Abstract
Information about nutrient dynamics is of upmost importance in order to contribute to the restoration of degraded forest environments in the Andes of southern Ecuador. This study aims to investigate the differences of nutrient dynamics between a native alder (Alnus acuminata) and an exotic pine (Pinus patula) tree species in this region. Based on litterfall, forest floor and mineral topsoil (0–20 cm) of two pine and two alder plantations, we studied the litterfall production and its seasonality; temporal variations of nutrient concentrations, stoichiometric ratios and potential nutrient return (PNR) of leaf-litterfall; mean residence times (MRT) of nutrients in the forest floor; and assessed soil biogeochemical properties. Our results showed that total litterfall production in pine was twice as high as in alder. Litterfall biomass seasonality was similar for both species and highly associated to periods with less precipitation. Pine exhibited the highest seasonality of nutrient concentrations and stoichiometric ratios. PNR of N, K, Ca, and Mn exhibited the major differences between the species. The annual PNR of N and Ca were higher in alder, while those of K and Mn were higher in pine. Pine exhibited higher MRT values for C, N, P, S, Cu, and Zn, while alder showed the higher for Mg, K, Mn, and Ca. In soils, alder exhibited higher concentrations and stocks of nutrients, but not for C. Although, the soil microbial biomass was similar under both species, microbial activity was different. C and net N mineralization were higher in alder, and nitrification dominated over ammonification processes. In general, our findings show a faster cycling of nutrients in alder than in pine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A high anthropogenic pressure through deforestation is affecting montane forests in tropical regions (Jacobs et al. 2018). Ecuador, as a tropical country, was in the top ten list of countries with the greatest loss of primary forest area between 1990 and 2015 (Morales-Hidalgo et al. 2015) and until recently exhibited the highest deforestation rate in South America (Mosandl et al. 2008). In Ecuador, the use of exotic species for afforestation programs has been implemented on large scale since the beginning of the previous century (Brandbyge 1991; Farley 2007). In this country, more than 90% of all forest plantations consist of exotic species, primarily Eucalyptus spp. and Pinus spp. established because of the lack of information about aut- and synecological requirements of the native species (Günter et al. 2009). However, they have been established without intensive baseline studies (van Voss et al. 2001). In the Andean region of Ecuador (Sierra region), pines have been commonly used for plantations (Ministerio del Ambiente de Ecuador 2006; Mejía and Pacheco 2013; Knoke et al. 2014), and reasons for their establishment have been mainly wood production, followed by carbon sequestration, erosion prevention and water regulation and supply, especially in the highlands (Dahik et al. 2018). However, pine plantations have negatively affected the forest restoration in the Ecuadorian Andes (Middendorp et al. 2016) and reduced environmental quality (Farley and Kelly 2004; Farley 2007; Buytaert et al. 2007; Chacón et al. 2009; Quichimbo et al. 2012; Bonnesoeur et al. 2019). Therefore, there is a growing interest in Ecuador for the use of native species for forest recovery (Palomeque et al. 2017). In this context, Alnus acuminata Kunth, also known as Andean alder, is one candidate tree species. This species from the Betulaceae family is growing in the southern hemisphere (Aceñaloza and Gallardo-lancho 1994; Weng et al. 2004). In the Sierra region of Ecuador, this alder species is present in natural forests as well as in plantations (Dunn et al. 1990), since this species has been recognized as a catalyst for forest regeneration in the Andes (Murcia 1997; Kattan and Murcia 2012). Alder shows fast growth rates, even higher than the typical exotic species (Günter et al. 2009, Weber et al. 2011). In addition, the importance of this species due to its N-fixing capacity that progressively improves soil quality is recognized (Knoke et al. 2014; Bare and Ashton 2016). Information about the dynamics of nutrients and biogeochemical processes in stands of this species is still missing for the tropical Andes. This knowledge is a crucial prerequisite to evaluate the need for improving site fertility of alder plantations (Alvarado 2016) or in general to support the restoration/rehabilitation of forests of abandoned or degraded lands (Knoke et al. 2016, Silva et al. 2019), as a first step to establish forest ecosystems with a higher biodiversity and also to ensure ecosystem function for the future (Löf et al. 2019). Furthermore, understanding how forest species influence nutrient cycling is relevant to inform land managers about the potential ecosystem effects of species selection (Hobbie 2015).
In forest ecosystems, the dynamic of organic matter and nutrients is highly dependent on quantity and quality of litterfall. Detailed knowledge about litter chemical composition can help to estimate humus buildup as well as nutrient storage and release in the organic layer and mineral topsoil (Berg and Laskowski 2005). Knowledge about potential nutrient return (PNR) by litterfall material and mean residence time (MRT) of nutrients in forest floor can support our understanding of nutrient cycling in the tropical montane forest ecosystems in the Andes (Wilcke et al. 2002). A remarkable topic is the seasonality of litterfall as a key factor affecting the dynamics of ecosystem C and N cycling (Zhang et al. 2014). Litterfall seasonality and the temporal variability of its chemical composition are still poorly studied in the tropics (Parsons et al. 2014). Chave et al. (2010) studied the seasonal patterns in tropical South American forests. They found significant correlations between litterfall and rainfall seasonality, but most of the studied sites were located in lowland rainforests. Tropical montane forest ecosystems are deficiently studied.
Dynamics of organic matter and nutrients are also highly dependent on the decomposition of litterfall material in soils (Berg and McClaugherty 2014). Thus, the study of soil biogeochemical properties related to microbial mediated mineralization processes is important (Cronan 2018).
Therefore, this study aimed to investigate the differences of nutrient dynamics between the native tree species (alder) and the exotic species (pine) in a montane forest ecosystem in the Andes of southern Ecuador. The specific objectives were to: (1) assess the litterfall production and its seasonality in pine and alder, (2) compare the temporal variation of nutrient concentrations, stoichiometric ratios and PNR of leaf-litterfall between both species, (3) examine the MRT of nutrients in the forest floor of both species, and (4) assess the soil biogeochemical properties of both species. The findings will contribute to a better understanding of the contrasts and ecosystem consequences of nutrient dynamics of both tree species in the study area.
