Abstract
Aims
Investigations into the nutrient contents and changes in the stoichiometry and nutrient resorption strategies of different original forests during their development are of great significance toward the establishment of healthy, high-quality forest ecosystems.
Methods
A total of 24 sample plots with age gradients were established in natural Larix principis-rupprechtii forests and plantations, from which mature and senescent leaves, new branches, fine roots, and soil were collected and analyzed. The carbon (C), nitrogen (N), and phosphorus (P) contents were determined, and the stoichiometric ratios and resorption efficiencies were calculated.
Results and conclusions
The soil organic carbon and total nitrogen of the forests accumulated with advancing age, whereas the total phosphorus decreased because of higher consumption. Plantation trees tended to reduce the storage of nutrients in inactive plant structures to prevent nutrient loss, whereas natural forest trees maintained a stable nutrient concentration within their active organs. Plantation and natural forest trees adopted highly conservative N and P utilization strategies through improved resorption efficiencies. However, plantation forests were more conservative than their natural counterparts. The mature leaves, litter, branches, and fine roots of natural forests were influenced to a greater degree by the soil P, whereas those of plantation forests were more affected by the soil N. Nutrient concentrations within the active organs of plantation trees were more strongly dependent on soil than natural forests. We considered that natural forests likely possessed a more extensive range of strategies to deal with the variable compositions and concentrations of soil nutrients, which might strengthen their resilience against the effects of aging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As they age, forests of various origins often have distinct external manifestations largely caused by differences in the absorption, utilization, and storage of nutrients by trees. Prudent and comprehensive management practices are considered key to the development of healthy and sustainable forest ecosystems (Dixon et al. 1994). Nevertheless, management principles cannot be standardized for forest ecosystems of different origins at various stages of growth and development. In contrast to natural forests, plantation forests have erratic resistance against disease and consume additional soil nutrients, albeit with the result of higher productivity (Perry and Maghembe 1989). Conversely, natural forests are superior to their artificial counterparts as regards resisting pests and diseases, maintaining soil fertility, and preserving species (Xu 1991). These differences in plant nutrient utilization strategies and soil nutrient contents are likely age-related (Lambers et al. 2008). Because of the variable growth rates between natural and plantation forests (White et al. 2021), the soil composition (Liao et al. 2012; Cai et al. 2019; Osuri et al. 2020; Parhizkar et al. 2021), diversity of understory vegetation (Tripathi and Singh 2009; Gong and Tang 2016; Zhang et al. 2021a, b), and other aspects are quite diverse. Standardized management strategies applied to different types of forests often lead to stunted growth, excessive competitive pressure, and premature tree aging. The explanation may be that unsuitable management strategies result in limited nutrient utilization in developing forests and imbalances or deficiencies in tree and organ elements. Thus, management principles should be implemented that align with the nutrient utilization strategies and other unique attributes of forests with diverse origins at each growth and developmental stage (Xu et al. 2021).
Carbon (C), nitrogen (N), and phosphorus (P) influence the growth of vegetation and the quality of soils in forest ecosystems while maintaining element cycling and stability (Han et al. 2005; Niklas et al. 2005; Qiu et al. 2019). Ecological stoichiometry has been used extensively to elucidate plant growth (Elser et al. 2000, 2007; Niklas et al. 2005), reveal plant nutrient allocation strategies and allometric growth (Yang et al. 2014; Zhao et al. 2016), and identify limiting elements (Koerselman and Meuleman 1996). The soil substrate age hypothesis (Reich and Oleksyn 2004) proposes that the developmental age of soil impacts the nutrient supply capacities of parent soil materials, which affects plant stoichiometry. Two components appear to be involved: (1) the composition and proportion of soil nutrients vary with advancing age, and (2) plant nutrient stoichiometry attributes fluctuate with increasing age. As age-related changes in forests may be considered complex disturbances, the soil carbon levels (Ewel et al. 1987; Klopatek 2002), carbon sinks (Zhou et al. 2006; Luyssaert et al. 2008), litter nutrient contents (Kelliher et al. 2004), and other factors related to the soil and plants will be affected by aging.
However, there is a profound covariation relationship between the changes in soil and plants. When the concentrations of external nutrients are altered, the nutrient absorption and storage strategies of plants will be modified accordingly (Chapin 1991; Wang et al. 2017; Castellanos et al. 2018). For example, in the context of N deposition on a global scale, the N:P ratios of plants in terrestrial and freshwater ecosystems were observed to increase. However, the C:N ratios of both plants and organic soils and the N2 fixation capacities of soil and water decreased (Sardans et al. 2012). Based on six consecutive years of research, Mao et al. (2016) proposed that exogenous P inputs induced the C:N, C:P, and N:P ratios of plants to simultaneously decrease, whereas N and P exhibited synergistic increases. Variable soil nutrient levels and other disturbances are essential for strategic use by forest trees, where higher soil nutrient concentrations translate to their increased acquisition and storage in leaves (Yan et al. 2006). Historically, ecologists have proposed several hypotheses to support these phenomena, including the Law of the Minimum, the multiple limitation hypothesis, and the stability of limiting elements hypothesis (Baar 1994; Han et al. 2011; Agren et al. 2012). Further, perennial plants use a strategy for the redistribution and absorption of nutrients back into living tissues, which is referred to as nutrient resorption (RE) (Niinemets and Tamm 2005; Hayes et al. 2014a, b). It is generally believed that the resorption rates of N and P in plants are ~ 50%, whereas the resorption efficiency of K is slightly higher. Nevertheless, resorption efficiencies are often influenced by the attributes of plants (Lal et al. 2001), climatic factors (Oleksyn et al. 2003; Yuan and Chen 2009), soil fertility (Drenovsky et al. 2010; Yuan and Chen 2015), and the plant development stage (Brant and Chen 2015).
In summary, we consider that plants maintain internal stability by regulating the uptake and loss of nutrients and elements through various response strategies. Further, under changing environmental conditions, plants adopt element compensation or absorption mechanisms through a series of responses. The steady state tends to shift in a certain direction to reconfigure the plant element content, stoichiometric ratio, and resorption characteristics. Forests of different origins undergo continuous changes and the differentiation of other environmental factors within their ecosystems during growth and development. Therefore, we speculated that their nutrient utilization and storage strategies would also undergo responsive changes to create differences; however, this has not been fully elucidated as yet.
