Abstract
The distribution of nutrients, both vertically and horizontally in a forest, has long been theorized to influence primary productivity. Working at La Selva Biological Station, Costa Rica, we gathered the most comprehensive foliar samples to date for a lowland tropical rainforest to measure horizontal and vertical trends in foliar nutrients. The mean traits of foliage from forest floor to top-of-canopy were determined at 45 plots placed across the landscape in a stratified random design. Area-basis foliar N and P for these vertically integrated columns varied by a factor of 3, while foliar N:P and mass-basis foliar N and P varied by a factor of 2. The variance in plot-level foliar N:P and P was best explained by total soil P, while variance in foliar N was best explained by soil pH (regression trees: r2 \(\ge \) 0.20, p \(\le \) 0.01). Other soil, topographic, and forest structure factors offered no additional explanatory power for variation of foliar nutrients from plot to plot. To explore vertical trends, we aggregated the data across the landscape into ~ 2 m vertical segments. We found that foliar N:P was unrelated to height in the canopy, and that area-basis foliar N and P increased with height in the canopy (linear regression: r2 = 0.82 and r2 = 0.65 respectively, p < 0.0001 for both). We compared these vertical trends to those of the eight other elements quantified in the leaves, and the only other element enriched with height was potassium (K). Vertical nutrient enrichment was driven by increases in leaf mass per area (LMA), not mass-basis concentrations. Altogether, these findings suggest that, even in diverse tropical rainforests, foliar chemistry may reflect environmental constraints.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
45 vertical transects reveal tropical forest landscape foliar N:P of 21.8 ± 0.5.
-
Soil P explains 23% of horizontal variance in plot-level foliar N:P.
-
Foliar N, P, and K increase with height on an area-basis but not on a mass-basis.
Introduction
Variation in the nitrogen to phosphorus (N:P) stoichiometry of live photosynthetic tissue may reflect nutrient limitation to net primary productivity (NPP) in some ecosystems (Redfield 1958; Koerselman and Meuleman 1996; Falkowski and others 1998; Sterner and Elser 2002). In lowland tropical rainforests, which represent less than a tenth of the land surface yet nearly a third of terrestrial NPP, it has been difficult to interpret the relationship between foliar chemistry and nutrient availability (Bonan 2008; Cleveland and others 2011). Adjacent tropical plants vary substantially in foliar stoichiometry (Townsend and others 2007; Sullivan and others 2014; Asner and Martin 2016), and this variation demands a more comprehensive foliar sampling. In particular, the vertical distribution of leaves can be highly related to the NPP of a lowland tropical rainforest (Cushman and Kellner 2019), and tropical forest trees partition foliar nutrients vertically (Leuning and others 1991; Lloyd and others 2010). Yet, to our knowledge, the vertical distribution of foliar nutrients has never been sampled systematically to capture the topographic and substrate variability of a lowland tropical rainforest landscape. Thus, the degree to which foliar nutrients are indicative of other ecosystem properties in lowland tropical rainforests remains an open question.
Despite very limited sampling relative to other biomes, some authors have interpreted high N:P in tropical foliage as an indication of P limitation to NPP, low levels of available P in tropical soils, or both (McGroddy and others 2004; Reich and Oleksyn 2004). Highly weathered, acidic, and P-poor soils are indeed relatively abundant in tropical rainforests (Sanchez 2019). Resorption of P is also higher relative to N during foliar senescence in tropical rainforests (Vitousek 1984; Reed and others 2012). Further evidence of the importance of P comes from recent MODIS-derived aboveground NPP estimates in the neotropics, which were positively correlated with the foliar P concentrations of the region’s woody plants, but not with their foliar N content (Šímová and others 2019). On the other hand, the few fertilization experiments in mature lowland tropical rainforests do not demonstrate P limitation to NPP (Townsend and others 2011; Alvarez-Clare and others 2013; Wright and others 2018). Even a lowland Amazonian site with a high tree foliar N:P of 25 (55 molar) was found to be co-limited by N and P in a fertilization study, rather than limited by P alone (Fisher and others 2013).
Tropical foliar stoichiometry interpretation is also complicated by the controls of different foliar nutrients. Much of the variance across individuals in foliar N results from phylogenetic variation, whereas much of the variance in foliar P (and other rock-derived nutrients) is best explained by location, and thus in part by local soil conditions (Fyllas and others 2009; Asner and others 2015a; Asner and Martin 2016). Chemical variation among individuals in a single tropical rainforest site is often as large as the variation among sites (Townsend and others 2007; Sullivan and others 2014; Asner and Martin 2016; Balzotti and others 2016). Thus, without comprehensive sampling of the entire photosynthetic community, it remains unclear whether and how foliar nutrients yield meaningful information about lowland tropical forest nutrient status.
The distribution of foliar nutrients within a canopy also contributes to the relationship between productivity and nutrient resources. The photosynthetic benefit of redistributing N to upper, well-lit leaves has long been thought to exceed the cost of amino acid transport (Field 1983). However, among the leaves of tropical and subtropical woody plants, no vertical trend of mass-basis enrichment of N, P, K, calcium (Ca), or magnesium (Mg) has been observed vertically (Lloyd and others 2010; Niinemets and others 2015), nor do mass-basis foliar N or P increase with light intensity (Poorter and others 2019). In North American mixed spruce and fir stands, mass-basis foliar Ca was significantly higher in sun leaves than shade leaves, but this was not observed for N, P, K, Zinc (Zn), or Mg (Richardson 2004). Thus, despite a theoretical benefit to photosynthetic optimization, mass-basis vertical nutrient enrichment does not seem to be common (Hirose and Werger 1987; Hikosaka and others 2016).
Nonetheless, an increase in the leaf mass supporting each square centimeter of leaf surface (leaf mass per area, LMA) can enrich the nutrient resources of the photosynthetic surface without changing stoichiometry (Lloyd and others 2013; Osnas and others 2013). LMA increases with height in every forest where it has been studied, including at our study location in northeastern Costa Rica (Cavaleri and others 2010), but whether there is mass-basis vertical nutrient enrichment remains an open question for the complex canopies characteristic of tropical rainforests. Plants with better light access are better situated to acquire nutrients via improved mycorrhizal relations (Konvalinková and Jansa 2016), symbiotic N-fixation (Taylor and Menge 2018), and investment in root biomass (Poorter and others 2019). Competition for full-sun positions between trees and lianas could alter vertical trends in foliar chemistry (Asner and Martin 2012), as could the sensitivity of palms’ canopy position to soil nutrient conditions (Cámara-Leret and others 2017). Sampling foliage of all major plant functional groups, and accounting for the relative abundance of their foliage, is therefore necessary to rigorously test for mass-basis foliar nutrient enrichment with height.