Materials and methods
Study area
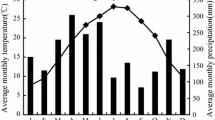
The research was conducted in Loja province in the southern Andean region of Ecuador (at the western side of the Cordillera Real). Four forest sites were selected close to Loja city (4°03ʹS; 79°11ʹW), two pine (Pinus patula) plantations: Zamora-Huayco (ZAM) and Dos Puentes (DOS); and two alder (A. acuminata) plantations: El Carmen (CAR) and Rumizhitana (RUM). CAR was close to ZAM, and RUM close to DOS. The distance between alder and pine sites (alder-pine sites: CAR-ZAM and RUM-DOS) was approximately 4.5 km. The climate of the study area is characterized by an annual mean precipitation of 912 mm, with a main rainy season between December and April, and an annual mean temperature of 15.7 °C (period 1965–2014, Online resource 1) (Oñate-Valdivieso et al. 2018). Soils are dominantly Umbrisols (Quichimbo et al. 2017). Altitude ranges from 2200 to 2350 m a.s.l. Natural vegetation is considered as evergreen montane forests (Günter et al. 2008; Tapia-Armijos et al. 2015), with dominant tree species of the genera Araliceae, Melastomataceae, Clusiaceae, Rubiaceae, Rosaceae, and Primulaceae (Jiménez and Aguirre 2017). The natural vegetation has been affected by common practices in the Andes of slash-and-burn activities for pasture or cropland establishment (Hamer et al. 2013; Ochoa-Cueva et al. 2013). The former land uses in alder and pine plantations were abandoned pastures established after the replacement of native vegetation. Alder sites are approximately 15-year-old plantations (RUM and CAR), while pines are approximately 22 and 18-year-old plantations, in DOS and ZAM, respectively. Stand structural characteristics have been obtained for each forest site according to Quichimbo et al. (2017) in an area of 24 m × 24 m (forest inventory plot) and they are described in Online resource 2.
Installation of litterfall traps
A central square plot of 144 m2 was installed in each of the forest inventory plots (of 24 m × 24 m). Then, 16 litterfall traps were placed (horizontally leveled) in a systematic arrangement at regular intervals (3 m approximately). Each trap had a capture area of 30 × 30 cm (Wilcke et al. 2002). Litterfall material was collected biweekly over 1 year (October 2013 to September 2014). According to Proctor (1983), 1 year of collection provides reliable information; and it enables to characterize the general trend of litterfall production (Chave et al. 2010). Litterfall material was fractioned in leaves, reproductive parts, twigs, and miscellaneous material. All fractions were dried at 40 °C (MEMMERT UFE600, Germany) until constant weight was reached.
Forest floor and soil sampling
Soil sampling was carried out in the same plots of the litterfall monitoring during September–October of 2013. Each plot was divided in 4 subplots. From each subplot five samples were taken and mixed to obtain one representative sample per subplot, from the forest floor and the mineral topsoil down to 20 cm. The forest floor was divided into Oi, Oe, and Oa organic horizons for alder. For pine the material was combined in Oe/Oa horizon (a separate sampling of Oe and Oa was not possible). Forest floor was collected on a volume basis using a square frame sampler of 100 cm2. The mineral soil layer was sampled using a cylindrical soil corer (6 cm diameter). This soil core was split into 0–5, 5–10 and 10–20 cm (Hamer et al. 2013).
Laboratory analyses
Nutrient determination in litterfall
Considering that leaf-litterfall fraction is the biggest contributor to the total litterfall and more active for the decomposition processes than the other components (cones, twigs and barks) only this fraction of litterfall was sent to Germany for chemical analyses. Samples were pooled on a monthly basis to reduce the number of analyses. Then, the pooled samples were grinded (Vibratory Disc Mill RS 200 Retsch, Haan, Germany); for pine needles with a high amount of resin a Mixer Mill MM 400 (Retsch, Germany) was used. The grinded material was transformed to pellets with a force of 10 tons using a CrushIR Digital Hydraulic Press (PIKE Technologies, Wisconsin, US). These pellets were used to determine the nutrient content by Wavelength Dispersive X-ray Fluorescence (XRF spectrometer, ZSX Primus II, Rogaku Corp.) to determine: P, S, K, Ca, Mg, Mn, Cu, and Zn. C and N concentrations were measured with a Euro EA 3000 CHNS-O Elemental Analyser (HEKAtech GmbH, Germany).
Nutrient determination in forest floor and soils
An aliquot of each sample was dried (40 °C) and ground to analyze soil organic carbon (SOC) and total nitrogen (TN) with a CNS-Analyzer (vario EL III/elementar, Heraeus, Germany). NO3−-N and NH4+-N (inorganic N forms) were determined after extraction with 0.1 M KCl in a continuous-flow auto analyzer (Skalar Analytik GmbH, Erkelenz, Germany). The available phosphorous (PO4-P) was determined by extraction with Bray I solution (0.03 M NH4F + 0.025 M HCl) (Bray and Kurtz 1945). Samples were shaken (180 rpm for 1 min) and filtered. Inorganic P (Pi-NH4F) content in the extracts was measured photometrically in the continuous-flow auto analyzer at 880 nm. Meanwhile, the organic P (Po-NH4F) was computed as the difference of the total P (P-NH4F) and the Pi-NH4F, the total P (P-NH4-F) was determined by ICP-OES measurements (CIROS-Spectro). The total amount of elements (P, K, Ca, Mg, S, Mn, Cu, and Zn) was determined from aliquots of dried soil samples after acid digestion (HNO3/HF/HClO4) in a microwave (Kingston and Jassie 1986) and measured with ICP-OES. The soil pH of the samples was measured with a glass membrane electrode in H2O (soil:solution ratio mineral soil 1:2.5; forest floor 1:10) and also the moisture content (105 °C) was determined.