Larix principis-rupprechtii is distinct from common evergreen coniferous plants in that its annual litter generation far exceeds that of common evergreen tree species, and it “frequently interacts” with forest soils (Liu et al. 2021). Further, it is a commercially valuable wood that is extensively planted in the mountainous regions of northern, northeastern, and southwestern China, which cumulatively comprise the largest area of L. principis-rupprechtii plantations worldwide (Chen et al. 2016). This study focused on the age-related changes of plants (e.g., nutritional aspects and utilization strategies) and soils (e.g., nutrient contents and quantitative attributes) in L. principis-rupprechtii forests of different origins, as the relationships between the two are unclear. Consequently, we proposed two hypotheses: (1) soil (nutrient contents and stoichiometry) and vegetation (nutrient contents, stoichiometry, and nutrient use strategies) exhibit age-related changes, and (2) plantations and natural forests differ as regards their nutrient use levels and dependence on soil nutrient resources.
Materials and methods
Study area
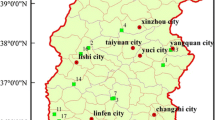
This research was performed primarily at the Xiaowenshan Forestry Farm in the Guandishanshan Mountains, which is ~ 22 km northwest of Jiaocheng County, Shanxi Province, China (Fig. 1). The study area is situated in the mid-region of the Lvliang Mountains, which includes the Pangquangou National Natural Reserve as its center, with six forest farms that surround it. The total area is 57,200 ha with altitudes that range from 1,360 to 2,839 m. This region has an inner-continental mountain monsoon climate, with a mean annual temperature and precipitation of 4.2 °C and 822.6 mm, respectively.
The soil originated from the base and gneissic granite of the Archean Era, which includes brown mountain soil. There are large expanses of L. principis-rupprechtii plantations and natural forests in the study area, wherein the natural forests are secondary forests regenerated spontaneously on secondary bare land following logging or burning since the 1930s. The plantation area was previously dominated by broadleaved forests or mixed shrub and herbaceous forests, where seedlings were planted following clear-cutting.
Plot setting and sample collection
To investigate the impacts of stand origin and age on leaves, litter, and soil C, N, and P, spatial substitution time and stratified random sampling techniques were used on three selected plantations and five selected natural forest sub-compartments. Plantation forests have been successively planted in the study area since the 1970s after resolving the issue of the survival rate of seedlings; thus, the age range is small. The natural forests are inherently regenerated and have a long history with a wide range of stand ages. As many age groups as possible were selected within the optional range. Although these sub-compartments were of different ages, the other site conditions were essentially identical (Table 1). There were a total of eight sub-compartments (including natural forests and plantations), which ranged from infancy to maturity, and each sub-compartment was a pure L. principis-rupprechtii forest. The distance between each sub-compartment was greater than 150 m to reduce spatial autocorrelation. Within each sub-compartment, three plots measuring 20 × 20 m were established and subjected to surveys and sampling to ensure that the intervals between plots were greater than 40 m. To determine the ages of the natural forests, 15 dominant trees were selected from each plot, and a core from each tree was extracted at breast height along the slope parallel to the vertical direction, for a total of 30 cores, which then underwent tree core scanning analyses (EPSON 10000XL) and WinDENDRO software calculations (Shumilov et al. 2013) (Table 1).
At the end of July and early August 2018, leaf samples, new branches, litter, and soil were collected while randomly selecting five trees from each plot from which to collect the leaves and branches. Mature leaves and new shoots were extracted from the top, middle, and bottom three crowns (the three samples were combined as one) of healthy and evenly spaced dominant trees. One compound leaf and one compound branch sample were obtained from each plot. A soil core drill (Ø10 cm ID) (Ø = diameter, ID = inside diameter) was used to sample the uppermost leaf litter and topsoil to a depth of 30 cm at 1.0 m from the five sample trees. Soil cores were extracted from the upper (0–10 cm), middle (10–20 cm), and lower (20–30 cm) strata of each soil profile to determine the potential heterogeneity of the soil layers (Baldrian et al. 2012). Akin to the leaf samples, the soil and litter samples from each plot were mixed as composites. After their transfer to the laboratory, the roots were sorted to obtain fine root samples (Ø < 2 mm). Finally, 72 soil samples and 96 active L. principis-rupprechtii organ samples (24 each of leaves, branches, litter, and fine roots) were obtained.
When calculating the resorption efficiency, we considered the leaves in the litter as senescent (Killingbeck 1996), which were obtained by shaking and collecting the fallen leaves of living plants. As it was challenging to extract senescent leaves from living plants, they were collected from the litter that recently fell directly under the trees. The leaf samples selected from the litter had not undergone obvious oxidation and decomposition and were not affected by significant precipitation erosion during the period from litterfall to collection (Hayes et al. 2014a, b), and their degree of photodegradation was very low (Austin and Vivanco 2006). Thus, any nutrient loss induced otherwise was negligible. All samples were placed in plastic bags and stored at 4 °C for laboratory analysis.
Laboratory analyses
The plant and litter samples were dried at 65 °C to a constant weight (the plant samples were initially dried at 105 °C for 15 min), after which the dried material was pulverized and passed through a 100-mesh screen. The soil samples were air-dried, ground, and then sifted using a 100-mesh sieve. The total carbon, total nitrogen, and total phosphorus (TP) of plant organs, and soil organic carbon (SOC), total nitrogen, TP, ammonium nitrogen (AN), nitrate nitrogen (NN), and available phosphorus (AP) of the soil were determined. The measurement techniques and instruments are presented in Table 2.
Data analysis
Before data analysis, we performed normality and homogeneity of variance tests on all variables. We used analysis of variance and multiple comparisons (least significant difference) to determine the nutrient contents and stoichiometric characteristics of age, origin, and their interactions with active plant organs, soil, and the impacts of resorption efficiencies. We used redundancy analysis (RDA) to determine the relationships between the nutrient contents of the soil and plant organs. To avoid multicollinearity, we initially screened the soil nutrient index (VIF < 10) and finally retained four variables (TP, TN, AP, and SOC) (Brian et al. 2001). First, we considered that relative and absolute nutrient contents are not equivalent in an ecological sense for the nutrient utilization strategies of trees. Therefore, we treated soil nutrients and stoichiometric characteristics separately and discussed those of common interpretation. Second, if the nutrient contents and quantitative characteristics were to be discussed together, the ecological significance of the ordination axis would be challenging to explain even with factor loadings because of the different orientations of their ecological significance. Therefore, we differentiated the nutrient contents and proportion of soil when performing RDA. We performed all statistical analyses in r 4.0.1 (Team 2009).
We calculated the RE (Nutrient resorption) efficiencies according to the percentage of the leaves reduced by the corresponding elements, which was a percentile of the difference between the elemental contents of the senescent and mature leaves (Eq. 1). To test the effects of age (A-continuous variable) and origin (O-natural forest or plantation) on the soil, litter, plant stoichiometric characteristics, and resorption efficiency variables, we used the following model (Eq. 2):
where Nm is the nutrient concentration of mature leaves and Ns is the nutrient concentration of litter for leaves that were beginning to senesce (Aerts and Chapin 1999). The RE capacity of the litter is correlated with the nutrient concentration.