In tropical forests, investigators rarely harvest whole plots because the effort involved is substantial (but see Golley 1975; Delitti and Burger 2000). Instead, investigators select mature, undamaged leaves for chemical analysis. However, the exclusion of old leaves from analyses can lead to overestimates of mass-basis foliar N (Albert and others 2018), the exclusion of senescing leaves results in a foliar stoichiometry that is less sensitive to soil nutrient availability (Vitousek 1984; Reed and others 2012; Tully and others 2013), and the exclusion of herbivory-damaged leaves likely results in oversampling of foliage with reduced N concentrations (Tao and Hunter 2011) and perhaps reduces the detected value for foliar N:P as well (Awmack and Leather 2002). Scaling from foliar samples to the landscape is also a challenge. At larger spatial scales, it becomes intractable to collect leaves from each plant in a plot and estimate the abundance of leaves on each plant (sensu Grubb and Edwards 1982). A recent innovation is to regress average foliar nutrition on visible to shortwave infrared spectra detected for calibration plots, and then scale that relationship to the landscape by using the spectra detected via airborne remote sensing (Asner and others 2015b). This method shows promise for resolving the environmental factors associated with foliar trait heterogeneity across a landscape (Asner and others 2015a), but only assesses the traits of the uppermost sunlit canopy.
In this context, we sought to comprehensively characterize horizontal and vertical heterogeneity in foliar chemistry across a continental lowland tropical rainforest, and probe whether that heterogeneity reflects the biotic and abiotic differences that have been proposed as important for plant nutrient status. We designed a method analogous to dragging a plankton net from the bottom to the top of the photosynthetic zone in an aquatic system (sensu Redfield 1958). We constructed 45 modular towers at representative sites across the mature forest at La Selva Biological Station, Costa Rica, and collected all leaves intersecting each tower. We used this, the largest systematic, stratified random sample of ground to top-of-canopy tropical forest foliage to date, to ask: (1) How does foliar chemistry vary horizontally across the landscape? (2) How does foliar chemistry vary with height in the canopy? and (3) What abiotic and biotic variables, if any, explain horizontal variation?
Methods
Site Description
Data were collected at La Selva Biological Station, a 1536 ha forest preserve in northeastern Costa Rica (10° 26′N 84° 1′W, 37–150 m asl) with 515 ha of continuous mature tropical forest. La Selva has undulating topography, with relatively flat ridges descending to stream channels with a few tens of meters of topographic relief. Many ridgetop soils in the landscape are Ultisols or Oxisols, with Inceptisols found on steeper slopes and in alluvial valleys (Sollins and others 1994; Porder and others 2006). Soils on ridges retain a relatively high fraction of rock-derived nutrients, as has been observed in other relatively young geomorphic settings of the lowland tropics (Bern and others 2005; Porder and others 2006). Although in this region, as in other lowland tropical sites, ridgetops have substantially lower rock-derived nutrient availability than slopes and alluvial areas, La Selva has fairly high rock-derived nutrients in the soil compared with more stable, geologically quiescent lowland tropical forests. For example, total soil P to 100 cm depth ranges from ~ 500 ppm on ridges to over 1600 ppm in alluvial soils (Porder and others 2006, data collected as part of this study), whereas on the Brazilian shield total P concentrations may be as low as 100 ppm (Quesada and others 2010).
Annual rainfall at La Selva is ~ 4000 mm with no month receiving less than 200 mm of precipitation, and the mean annual temperature is ~ 26 °C (Sanford and others 1994). There were 113 species of palm, tree, and liana identified among the plants whose branches we sampled at La Selva. As in many Central American forests with year-round precipitation, the leguminous tree Pentaclethra macroloba (Willd.) Kuntze is abundant; at La Selva, Pentaclethra is by far the most common plant species (Hartshorn 1983). Previously reported results from our sampling campaign showed that this species makes up 14 ± 2% of leaf area at the 45 locations where we sampled, and 35% of total basal area within a 10 m radius of these locations (Clark and others 2008).
Sampling & Analyzing Photosynthetic Tissue
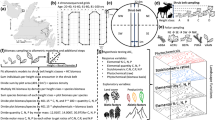
In order to span the variability in soils at La Selva, 45 plots were identified via stratified random design (based on predictions of topsoil total soil P and slope) across the 515 ha of mature forest (Clark and others 2008). Forest heights at these locations ranged from 4 to 45 m. Between June 2003 to March 2005, we constructed a modular tower at each plot (Figure 1). All foliage intersecting the tower footprint was collected, from the ground to the canopy top. The sample area was 2.42 m2 for the first 4 plots, after which we obtained a side platform that increased the tower footprint to 4.56 m2 for the remaining 41 plots. Leaf area index (LAI) ranged from 1.2 to 12.9 m2m−2, with an overall average across all tower transects of 6.0 ± 0.3 m2m−2 (Clark and others 2008).
Within each 1.86 m tall tower section, we grouped leaves by functional group to measure LMA, LAI, and elemental concentrations. In the ground level tower section of plot 1 in this example, the traits of the liana leaves would be measured and recorded separately from those of the palm leaves. In plot 2, all leaves of the ground level segment would be analyzed together because they are all palm leaves. The data from these samples were aggregated by plot and by canopy level. LAI-weighted average of area-basis nutrient concentrations and leaf-mass-weighted average of mass-basis concentrations were calculated for both plots and canopy levels. For illustration purposes, here we show 2 plots spanning a maximum of 5 canopy layers. Our study encompassed 45 plots and 22 canopy layers. The unweighted, arithmetic mean of those two plot values would describe the “landscape” in this example.
For each 1.86 m tall tower segment, leaves were collected and categorized into eight plant functional groups: Pentaclethra, other trees, palms, lianas, herbs, herbaceous vines, ferns, and epiphytes. Herbs, herbaceous vines, ferns, and epiphytes contributed only 12 ± 1% of LAI across all 45 plots, and leaf chemistry was not measured for these groups at all plots. For the purposes of our analyses (described in Data Analysis and Statistics), we excluded the contribution of these minor functional groups to the total LAI and leaf mass of a tower or tower section and reweighted the four major functional groups (Pentaclethra, other trees, palms, and lianas) to 100%.