Estimation of microbial biomass
Microbial biomass carbon (MBC) and nitrogen (MBN) were estimated according to the chloroform fumigation-extraction procedure using a 0.5 M K2SO4 solution (Vance et al. 1987). Five g of dry weight (dw) equivalent forest floor material was extracted with 100 ml K2SO4 solution, whereas 20 g of dw mineral soil was extracted with 80 ml K2SO4 solution. Extracts from fumigated and non-fumigated samples (after shaking and filtering) were analyzed for extractable C and N content with a multi-NC-Analyzer (Analytic Jena, Germany) (Potthast et al. 2010). MBC and MBN concentrations were computed as the difference between fumigated and non-fumigated extracts. A kEC of 0.45 and kEN of 0.54, respectively, were used as the conversion factors (Tischer et al. 2015).
Microbial biomass phosphorus (MBP) was estimated in accordance with the chloroform-extraction procedure adapted for acid soils (Chen and He 2004). Two subsamples were taken from each sample. One subsample was extracted with 0.03 NH4F and 0.025 M HCl (1:5 soil:solution ratio). The second subsample was extracted after 24 h of fumigation. Inorganic P content in the extracts was measured photometrically using a continuous flow auto analyzer (Skalar Analytik GmbH, Germany). Phosphate adsorption after cell lysis of microbial biomass was kept in mind for the MBP computations of each site and soil depth, by adding a P-spike—25 μg KH2PO4-P g−1 soil and the respective extraction—(Tischer et al. 2014). MBP was estimated by the difference between the fumigated and non-fumigated extracts using a kEP of 0.4 (Brookes et al. 1982). Microbial nutrient concentrations and ratios were computed on a molar basis.
Estimation of the microbial activity
Rates of C and net N mineralization were determined by incubation of field-moist soil (20 g and 10 g for mineral and organic soil samples, respectively) using glass bottles (Schott Duran®, Germany) at 22 °C for 14 days in darkness. Throughout incubation, soil C mineralization was quantified by capturing evolved CO2 in 0.2 M NaOH, precipitation with 1 M BaCl2, and titration against 0.1 M HCl. NH4+-N and NO3−-N concentrations were determined before and at the end of the incubation by 0.1 M KCl extraction (Potthast et al. 2012). Rates of net N mineralization were calculated by subtracting the sum of the initial concentrations (NH4+-N + NO3−-N) from the sum of the final concentrations (after 13 days) (Potthast et al. 2012).
Data analyses
The production of total litterfall and its fractions was computed by cumulated values for the year, based on the following equation (Berg and Laskowski 2005):
where DM is the monthly dry matter biomass per area (kg ha−1), m1 the weight of the container plus the dried sample (40 °C) (g), m0 the weight of the empty container weight (g), a the trap capture area (0.09 m2), and b the transformation factor to convert the values in kg ha−1 (10).
Seasonality of the litterfall and their fractions was assessed by the Seasonality Index (SL) as stated by Chave et al. (2010) and Parsons et al. (2014). This index is an estimation of the evenness of the litterfall distribution throughout the year; thus if the index is close to 1, the litterfall production is highly concentrated in a given period of the year, and if the index has a value close to 0, the litterfall is approximately evenly distributed through the year. This index was computed as follows:
where L is the annual litterfall (Mg ha−1 year−1), and \(\parallel m\parallel\) is the mean vector obtained by the components:
\(L_{i}\) belongs to the litterfall per month expressed in Mg ha−1 year−1. The SL was used to characterize the difference between the sites and forest species. Additionally, coefficient of variation (C.V.) was used to describe the statistical variation of data.
PNR corresponds to the nutrient stocks in leaf-litterfall and was computed by multiplying the values of leaf-litterfall biomass with its corresponding nutrient concentration (monthly and annual basis).
The temporal (monthly) variation of nutrient concentrations, C:N:P (C:elements, N:elements and P:elements) stoichiometric molar ratios, and PNR of leaf-litterfall was evaluated using the same computation of the SL described above and also the C.V. Additionally Spearman correlation (ρ) was applied to identify associations between nutrients and climate variables (precipitation and temperature).
MRT (expressed in years) of C and nutrients were calculated according to Wilcke et al. (2002) by the quotient of the respective element stocks (kg ha−1) in the organic layer and the rate of element return by the needle litterfall (kg ha−1 year−1). Since the element stocks in the organic layers are the result of the contribution not only from needles but also from other plant organs (e.g. reproductive organs, twigs, barks, etc.), a correction factor was used to estimate MRT of C and nutrients. This factor was calculated on the basis of data from other pine and alder forests in the same study region (unpublished data from the authors) and represent the percentage of nutrient contribution by the needles to the total nutrient stocks of litterfall (based on proportions of each type of litter that contribute to the total mass of litterfall). For the different elements the following factors were applied for pine: 0.67, 0.77, 0.55, 0.73, 0.49, 0.69, 0.59, 0.81, 0.26 and 0.61 for C, N, P, K, Ca, Mg, S, Mn, Cu and Zn, respectively. Whereas for alder, the factors were: 0.55, 0.59, 0.43, 0.59, 0.48, 0.61, 0.54, 0.73, 0.33, 0.41 for C, N, P, K, Ca, Mg, S, Mn, Cu and Zn, respectively.
Non-parametric tests were applied due to data did not fulfill the assumptions of normality (Shapiro–Wilk test, p < 0.05). Differences in soil biochemical properties between alder and pine sites were evaluated by the Mann–Whitney (M–W) test (α = 0.05), and differences between soil mineral layers by the non-parametric Kruskal–Wallis (K–W) test (α = 0.05) with the post hoc Benjamini and Hochberg pairwise procedure (HB) (Benjamini and Hochberg 1995).
