004). 0.2 g fresh seedlings were homogenated with chilled distilled water for 3 h at 4 °C and the mixture was centrifuged at 8000 g for 10 min at 4 °C. INV activity was assayed using the DNS method, which measures reducing sugar content at 540 nm.
To measure sucrose synthase (SS) and sucrose phosphate synthase (SPS) activities, 0.2 g fresh seedlings were homogenized with 50 mM HEPES–NaOH (pH 7.5) and the mixture was centrifuged at 12000 g for 10 min at 4 °C. The assay mixture of SS containing the supernatant, 50 mM HEPES–NaOH (pH 7.5), 50 mM MgCl2, 100 mM uridine diphosphoglucose (UDPG) and 100 mM fructose. After incubation at 30 °C for 30 min, 2 M NaOH was added to the assay mixture to terminate the reaction, then, the mixture was boiled for 10 min and cooled. After that, 30% (w/v) HCl and 0.1% (w/v) m-dihydroxybenzene were added, the mixture was shaken thoroughly, kept in a water bath for 10 min at 80 °C, and once the mixture was cooled, its absorbance was measured at 480 nm. SPS activity was determined in the same way as SS activity; the only difference is that fructose was substituted by fructose-6-phosphate (Xie et al. 2021).
To measure nitrate reductase (NR) activity, 0.2 g fresh seedlings were homogenized with 3 mL of 25 mM potassium phosphate buffer (pH 7.5) containing 10 mM L-cysteine and 1 mM EDTA-Na2 and centrifuged at 8300 g for 10 min at 4 °C. The NR activity in the supernatant was quantified photometrically at 540 nm based on the reduction of nitrate to nitrite during 30 min at 25 °C (Du et al. 2008).
To measure the activities of glutamine synthetase (GS) and glutamate synthetase (GOGAT), 0.2 g fresh seedlings were homogenized with 3 mL of 50 mM Tris–HCl buffer (pH 8.0, containing 2 mM Mg2+, 2 mM DTT, and 0.4 M sucrose) and the mixture was centrifuged at 8300 g for 10 min at 4 °C, and the supernatant as enzyme extract. GS activity was assayed by monitoring the formation of glutamyl hydroxamate at 540 nm after reacting with acidified ferric chloride (Liu et al. 2014a, b). GOGAT activity was measured by estimating the oxidation of NADH at 340 nm was assayed according to Wang et al. (2018).
Determination of glycyrrhizic acid, liquiritin and licochalcone A contents
The determination was carried out by HPLC on the C18 column (4.6 mm × 15 cm, 5 μm). The mobile phase consisted of acetonitrile-1% acetic acid with 0.6 mL·min−1 of the flow rate, and the temperature of column was 25 °C. To determine the quantitative assay using the external standard method.
Determination of total flavonoids, total saponins and total polysaccharide contents
For determination of total flavonoids, the powder sample (0.05 g) was extracted with 80% ethanol for 40 min at 40 °C in an ultrasonic bath and the supernatant was used as the extract. 400 μL extract contained 1 mL 70% methanol and 2 mL 10% sodium hydroxide for 10 min, then added 1 mL 70% methanol again, finally the supernatant was measured photometrically at 410 nm.
For determination of total saponins, the powder sample (0.05 g) was extracted with 80% (containing 0.3% ammonia water) ethanol for 80 min at 40 °C in an ultrasonic bath and the supernatant used as the sample extract. 200 μL extract contained 0.25 mL 5% vanillin glacial acetic acid solution and 0.8 mL perchloric acid, react at 55 °C for 20 min in a water bath, then added 2 mL glacial acetic acid under temperature, the absorbance of the supernatant at 590 nm was measured.
For determination of total polysaccharide, the powder sample (0.05 g) was extracted with 80% ethanol for 80 min at 40 °C in an ultrasonic bath and the supernatant was used as the sample extract. 40 μL extract contained 0.5 mL 5% phenol solution, then added 2.5 mL concentrated sulfuric acid, finally the supernatant was measured photometrically at 490 nm.
Statistical analysis
Each treatment of the experiment was designed completely randomly. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for principal component analysis (PCA) and Pearson’s correlation analysis. Significant differences were tested using the least significant difference (LSD) test at P < 0.05. Mean values and standard errors (SEs) were presented.