We measured leaf area with a LI-3100 benchtop leaf area meter (LI-COR Biosciences, Lincoln, NE, USA). Foliar samples were taken from each functional group at each height, homogenized with a Wiley mill and 20-mesh sieve, and weighed. For each foliar sample, a subsample was analyzed for foliar N at Colorado State University with a TruSpec CN Determinator (LECO, St. Joseph, MI, USA), and another subsample was digested by nitric acid and hydrogen peroxide to determine foliar P at MDS Harris Laboratories on a 4300 Optima Dual View inductively coupled plasma (ICP) spectrometer (PerkinElmer, Waltham, MA, USA). For two periods of foliar sampling (June 2003 to Mid-October 2003 and February 2004 to June 2004), only N and P were measured. For the rest of the study (Mid-October 2003 to January 2004 and July 2004 to Mid-March 2005), foliar concentrations were also determined for Potassium (K), Magnesium (Mg), Calcium (Ca), Sulfur (S), Sodium (Na), Zinc (Zn), Manganese (Mn), and Aluminum (Al). Thus, we have a full suite of foliar concentrations for 28 towers. Light conditions were highly correlated with the height from which a sample was collected in the canopy (log-linear regression: r2 = 0.99; Cavaleri and others 2010), so in this study we use higher leaves as a proxy for well-lit leaves.
Quantifying Soil and Topographic Variables
At the time of foliar sampling, we collected eight to ten 100 cm soil cores of 3 cm diameter within 2 m of each tower base and homogenized them. Prior work at La Selva on total soil C, N, and P suggests that soil chemistry is likely similar for tens of meters beyond this soil sampling area (Espeleta and Clark 2007; Clark and others, unpublished data). Total P in the top 10 cm of soil was correlated at r2 = 0.96 with the 0–100 cm value. We use the latter in this analysis. Soil C and soil N were quantified by Vario EL Elemental Analyzer CNS (Elementar, Langenselbold, Germany). All other elements were analyzed by nitric acid digest followed by inductively coupled plasma spectrometry (SPECTRO Analytical Instruments, Kleve, Germany). Cation exchange capacity was recorded as the sum of charges from exchangeable H, Na, K, Ca, Mg, Mn, Fe, and Al (McKenzie and others 2004), and base saturation as the fraction of this occupied by Na, K, Ca and Mg. Dry soil was mixed with 1 M KCl in a 1:2.5 ratio for pH measurement with a combined electrode (Schlichting and others 1995). In addition, we used a high-resolution digital elevation model generated by aircraft-based LiDAR (Kellner and others 2009) for a post hoc categorization of the topographic position (ridge, slope, or valley) and to determine elevation below the local topographic high (Porder and others 2005).
Data Analysis and Statistics
For a given foliar sample (i), we measured leaf area (Li), LMA (Hi), mass-based foliar N (Nmassi), and mass-based foliar P (Pmassi). The fundamental unit of analysis in this study is all foliar samples collected within the same 1.86 m tall section of the same collection tower (Figure 1). We calculated mean foliar chemistry by grouping these 1.86 m tall height sections (a) with the others from the same collection tower (plot) and (b) by aggregating 1.86 m tall sections with all others from the same height (canopy level). (a) To scale foliar measurements up to the plot, mass-basis foliar N (Eq. 1) and mass-basis foliar P (Eq. 2) values were weighted based on the relative contribution of the foliar sample leaf mass (the product of leaf area, L, and LMA, H) to the total leaf mass captured in the tower (\(\sum\nolimits_{i = 1}^s {{L_i}{H_i}} \) where s is all foliar samples in a plot). To determine the resources available to the total (t) photosynthetic surface at a plot, area-basis foliar N (Eq. 3), area-basis foliar P Eq. 4), and N:P (Eq. 5) values were weighted based on the relative contribution of Li to the total leaf area captured in the collection tower (\(\sum\nolimits_{i = 1}^s {{L_i}} \) where s is all foliar samples in a plot). Because plots were placed at representative locations across the mature forest at La Selva, the unweighted arithmetic mean across plots was taken to represent the landscape-scale foliar trait. (b) Weighted averages were also calculated for the mass of tissue at each canopy level (Eqs. 1 and 2, where s is all foliar samples in a canopy layer), and for the photosynthetic surface at each canopy level (Eqs. 3, 4, and 5, where s is the index of foliar samples in a canopy layer).
We focus on area-weighted values for horizontal analyses, but our results did not differ substantially when mass-weighted values were considered instead (Figure S1). This analysis excluded any samples for which one or more measurements were missing from any calculation that required the missing factor or factors, but these samples were included in all other calculations. Less than 1% of data was missing for leaf mass per area, but 3% of Nmass measures and 9% of Pmass were missing due to the additional logistics involved in performing these measures on rainforest foliage over years of sampling. There was no significant difference between the sampling heights of missing data and those of recorded data.
Finally, we used regression tree analysis (Breiman and others 1983; De’Ath and Fabricius 2000) to partition horizontal variation in foliar N, P, and N:P using the following independent variables: soil C:N, soil N, soil P, soil pH, cation exchange capacity, base saturation, topographic position, elevation difference from the local topographic maximum, forest height, and LAI. A regression tree classifies data dichotomously along a single categorical or continuous variable, to explain variation in the dependent variable, then continues splitting data to create a hierarchical model. Because this method can overfit, we only included splits that reduced the Akaike Information Criterion (AIC) of the model and that resulted in subsets that were significantly different (p \(\le \) 0.05, as indicated by a 2-tailed heteroscedastic t-test for a single split tree, or by a one-way ANOVA followed by Tukey’s HSD for a tree with multiple splits).
Differences in foliar nutrients could result from flexibility within functional groups, from functional group turnover, or both. To explore the possibility of functional group turnover as a mediating factor in horizontal trends, we quantified the relative contribution of each functional group to the foliage of each plot (percent of \(\sum\nolimits_{i = 1}^s {{L_i}{H_i}} \) for mass-basis foliar traits and percent of \(\sum\nolimits_{i = 1}^s {{L_i}} \) for area-basis foliar traits), and tested for a difference between plot groups identified by the regression tree model. We also used linear regression to see if functional group turnover was associated with foliar chemistry across all plots. The strength and direction of vertical trends within the canopy was assessed by linear regression as well. Where weighted means are reported, weighted mean standard errors are used (Cochran 1977; Gatz and Smith 1995). All statistics were performed in R version 1.1.453 (R Core Team 2013), using packages dplyr (Wickham and others 2021), rpart (Therneau and others 2019), regclass (Petrie 2020), weights (Pasek and others 2020), and Hmisc (Harrell and Dupont 2020). Figures 2, 3, and 4 were created with package ggplot2 (Wickham and others 2020). All R code is included in the supplemental material.
Boxplots showing LAI-weighted plot-level average A foliar N:P for the full landscape, B area-basis foliar N, and C area-basis foliar P, then for subsets of plots identified by regression tree analysis. Removal of the one anomalously high point (N:P = 35) does not eliminate the significant difference between the foliar N:P values on high vs. low P soils. See Figure S1 for boxplots of leaf-mass-weighted values.