We used the following model equation to test the effects of age (A) and origin (O, natural forest vs. plantation) on the soil, litter, plant index, and resorption efficiencies.
where Yij is the given plant or soil index, e(ij) is the sampling error nested within A and O, and i and j represent the given age and origin, respectively.
Results
Changes in the elemental content of active organs
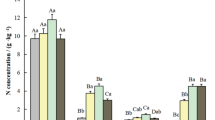
We analyzed the C, N, and P contents in the organs of differently aged plants. The C content of mature leaves decreased with age, whereas the N content increased in plantations at a significantly faster rate than that in natural forests. The P content decreased with age; however, natural forests were more resistant in this regard. The C content in plantation forest litter initially increased and then decreased over time, whereas that in the natural forest litter decreased slightly because of fluctuations. The N content of senescent plantation leaves stabilized after increasing, whereas that in natural forests decreased slightly. The P content of litter decreased in plantations but followed the general trend of natural forests, where it decreased before the age of 40 years but then increased.
Age-related changes in new branches were not obvious, whereas the C content of the fine roots in plantations increased with age. Natural forests followed the same trend as 40-year-old plantations but declined thereafter. The N content of the fine roots in plantations increased and then decreased, with its peak value at ~ 30 years. The fine root P content exhibited a downward trend with advancing age, whereas in plantations, it initially decreased and then increased; however, it was even lower during the immature period (Fig. 2).
Age-related changes in the nutrient contents of active organs in forests of different origins. The uppercase letters in the upper left corner are the graphic numbers. N indicates natural forest, and P indicates plantation. Error bars indicate standard deviation. * and ns represent statistical significance and non-significant, respectively. * P < 0.05; ** P < 0.01; *** P < 0.001
Changes in the elemental content of the soil
The content of each nutrient index decreased with soil depth. The soil resident SOC, TN, NN, AN, and AP of natural forests increased with age, whereas the TP exhibited a certain downward trend. In plantations, the SOC increased with age, whereas the soil TN, AN, and NN peaked at ~ 30 years, and the TP and AP exhibited no obvious changes (Fig. 3).
Age-related changes in soil nutrient contents in forests of different origins. The uppercase letters in the upper right corner are the graphic numbers. The green histogram represents a 0–10-cm soil layer, the yellow represents a 10–20-cm soil layer, and the purple represents a 20–30-cm soil layer. Error bars indicate standard deviation. Different uppercase letters (A, B, and C) indicate significant differences between different ages. Different lowercase letters (a, b, and c) indicate significant differences between various soil layers. SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; NN, nitrate nitrogen; AN, ammonium nitrogen; AP, available phosphorus
Changes in active organ stoichiometry
The C:N ratio of mature leaves exhibited a downward trend with increasing age, whereas the C:P and N:P ratios exhibited an upward trend, where the rate of change in plantation forests was significantly higher than that of natural forests. The C:N ratio of leaf litter in the plantations decreased with age, whereas that of natural forests remained stable. The C:P ratio of the plantations increased slightly with age and then remained stable with higher N:P ratios. The natural forests exhibited no obvious trend and were observed to fluctuate within a stable range. The C:N and C:P ratios of plantation tree branches initially decreased and then increased with advancing age, which was similar to natural forests. Changes in the C:P ratio were relatively negligible, with those in natural forests rising and falling slightly in the plantations. The fine root C:N ratio in the plantations decreased with age, whereas the C:P and N:P ratios were essentially the same, as they initially increased and then decreased with age. However, both were higher than those during the juvenile period. The stoichiometry of fine roots in natural forests was stable within a certain range, and its propensity for change was weak (Fig. 4).
Age-related changes in the stoichiometry of active organs in forests of different origins. The uppercase letters in the upper left corner are the graphic numbers. N indicates natural forest, and P indicates plantation. Error bars indicate standard deviation. * and ns represent statistical significance and non-significant, respectively. * P < 0.05; ** P < 0.01; *** P < 0.001
Changes in soil stoichiometry
The soil C:N ratio (0–30 cm) decreased to a certain extent with advanced age in plantation forests, whereas in natural forests, it appeared to be more stable. The C:P ratio of soil initially increased and then stabilized in plantation forests, whereas in natural forests, it initially increased and then decreased, where changes in the N:P and C:P ratios were roughly the same (Fig. 5).
Age-related changes in the stoichiometry of soil (0–30 cm) in forests of different origins. The uppercase letters in the upper left corner are the graphic numbers. N indicates natural forest, and P indicates plantation. Error bars indicate standard deviation. * and ns represent statistical significance and non-significant, respectively. * P < 0.05; ** P < 0.01; *** P < 0.001
Age-related changes in RE
As regards RE, we observed that the nitrogen resorption efficiency increased with age and that natural forests were significantly slower in this regard than plantations. The phosphorus resorption efficiency initially increased and then slowly decreased with age but was always higher than that of immature forests (Fig. 6).
Age-related changes in nutrient resorption in forests of different origins. The uppercase letters in the upper left corner are the graphic numbers. N indicates natural forest, and P indicates plantation. Error bars indicate standard deviation. * and ns represent statistical significance and non-significant, respectively. * P < 0.05; ** P < 0.01; *** P < 0.001. NRE, nitrogen resorption efficiency; PRE, phosphorus resorption efficiency
Effects of forest age and origin
Multivariate analysis of variance was used to further confirm the role of aging in the various changes described earlier. The results indicated that the ages of forest stands had significant effects on the fine root C, mature leaf C and N, SOC, and soil TP and AN, whereas the ages of forest stands had specific, albeit not significant, effects on the senescent leaf N, fine root P, mature leaf P, branch C and N, and soil TN. The forest origins significantly impacted the fine root N, mature leaf C and N, branch C and N, and soil TN, TP, and AP. The interactions between age and origin significantly impacted the leaf litter N, fine root C, mature leaf N, SOC, and soil AP. Regarding stoichiometric characteristics, age significantly affected the C:P ratio of leaf litter, the C:N and N:P ratios of mature leaves, and the C:P and N:P ratios of soil. The stand origin significantly impacted the litter C:P and N:P ratios, fine root C:N and N:P ratios, C:N and C:P ratios of new branches, and soil SOC (Table 3).
Effects of soil characteristics on plant organs
We selected the surface soil as an exemplar and used RDA to determine the relationships between the soil nutrient contents and plant organs. The RDA results revealed (all full-model P values of RDAs were < 0.05) that the soil nutrient contents could explain 72.93% and 45.41% of the variations in the nutrient contents of the active organs of the plantations and natural forests, respectively. The highest degree of explanation for TP in the natural forest was 17.18%, whereas that for TN in the plantations was 46.57% (Fig. 7A and D). The soil C:N, C:P, and N:P ratios explained 34.84% of the variations in the nutrient contents of the natural forest organs, which was 73.31% in the plantation forests. Among these, the highest degree of explanation was the N:P ratio, which was negatively correlated with almost all variables of the active organ content of L. principis-rupprechtii (the angle was > 90°), which explained 20.52% of the natural forest variables and 54.81% of the plantation variables (Fig. 7B and E).