Results
Effects of MeJA on growth in salt-stressed G. uralensis seedlings
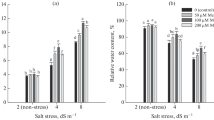
Exposure of G. uralensis plants to salt stress leads to a significant decline in growth parameters including main root length, main root diameter and secondary root number (Fig. 1), and reduced by 28.11%, 23.04% and 48.01%, respectively compared with control. Salt stress also decreased plant height, leaf number and biomass yield by 46.67%, 31.75% and 56.19%, respectively, relative to control. Supply of MeJA showed an enhancement by 23.61%, 16.04% and 24.58% at axial root length, secondary root numbers and biomass yield as compared with salt stress, respectively (Table 1).
A significant negative correlation was observed between soluble sugar and main root length, main shoot diameter, secondary root numbers and leaf number, respectively. A significant negative correlation was observed between sucrose and main root diameter, also a significant negative correlation was observed between NO3− and plant height, leaf number, respectively. In addition, a significant positive correlation was observed between NR and plant height, a significant negative correlation was observed between NR and shoot diameter (Table 2).
A significant positive correlation was observed between leaf number and glycyrrhizic acid and glycyrrhizin, while a significant positive correlation was observed between biomass and glycyrrhizic acid, glycyrrhizin and licochalcone A. A significant positive correlation was observed between total flavonoids and leaf number and biomass yield (Table 3).
Effects of MeJA on carbohydrates contents in salt-stressed G. uralensis seedlings
Salt stress enhances soluble sugar and sucrose contents by 69.74% and 36.05% in salt-stressed G. uralensis plants relative to control. MeJA further induces an enhancement of soluble sugar and sucrose contents by 7.80% and 8.69%, respectively, as compared with plants treated with salt only (Fig. 2).
Effect of MeJA on the soluble sugar, sucrose, SS, SPS and INV in G. uralensis grown under salt stress. Values are means ± SE (n = 6). CK, control group; S (75 mM NaCl), salt stress group; S + MeJA (75 mM NaCl + 30 μM MeJA), salt stress combined with methyl jasmonate group. The different letters within the different treatments indicate the significant difference at P ≤ 0.05
A significant positive correlation was observed between soluble sugar, SS and SPS (Table 2).
Effects of MeJA on the activities of INV, SS and SPS in salt-stressed G. uralensis seedlings
The activities of SS, SPS and INV in G. uralensis plants were not affected by salt stress compared to control. MeJA triggered the enhancement of SS and SPS activities compared to salt stress, and increased by 43.08% and 66.74%, respectively, compared to salt stress (Fig. 2).
Effects of MeJA on NR, GS and GOGAT activities in salt-stressed G. uralensis seedlings
The results regarding the impacts of MeJA on NR, GS and GOGAT activities in salt-stressed G. uralensis plants are depicted in Fig. 4. Reduction in NR and GOGAT activities were observed in G. uralensis plants under salt condition compared to control. MeJA increases NR, GS and GOGAT activities by 31.06%, 8.42% and 47.38% in G. uralensis plants, respectively, as compared with salt alone (Fig. 3).
Effect of MeJA on NO2−, NO3−, GOGAT, NH4+, GS and NR in G. uralensis grown under salt stress. Values are means ± SE (n = 6). CK, control group; S (75 mM NaCl), salt stress group; S + MeJA (75 mM NaCl + 30 μM MeJA), salt stress combined with methyl jasmonate group. The different letters within the different treatments indicate the significant difference at P ≤ 0.05
Effects of MeJA on NO2 −, NO3 − and NH4 + concentrations in salt-stressed G. uralensis seedlings
Salt stress triggered the increase of NO2− and NH4+ contents by 21.76% and 47.43%, respectively, compared to control. Supplementation of MeJA to G. uralensis plants displayed a remarkable increase in NO3− and NO2− contents, which rose by 21.67% and 28.99%, respectively, as compared to salt alone. In addition, MeJA effectively reduced NH4+ content compared to salt alone (Fig. 3).
Effects of MeJA on the contents of glycyrrhizic acid, liquiritin and licochalcone A in salt-stressed G. uralensis seedlings
Salt stress induced the decreased of glycyrrhizic acid content by 12.72%, respectively, compared to control in G. uralensis plants, but glycyrrhizin and licochalcone A contents unchanged compared with the control. MeJA significantly increased glycyrrhizic acid and licochalcone A contents by 12.04% and 10.01%, respectively, compared to salt stress (Fig. 4).