It must be mentioned that soil MBN concentrations were only evaluated for the topsoil mineral layer, due to unavailable data for the forest floor.
All statistical analyses were applied using R software (R Core Team 2018).
Results
Litterfall production and seasonality of its fractions
Total litterfall production in alder was 8.08 Mg ha−1 year−1. It was only half the amount of that in pine (16.3 Mg ha−1 year−1). Leaf-litterfall contributed with approximately 68% to the total litterfall in alder, whereas in pine its contribution was about 63% (Fig. 1a). According to the SL values, the seasonality of the total litterfall and its main fraction (leaf-litterfall) was similar between both species (Online resource 3) and the effect of site within the same tree species was minimum as we noted from their patterns in the Fig. 1b. For this reason, we conducted our analysis based on the differences between the forest species—showing mean values of the plots for each species in the subsequent results.
Annual litterfall and leaf-litterfall production for alder (A. acuminata) and pine (P. patula) plantations in the Andes of southern Ecuador (mean values, n = 2) (a) and seasonality of total litterfall and leaf litterfall (b) during the period of October 2013 to September 2014 (mean values, n = 2 and individual values for the four sites CAR and RUM with alder plantations and DOS and ZAM with pine plantations)
Seasonality of leaf-litterfall element nutrient characteristics
Element concentrations
Concentrations of all nutrients were higher in alder. Briefly, the N concentration in alder was more than three times higher compared with pine, while concentrations of P, Ca and S were more than two times higher (M–W test, p < 0.05) (Fig. 2). For the micronutrients, Cu was the element showing the highest difference in concentrations between the species (Fig. 2). Only C and Mn did not show differences (M–W test, p = 0.07 and 0.13, for C and Mn, respectively).
Temporal variation in monthly basis of nutrient concentrations and potential nutrient return (PNR) of nutrients in leaf litterfall in alder (A. acuminata) and pine (P. patula) plantations in the Andes of southern Ecuador during the period of October 2013 to September 2014. Mean nutrient concentration values are given based on the study period (12 months) for alder [\({\bar{\mathbf{x}}}_{{\mathbf{a}}}\)] and pine [\({\bar{\mathbf{x}}}_{{\mathbf{p}}}\)]. The seasonality of nutrient concentrations is based on the seasonality index [SL]. Leaf litterfall potential nutrient return is also presented on an annual cumulative basis for alder [PNRa] and pine [PNRp]. Mean residence times (years) for alder (MRTa) and pine (MRTp) are provided
C exhibited lowest seasonality compared with the other elements in both species (only Cu in alder presented a lower SL value than those of C, Fig. 2). From the macronutrients, Ca exhibited the highest seasonality in both species. In pine, the Ca seasonality was more than four times higher than that of alder (Fig. 2). In the group of micronutrients, Mn showed the highest SL value in alder, and Zn the highest in pine, whereas Cu showed the lowest SL in both species, particularly alder exhibited the lowest SL (Fig. 2). In general, pine exhibited the highest nutrient seasonality, in particular for macronutrients as can be noted from the average SL values of all nutrients.
Stoichiometric molar ratios
A higher seasonality of stoichiometric ratios were found in pine according to the average value of SL for all C:elements, N:elements and P:elements ratios (Online resource 4). In both species, ratios related with Ca and Mn (C:Ca, N:Ca, P:Ca, C:Mn, N:Mn and P:Mn) presented the highest seasonality for macronutrients and micronutrients, respectively (with the exception of alder, which presented a higher P:K ratio instead of the P:Ca ratio, see Online resource 4). In both species, C:P, N:P and P:S exhibited the broadest ratios for macronutrients and C:Cu, N:Cu and P:Cu for micronutrients. However, the seasonality for this group of ratios (accounting as the averaged SL) was about two-third lower than the group of the highest seasonality (Ca and Mn related ratios) (Online resource 4).
Potential nutrient return (PNR) in leaf-litterfall
Despite the lower annual leaf-litterfall of alder (Fig. 1b), this species showed a higher annual PNR compared to pine. In total 227 kg ha−1 year−1 of nutrients (sum of the total amount of the returned nutrients excluding C) were returned in alder vs 176 kg ha−1 year−1 in pine, via leaf-litterfall. Annual PNR of C by pine leaf-litterfall was 1.8 times higher than alder. In the group of macronutrients: N, K, and Ca exhibited the major differences between the species. The annual PNR of N and Ca were higher in alder, while that of K was higher in pine (Fig. 2). In the micronutrient group, Mn showed the highest PNR value and pine showed the largest return.
According to the average SL values, the seasonality of PNR was higher than that of nutrient concentrations. Between species, the average of SL values of PNR of all studied nutrients, was higher in alder than pine, however this difference is small (SL: 0.23 and 0.21, for alder and pine, respectively). For each species, the element seasonality varied. Briefly, in alder the highest SL value was observed for P, whereas highest SL was observed for Ca in pine; and the lowest was for K in alder and N in pine (Fig. 2). For micronutrients, alder in general presented a higher seasonality than pine, with Mn showing the maximum in alder, and Cu in pine (Fig. 2). C seasonality in both species was different (although this difference was small, see Fig. 2).
Mean residence time (MRT) of nutrients in the forest floor
Pine exhibited a higher MRT average value for all nutrients than alder (6.5 years and 5.8 years for pine and alder, respectively). However, for each nutrient the MRTs were different between species (Fig. 2). Pine showed about three times higher values of MRT for Cu and two times higher values of MRT for N and S. The MRT for C and P is about 2.5 years longer in pine than in alder plantations. Alder showed about three times higher MRT values for Mg, K and Mn and almost four times higher values for Ca (Fig. 2).