Redundancy analysis of soil stoichiometry and nutrient contents of plant organs in forests of different origins. Red arrows indicate soil factors, and green arrows indicate tree organ nutrient contents. MLC, mature leaf carbon; MLN, mature leaf nitrogen; MLP, mature leaf phosphorus; LC, litter carbon; LN, litter nitrogen; LP, litter phosphorus; BC, branch carbon; BN, branch nitrogen; BP, branch phosphorus; RC, fine root carbon; RN, fine root nitrogen; RP, fine root phosphorus. Numbers in the legend indicate the ages of the forests
Overall, the soil nutrient contents and soil C:N, C:P, and N:P ratios explained 70.41% of the changes in the active organ nutrient contents of natural L. principis-rupprechtii forests and 99.2% of those in the plantations. The combined soil nutrient contents and stoichiometric indices of the natural forests explained 9.84%, whereas the plantations accounted for 47.04%. The explanatory levels of the soil nutrient contents, soil stoichiometric index, and total explanatory degree were much higher for the plantations than for natural forests (Fig. 7C and F).
Discussion
Age-related nutrient storage strategies for active organs in forests of different origins
Age-related changes in plantations and natural forests were distinctly summarized. Practically all active organs exhibited a certain reduction in their P concentrations (Fownes 1995; Vitousek et al. 2010), which was likely caused by the limited supply of soil P during forest growth and supported by the soil nutrient contents. The soil TP content of the study area was ~ 0.4 g/kg, which was significantly lower than the national average (Liu et al. 2016), and decreased with stand age. However, the soil AP increased, which was directly utilized by soil microorganisms and absorbed by plants, inevitably leading to competition between plants and soil microorganisms (Kirkby et al. 2013).
In forest ecosystems, leaves, litter, and fine roots have rapid turnover rates, which are also functional drivers of nutrient cycling (Zhang et al. 2020). Interestingly, with advanced stand age, the C content of natural forests (except for mature leaves) was observed to decrease significantly, whereas the C, N, and P contents of other organs were considerably more stable in contrast to those of the plantations. Without considering the case for entire ecosystems, with advancing age, plantations tend to store scarce nutrients within inactive structures to prevent nutrient loss (Liu and Wang 2021). In contrast, the trees of natural forests appear to have the capacity to combat this age-associated trend or are more able to maintain stable nutrient contents within their active organs.
It is well known that when specific nutritional elements in forest ecosystems are lacking to a certain extent, the active organs of trees will implement absorption and compensation strategies to synergistically increase them or maintain internal stability (Peñuelas et al. 2012), as has been observed in plantation trees (Harpole et al. 2011; Agren et al. 2012). This might be the reason behind the significant fluctuations in the nutrient contents of the active organs of plantation trees.
Age-related nutrient utilization and resorption strategies in forests of different origins
The findings of this study revealed that the nutrient contents of forest trees determined their stoichiometry, which indicated their general condition and feedback strategies (Elser 2000; Elser et al. 2007; Sterner and Elser 2008). With advancing age, the C:P and N:P ratios of the mature leaves and litter of plantation trees increased significantly (Xu et al. 2016), although a suitable threshold could not be identified to determine whether the plants or soil were restricted. However, it was clear that the availability of P in the soil was insufficient in this region; thus, the local plants were also P-limited (Gusewell 2004; Cleveland and Liptzin 2007). In this study, the N:P ratios of the mature leaves and litter of almost all forest stands were higher than those in other areas of China (Jiang and Guo 2019) and globally (Post et al. 1985), with levels of 5.6 and 12.15, respectively. The higher C:P and N:P ratios indicated that the trees were more limited by P (Wang et al. 2017). The stoichiometric ratios in the active organs of natural forest trees (determined by the N and P contents) were relatively stable, which explained why the N and P resorption efficiencies of plantations increased with age.
To better conserve N and P with advancing age, plantation forests tend to initiate relatively low and high resorption strategies, respectively, whereas resorption in natural forests is less affected. The age-related strategies of plantations and natural forests differed and became increasingly evident with maturation. This was supported by Yan et al. (Yan et al. 2016), who revealed that plantations had higher RE efficiencies than natural forests.
In summary, the nutrient utilization strategies of plantation trees have greater age sensitivity and tend to adopt highly conservative N and P utilization measures as trees mature. Although natural forests are increasingly subject to conservation, they are better able to combat age-related trends.
Soil changes and causes
Changing trends and factor analyses revealed that the concentrations of soil nutrients in forests were significantly influenced by their age, where the soil C and N increased (Zeng et al. 2017; Vittori Antisari et al. 2018). The main reason behind this is that during tree/plant development, the soil, roots, and microbes have multiple strategies for fixing C and N and other elements (Belnap 2002; Chen et al. 2003). However, it is difficult for trees to obtain P from external sources, as it is typically supplied through the decomposition of onsite minerals (Jenny 1941). Consequently, the soil SOC and TN of L. principis-rupprechtii forests generally positively accumulated, whereas the TP was consumed in the negative direction. The TN, NN, and AN contents of plantation soils peaked at ~ 30 years. We surmised that during the middle stages of the concentrated growth and development cycle in plantations, root turnover, microbial activities, and cumulative nutrient utilization reached saturation/supersaturation.
In the case of limited overall environmental resources, a downward trend occurred in the follow-up, as age was observed to significantly impact (P < 0.05) the nutrient contents and stoichiometry of the soil. The C:P and N:P ratios in the soil of natural forests and plantations increased with age, which highlighted the insufficient availability of P (Post et al. 1985; Liu et al. 2016). However, this did not necessarily represent the lack of P in the soil (Turner and Engelbrecht 2010; Yin et al. 2021), as it is likely that during plant growth, significant quantities of P are stored within them (Tsujii et al. 2017; Turner et al. 2018). As the soil itself lacked a way to fix exogenous P (Vincent et al. 2010), it decreased relative to C and N, where interestingly, there was no noticeable decrease in the AP.
It is understood that the soil TP is equivalent to a “deposit,” whereas AP is equivalent to a “circulating fund” (Darch et al. 2016), which indicates that although the soil P content decreases, it does not impact the normal “circulating fund.” Put differently, the soil TP is efficiently converted to soil AP via extracellular soil enzymes for use by plants and microorganisms, the extent to which may vary from region to region. The upward trend of C:P and N:P ratios in natural forests was slower than that of plantation forests; however, their volatility was significant. We considered that the quantitative state of soil in plantations was more strongly responsive to advancing age. As soil exists in a “passive” state in contrast to dynamic plants in ecosystems, there are fewer known active coping strategies; thus, the nutrient contents and stoichiometric ratios can change significantly (Zhang et al. 2021a, b).