Effect of MeJA on the contents of glycyrrhizic acid, liquiritin and licochalcone A, and total flavonoids, saponins and polysaccharide in G. uralensis grown under salt stress. Values are means ± SE (n = 6). CK, control group; S (75 mM NaCl), salt stress group; S + MeJA (75 mM NaCl + 30 μM MeJA), salt stress combined with methyl jasmonate group. The different letters within the different treatments indicate the significant difference at P ≤ 0.05
A significant positive correlation was observed between glycyrrhizic acid and licochalcone A and glycyrrhizin (Table 3).
Effects of MeJA on contents in total flavonoids, total saponins, total polysaccharide in salt-stressed G. uralensis seedlings
Salt stress triggered the decrease of total flavonoids and polysaccharides contents by 27.83% and 37.01%, respectively, compared to control in G. uralensis plants, while salt stress also increased total saponin content. MeJA increased total flavonoids and total polysaccharides contents by 15.75% and 41.82%, respectively, compared to salt stress (Fig. 4).
PCA of growth and physio-biochemical characteristics in G. uralensis
PCA divided the total variance into five PCs with the largest contribution (88.799%) (Table 4). The higher eigenvectors of PC I are soluble sugar, SS, SPS and GS. The higher eigenvectors of PC II are INV and NR. The higher eigenvectors of PC III is NO3−. The higher eigenvectors of PC IV are sucrose and NO2−. The higher eigenvectors of PC V is GOGAT (Fig. 5).
Effect of MeJA in salt-stressed G. uralensis seedlings by carbon and nitrogen metabolism. Sucrose synthase (SS), sucrose phosphate synthase (SPS) and sucrose of carbon metabolism were analyzed, and nitrate (NO3−), ammonium (NH4+), nitrate reductase (NR), glutamate synthase (GOGAT), nitrite (NO2−) and glutamine synthetase (GS) of nitrogen metabolism were also detected. The pointing of red arrows in the figure represent the changes of enzymes and substances in carbon and nitrogen metabolism which were mainly affected by MeJA
Discussion
Salt stress usually causes a severe reduction in plant biomass. In this study, the length and diameter of main root and the number of secondary root of G. uralensis seedling significantly reduced by salt stress, which is consistent with previous reports on various plants such as wheat (Bot et al. 2013), tomato (Latef et al. 2011), pepper (Latef et al. 2014) and others (Duan et al. 2013; Mostofa et al. 2015; Ahmad et al. 2016). MeJA can alleviate the inhibition effect of salt stress on plant growth (Ahmadi et al. 2018). The present results showed that MeJA significantly increased main root length, secondary root numbers and biomass while did not have a significant effect on shoot diameter of G. uralensis seedling subjected to salt stress, indicating that MeJA application alleviates the inhibited effect of salt to the growth of root and biomass, which is consistent with previous results in cowpea (Sadeghipour 2017) and strawberry (Faghih et al. 2017).
Osmotic stress affects plant growth and biomass under salt stress condition. To respond to the harmful impact of osmotic stress induced by salt stress, plants produce and accumulate higher levels of compatible solutes in the cytosol and other organelles (Latef and Miransari 2014). Compatible solutes such as proline, soluble sugar and soluble protein are mainly accumulated in response to salt stress in G. uralensis (Lu et al. 2013; Zhang et al. 2021). Among them, soluble sugar maintains cell homeostasis and improves plant tolerance to osmotic stress (Ahmad et al. 2016; Bai et al. 2013). In which sucrose plays an important role in plant structure and metabolism and it is also involved in responses to various stresses (Qiu et al. 2014). In this study, a significant accumulation trend in soluble sugar and sucrose was recorded in G. uralensis seedling exposed to salt stress (Fig. 2), which is similar to previous results in Zea mays L. (Feng et al. 2002), Lupinus albus L. (Fernandes et al. 2004) and Nitraria tangutorum (Liu et al. 2016). A previous study reported that MeJA enhanced the protective property of the osmolytes in peach (Yu et al. 2016). Moreover, the present results showed that the increase in contents of soluble sugars accompanied by the decrease in growth parameters under salt treatment, which explain the negative Pearson’s coefficient of soluble sugars for the growth parameters (Table 2). Our results also showed that application of MeJA to salt-stressed G. uralensis seedlings induced an increase in sucrose level, which perhaps provide better protection to G. uralensis seedlings subjected to osmotic stress. Therefore, MeJA may be an effective method for protecting plants against osmotic stress through regulating carbohydrate accumulation.