Plot-level, area-basis foliar N is associated with the relative leaf area contribution of Pentaclethra (A, p < 0.0001, r2 = 0.43) and other tree foliage (B, p = 0.02 r2 = 0.13). Plot-level, area-basis foliar P is also associated with the relative leaf area contribution of Pentaclethra (C, p = 0.003, r2 = 0.19) and other tree foliage (D, p = 0.05, r2 = 0.09).
Height versus canopy-level A LAI-weighted average foliar N:P, B mass-weighted average foliar N:P, C LAI-weighted average area-basis foliar N, D mass weighted mean mass-basis foliar N, E LAI-weighted average area-basis foliar P, F mass-weighted average mass-basis foliar P, G LAI-weighted average area-basis foliar K, and H mass-weighted average mass-basis foliar K. Each point shows a canopy level, binning leaf height by 1.86 m increments (the height of a sampling tower module). Error bars show ± 1 SE, calculated with weighted variance (Cochran 1977; Gatz and Smith 1995). Black lines indicate significant regression at p < 0.05. Significant linear trends were found for area-basis N (r2 = 0.83, slope = 0.04 { g m−2 leaf} m−1), area-basis P (r2 = 0.67, slope = 0.002 { g m−2 leaf} m−1), area-basis K (r2 = 0.30, slope = 0.009 { g m−2 leaf} m−1), and mass-basis K (r2 = 0.58, slope = -0.008 {% by mass leaf} m−1). All regression results for all 10 foliar concentrations are available in Table S3. LMA trends with height were previously reported (Cavaleri and others 2010), as were LAI distribution with height (Clark and others 2008).
Results
Horizontal Trends
Among individual foliar samples in this tropical rainforest, foliar N:P spanned 38% of the log-transformed global range (Wright and others 2004, see Isles 2020 for the reasons for log-transformation in stoichiometric comparisons). However, there was only modest variation among plots (that is, comparing all foliage captured in one sampling tower to all foliage captured in another). The arithmetic mean of plot-level foliar N:P was 21.8 ± 0.5 (48 ± 1 molar). The highest plot-level foliar N:P we found in the canopy exceeded the lowest by about a factor of 2 (N:P molar-ratio range: 34 to 78, N:P mass-ratio range: 15.5 to 35.3). This variance in N:P was partially explained by total soil P (0–100 cm). Plots on soils with < 670 ppm soil P had foliage with significantly higher foliar N:P (r2 = 0.23, p = 0.008, Figure 2) than those on more P-rich soils. No additional explanatory power resulted from including any other soil variables (C:N, total N, pH, cation exchange capacity, base saturation), topographic position, elevation difference from the local topographic maximum, forest height, or LAI.
As with foliar N:P, total soil P best explained variance from plot to plot in foliar P (r2 = 0.26, p = 0.0003, Figure 2). The optimal split selected by the regression tree model was 680 ppm, very similar to the result for foliar N:P. Notably, splitting the data by soil N did not significantly explain plot-level foliar N on a mass- (p = 0.21) or area-basis (p = 0.37). Plot-level foliar N variance was best explained by soil pH (r2 = 0.25, p = 0.0003, Figure 2). Soils of pH ≥ 4.1 supported more N-rich foliage, in comparison to the more acidic sites of pH 3.82–4.08. These soil conditions were not associated with one another (χ2 of regression tree split: p = 0.51, linear regression: p = 0.52). Foliar N, P, and N:P were thus each explained by a single factor, with no other factor adding explanatory power, and the results for mass-basis foliar traits were nearly identical (Figure S1).
The four plant functional groups differed in foliar chemistry (Table 1), so we asked whether functional group turnover was associated with the soil condition thresholds determined above. At total soil P < 670 ppm, 10% of leaf area was from Pentaclethra, but Pentaclethra became relatively more important at high soil P: 20% from Pentaclethra (p = 0.05). When comparing functional groups’ relative contribution to leaf mass, the change in Pentaclethra was similar (from 11% of leaf mass at low soil P to 25% at high soil P, p = 0.04). For soil pH, the only significant association was that Pentaclethra accounted for 15% of leaf mass at the most acidic plots and 38% of leaf mass at soil pH ≥ 4.1 (p = 0.04).
Across all plots, the relative abundance of Pentaclethra foliage was positively correlated with plot-level area-basis foliar N (p < 0.0001, r2 = 0.43, Figure 3) and P (p = 0.003, r2 = 0.19, Figure 3). This corresponded to a weak, but significant, decrease in the relative abundance of other tree foliage (p = 0.02, r2 = 0.13 and p = 0.05, r2 = 0.09, respectively, Figure 3). On a mass-basis, the correlation of Pentaclethra abundance with plot-level foliar N was weaker (p = 0.008, r2 = 0.15, Table S5), and rather than supplanting other trees (p = 0.28), there was a decrease in the percent of leaf mass from palms as plot-level mass-basis foliar N increased (p = 0.009, r2 = 0.15). There was no significant association of any functional group with plot-level foliar N:P (p ≥ 0.09), nor with mass-basis P (p ≥ 0.16). It is worth noting that there is less variance to be explained for mass-basis foliar chemistry. From plot to plot, area-basis foliar P (0.096 ± 0.004 g m−2, range: 0.055–0.168 g m−2) and area-basis N (2.0 ± 0.06 g m−2, range: 0.97–3.1 g m−2) varied by a factor of ~ 3, whereas mass-basis P (0.10 ± 0.003%, range: 0.06–0.15%) and mass-basis N (2.1 ± 0.04%, range: 1.6–2.8%) were more constrained, with the highest plot-level value exceeding the lowest by a factor of ~ 2.
Vertical Trends
Weighted averages of area-based foliar N (g m−2 leaf) and P (g m−2 leaf) increased with height in the canopy (r2 = 0.82, p < 0.0001 and r2 = 0.65, p < 0.0001, respectively; Figure 4). In contrast, the weighted average of mass-based foliar N (percent of leaf mass) and P (percent of leaf mass) were not significantly correlated with height (p = 0.87, p = 0.79, respectively; Figure 4). K was the only other leaf nutrient whose area-based concentration increased with height (r2 = 0.26, p = 0.01; Figure 4), and there was significant vertical depletion of mass-basis K (percent of leaf mass; r2 = 0.55, p < 0.0001; Figure 4). In fact, N and P were the only foliar nutrients that were not significantly depleted with height on a mass-basis, even though N and P were analyzed in ~ 50% more foliar samples than the other elements and thus, we had greater statistical power to detect a relationship with height (Tables S3-S4).
Discussion
Measures of foliar chemistry, combined with vertical structure of leaves and light, has promise to efficiently model NPP across tropical rainforest landscapes (Wieczynski and others 2021). However, to our knowledge, ours is the first systematic sampling of vertical nutrient distribution in lowland tropical rainforest foliage. This comprehensive foliar sampling (Figure 1) shows that LMA increase is the driver of vertical nutrient distribution, and that plot-level foliar N:P is marginally sensitive to total soil P, at least across the La Selva landscape.