Soil biogeochemical properties
Soil element concentrations
SOC and nutrient concentrations in soil organic horizons were more variable than those of the mineral topsoil layers, according to the statistical differences (M–W test, α = 0.05, Online resource 1). In the organic horizons, SOC and nutrients’ concentrations were statistically different between species, alder showed higher concentrations for all nutrients but not for SOC (Online resource 5). In the mineral topsoil, SOC was not different between tree species for all layers, total N was different between species only for the top mineral layer (0–5 cm), Ca was similar only in the layer of 5–10 cm. The remaining macronutrients and extractable N forms showed significant differences between species in all mineral layers, with alder showing the highest concentrations (Online resource 5).
The effect of tree species along soil depth (mineral layers) evidenced in pine a decrease of SOC, N and S, and NH4+-N concentrations (K–W test, p < 0.05), and an increase of NO3−-N (K–W test, p < 0.05). In alder, concentrations of N, NO3−-N, NH4+-N, and Pi decreased along soil depth (K–W test, p < 0.05), while Po showed an increase especially from 5 to 20 cm of soil depth (Online resource 5).
Soil nutrient stocks
The total SOC stock was higher in pine than alder with consistently higher values in the forest floor and in the mineral topsoil layers (Table 1). N and forms of plant available N (NO3−-N + NH4+-N) were higher in Alder, especially the nitrate form (NO3−-N) (Table 1). Total P stocks were twice as high in the mineral topsoil in alder than in pine. Available and easily mineralized forms of P (Pi-NH4F and Po-NH4F) were also higher in alder, but differences become non-significant with increasing soil depth. Additionally, alder exhibited highest total stocks of the other nutrients (including the micronutrients), specifically those of the cations (K, Ca and Mg); particularly Ca was the highest with approximately four times higher amounts than in the forest floor and mineral topsoil of pine stands (Table 1). The effect of tree species along soil mineral layers evidenced that alder showed the higher stocks of nutrients in the first mineral layers (0–10 cm) compared with the layer of 10–20 cm. While for pine higher stocks of nutrients (with the exception of SOC and N) were in the layer of 10–20 cm (Table 1).
Soil stoichiometric ratios
For most of stoichiometric ratios, pine exhibited broader ratios than alder, specifically for all C:elements ratios (Online resource 6). However in the group of N:elements, alder exhibited broader values than pine for N:P, N:K, N:S and N:Cu (and additionally P:S, and P:Cu). Ratios related with Ca resulted in the broadest ratios in the group of macronutrients, while Cu was for micronutrients (Online resource 6).
Soil microorganisms: biomass and activity
Microbial biomass C and P concentrations for the total soil (forest floor + mineral topsoil 0–20 cm) were higher in pine. A high proportion of microbial biomass was found in the forest floor compared to mineral soil, with approximately five and three times higher MBC and MBP concentrations, respectively. However, MBC and MBP stocks in the total soil (forest floor + mineral topsoil 0–20 cm) were similar at both sites (MBC: 0.39 Mg ha−1 and 0.41 Mg ha−1 for alder and pine respectively; MBP: 0.041 Mg ha−1 and 0.038 Mg ha−1, for alder and pine, respectively). In contrast, MBN (mineral topsoil) in alder was about two times higher than in pine. In general, MBC:MBN and MBC:MBP ratios (mineral topsoil) were lower in alder than pine, but MBN:MBP ratios were higher in alder (Table 2).
A fast C mineralization rate was observed in alder (approximately 1.4 times faster than pine) in the total soil (forest floor + mineral topsoil 0–20 cm). Net N mineralization rate was approximately six times faster in alder than pine, especially in the organic horizons. Nitrification processes dominated over those of ammonification in alder, whereas in pine the opposite was observed (Table 2).
Discussion
Litterfall dynamics
Our study reports amounts of litterfall and litter characteristics of pine and alder plantations in the tropical Andes, which is for this alder species, to our knowledge, the first dataset. The mean litterfall value for alder reported in our study (8.08 Mg ha−1 year−1) is close to the global average value for forests of tropical regions. It is comparable to amounts reported by Liu et al. (2004) for Eurasian tropical broadleaf forests, 8.52 Mg ha−1 year−1, but higher than the amount of 7.06 Mg ha−1 year−1 mentioned by Chave et al. (2010) for a tropical montane deciduous forest in South America. In comparison with data of alders in mixed forest areas close to the tropics (Sharma and Ambasht 1987; Sharma 2002; Wang et al. 2007; Tripathi et al. 2009) our estimations are higher. In contrast, litterfall production for pines has been widely studied from boreal to tropical regions (Quichimbo et al. 2016). Our results show amounts considerably higher (16.3 Mg ha−1 year−1) than that reported by Ramírez et al. (2014) for the same pine species but of higher stand age (48 year-old plantations) in a tropical montane forest region in Colombia (7.77 Mg ha−1 year−1). The latter was close to the mean value for conifers in other tropical regions (5.01 Mg ha−1 year−1, Liu et al. 2004). It is likely that there is a decrease in litterfall production due to an increase of the stand age (Delzon and Loustau 2005). However, high amounts of litterfall have also been reported in the tropics—e.g. 14.3 Mg ha−1 year−1, in P. caribaea (Cuevas and Lugo 1998); 14.8 Mg ha−1, in a tropical secondary forest (Zhu et al. 2019). Despite the observation that montane forest ecosystems are characterized by a lower litterfall production than lowland tropical ecosystems (Chave et al. 2010; Zhang et al. 2014). However, litterfall productivity is higher in sites of high soil fertility (Berg and Laskowski 2005); in this regard, pine plantations in this research are producing high amounts of litterfall comparable to those of lowlands, this could be explained by the higher soil fertility of the soils in the study area (young soils of low weathering, dominantly Umbrisols, see Quichimbo et al. 2017) compared with the more intensively weathered soils of lowlands (Quesada et al. 2010).