Accumulation of carbohydrates is closely relevant to the activities of INV, SS and SPS that are involved in sucrose synthesis and metabolism. Salt stress affected the levels of INV, SS and SPS activities in various plants such as Cicer arietinum (Kaur et al. 2003), Lotus japonicus (Miguel et al. 2008), and Triticum aestivum L. (Fresneau et al. 2007). In the present study, INV activity increased in G. uralensis under salt stress with the increased sucrose content, while the activities of SS and SPS had slight change in G. uralensis, indicating that INV plays a vital role in buffer regulation of the carbohydrates contents for salt-induced osmotic stress. This is supported by the previous results in Dwarf bamboo (Liu et al. 2014a, b) and Cicer arietinum (Kaur et al. 2003). Moreover, MeJA markedly enhanced INV, SS and SPS activities, which is in harmony with the results in Hericium erinaceus (Wang et al. 2011). These results suggested that MeJA could further alleviate salt stress by inducing INV, SS and SPS activities, and then these enzymes could affect carbohydrate levels of G. uralensis under salt stress (Table 2).
The regulation of N metabolism is an important property to responding to stress, which affects almost all physiological processes in plants. Salt stress interferes with NO3− uptake and decreases NO3− contents in Triticum aestivum L. (Elbaki et al. 2017), Citrullus lanatus (Yang et al. 2013), Helianthus annuus L. (Silva et al. 2014) and Cucumis sativus L. (Shao et al. 2015). In this study, salt stress has no effect on NO3− content in G. uralensis seedlings, this may be due to the NO3−supply and transfer rate from the vacuole into the cytoplasm, which is supported by the previous found in Fargesia denudata (Liu et al. 2014a, b). Increased NO3− assimilation is beneficial for improving the resistance of plants under stresses via regulating stomatal opening and the synthesis of osmotic substances (Yi et al. 2014; Wilkinson et al. 2007). Interestingly, MeJA application significantly increased NO3− content in G. uralensis under salt stress. It suggests that MeJA enhanced NO3− absorption in G. uralensis roots which might improve osmotic regulation, thus strengthen the adaptation to salt stress. NR convert NO3− to NO2−, then NiR catalyze the conversion of NO2− into NH4+, further supply more substrate for proline synthesis (Khan et al. 2015). Previous studies showed that salt stress decreased the NR activity in Populus simonii (Meng et al. 2016), soybean (Farhangi-Abriz and Torabian 2017) and wheat (Elbaki 2017) under salt stress. Moreover, MeJA can increase N assimilation in Triticum aestivum L. by increasing NR and glutamate synthase (GS) activity (Kaya et al. 2021). Present study results found that salt stress significantly decreased NR activity but increased NO2− content in G. uralensis seedlings. However, MeJA application increased NR activity and NO2− content in salt-stressed G. uralensis, which in harmony with the results in Solanum lycopersicum (Singh et al. 2016). These results suggest that MeJA can promote the conversion of NO3− to NO2− by increasing the activity of NR, which might facilitating the assimilation of NO3− and the synthesis of amino acid subsequently (Kaya et al. 2021).
Excessive NH4+ from both NO3− reduction and photo-respiration is toxic to plant cells (Thomas and Hilker 2000). In the present study, salt stress caused a significant increase in NH4+ content, which is consistent with the results in tomato (Manai 2012) and cucumber (Shao et al. 2015). Interestingly, MeJA application decreased NH4+ content in salt-stressed G. uralensis and this may mitigate the cytotoxicity of excess NH4+ induced by salinity. The conversion of non-toxic organic N is closely associated with GS/GOGAT cycle, which is the major route for NH4+ assimilation (Yang et al. 2010). Our result observed decreased GOGAT activity in G. uralensis under salt stress, the results were consistent with those in cucumber (Shao et al. 2015) and soybean (Zilli et al. 2008). These results suggested that the GS/GOGAT cycle is severely blocked in plants subjected to salt stress condition. Whereas, MeJA remarkably improved GOGAT and GS activities in G. uralensis under salt stress, suggesting that MeJA could reduce NH4+ accumulation by accelerating the interconversion of glutamine (Glu) and glutamic acid (Gln). Taken together, we concluded that MeJA promoted N assimilation and removal of the excess NH4+ by increasing the activities of NR, GS and GOGAT under salt stress, which helps to maintain the balance of N metabolism in G. uralensis exposed to salt stress. In addition, MeJA significantly up-regulated GDH in rice, which plays a role in the re-assimilation of the excess ammonium induced exposed to stress (Wu et al. 2019). The effect of MeJA on GDH activity should be considered subsequently in this study to help clarify in more deeply the mechanisms by which MeJA improves nitrogen metabolism under salt stress.