Horizontal Trends
Latitudinal differences in foliar stoichiometry have been invoked to suggest that P is particularly important to the tropics. However, lowland tropical rainforest is highly heterogeneous as a biome, and the assumption of P limitation across the biome is likely an over-generalization (Cuevas and Medina 1986; Porder and Hilley 2011; Sanchez 2019; Quesada and others 2020). Soils at La Selva, for instance, have higher total P than many other lowland tropical sites (Porder and others 2006). Yet even across La Selva’s relatively P-rich soils, landscape foliar N:P was 21.8 ± 0.5 (48 ± 1 molar). This is almost double the estimate for temperate broadleaf forests, 12.6 ± 0.7 (28.2 ± 1.5 molar, 18% lower than our value on a logarithmic scale, McGroddy and others 2004). From plot to plot across La Selva, foliar stoichiometry responded to total soil P (regression tree: r2 = 0.23). That said, total soil P explained a greater portion of foliar N:P variance in a temperate rainforest (linear regression: r2 = 0.55, Richardson and others 2008). Thus, our data do not suggest that lowland tropical foliar stoichiometry is unusually responsive to soil P.
We recognize that total P is not the only metric for soil P status. However, in many cases, total P and available P are correlated, including in a survey of the isthmus of Panama (r2 = 0.68, Turner and others 2018). Across our plots, which have relatively homogenous parent material (Porder and others 2006), we also expect available and total soil P to be well correlated. Furthermore, the elevated foliar N:P that we observed at P-poor plots was driven by lower foliar P rather than higher foliar N (Figure 2). This landscape-scale finding is consistent with analyses of individual plants (Reich and others 2010) and plant communities (Guo and others 2020; Sardans and Peñuelas 2015), which have shown that decreases in mass-basis foliar N are accompanied by steeper decreases in foliar P. Thus, given our results, we posit that plots with even lower soil P, which are relatively common in tectonically stable, ancient cratons on granitic material (Porder and Hilley 2011; Porder and Ramachandran 2013), might have even higher foliar N:P.
Community shifts in response to the environment can alter stoichiometry (Sterner and Elser 2002). Given the low foliar N:P of palms (Table 1), a pronounced increase in palm abundance could theoretically reduce landscape foliar N:P. With the more frequent and intense hurricanes expected with climate change (Fraza and Elsner 2013; Emanuel 2017), palms may become more abundant in affected tropical forests (Comita and others 2018). However, in our study, the relative abundance of functional groups did not have a clear effect on plot-level foliar N:P. Both foliar N and foliar P (g m−2 leaf) tended to be higher in plots with a greater percent of leaf area from Pentaclethra (Figure 3). Pentaclethra abundance also contributed to the relationship between soil and foliar chemistry: the average percent of leaf mass from Pentaclethra doubled across the two unrelated soil thresholds selected by our model to explain plot-level foliar N and P (soil pH of 4.1 and 670 ppm soil P respectively, p = 0.04 for each). Pentaclethra form a large majority of the symbiotic N-fixers at La Selva (Menge and Chazdon 2016), suggesting a possible mechanism of this species effect. N-fixation demands P so that the plant’s rhizobial partners can incorporate N2 from the air into foliage (Houlton and others 2008), and the symbiosis is stressed below a soil pH of 4 (Bordeleau and Prévost 1994). Because we did not separate the leaves of symbiotic N-fixers from the rest of trees and lianas, we can only speculate that overall N-fixer abundance tracks Pentaclethra abundance.
Community shifts have been shown to maintain tropical forest productivity across soil conditions (Turner and others 2018), with a corresponding turnover in some plant functional traits (Umaña and others 2021). Our findings beg the question of whether foliar stoichiometry changes across soil nutrient gradients as well. Nutrient addition to tropical seedlings has shown little flexibility of foliar N:P within species, though the stoichiometry of stems and roots was more responsive to nutrients (Schreeg and others 2014). This suggests that, without community turnover, strict foliar stoichiometric requirements might lead to nutrient storage in other tissues. In a group of temperate forests with only 26 tree species, large trees appeared to store P in wood rather than substantially alter foliar stoichiometry (Sardans and Peñuelas 2014). It is plausible that, with the diversity of lowland tropical rainforests, community turnover could help to align foliar stoichiometry with soil conditions. Rather than denoting limitation to productivity, as has long been theorized, foliar stoichiometric differences could be another mechanism by which lowland tropical rainforests maintain relatively constant productivity across a wide range of soil conditions.
Vertical Trends
Many studies have theorized that foliar nutrients (especially N) are optimized across vertical gradients of forest canopies in response to light environment (Takenaka 1989; Hikosaka and others 2016), but others have found weak evidence of this optimization theory (Bond and others 1999) and some suggest that vertical gradients in nutrients minimize the damage due to excess light, rather than optimize light utilization (Kitao and others 2018). At La Selva, however, the remarkably consistent vertical gradients of LMA across the tower transects were more strongly related to height than to light, which points to hydraulic constraints on leaf morphology due to gravity rather than optimization due to light (Cavaleri and others 2010). Because the area-basis concentrations of N, P, and K increase with height, but the mass-basis concentrations do not, these patterns are driven by the LMA gradient. This has also been observed in individual tropical plants (Niinemets and others 2015; Poorter and others 2019). LMA increase with height, in turn, is likely driven by hydraulic constraint. This consistent pattern of LMA increases with height, which drives N, P, and K (Figure 4), as well as foliar respiration (Cavaleri and others 2008), has the potential to greatly simplify the modeling of physiological processes in tropical forests.
In contrast to N and P, foliar concentrations of all other elements decreased with height on a mass-basis (Tables S3 and S4). This is similar to findings from across the Amazon (Lloyd and others 2010) and from a montane rainforest in New Guinea (Grubb and Edwards 1982) where shorter trees had greater mass-basis foliar Ca and Mg. In the Amazon, Mg was enriched on an area-basis in the taller trees, but in our study at La Selva, only N, P, and K were vertically enriched. In the case of K, the mass-basis decrease was small enough relative to the change in LMA that area-basis K increased with height, both across the Amazon and in our study at La Selva. Potassium leaches more readily than foliar N or foliar P, presumably because of the very small size of K+ (Sardans and Peñuelas 2015), and the uppermost leaves experience the most leaching (Adriaenssens and others 2012). Given the importance of these elements for photosynthesis (Mercer and others 1962; Epstein and Bloom 2005), these results bear further consideration as the vertical distribution of leaves and their chemistry is integrated into photosynthetic models.