Impacts of soil changes on plants
Soil serves as an essential substrate and nutrient pool for the growth of plants and forests, and its physical and chemical properties undergo certain changes over time. The stoichiometry of soil directly impacts the nutrient utilization efficiencies of plants (Cui et al. 2019). Thus, we queried whether changes in the nutrient contents of plant leaves, litter, new branches, and fine roots were correlated with soil nutrient concentrations and stoichiometry characteristics. Further, were there differences in these correlations between forest stands of different origins? Toward addressing these two issues, the RDA results revealed two important pieces of information: (1) the soil TP in natural forests had a higher degree of nutrient contents in active vegetation organs, whereas in plantations, it was TN; (2) the explanatory degree of the soil nutrient contents, soil stoichiometric index, and total explanatory level were much higher in plantations than in natural forests.
When studying a tropical rainforest in Costa Rica, Massmann et al. (Massmann et al. 2021) revealed that the soil TP aligned best with the N:P ratio and P content of plant leaves, which agreed with the results of natural forests in this study. In general, soil characteristics had little effect on the nutrient contents of natural forest organs, and the most significant effect was the soil P content. This may have been due to the preference of natural forests to utilize P in the soil in the absence of apparent nutrient limitations or deprivation. Compared with those of natural forests, the soil attributes were more explanatory for the plant organ nutrient contents of plantation forests, with soil N being the most powerful factor (46.57%).
We considered that because trees in plantations grow faster than those in natural forests and that the soil N content guarantees plant uptake and growth, plantations require higher supplies of soil N (Proe and Millard 1994; Emmett 1999; Solberg et al. 2004), which have a greater impact on plantation tree organs. Additionally, the law of minimum factors and the common restriction hypothesis may assist with explaining these results, as restriction factors often play a decisive role (Baar 1994). The organizer nutrient contents of L. principis-rupprechtii plantations were more affected by and had more dependence on the soil (Maeda et al. 2018), whereas the feedback was also more direct.
It is acknowledged that soils exhibit particular changes with advancing age (Barbato et al. 2020), and the active organs of natural forests possess more robust strategies to deal with the changes than do plantation forests, which may operate as follows: (1) the trees of natural forests have stronger root absorption or element storage capacities, which enables them to cope with changes in soil nutrients without excessively adjusting their organ element content to achieve normal growth and balance. (2) Because of higher element utilization efficiencies, it is difficult for trees in natural forests to adjust their strategies to limiting soil changes. However, these two mechanisms require further confirmation. We considered that these factors might explain why the nutrient concentrations and stoichiometry of the active organs of natural forest trees better resisted the impacts of aging, in contrast to those of plantations. This led to further insights into a stronger dependence on soil nutrients, more conservative nutrient utilization strategies, and soil N requirements exhibited by plantation forests as they age. The specific timing of N fertilization for plantations might facilitate their capacity to better conserve tree nutrients and stabilize active organs to potentially prevent or delay their degradation.
Conclusion
There was a general accumulation of soil SOC and TN in L. principis-rupprechtii forests with advancing age, whereas TP was negatively consumed. The soil C:P and N:P ratios of both natural forests and plantations increased with advancing age, with the relative scarcity of P being prominent; however, the available soil P was minimally affected. Increases in the soil C:P and N:P ratios of natural forests were slower than those of plantations; however, the fluctuations were acute. The C, P, and litter P of mature leaves and the fine root P of plantations exhibited a certain downward trend with age, whereas the N content of mature leaves and the C and N contents of litter and fine roots increased with age. The N and P of branches decreased after attaining a peak at ~ 30 years, and almost all active organs exhibited a certain level of decreased P content. With advancing age, the C, N, and P contents of natural forest trees (except for mature leaves) decreased significantly, whereas the C, N, and P contents of other organs were much more stable than those of plantation forests. Plantation forests tended to store scarce nutrients in inactive plant structures to prevent nutrient loss, whereas natural forests appeared to have an improved capacity to combat this age-related trend by maintaining stable nutrient concentrations within their active organs. To better conserve N and P, plantation trees were inclined to engage relatively low and high resorption strategies, respectively, with age, whereas the resorption strategies of natural forests were less affected. The consumption of nutrients in plantation forests was more age sensitive and tended to adopt highly conservative N and P utilization strategies with advancing age. Although natural forests are increasingly subject to conservation, their nutrient utilization strategies make them more resilient against typical age-related degradation. The organs of natural forest L. principis-rupprechtii trees were observed to be more susceptible to the soil P content, whereas plantation trees tended to be affected by the soil N content. The active organizer nutrient contents of plantations were more affected by and dependent on the soil, whereas the feedback was also more direct. It was confirmed that soils exhibit certain changes with advancing age and that the active organs of natural forests have more robust strategies to deal with these changes. This may be one of the reasons why the nutrient contents and stoichiometry of the active organs of natural forests can resist the impacts of aging.
Data availability
Not Applicable.