The seasonality of the litterfall and leaf-litterfall was highly associated to the period of dryness. According to the calculations by Oñate-Valdivieso et al. (2018) there is a period of 7 months (from May to November) that corresponds to the dry season in the study area, where 42% of the total annual precipitation falls (August and September being the driest months, see Online resource 1). 66% of the total litterfall and leaf-litterfall production for both species were observed within this long lasting dry period leading to low seasonality values (mean SL = 0.19 for the total litterfall and 0.16 for the leaf-litterfall) as also found by Chave et al. (2010) for tropical South American forests (mean SL: 0.17). In accordance with a study by Zhang et al. (2014) in tropical forests, a high litterfall production occurs in dry seasons as a consequence of moisture stress.
One limitation of our research could be the duration of the litterfall monitoring, since seasons (dry or rainy) could be influenced by shifts in climate and also the litterfall production could be affected by the plantation’s age, and therefore longer periods of litterfall monitoring including forest stands of different ages could be necessary for a better understanding of the litterfall dynamics. However, our results could be a good starting point for long-term research in montane forest ecosystems in the tropical Andes.
Temporal variation of nutrients in leaf-litterfall
In our study the seasonal patterns of nutrient concentrations and stoichiometric ratios varied between both species which could be mainly due to the environmental factors of precipitation and temperature as mentioned by Liu et al. (2004). In the study area, precipitation showed a higher variation than temperature (C.V.: 41% and 3%, for precipitation and temperature, respectively, see Online resource 1). Therefore, in our study the seasonality of litterfall and associated nutrients depends on precipitation. However, the effect of precipitation is differently acting on the studied species. For pine the effect is stronger—by controlling the nutrient variability—compared to alder, as can be demonstrated by the higher correlation of the main nutrients (macronutrients) with precipitation (average values of coefficient of spearman, ρ: 0.29 and 0.18, for pine and alder respectively). This suggests that pine productivity is more vulnerable to precipitation changes than that of alder. The highest seasonality was observed for Ca concentrations and Ca related stoichiometric ratios. This high seasonality is linked to the rainy season in the study area—(e.g. Ca most correlated with precipitation, ρ: 0.50, also see Online resource 1 and Fig. 2). This can be explained by the higher demand of Ca (aside from other macronutrients like N and P) by trees to increase their biomass during the rainy season (Feng et al. 2012). Ca movement is unidirectional, once it reaches the leaf-tissues forced by transpiration, it is hardly translocated to other organs due to its immobile nature in phloem (Kumar et al. 2015). Thus, Ca has a lower nutrient resorption efficiency compared to the other macronutrients (Vergutz et al. 2012), for instance in the pine sites under study (DOS and ZAM), the average value of resorption efficiency for N and P were 9% and 5%, respectively, while that of Ca was negligible (unpublished data from the authors). Therefore, large amounts of Ca can be returned to the soil via litterfall (leaf-litterfall with highest concentrations of Ca, see Fig. 2)—associated to months of high precipitation (Online resource 1). The temporal variation of Ca concentrations was higher in pine. This influences the seasonality of the stoichiometric ratios of this element with the others. Therefore, an environment of plant nutrient imbalance can occur, which was more pronounced in the stoichiometric ratios in pine than in alder.
However, it is widely known that N and P are the most limiting nutrients for vegetal growth across the world (Čapek et al. 2018); in the studied species, N:P ratio was one of the broadest ratios but exhibited a lower seasonality than ratios related with Ca and Mn (see Sect. 3.2.2). The temporal dynamics of N and P in litterfall are more affected by the tree physiology (before falling leaves) affecting their stoichiometric ratios, because N and P are previously reabsorbed to a higher degree than the other plant nutrients (Vergutz et al. 2012). However, clear differences in stoichiometry of these nutrients appear between species. In alder the higher N:P ratio indicates that alder is reabsorbing less N compared to pine. Killingbeck (1993) emphasizes the inefficiency of N resorption of actinorhizal tree species hosting the N-fixing bacteria of the genus Frankia (e.g. Alnus species). N is less conserved in leaves during senescence, because this nutrient is readily available for trees by the biochemical fixation.
Dynamic of nutrients between leaf-litterfall and forest floor
Despite the higher mass of litterfall produced by pines, a higher return of macronutrients (N, P, Ca and S) by alder was shown. Cuevas and Lugo (1998) described the lowest nutrient return by pine (Pinus caribaea) under a comparison of several tropical exotic and native species indicating a high resorption potential or a lower uptake from soil. The high return (especially of N and P) by alder species compared to conifers has also been pointed out by other studies (Radwan et al. 1984; Binkley et al. 1992; Wang et al. 2007; Tripathi et al. 2009), but our values are higher.
This high nutrient return by alder can be a function of the higher leaf-litterfall biomass produced in our study area rather than other factors like alder species, latitude and quality of leaf-litterfall. For example in a study with Alnus rubra growing in a temperate latitude in North America (Radwan et al. 1984), concentrations of nutrients in the leaf-litterfall were similar to those of our study, but their results of nutrient returns were lower, although Ca had a higher leaf concentration than in our study.
In the case of pine, under a comparison with the unique reported reference for pines in a tropical montane forest ecosystem region in the Andes (Colombia) (Ramírez et al. 2014), nutrient return was lower in their study. This is a consequence of a lower amount of litterfall together with lower nutrient concentrations.
The return of base cations, particularly K and Ca showed contrasting trends between species. Calcium showed a higher return in alder, whereas K in pine. The Ca demand of pines seems to be different than that of alders. The normal range of foliar Ca concentration starts from 2 mg g−1 for Pinus sylvestris (Mellert and Göttlein 2012), and 1.5 mg g−1 for A. acuminata (Alvarado et al. 2015). However, the width of the normal range for this nutrient concentration is shorter in pine (1.9 mg g−1) than in alder (12.5 mg g−1). This can lead to a lower intake of Ca by pine compared to alder. Additionally, the water use capacities of trees can contribute to the differences in Ca accumulation in leaves. Alder can be a stronger accumulator of Ca than pine, since alder species have a weak transpiration-control (as a water-demanding species) (Claessens et al. 2010). Transpiration is the mechanism that allows the upward movement of Ca in trees (Hanger 1979; Kumar et al. 2015). On the other hand for K, an opposite situation was denoted related to the nutrient demand by trees, since pine is more demanding in K than alder (Mellert and Göttlein 2012; Alvarado et al. 2015), explaining the higher potential K return by leaf-litterfall.