Conclusion
Our comprehensive foliar sampling of a lowland tropical rainforest suggests that plot-level variability of foliar chemistry reflects soil conditions, and that plot-level foliar N:P in particular is an indicator of soil P status. Furthermore, we show that landscape-scale vertical nutrient enrichment is the result of LMA increase, as has previously been reported within individual tropical plants. Landscape photosynthetic models often consider foliar N alone, but we show concomitant vertical increase of foliar P and K. Finally, our data hint that stoichiometric flexibility may help to maintain productivity on low P soils. Testing this idea will require additional work in lower P sites.
References
Adriaenssens S, Hansen K, Staelens J, Wuyts K, De Schrijver A, Baeten L, Boeckx P, Samson R, Verheyen K. 2012. Throughfall deposition and canopy exchange processes along a vertical gradient within the canopy of beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst). Sci Total Environ 420:168–182.
Albert LP, Wu J, Prohaska N, de Camargo PB, Huxman TE, Tribuzy ES, Ivanov VY, Oliveira RS, Garcia S, Smith MN, Oliveira Junior RC, Restrepo-Coupe N, da Silva R, Stark SC, Martins GA, Penha DV, Saleska SR. 2018. Age-dependent leaf physiology and consequences for crown-scale carbon uptake during the dry season in an Amazon evergreen forest. New Phytol 219:870–884.
Alvarez-Clare S, Mack MC, Brooks M. 2013. A direct test of nitrogen and phosphorus limitation to net primary productivity in a lowland tropical wet forest. Ecology 94:1540–1551.
Asner GP, Martin RE. 2012. Contrasting leaf chemical traits in tropical lianas and trees: implications for future forest composition. Ecol Lett 15:1001–1007.
Asner GP, Martin RE. 2016. Convergent elevation trends in canopy chemical traits of tropical forests. Glob Change Biol 22:2216–2227.
Asner GP, Anderson CB, Martin RE, Tupayachi R, Knapp DE, Sinca F. 2015a. Landscape biogeochemistry reflected in shifting distributions of chemical traits in the Amazon forest canopy. Nat Geosci 8:567–573.
Asner GP, Martin RE, Anderson CB, Knapp DE. 2015b. Quantifying forest canopy traits: Imaging spectroscopy versus field survey. Remote Sens Environ 158:15–27.
Awmack CS, Leather SR. 2002. Host Plant Quality and Fecundity in Herbivorous Insects. Annu Rev Entomol 47:817–844.
Balzotti CS, Asner GP, Taylor PG, Cleveland CC, Cole R, Martin RE, Nasto M, Osborne BB, Porder S, Townsend AR. 2016. Environmental controls on canopy foliar nitrogen distributions in a Neotropical lowland forest. Ecol Appl 26:2451–2464.
Bern CR, Townsend AR, Farmer GL. 2005. Unexpected Dominance of Parent-Material Strontium in a Tropical Forest on Highly Weathered Soils. Ecology 86:626–632.
Bonan GB. 2008. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 320:1444–1449.
Bond BJ, Farnsworth BT, Coulombe RA, Winner WE. 1999. Foliage physiology and biochemistry in response to light gradients in conifers with varying shade tolerance. Oecologia 120:183–192.
Bordeleau L, Prévost D. 1994. Nodulation and nitrogen fixation in extreme environments. Plant Soil 161:115–125.
Breiman L, Friedman JH, Olshen RA, Stone C. 1983. Classification and Regression Trees. Belmont, CA: Wadsworth.
Cámara-Leret R, Tuomisto H, Ruokolainen K, Balslev H, Munch Kristiansen S. 2017. Modelling responses of western Amazonian palms to soil nutrients. Wurzburger N, editor. J Ecol 105:367–381.
Cavaleri MA, Oberbauer SF, Ryan MG. 2008. Foliar and ecosystem respiration in an old-growth tropical rain forest. Plant Cell Environ 31:473–483.
Cavaleri MA, Oberbauer SF, Clark DB, Clark DA, Ryan MG. 2010. Height is more important than light in determining leaf morphology in a tropical forest. Ecology 91:1730–1739.
Clark DB, Olivas PC, Oberbauer SF, Clark DA, Ryan MG. 2008. First direct landscape-scale measurement of tropical rain forest Leaf Area Index, a key driver of global primary productivity. Ecol Lett 0:163–72.
Cleveland CC, Townsend AR, Taylor P, Alvarez-Clare S, Bustamante MMC, Chuyong G, Dobrowski SZ, Grierson P, Harms KE, Houlton BZ, Marklein A, Parton W, Porder S, Reed SC, Sierra CA, Silver WL, Tanner EVJ, Wieder WR. 2011. Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis: Nutrients, climate and tropical NPP. Ecol Lett 14:939–947.
Cochran W. 1977. Sampling Techniques, 3rd edn. New York: Wiley.
Comita L, Uriarte M, Forero-Montaña J, Kress W, Swenson N, Thompson J, Umaña M, Zimmerman J. 2018. Changes in Phylogenetic Community Structure of the Seedling Layer Following Hurricane Disturbance in a Human-Impacted Tropical Forest. Forests 9:556.
Cuevas E, Medina E. 1986. Nutrient dynamics with in Amazonian forest ecosystems: I. Nutrient flux in fine litter fall and efficiency of nutrient utilization. Oecologia 68:466–472.
Cushman KC, Kellner JR. 2019. Prediction of forest aboveground net primary production from high-resolution vertical leaf-area profiles. Uriarte M, editor. Ecol Lett 22:538–546.
De’Ath G, Fabricius KE. . 2000. Classification and Regression Trees: A Powerful Yet Simple Technique for Ecological Data Analysis. Ecology 81:3178–3192.
Delitti WBC, Burger DM. 2000. Carbon and mineral nutrient pools in a gallery forest at Mogi Guaçu River, Southeast Brazil. Ann For Sci:39–47.
Emanuel K. 2017. Assessing the present and future probability of Hurricane Harvey’s rainfall. Proc Natl Acad Sci 114:12681–12684.
Epstein E, Bloom A. 2005. Mineral Nutrition of Plants, 2nd edn. Sunderland, MA: Sinauer Associates Inc.
Espeleta JF, Clark DA. 2007. Multi-scale variation in fine-root biomass in a tropical rain forest: a seven-year study. Ecol Monogr 77:377–404.
Falkowski PG, Barber RT, Smetacek V. 1998. Biogeochemical Controls and Feedbacks on Ocean Primary Production. Science 281:200–206.
Field C. 1983. Allocating leaf nitrogen for the maximization of carbon gain: Leaf age as a control on the allocation program. Oecologia 56:341–347.