References
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. In: Fitter AH, Raffaelli DG (eds) Advances in ecological research. Academic Press, pp 1–67
Agren GI, Wetterstedt JA, Billberger MF (2012) Nutrient limitation on terrestrial plant growth–modeling the interaction between nitrogen and phosphorus. New Phytol 194(4):953–960. https://doi.org/10.1111/j.1469-8137.2012.04116.x (Available from https://www.ncbi.nlm.nih.gov/pubmed/22458659)
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442(7102):555–558. https://doi.org/10.1038/nature05038 (Available from https://www.ncbi.nlm.nih.gov/pubmed/16885982)
Baar H (1994) Von liebig’s law of the minimum and plankton ecology (1899–1991). Prog Oceanogr 33(4):347–386
Baldrian P, Kolarik M, Stursova M, Kopecky J, Valaskova V, Vetrovsky T, Zifcakova L, Snajdr J, Ridl J, Vlcek C, Voriskova J (2012) Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J 6(2):248–258. https://doi.org/10.1038/ismej.2011.95 (Available from https://www.ncbi.nlm.nih.gov/pubmed/21776033)
Bao S (2000) Soil and agricultural chemistry analysis. 3rd edn. China Agriculture Press, Beijing (in Chinese)
Barbato D, Benocci A, Manganelli G (2020) Does forest age affect soil biodiversity? Case study of land snails in mediterranean secondary forests. For Ecol Manag 455:117693. https://doi.org/10.1016/j.foreco.2019.117693
Belnap J (2002) Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol Fertil Soils 35(2):128–135. https://doi.org/10.1007/s00374-002-0452-x
Brant AN, Chen HYH (2015) Patterns and mechanisms of nutrient resorption in plants. Crit Rev Plant Sci 34(5):471–486. https://doi.org/10.1080/07352689.2015.1078611
Bremner JM (1960) Determination of nitrogen in soil by the kjeldahl method. J Agric Sci 55(1):11–33
Cai H, Li F, Jin G (2019) Fine root biomass, production and turnover rates in plantations versus natural forests: Effects of stand characteristics and soil properties. Plant Soil 436(1–2):463–474. https://doi.org/10.1007/s11104-019-03948-8
Castellanos AE, Llano-Sotelo JM, Machado-Encinas LI, López-Piña JE, Romo-Leon JR, Sardans J, Peñuelas J (2018) Foliar c, n, and p stoichiometry characterize successful plant ecological strategies in the sonoran desert. Plant Ecol 219(7):775–788. https://doi.org/10.1007/s11258-018-0833-3
Chapin FS (1991) 3 - effects of multiple environmental stresses on nutrient availability and use. In: MooneyW HA, Winner E, Pell EJ (eds) Response of plants to multiple stresses. Academic Press, San, Diego, pp 67–88
Chen G, Zhu H, Zhang Y (2003) Soil microbial activities and carbon and nitrogen fixation. Res Microbiol 154(6):393–398. https://doi.org/10.1016/s0923-2508(03)00082-2
Chen D, Huang X, Sun X, Wu M, Zhang S (2016) A comparison of hierarchical and non-hierarchical Bayesian approaches for fitting allometric larch (larix.Spp.) biomass equations. Forests 7(1):18
Cleveland CC, Liptzin D (2007) C:N: P stoichiometry in soil: Is there a “redfield ratio” for the microbial biomass? Biogeochemistry 85(3):235–252. https://doi.org/10.1007/s10533-007-9132-0
Cui Y, Bing H, Fang L, Jiang M, Shen G, Yu J, Wang X, Zhu H, Wu Y, Zhang X (2019) Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 458(1–2):7–20. https://doi.org/10.1007/s11104-019-04159-x
Darch T, Blackwell MS, Chadwick D, Haygarth PM, Hawkins JM, Turner BL (2016) Assessment of bioavailable organic phosphorus in tropical forest soils by organic acid extraction and phosphatase hydrolysis. Geoderma 284:93–102. https://doi.org/10.1016/j.geoderma.2016.08.018 (Available from https://www.ncbi.nlm.nih.gov/pubmed/27990026)
Dixon RK, Solomon AM, Brown S, Houghton RA, Trexier MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263(5144):185–190
Drenovsky RE, James JJ, Richards JH (2010) Variation in nutrient resorption by desert shrubs. J Arid Environ 74(11):1564–1568. https://doi.org/10.1016/j.jaridenv.2010.05.030 (Available from https://www.sciencedirect.com/science/article/pii/S0140196310001722)
Elser JJ (2000) Ecological stoichiometry: From sea to lake to land. Trends Ecol Evol 15(10):393–394
Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, Interlandi S, Kilham SS, Mccauley E, Schulz KL (2000) Nutritional constraints in terrestrial and freshwater food webs. Nature 408(6812):578–580
Elser JJ, Bracken ME, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10(12):1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x (Available from https://www.ncbi.nlm.nih.gov/pubmed/17922835)
Emmett BA (1999) The impact of nitrogen on forest soils and feedbacks on tree growth. Water Air Soil Pollut 116(1/2):65–74
Ewel KC, Cropper WP, Gholz HL (1987) Soil co2 evolution in florida slash pine plantations. I. Changes through time. Can J For Res 17(4):325–329
Fownes H (1995) Phosphorus limitation of forest leaf area and net primary production on a highly weathered soil. Biogeochemistry 29(3):223–235
Gong ZL, Tang Y (2016) Impacts of reforestation on woody species composition, species diversity and community structure in dry-hot valley of the Jinsha river, southwestern China. J Mt Sci 13(12):2182–2191. https://doi.org/10.1007/s11629-015-3449-2
Gusewell S (2004) N: P ratios in terrestrial plants: Variation and functional significance. New Phytol 164(2):243–266. https://doi.org/10.1111/j.1469-8137.2004.01192.x (Available from https://www.ncbi.nlm.nih.gov/pubmed/33873556)
Han W, Fang J, Guo D, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytologist 168(2):377–385
Han WX, Fang JY, Reich PB, Ian Woodward F, Wang ZH (2011) Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in china. Ecol Lett 14(8):788–796. https://doi.org/10.1111/j.1461-0248.2011.01641.x (Available from https://www.ncbi.nlm.nih.gov/pubmed/21692962)
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken ME, Elser JJ, Gruner DS, Hillebrand H, Shurin JB, Smith JE (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14(9):852–862. https://doi.org/10.1111/j.1461-0248.2011.01651.x (Available from https://www.ncbi.nlm.nih.gov/pubmed/21749598)
Hayes P, Turner BL, Lambers H, Laliberte E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102(2):396–410
Hayes P, Turner BL, Lambers H, Laliberté E, Bellingham P (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102(2):396–410. https://doi.org/10.1111/1365-2745.12196
Jenny H (1941) Factors of soil formation: a system OF quantitativ[J]. Soil Science 52(5):415. https://doi.org/10.1097/00010694-194111000-00009
Jiang Y, Guo X (2019) Stoichiometric patterns of soil carbon, nitrogen, and phosphorus in farmland of the poyang lake region in southern China. J Soils Sediments 19:3476–3488