Despite the lowest return of nutrients in pine, the coupling to longest residence times of nutrients, especially those related to organic matter (N, P, and S), indicates a slower nutrient cycling process for this exotic species under tropical Andean environments. Although comparative studies with alder and pine have not been carried out in the tropics, this fast cycling of nutrients under alder has been evidenced in other regions comparing other alder species with conifers—both in individual plantations and mixed forests, largely explained by the lower C:N ratios of the alder leaf-litterfall (Binkley et al. 1992; Lavery et al. 2004; Wang et al. 2007; Tripathi et al. 2009).
Relationships between soil microorganisms and C, N, and P dynamics
Between alder and pine forests, the quality and quantity of litterfall material is affecting the soil characteristics. Our study shows a slower nutrient cycling of the organic matter in pine plantations as indicated by higher stocks of C and N in the forest floor, higher residence times of those elements in the forest floor, and lower mineralization rates of the soil organic matter. The N cycle in pine stands is dominated by ammonification processes, releasing more ammonia potentially available for tree growth. This can be noted by the higher stocks of NH4+-N in the pine soil (forest floor + mineral topsoil) The NH4+-N:NO3−-N ratio in pine is 2.5 times higher than in alder indicating the preferential uptake of ammonium-nitrogen (Scholes and Nowicki 1998). In the case of alder, the normal range of foliar N is between 2.0 and 4.0% (Alvarado et al. 2015), whereas for pine (Pinus sylvestris) it is between 1.4 and 1.8% (Mellert and Göttlein 2012). Thus alder is more N demanding than pine, but due to its N2-fixing capacity by the bacterial genus Frankia—featured by its tolerance to acidic soils (Cronan 2018)—its susceptibility to N deficiency is of less importance than that of pine. Since the alder trees get significant amounts of N from the symbioses with Frankia the competition among nitrifying microbes and trees for the ammonium substrate is lower and nitrification increases. The higher plant available nitrogen (ammonium + nitrates) stocks in alder compared to pine can evidence this (Table 1).
The dynamic of P in the litterfall-soil system was similar to that of N in terms of PNR and MRT (higher PNR and lower MRT in alder than pine). Although the amounts returned by leaf-litterfall in both species were the lowest for P (compared to the other macronutrients), alder is less P-demanding than pine—according to their levels of foliar concentrations (Mellert and Göttlein 2012; Alvarado et al. 2015). Besides, alder is promoting the larger stocks of total P and plant available P (Pi-NH4F + Po-NH4F) by exhibiting higher returns of P via litterfall. The faster mineralization of organic matter, deduced from the lower resident time, is favored by the highest stocks of Ca (which is highly related to the decomposition of litter material Berg et al. 2017), thereby releasing potentially more nutrients for tree growth. All these features are contributing to the rapid ecosystem cycling of P in alder compared to that in pine.
In general, the use of alder as N-fixing forest species can promote a faster cycling of nutrients by the increment of forest biomass and an allocation of nutrients into tree organs such as leaves. Leaves return more nutrients—compared to pine needles—via litterfall. This in turn modifies the soil fertility by enhancing the availability of nutrients for tree growth. In zones with soils of low to medium forest productivity, as in the present study (Quichimbo et al. 2017) the better litter quality and mineralization rates of alder can be beneficial for underplanting with seedlings from other native species. This is of special importance under the N limiting conditions occurring in the study area (unpublished data from the authors). Although, both species might be used as an immediate and supporting step towards the reestablishment of forest composed of native species, which grow poorly on non-forested and degraded sites depending especially on their nutrient demands. However, it is most likely alder shows better preconditions as shelter tree species than pine on sites with low to medium site productivity conditions. Our findings are in line with Knoke et al. (2014), who mention the benefits of alder against pines in the context of ecological functions especially in the southern Andean region of Ecuador. Thus, alder can be a good alternative towards a faster rehabilitation/restoration of mixed forests in areas of abandoned pastures for establishing mixed plantations in the future. Nevertheless, further research is necessary to know if alders perform well under other site conditions.
Conclusions
Soil nutrient dynamics differ between the exotic and native studied species. Alder demonstrated faster nutrient dynamics than pine, mediated especially by the effects on soil microbial activity. Particularly with alders the nitrification rates are of remarkable importance to improve fertility of the soils of medium to low fertility. The nitrification rate in alder soils was even higher than those of the most fertile soils under pine stands with the highest productivity in the study area (1.7 times higher approximately, unpublished data from the authors). The higher PNR of nutrients by alder also demonstrates its capacity to improve soil fertility; in this sense alder trees are able to act as nutrient pump faster and more efficiently compared with pine. Seasonal variations in quantity and quality of litterfall material may create a different habitat heterogeneity in alder versus pine plantations. Thus, a broad set of ecological niches for various native tree species as well as diverse decomposer communities could be available. To decide which of the native tree species with higher ecological and/or economic stability (e.g. Cedrela montana, Tabebuia chrysantha, Cupania sp., Heliocarpus americanus, Isertia laevis, Myrica pubescens, Piptocoma discolor, and Cinchona officinalis Aguirre et al. 2006) should be planted under the shelter of pine versus alder further knowledge on the nutritional demands of these native tree species are necessary.