Fisher JB, Malhi Y, Torres IC, Metcalfe DB, van de Weg MJ, Meir P, Silva-Espejo JE, Huasco WH. 2013. Nutrient limitation in rainforests and cloud forests along a 3,000-m elevation gradient in the Peruvian Andes. Oecologia 172:889–902.
Fraza E, Elsner JB. 2013. A spatial climatology of North Atlantic hurricane intensity change. Int J Climatol 34:2918–2124.
Fyllas NM, Schwarz M, Horna V, Mercado LM, Santos A, Arroyo L, Jimenez EM, Luizao FJ, Neill DA, Silva N, Prieto A, Rudas A, Silviera M, Vieira ICG, Lopez-Gonzalez G, Malhi Y, Phillips OL, Lloyd J. 2009. Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences 6:2677–2708.
Gatz DF, Smith L. 1995. The standard error of a weighted mean concentration—I. Bootstrapping vs other methods. Atmos Environ 29:1185–1193.
Golley F. 1975. Mineral cycling in a tropical moist forest ecosystem, 1st edn. Athens, GA: University of Georgia Press.
Grubb PJ, Edwards PJ. 1982. Studies of Mineral Cycling in a Montane Rain Forest in New Guinea: III. The Distribution of Mineral Elements in the Above-Ground Material. J Ecol 70:623–648.
Guo Y, Yan Z, Gheyret G, Zhou G, Xie Z, Tang Z. 2020. The community-level scaling relationship between leaf nitrogen and phosphorus changes with plant growth, climate and nutrient limitation. Wardle D, editor. J Ecol:1365–2745.13369.
Harrell F, Dupont C. 2020. Hmisc. https://CRAN.R-project.org/package=Hmisc
Hartshorn G. 1983. Species Accounts: Pentaclethra macroloba. In: Costa Rican Natural History. pp 301–3.
Hikosaka K, Anten NPR, Borjigidai A, Kamiyama C, Sakai H, Hasegawa T, Oikawa S, Iio A, Watanabe M, Koike T, Nishina K, Ito A. 2016. A meta-analysis of leaf nitrogen distribution within plant canopies. Ann Bot 118:239–247.
Hirose T, Werger MJA. 1987. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72:520–526.
Houlton BZ, Wang Y-P, Vitousek PM, Field CB. 2008. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330.
Isles PDF. 2020. The misuse of ratios in ecological stoichiometry. Ecology 101:1–7.
Kellner JR, Clark DB, Hofton MA. 2009. Canopy height and ground elevation in a mixed-land-use lowland Neotropical rain forest landscape: E090–233. Ecology 90:3274–3274.
Kitao M, Kitaoka S, Harayama H, Tobita H, Agathokleous E, Utsugi H. 2018. Canopy nitrogen distribution is optimized to prevent photoinhibition throughout the canopy during sun flecks. Sci Rep 8:503.
Koerselman W, Meuleman AFM. 1996. The Vegetation N: P Ratio: a New Tool to Detect the Nature of Nutrient Limitation. J Appl Ecol 33:1441–1450.
Konvalinková T, Jansa J. 2016. Lights Off for Arbuscular Mycorrhiza: On Its Symbiotic Functioning under Light Deprivation. Front Plant Sci 7.
Leuning R, Cromer RN, Rance S. 1991. Spatial distributions of foliar nitrogen and phosphorus in crowns of Eucalyptus grandis. Oecologia 88:504–510.
Lloyd J, Patiño S, Paiva RQ, Nardoto GB, Quesada CA, Santos AJB, Baker TR, Brand WA, Hilke I, Gielmann H, Raessler M, Luizão FJ, Martinelli LA, Mercado LM. 2010. Optimisation of photosynthetic carbon gain and within-canopy gradients of associated foliar traits for Amazon forest trees. Biogeosciences 7:1833–1859.
Lloyd J, Bloomfield K, Domingues TF, Farquhar GD. 2013. Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? New Phytol 199:311–321.
McGroddy ME, Daufresne T, Hedin LO. 2004. Scaling of C:N: P Stoichiometry in Forests Worldwide: Implications of Terrestrial Redfield-Type Ratios. Ecology 85:2390–2401.
McKenzie, Jacquier, Isbell, Brown. 2004. Australian soils and landscapes: an illustrated compendium.
Menge DNL, Chazdon RL. 2016. Higher survival drives the success of nitrogen-fixing trees through succession in Costa Rican rainforests. New Phytol 209:965–977.
Mercer FV, Nittim M, Possingham JV. 1962. The effect of Manganese deficiency on the structure of spinach chloroplasts. J Cell Biol 15:379–381.
Niinemets Ü, Keenan TF, Hallik L. 2015. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol 205:973–993.
Osnas JLD, Lichstein JW, Reich PB, Pacala SW. 2013. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. Science 340:741–744.
Pasek J, Tahk A, Cutler G, Schwemmle M. 2020. weights. https://CRAN.R-project.org/package=weights
Petrie A. 2020. regclass. In: Vol. Version 1.6. https://CRAN.R-project.org/package=regclass/
Poorter H, Niinemets Ü, Ntagkas N, Siebenkäs A, Mäenpää M, Matsubara S, ThijsL Pons. 2019. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol 223:1073–1105.
Porder S, Hilley GE. 2011. Linking chronosequences with the rest of the world: predicting soil phosphorus content in denuding landscapes. Biogeochemistry 102:153–166.
Porder S, Ramachandran S. 2013. The phosphorus concentration of common rocks—a potential driver of ecosystem P status. Plant Soil 367:41–55.
Porder S, Asner GP, Vitousek PM. 2005. Ground-based and remotely sensed nutrient availability across a tropical landscape. Proc Natl Acad Sci 102:10909–10912.
Porder S, Clark DA, Vitousek PM. 2006. Persistence of Rock-Derived Nutrients in the Wet Tropical Forests of La Selva, Costa Rica. Ecology 87:594–602.
Quesada CA, Lloyd J, Schwarz M, Nardoto GB, Schmerler J, Santos AJB, Hodnett MG, Herrera R, Luizao FJ, Arneth A, Lloyd G, Dezzeo N, Hilke I, Kuhlmann I, Raessler M, Brand WA, Geilmann H, Filho JOM, Carvalho FP, Filho RNA, Chaves JE, Junior OFC, Pimentel TP, Paiva R. 2010. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences Discuss:3923–92.
R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Redfield AC. 1958. The Biological Control of Chemical Factors in the Environment. Am Sci 46:230A-A221.
Reed SC, Townsend AR, Davidson EA, Cleveland CC. 2012. Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytol 196:173–180.
Reich PB, Oleksyn J. 2004. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci 101:11001–11006.