Kelliher FM, Ross DJ, Law BE, Baldocchi DD, Rodda NJ (2004) Limitations to carbon mineralization in litter and mineral soil of young and old ponderosa pine forests[J]. Forest Ecology and Management. 191(1–3):201–213
Killingbeck KT (1996) Nutrients in senesced leaves: Keys to the search for potential resorption and resorption. Ecology 77(6):1716–1727
Kirkby CA, Richardson AE, Wade LJ, Batten GD, Kirkegaard JA (2013) Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol Biochem 60:77–86
Klopatek JM (2002) Belowground carbon pools and processes in different age stands of douglas-fir. Tree Physiol 22(2–3):197
Koerselman W, Meuleman A (1996) The vegetation n: P ratio: A new tool to detect the nature of nutrient limitation. J Appl Ecol 33(6):1441–1450
Lal CB, Annapurna C, Raghubanshi AS, Singh JS (2001) Effect of leaf habit and soil type on nutrient resorption and conservation in woody species of a dry tropical environment. Rev Can Bot 79(9):1066–107500
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23(2):95–103. https://doi.org/10.1016/j.tree.2007.10.008 (Available from https://www.ncbi.nlm.nih.gov/pubmed/18191280)
Liao C, Luo Y, Fang C, Chen J, Li B (2012) The effects of plantation practice on soil properties based on the comparison between natural and planted forests: A meta-analysis. Glob Ecol Biogeogr 21(3):318–327. https://doi.org/10.1111/j.1466-8238.2011.00690.x
Liu R, Wang D (2021) C:N: P stoichiometric characteristics and seasonal dynamics of leaf-root-litter-soil in plantations on the loess plateau. Ecol Ind 127(1):107772
Liu J, Gu Z, Shao H, Zhou F, Peng S (2016) N–p stoichiometry in soil and leaves of pinus massoniana forest at different stand ages in the subtropical soil erosion area of China. Environ Earth Sci 75(14):1–9. https://doi.org/10.1007/s12665-016-5888-7
Liu T, Peng D, Tan Z, Guo J, Zhang Y (2021) Effects of stand density on soil respiration and labile organic carbon in different aged larix principis-rupprechtii plantations. Ecol Process 10(1):1–15. https://doi.org/10.1186/s13717-021-00301-9
Luyssaert S, Schulze ED, Borner A, Knohl A, Hessenmoller D, Law BE, Ciais P, Grace J (2008) Old-growth forests as global carbon sinks. Nature 455(7210):213–215. https://doi.org/10.1038/nature07276 (Available from https://www.ncbi.nlm.nih.gov/pubmed/18784722)
Maeda Y, Tashiro N, Enoki T, Urakawa R, Hishi T (2018) Effects of species replacement on the relationship between net primary production and soil nitrogen availability along a topographical gradient: Comparison of belowground allocation and nitrogen use efficiency between natural forests and plantations. For Ecol Manage 422:214–222. https://doi.org/10.1016/j.foreco.2018.03.046
Mao R, Chen HM, Zhang XH, Shi FX, Song CC (2016) Effects of p addition on plant c:N: P stoichiometry in an n-limited temperate wetland of northeast China. Sci Total Environ 559:1–6. https://doi.org/10.1016/j.scitotenv.2016.03.158 (Available from https://www.ncbi.nlm.nih.gov/pubmed/27045368)
Massmann A, Cavaleri MA, Oberbauer SF, Olivas PC, Porder S (2021) Foliar stoichiometry is marginally sensitive to soil phosphorus across a lowland tropical rainforest. Ecosystems. https://doi.org/10.1007/s10021-021-00640-w
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: A comment on distanceâbased redundancy analysis. Ecology 82(1):290–297
Niinemets U, Tamm U (2005) Species differences in timing of leaf fall and foliage chemistry modify nutrient resorption efficiency in deciduous temperate forest stands. Tree Physiol 25(8):1001–1014. https://doi.org/10.1093/treephys/25.8.1001 (Available from https://www.ncbi.nlm.nih.gov/pubmed/15929931)
Niklas KJ, Owens T, Reich PB, Cobb ED (2005) Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecol Lett 8(6):636–642. https://doi.org/10.1111/j.1461-0248.2005.00759.x
Oleksyn J, Reich PB, Zytkowiak R, Karolewski P, Tjoelker MG (2003) Nutrient conservation increases with latitude of origin in european pinus sylvestris populations. Oecologia 136(2):220–235. https://doi.org/10.1007/s00442-003-1265-9 (Available from https://www.ncbi.nlm.nih.gov/pubmed/12756524)
Osuri AM, Gopal A, Raman TRS, DeFries R, Cook-Patton SC, Naeem S (2020) Greater stability of carbon capture in species-rich natural forests compared to species-poor plantations. Environ Res Lett 15(3):034011. https://doi.org/10.1088/1748-9326/ab5f75
Parhizkar M, Shabanpour M, Khaledian M, Asadi H (2021) The evaluation of soil detachment capacity induced by vegetal species based on the comparison between natural and planted forests. J Hydrol 595:126041. https://doi.org/10.1016/j.jhydrol.2021.126041
Peñuelas J, Sardans J, Rivas-ubach A, Janssens IA (2012) The human-induced imbalance between c, n and p in earth’s life system. Glob Change Biol 18(1):3–6. https://doi.org/10.1111/j.1365-2486.2011.02568.x
Perry DA, Maghembe J (1989) Ecosystem concepts and current trends in forest management: Time for reappraisal. For Ecol Manage 26(2):123–140
Post WM, Pastor J, Zinke PJ, Stangenberger AG (1985) Global patterns of soil nitrogen storage. Nature 317(6038):613–616
Proe MF, Millard P (1994) Relationships between nutrient supply, nitrogen partitioning and growth in young sitka spruce (picea sitchensis). Tree Physiol 14(1):75–88
Qiu X, Peng D, Wang H, Wang Z, Cheng S (2019) Minimum data set for evaluation of stand density effects on soil quality in larix principis-rupprechtii plantations in north China. Ecol Ind 103:236–247. https://doi.org/10.1016/j.ecolind.2019.04.010
Reich PB, Oleksyn J (2004) Global patterns of plant leaf n and p in relation to temperature and latitude. Proc Natl Acad Sci U S A 101(30):11001–11006. https://doi.org/10.1073/pnas.0403588101
Sardans J, Rivas-Ubach A, Peñuelas J (2012) The c:N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect Plant Ecol Evol Syst 14(1):33–47. https://doi.org/10.1016/j.ppees.2011.08.002
Shumilov O, Kanatjev A, Kasatkina E (2013) A measuring tool for tree-rings analysis. EGU General Assembly Conference Abstracts
Solberg S, Andreassen K, Clarke N, Tørseth K, Tveito OE, Strand GH, Tomter S (2004) The possible influence of nitrogen and acid deposition on forest growth in norway. For Ecol Manage 192(2–3):241–249. https://doi.org/10.1016/j.foreco.2004.01.036
Sterner RW, Elser JJ (2008) Ecological stoichiometry: Overview [M]//Fath B. Encyclopedia of Ecology, 2nd edn. Oxford; Elsevier. p 331–345
Team R. (2009) R: A language and environment for statistical computing. R foundation for statistical computing:Vienna, austria. Computing 14:12–21
Tripathi KP, Singh B (2009) Species diversity and vegetation structure across various strata in natural and plantation forests in katerniaghat wildlife sanctuary, north India. Trop Ecol 50(1):191–200