Secondary metabolism is the result that the interaction between plants and environments in the long-term evolution, and it is closely correlated with the strengthening of plant tolerance to stresses (Xiao-Hong et al. 2005; Wang et al. 2015). The production of anthraquinone, phenolics and flavonoids was increased in adventitious roots of Morinda citrifolia under salt stress (Baque et al. 2010). The contents of secondary metabolites in Swertia chirata Buch.-Ham were remarkably increased under salt stress (Abrol et al. 2012). Salt stress also remarkably enhanced the total phenolics and γ- and δ-tocopherols levels in wild almond species (Sorkheh et al. 2012). In this study, the results found that salt stress significantly decreased glycyrrhizic acid content in G. uralensis plants, but has no effect on glycyrrhizin and licochalcone A contents. Salt stress also decreased total flavonoids and polysaccharides contents in G. uralensis plants, suggesting that salt stress remarkably regulated the synthesis and accumulation of secondary metabolites. However, salt stress significantly increased the total saponin content that perhaps a responsive strategy for G. uralensis seedlings adapt to salt stress by producing more secondary metabolites, further promoting plant growth. Previous studies have found that the application of exogenous substances (plant growth regulators) can effectively promote the synthesis of secondary metabolites in plants (Yu et al. 2018). In Catharanthus roseus L., MeJA promoted the accumulation of secondary metabolites (Ruiz-May et al. 2011). In Polygonum multiflorum, MeJA enriches arachidonic acid and linoleic acid metabolisms, and biosynthesis of stilbenoid by inducing transcriptome changes (Liu et al. 2015). Our results showed that MeJA remarkably enhanced glycyrrhizic acid, licochalcone A contents, total flavonoids and polysaccharides contents compared in salt-stressed G. uralensis seedlings. These results indicated that MeJA could induce the accumulation of secondary metabolites in G. uralensis seedlings, the possible reason is that MeJA regulates the key enzymes related to the secondary metabolites and its gene expression in G. uralensis which need further research.
Conclusions
MeJA could relieve the harmful effects caused by salt stress on G. uralensis seedlings through affecting C and N metabolisms that further trigger the accumulation of secondary metabolites. Specifically, MeJA significantly increased carbohydrate contents by enhancing the activities of related enzymes in C metabolism in G. uralensis. Moreover, the increase in soluble sugar and sucrose induced by MeJA plays a protective role in osmotic regulation of salt-stressed G. uralensis. Meanwhile, MeJA enhanced the N metabolism that could induce G. uralensis seedlings produce more amino acids and total N to overcome osmotic damage. Furthermore, MeJA significantly increased glycyrrhizic acid, licochalcone A, total flavonoids and total polysaccharides contents in salt-stressed G. uralensis. Thus, MeJA can effectively regulate different metabolites in G. uralensis to relieve osmotic pressure under salt stress to improve growth. Further research should investigate mechanisms of molecular by which MeJA affect secondary metabolism, including signaling pathway to regulate biosynthesis gene expression. The positive MeJA effects on G. uralensis for growth and the accumulation of secondary metabolites also need to be tested under field conditions.
Author contribution statement
XH Z conceived and designed the experiments; X M, XX Y, GC C and DY L performed the experiments; X M wrote the manuscript; XX Y made the figure; ZG G provided guidance on the whole manuscript, and all authors read and approved the final manuscript and post no conflicting interest.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GS:
-
Glutamine synthetase
- GOGAT:
-
Glutamate synthetase
- NR:
-
Nitrate reductase
- GOT:
-
Glutamic-oxaloacetic transaminase
- GPT:
-
Glutamic-pyruvic transaminase
- N:
-
Nitrogen
- NH4 + :
-
Ammonium
- SS:
-
Sucrose synthase
- SPS:
-
Sucrose phosphate synthase
- MeJA:
-
Methyl jasmonate
- C:
-
Carbon
- NO3 − :
-
Nitrate
- NO2 − :
-
Nitrite
- GDH:
-