Reich PB, Oleksyn J, Wright IJ, Niklas KJ, Hedin L, Elser JJ. 2010. Evidence of a general 2/3-power law of scaling leaf nitrogen to phosphorus among major plant groups and biomes. Proc R Soc B Biol Sci 277:877–883.
Richardson AD. 2004. Foliar chemistry of balsam fir and red spruce in relation to elevation and the canopy light gradient in the mountains of the northeastern United States. Plant Soil 260:291–299.
Richardson SJ, Allen RB, Doherty JE. 2008. Shifts in leaf N: P ratio during resorption reflect soil P in temperate rainforest. Funct Ecol 22:738–745.
Sanchez PA. 2019. Properties and Management of Soils in the Tropics, 2nd edn. Cambridge, UK: Cambridge University Press.
Sanford R, Paaby P, Luvall J, Phillips E. 1994. Climate, geomorphology, and aquatic systems. In: McDade L, Bawa K, Hespenheide H, Hartshorn G, Eds. La Selva: Ecology and Natural History of a Neotropical Rain Forest, . Chicago, IL, USA.: University of Chicago Press. pp 19–33.
Sardans J, Peñuelas J. 2014. Trees increase their P: N ratio with size: Phosphorus; the treasure nutrient. Glob Ecol Biogeogr 24:147–156.
Sardans J, Peñuelas J. 2015. Potassium: a neglected nutrient in global change: Potassium stoichiometry and global change. Glob Ecol Biogeogr 24:261–275.
Schlichting E, Blume H, Stahr K. 1995. Bodenkundliches Praktikum. Berlin-Wien: Blackwell Wissenschaftsverlag.
Schreeg LA, Santiago LS, Wright SJ, Turner BL. 2014. Stem, root, and older leaf N: P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 95:2062–2068.
Šímová I, Sandel B, Enquist BJ, Michaletz ST, Kattge J, Violle C, McGill BJ, Blonder B, Engemann K, Peet RK, Wiser SK, Morueta-Holme N, Boyle B, Kraft NJB, Svenning J-C. 2019. The relationship of woody plant size and leaf nutrient content to large-scale productivity for forests across the Americas. J Ecol 00:1–13.
Sollins P, Sancho F, Mata R, Sanford R. 1994. Soils and Soil Process Research. In: McDade L, Bawa K, Hespenheide H, Hartshorn G, Eds. La Selva: Ecology and Natural History of a Neotropical Rain Forest, . Chicago, IL, USA.: University of Chicago Press. pp 34–53.
Sterner RW, Elser JJ. 2002. Ecological Stoichiometry. Princeton, NJ: Princeton University Press.
Sullivan BW, Alvarez-Clare S, Castle SC, Porder S, Reed SC, Schreeg L, Townsend AR, Cleveland CC. 2014. Assessing nutrient limitation in complex forested ecosystems: alternatives to large-scale fertilization experiments. Ecology 95:668–681.
Takenaka A. 1989. Optimal leaf photosynthetic capacity in terms of utilizing a natural light environment. J Theor Biol 139:517–529.
Tao L, Hunter MD. 2011. Effects of Insect Herbivores on the Nitrogen Economy of Plants. In: Ecological Aspects of Nitrogen Metabolism in Plants. Wiley-Blackwell. pp 257–79.
Taylor BN, Menge DNL. 2018. Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat Plants 4:655–661.
Therneau T, Atkinson B, Ripley B. 2019. rpart. https://CRAN.R-project.org/package=rpart
Townsend AR, Cleveland CC, Asner GP, Bustamante MMC. 2007. Controls Over Foliar N: P Ratios in Tropical Rain Forests. Ecology 88:107–118.
Townsend AR, Cleveland CC, Houlton BZ, Alden CB, White JW. 2011. Multi-element regulation of the tropical forest carbon cycle. Front Ecol Environ 9:9–17.
Tully KL, Wood TE, Schwantes AM, Lawrence D. 2013. Soil nutrient availability and reproductive effort drive patterns in nutrient resorption in Pentaclethra macroloba. Ecology 94:930–940.
Turner BL, Brenes-Arguedas T, Condit R. 2018. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 555:367–370.
Umaña MN, Condit R, Pérez R, Turner B, Wright SJ, Comita LS. 2021. Shifts in taxonomic and functional composition of trees along rainfall and phosphorus gradients in central Panama. J Ecol 109:51–61.
Vitousek PM. 1984. Litterfall, Nutrient Cycling, and Nutrient Limitation in Tropical Forests. Ecology 65:285–298.
Wickham H, Chang W, Henry L, Lin Pedersen T, Takahashi K, Wilke C, Woo K, Yutani H, Dunnington D. 2020. ggplot2. https://CRAN.R-project.org/package=ggplot2
Wickham H, François R, Henry L, Müller K. 2021. dplyr. https://CRAN.R-project.org/package=dplyr
Wieczynski D, Díaz S, Duran S, Fyllas N, Salinas N, Martin R, Shenkin A, Silman M, Asner G, Bentley L, Malhi Y, Enquist B, Savage V. 2021. Quantifying productivity at landscape scale using remotely-sensed foliar traits and canopy structure. https://doi.org/10.21203/rs.3.rs-98574/v1
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R. 2004. The worldwide leaf economics spectrum. Nature 428:821–827.
Wright SJ, Turner BL, Yavitt JB, Harms KE, Kaspari M, Tanner EVJ, Bujan J, Griffin EA, Mayor JR, Pasquini SC, Sheldrake M, Garcia MN. 2018. Plant responses to fertilization experiments in lowland, species-rich, tropical forests. Ecology 99:1129–1138.
Acknowledgements
This study was funded by the U.S. National Science Foundation (Geosciences #0223284). AM also received funding from the Institute at Brown for Environment and Society. We thank the TOWERS Project field crew and technicians, the laboratory staff and forest technicians Leonel Campos and William Miranda, and OTS, particularly Don Isaías Alvarado for construction advice and Ms. Cynthia Rossi for GIS assistance and LiDAR data processing. Loren Albert, John Burley, KC Cushman, James Kellner, Lindsay McCulloch, and Dafeng Zhang provided helpful feedback on early drafts of the manuscript. We also thank Christian Giardina and two anonymous reviewers for a highly constructive peer review process which greatly improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Author contributions
PCO and SFO designed the field study. MAC and PCO collected samples and performed lab analyses. AM, MAC, and SP analyzed data. AM, MAC, and SP wrote the paper. All authors participated in editing the paper.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Massmann, A., Cavaleri, M.A., Oberbauer, S.F. et al. Foliar Stoichiometry is Marginally Sensitive to Soil Phosphorus Across a Lowland Tropical Rainforest. Ecosystems 25, 61–74 (2022). https://doi.org/10.1007/s10021-021-00640-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-021-00640-w