Tsujii Y, Oikawa M, Kitayama K (2017) Significance of the localization of phosphorus among tissues on a cross-section of leaf lamina of bornean tree species for phosphorus-use efficiency. J Trop Ecol 33(3):237–240. https://doi.org/10.1017/s0266467417000141
Turner BL, Engelbrecht BMJ (2010) Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103(1–3):297–315. https://doi.org/10.1007/s10533-010-9466-x
Turner BL, Brenes-Arguedas T, Condit R (2018) Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 555(7696):367–370. https://doi.org/10.1038/nature25789 (Available from https://www.ncbi.nlm.nih.gov/pubmed/29513656)
Vincent AG, Turner BL, Tanner EVJ (2010) Soil organic phosphorus dynamics following perturbation of litter cycling in a tropical moist forest. Eur J Soil Sci 61(1):48–57. https://doi.org/10.1111/j.1365-2389.2009.01200.x
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20(1):5–15
VittoriAntisari L, Papp R, Vianello G, Marinari S (2018) Effects of douglas fir stand age on soil chemical properties, nutrient dynamics, and enzyme activity: A case study in northern Apennines, Italy. Forests 9(10):641. https://doi.org/10.3390/f9100641
Wang N, Fu F, Wang B, Wang R (2017) Carbon, nitrogen and phosphorus stoichiometry in pinus tabulaeformis forest ecosystems in warm temperate Shanxi province, north China. J For Res 29(6):1665–1673. https://doi.org/10.1007/s11676-017-0571-8
White DA, Silberstein RP, Balocchi-Contreras F, Quiroga JJ, Meason DF, Palma JHN, Ramírez de Arellano P (2021) Growth, water use, and water use efficiency of eucalyptus globulus and pinus radiata plantations compared with natural stands of roble-hualo forest in the coastal mountains of central chile. For Ecol Manag 501:119676. https://doi.org/10.1016/j.foreco.2021.119676
Xu HC (1991) Comparative evaluation of plantation and natural forest. World For Res 4(3):7 (In Chinese)
Xu X, Li D, Cheng X, Ruan H, Luo Y (2016) Carbon: Nitrogen stoichiometry following afforestation: A global synthesis. Sci Rep 6:19117. https://doi.org/10.1038/srep19117 (Available from https://www.ncbi.nlm.nih.gov/pubmed/26743490)
Xu M, Jian J, Wang J, Zhang Z, Yang G, Han X, Ren C (2021) Response of root nutrient resorption strategies to rhizosphere soil microbial nutrient utilization along robinia pseudoacacia plantation chronosequence. For Ecol Manag 489:119053. https://doi.org/10.1016/j.foreco.2021.119053 (Available from https://www.sciencedirect.com/science/article/pii/S0378112721001420)
Yan ER, Wang XH, Huang JJ (2006) Shifts in plant nutrient use strategies under secondary forest succession. Plant Soil 289(1–2):187–197
Yan T, Lü X, Yang K, Zhu J (2016) Leaf nutrient dynamics and nutrient resorption: A comparison between larch plantations and adjacent secondary forests in northeast China. J Plant Ecol 9(2):165–173. https://doi.org/10.1093/jpe/rtv034
Yang X, Tang Z, Ji C, Liu H, Ma W, Mohhamot A, Shi Z, Sun W, Wang T, Wang X, Wu X, Yu S, Yue M, Zheng C (2014) Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across northern China. Sci Rep 4:5448. https://doi.org/10.1038/srep05448 (Available from https://www.ncbi.nlm.nih.gov/pubmed/24965183)
Yin X, Zhao L, Fang Q, Ding G (2021) Differences in soil physicochemical properties in different-aged pinus massoniana plantations in southwest China. Forests 12(8):987. https://doi.org/10.3390/f12080987
Yuan Z, Chen HYH (2009) Global trends in senesced-leaf nitrogen and phosphorus. Glob Ecol Biogeogr 18(5):532–542. https://doi.org/10.1111/j.1466-8238.2009.00474.x
Yuan ZY, Chen HYH (2015) Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat Clim Chang 5(5):465–469. https://doi.org/10.1038/nclimate2549
Zeng Q, Lal R, Chen Y, An S (2017) Soil, leaf and root ecological stoichiometry of caragana korshinskii on the loess plateau of China in relation to plantation age. PLoS One 12(1):e0168890. https://doi.org/10.1371/journal.pone.0168890 (Available from https://www.ncbi.nlm.nih.gov/pubmed/28076357)
Zhang J, Wang Y, Cai C (2020) Multielemental stoichiometry in plant organs: A case study with the alpine herb gentiana rigescens across southwest China. Front Plant Sci 11:441
Zhang Y, Liu T, Guo J, Tan Z, Dong W, Wang H (2021) Changes in the understory diversity of secondary pinus tabulaeformis forests are the result of stand density and soil properties. Glob Ecol Conserv 28:e01628. https://doi.org/10.1016/j.gecco.2021.e01628
Zhang Y, Liu T, Guo J, Tan Z, Dong W, Wang H (2021) Changes in the understory diversity of secondary pinus tabulaeformis forests are the result of stand density and soil properties. Glob Ecol Conserv 28:e01628. https://doi.org/10.1016/j.gecco.2021.e01628 (Available from https://www.sciencedirect.com/science/article/pii/S2351989421001785)
Zhao N, Yu G, He N, Wang Q, Guo D, Zhang X, Wang R, Xu Z, Jiao C, Li N, Jia Y (2016) Coordinated pattern of multi-element variability in leaves and roots across chinese forest biomes. Glob Ecol Biogeogr 25(3):359–367. https://doi.org/10.1111/geb.12427
Zhou G, Liu S, Li Z, Zhang D, Tang X, Zhou C, Yan J, Mo J (2006) Old-growth forests can accumulate carbon in soils. Science 314(5804):1417–1417. https://doi.org/10.1126/science.1130168
Acknowledgements
We gratefully acknowledge the support of the Xiaowenshan Forest Farm for fieldwork. We also thank our colleagues for assistance with field measurements and laboratory work.
Funding
National Natural Science Foundation of China (30970480).
Author information
Authors and Affiliations
Contributions
Tairui Liu: Conceptualization, Formal analysis, Investigation, Data curation, Writing—original draft, Writing—review & editing. Fujing Bo: Investigation, Data curation, Writing—review & editing. Zhijie Tan: Investigation, Data curation, Validation, Visualization. Ruyuan Hu: Data curation. Xuming Ren: Investigation. Pingan Wang: Investigation. Jinping Guo: Conceptualization, Resources, Funding acquisition. Yunxiang Zhang: Conceptualization, Resources, Supervision, Project administration. Qiwu Wang: Resources.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
All authors have read and approved the manuscript being submitted, and agree to its submittal to this journal. The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Competing interests
The authors declare that they have no competing financial interests or personal relationships that could influence the work reported in this paper.
Additional information
Responsible Editor: Christopher Guppy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, T., Bo, F., Tan, Z. et al. Nutrient utilization strategies and age-related changes in Larix principis-rupprechtii forests. Plant Soil 494, 149–166 (2024). https://doi.org/10.1007/s11104-023-06259-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06259-1