Glutamine dehydrogenase
References
Abrol E, Vyas D, Koul S (2012) Metabolic shift from secondary metabolite production to induction of anti-oxidative enzymes during NaCl stress in Swertia chirata Buch.-Ham. Acta Physiol Plant 34:541–546
Ahmad P, Latef AA, Hashem A, Abdallah EF, Gucel S, Tran LS (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347–358
Ahmadi FI, Karimi K, Struik PC (2018) Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S Afr J Bot 115:5–11
Bai JH, Liu JH, Zhang N, Sa R (2013) Effect of salt stress on antioxidant enzymes, soluble sugar and yield of oat. J Food Sci Tech Mys 5:303–309
Baque AA, Lee EJ, Paek KYM (2010) Salt strength induced changes in growth, physiology and secondary metabolite content in adventitious roots of Morinda citrifolia: the role of antioxidant enzymes and phenylalanine ammonia lyase. Plant Cell Rep 29:685–694
Bot PJ, Kausar F, Shahbaz M (2013) Interactive effect of foliar application of nitric oxide (NO) and salinity on wheat (Triticum aestivum L.). Pak J Bot 45:67–73
Cuellar-Ortiz SM, Arrieta-Montiel MP, Acosta-Gallegos J, Covarrubias AA (2008) Correlationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ 31:1399–1409
Debouba M, Gouia H, Suzuki A, Ghorbel MH (2006) Salt stress effects on enzymes involved in nitrogen assimilation pathway in tomato Lycopersicon esculentum seedlings. J Plant Physiol 163:1247–1258
Du S, Zhang Y, Lin X, Wang Y, Tang C (2008) Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant Cell Environ 31:195–204
Duan L, Dietrich D, Ng CH, Chan PM, Bhalerao R, Bennett MJ (2013) Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25:324–341
Egamberdieva D, Wirth S, Li L, Abdallah EF, Lindström K (2017) Microbial cooperation in the rhizosphere improves liquorice growth under salt stress. Bioengineered 8:433–438
Elbaki GKA (2017) The productivity of wheat cultivars under salt stress not always linked with their nitrate reductase activity in leaves. J Adv Biol 6:2347–6893
Elzbieta S, Edward G, Demczuk A, Spiak Z (2008) Effect of salt and water stresses on growth, nitrogen and phosphorus metabolism in Cucumis sativus L. seedlings. Acta Soc Bot Pol 77:23–28
Faghih S, Ghobadi C, Zarei A (2017) Response of strawberry plant cv. ‘Camarosa’ to salicylic acid and methyl jasmonate application under salt stress condition. J Plant Growth Regul 36:1–9
Fan L, Wang Q, Lv J, Gao L, Zuo J, Shi J (2016) Amelioration of postharvest chilling injury in cowpea (Vigna sinensis) by methyl jasmonate (MaJA) treatments. Sci Hortic 203:95–101
Farhangi-Abriz S, Torabian S (2017) Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis 74:1–9
Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z (2002) Improved tolerance of maize plants to salt stress by Arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12:185–190
Fernandes FM, Arrabaça MC, Carvalho LM (2004) Sucrose metabolism in Lupinus albus L. under salt stress. Biol Plantarum 48:317–319
Flores P, Botella MA, Cerda A, Martinez V (2004) Influence of nitrate level on nitrate assimilation in tomato (Lycopersicon esculentum) plants under saline stress. Revue Canadienne De Botanique 82:207–213
Fresneau C, Ghashghaie J, Cornic G (2007) Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum duruml.): role of leaf internal CO2. J Exp Bot 58:2983–2993
Garg N, Singla R (2005) Nitrate reductase activity in roots and leaves of chickpea cultivars under salt stress. Span J Agric Res 3:248–252
Kaur S, Gupta AK, Kaur N (2003) Effect of kinetin on starch and sucrose metabolising enzymes in salt stressed chickpea seedlings. Biol Plantarum 46:67–72
Kaya C, Ugurlar F, Ashraf M, Noureldeen A, Darwish H, Ahmad P (2021) Methyl jasmonate and sodium nitroprusside jointly alleviate cadmium toxicity in wheat (Triticum aestivum L.) plants by modifying nitrogen metabolism, cadmium detoxification, and AsA-GSH cycle. Front Plant Sci 12:654780
Khan MI, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Kleinwächter M, Selmar D (2013) Influencing the product quality by applying drought stress during the cultivation of medicinal plants. Ind Crop Prod 42:558–566
Latef AA, He C (2011) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci Hortic 127:228–233
Latef AA, He C (2014) Does inoculation with Glomus mosseae improve salt tolerance in pepper plants. J Plant Growth Regul 33:644–653
LatefA A, Miransari M (2014) The role of arbuscular mycorrhizal fungi in alleviation of salt stress. Use of microbes for the alleviation of soil Stresses. Springer, New York, pp 23–38
Li Z, Wang WW, Li GL, Guo K, Harvey P, Chen Q, Zhao ZJ, Wei YL, Li JS, Yang HT (2016) MAPK-mediated regulation of growth and essential oil composition in a salt-tolerant peppermint (Mentha piperita L.) under NaCl stress. Protoplasma 253:1541–1556
Liu C, Wang Y, Pan K, Zhu T, Li W, Zhang L (2014a) Carbon and nitrogen metabolism in leaves and roots of dwarf bamboo ( Fargesia denudata) subjected to drought for two consecutive years during sprouting period. J Plant Growth Regul 33:243–255
Liu C, Wang Y, Pan K, Zhu T, Li W, Zhang L (2014b) Carbon and nitrogen metabolism in leaves and roots of dwarf bamboo (Fargesia denudata Yi) subjected to drought for two consecutive years during sprouting period. J Plant Growth Regul 33:243–255
Liu HC, Wu W, Hou K, Chen JW, Zhao Z (2015) Transcriptome changes in Polygonum multiflorum thunb. roots induced by methyl jasmonate. J Zhejiang Univ Sci 16:1027–1041 (in Chinese)
Liu W, Zhang Y, Yuan X, Xuan Y, Gao Y, Yan Y (2016) Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ J Plant Physiol 63:132–142
Loutfy N, El-Tayeb MA, Hassanen AM, Moustafa MF, Sakuma Y, Inouhe M (2012) Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J Plant Res 125:173–184
Lu J, Lv X, Liang Y, Lin H (2013) Salt tolerance of Glycyrrhiza inflata seedlings in Xinjiang and its ion response to salt stress. Chin J Plant Ecol 37:839–850 (in Chinese)
Manai J (2012) Role of nitric oxide in saline stress: implications on nitrogen metabolism. Afr J Plant Sci 6:376–382
Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129:1003–1018
Martin DM, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol 132:1586–1599
Meng S, Su L, Li Y, Wang Y, Zhang C, Zhao Z (2016) Nitrate and ammonium contribute to the distinct nitrogen metabolism of populus simonii during moderate salt stress. PLoS ONE 11(3):e0150354
Miguel L, Herrera-Cervera JA, Iribarne C, Tejera NA, Lluch C (2008) Growth and nitrogen fixation in lotus japonicus and medicago truncatula under NaCl stress: nodule carbon metabolism. J Plant Physiol 3:137–144
Mostofa MG, Fujita M, Tran LP (2015) Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 77:265–277
Niazian M, Sadat-Noori SA, Tohidfar M, Mortazavian S, Sabbatini P (2021) Betaine aldehyde dehydrogenase (BADH) vs flavodoxin (Fld): two important genes for enhancing plants stress tolerance and productivity. Front Plant Sci 12:650215
Qiu ZB, Wang YF, Zhu AJ, Peng FL, Wang LS (2014) Exogenous sucrose can enhance tolerance of Arabidopsis thaliana seedlings to salt stress. Biol Plantarum 58:611–617
Ruiz-May E, Delapeña C, Galazávalos RM, Lei Z, Watson BS, Sumner LW (2011) Methyl jasmonate induces atp biosynthesis deficiency and accumulation of proteins related to secondary metabolism in Catharanthus roseus L. hairy roots. Plant Cell Physiol 52:1401–1421
Sadeghipour O (2017) Amelioration of salinity tolerance in cowpea plants by seed treatment with methyl jasmonate. Legume Res 40:1100–1106
Salimi F, Shekari F, Hamzei J (2016) Methyl jasmonate improves salinity resistance in german chamomile (Matricaria chamomilla L.) by increasing activity of antioxidant enzymes. Acta Physiol Plant 38:1–14
Shao QS, Shu S, Du J, Xing WW, Guo SR, Sun J (2015) Effects of salt stress on nitrogen metabolism of cucumber seedlings. Russ J Plant Physiol 62:595–603
Silva MS, Feijão AR, Marques EC, Filho EG, Prisco JT (2014) Growth, accumulation of solutes and nitrogen metabolism in plants of sunflower under salt stress. Inovagri Int Meet II:4826–4835
Singh M, Singh VP, Prasad SM (2016) Responses of photosynthesis, nitrogen and proline metabolism to Salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol and Bio 109:72–83
Sorkheh K, Shiran B, Rouhi V, Khodambashi M, Sofo A (2012) Salt stress induction of some key antioxidant enzymes and metabolites in eight Iranian wild almond species. Acta Physiol Plant 34:203–213
Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312
Takahashi I, Hara M (2014) Enhancement of starch accumulation in plants by exogenously applied methyl jasmonate. Plant Biotechnol Rep 8:143–149
Thomas FM, Hilker C (2000) Nitrate reduction in leaves and roots of young pedunculate oaks (quercus robur) growing on different nitrate concentrations. Environ Exp Bot 43:19–32
Wang CL, Liang ZS, Dian-Rong LI, Yang JL (2011) Effects of salicylic acid and methyl jasmonate on sucrose metabolism and phenolic compounds accumulation in salvia miltiorrhiza bunge seedlings. Acta Botan Boreali-Occiden Sin 31:1405–1410 (in Chinese)
Wang Q, Eneji AE, Kong X, Wang K, Dong H (2015) Salt stress effects on secondary metabolites of cotton in correlation to gene expression responsible for aphid development. PLoS ONE 10:e0129541
Wang J, Fu Z, Chen G, Zou G, Song X, Liu F (2018) Runoff nitrogen (N) losses and related metabolism enzyme activities in paddy field under different nitrogen fertilizer levels. Environ Sci Pollut Res 25:27583–27593
Wilkinson S, Bacon MA, Davies WJ (2007) Nitrate signalling to stomata and growing leaves: interactions with soil drying, ABA, and xylem sap pH in maize. J Exp Bot 58:1705–1716
Wu X, Ding C, Baerson SR, Lian F, Lin X, Zhang L, Wu C, Hwang SY, Zeng R, Song Y (2019) The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L.). Plant Cell Environ 42:659–672
Xie Z, Lang D, Chu Y, Cui G, Jia X, Zhang X (2021) Bacillus pumilus improved drought tolerance in Glycyrrhiza uralensis G5 seedlings through enhancing primary and secondary metabolisms. Physiol Plant 171:388–399
Yang J, Zhang J, Wang Z, Xu G, Zhu Q (2004) Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physio 135:1621–1629
Yang X, Wang X, Wei M, Hikosaka S, Goto E (2010) Response of ammonia assimilation in cucumber seedlings to nitrate stress. J Plant Biol 53:173–179
Yang Y, Lu X, Yan B, Li B, Sun J, Guo S (2013) Bottle gourd rootstock-grafting affects nitrogen metabolism in salt-stressed watermelon leaves and enhances short-term salt tolerance. J Plant Physiol 170:653–661
Yi XP, Zhang YL, Yao HS, Zhang XJ, Luo HH, Gou L, Zhang WF (2014) Alternative electron sinks are crucial for conferring photoprotection in field-grown cotton under water deficit during flowering and boll setting stages. Funct Plant Biol 41:737–747
Yu L, Liu H, Shao X, Yu F, Wei Y, Ni Z (2016) Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol Tec 113:8–16
Yu XX, Zhang WJ, Zhang Y, Zhang XJ, Lang DY, Zhang XH (2018) The roles of methyl jasmonate to stress in plants. Funct Plant 46:197–212
Zaid A, Mohammad F (2018) Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J Plant Growth Regul 37:1331–1348
Zhang RM, Sun YK, Liu ZY, Jin W, Sun Y (2017) Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J Pineal Res 62:e12403
Zhang XH, Zhang WJ, Lang DY, Cui JJ, Li YT (2018) Silicon improves salt tolerance of Glycyrrhiza uralensis Fisch. by ameliorating osmotic and oxidative stresses and improving phytohormonal balance. Environ Sci Pollut R 25:25916–25932
Zhang Y, Cui G, Zhang W, Lang D, Li Z, Zhang X (2021) Bacillus sp. G2 improved the growth of glycyrrhiza uralensis fisch. related to antioxidant metabolism and osmotic adjustment. Acta Physiol Plant 43:1–16
Zilli CG, Balestrasse KB, Yannarelli GG, Polizio AH, Santa-Cruz DM, Tomaro ML (2008) Heme oxygenase up-regulation under salt stress protects nitrogen metabolism in nodules of soybean plants. Environ Exp Bot 6:83–89
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National Natural Science Foundation of China (31860343), the Ningxia Science and Technology Innovation Leader Program (2020GKLRLX12). The authors declare that no funding agency have any role in the design of the study, data collection, analysis, interpretation or manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by S. Renault.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, X., Yu, X., Cui, G. et al. Methyl jasmonate mitigates osmotic stress by regulating carbon and nitrogen metabolism of Glycyrrhiza uralensis seedlings subjected to salt stress. Acta Physiol Plant 45, 96 (2023). https://doi.org/10.1007/s11738-023-03574-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-023-03574-z