References
Aceñaloza PG, Gallardo-lancho JF (1994) Pérdida de peso seco en hojarasca de Alnus acuminata en la provincia de Tucumán (Argentina). Bosque 15:51–54
Aguirre N, Günter S, Weber M, Stimm B (2006) Enrichment of Pinus patula plantations with native species in southern Ecuador. Lyionia 10:33–45
Alvarado A (2016) Plant nutrition in tropical forestry. In: Pancel L, Köhl M (eds) Tropical forestry handbook. Springer, Berlin, pp 1113–1202
Alvarado A, Camacho ME, Fernández-Moya J et al (2015) Interpretación del análisis foliar de varias especies forestales latifoliadas del trópico americano. In: VIII Congreso Nacional de Suelos (Costa Rica). Asociación Costarricense de la Ciencia del Suelo, San José, pp 1–68
Bare MC, Ashton MS (2016) Growth of native tree species planted in montane reforestation projects in the Colombian and Ecuadorian Andes differs among site and species. New For 47:333–355. https://doi.org/10.1007/s11056-015-9519-z
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Berg B, Laskowski R (2005) Litter decomposition: a guide to carbon and nutrient turnover. Elsevier, Amsterdam
Berg B, McClaugherty C (2014) Plant litter: decomposition, humus formation, carbon sequestration, 3rd edn. Springer, Berlin
Berg B, Johansson MB, Liu C et al (2017) Calcium in decomposing foliar litter—a synthesis for boreal and temperate coniferous forests. For Ecol Manage 403:137–144. https://doi.org/10.1016/j.foreco.2017.08.022
Binkley D, Sollins P, Bell R et al (1992) Biogeochemistry of adjacent conifer and alder–conifer stands. Ecology 73:2022–2033
Bonnesoeur V, Locatelli B, Guariguata MR et al (2019) Impacts of forests and forestation on hydrological services in the Andes: a systematic review. For Ecol Manage 433:569–584. https://doi.org/10.1016/j.foreco.2018.11.033
Brandbyge J (1991) Reforestación de los Andes ecuatorianos con especies nativas. CESA-Intercooperation Suiza, Quito
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329. https://doi.org/10.1016/0038-0717(82)90001-3
Buytaert W, Iñiguez V, De Bièvre B (2007) The effects of afforestation and cultivation on water yield in the Andean páramo. For Ecol Manage 251:22–30. https://doi.org/10.1016/j.foreco.2007.06.035
Čapek P, Manzoni S, Kaštovská E et al (2018) A plant–microbe interaction framework explaining nutrient effects on primary production. Nat Ecol Evol 2:1588–1596. https://doi.org/10.1038/s41559-018-0662-8
Chacón G, Gagnon D, Paré D (2009) Comparison of soil properties of native forests, Pinus patula plantations and adjacent pastures in the Andean highlands of southern Ecuador: land use history or recent vegetation effects? Soil Use Manag 25:427–433. https://doi.org/10.1111/j.1475-2743.2009.00233.x
Chave J, Navarrete D, Almeida S et al (2010) Regional and seasonal patterns of litterfall in tropical South America. Biogeosciences 7:43–55. https://doi.org/10.5194/bg-7-43-2010
Chen GC, He ZL (2004) Determination of soil microbial biomass phosphorus in acid red soils from southern China. Biol Fertil Soils 39:446–451. https://doi.org/10.1007/s00374-004-0734-6
Claessens H, Oosterbaan A, Savill P, Rondeux J (2010) A review of the characteristics of black alder (Alnus glutinosa (L.) Gaertn.) and their implications for silvicultural practices. Forestry 83:163–175. https://doi.org/10.1093/forestry/cpp038
Cronan CS (2018) Ecosystem biogeochemistry: element cycling in the forest landscape. Springer, Cham
Cuevas E, Lugo AE (1998) Dynamics of organic matter and nutrient return from litterfall in stands of ten tropical tree plantation species. For Ecol Manage 112:263–279. https://doi.org/10.1016/S0378-1127(98)00410-1
Dahik CQ, Crespo P, Stimm B et al (2018) Contrasting stakeholders’ perceptions of pine plantations in the páramo ecosystem of Ecuador. Sustainability 10:1. https://doi.org/10.3390/su10061707
Delzon S, Loustau D (2005) Age-related decline in stand water use: sap flow and transpiration in a pine forest chronosequence. Agric For Meteorol 129:105–119. https://doi.org/10.1016/j.agrformet.2005.01.002
Dunn WW, Morgan P, Lynch AM (1990) Production of alder (Alnus jorullensis) to meet fuelwood demand in the Sierra of Ecuador. Agrofor Syst 10:199–211. https://doi.org/10.1007/BF00122912
Farley KA (2007) Grasslands to tree plantations: forest transition in the Andes of Ecuador. Ann Assoc Am Geogr 97:755–771
Farley KA, Kelly EF (2004) Effects of afforestation of a páramo grassland on soil nutrient status. For Ecol Manage 195:281–290. https://doi.org/10.1016/j.foreco.2003.12.015
Feng X, Vico G, Porporato A (2012) On the effects of seasonality on soil water balance and plant growth. Water Resour Res 48:1–12. https://doi.org/10.1029/2011WR011263
Günter S, Stimm B, Cabrera M, Diaz ML, Lojan M, Ordoñez E, Richter M, Weber M (2008) Tree phenology in montane forests of southern Ecuador can be explained by precipitation, radiation and photoperiodic control. J Trop Ecol 24(3):247–258
Günter S, Gonzalez P, Álvarez G et al (2009) Determinants for successful reforestation of abandoned pastures in the Andes: soil conditions and vegetation cover. For Ecol Manage 258:81–91. https://doi.org/10.1016/j.foreco.2009.03.042
Hamer U, Potthast K, Burneo JI, Makeschin F (2013) Nutrient stocks and phosphorus fractions in mountain soils of Southern Ecuador after conversion of forest to pasture. Biogeochemistry 112:495–510. https://doi.org/10.1007/s10533-012-9742-z
Hanger BC (1979) The movement of calcium in plants. Commun Soil Sci Plant Anal 10:171–193. https://doi.org/10.1080/00103627909366887
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30:357–363. https://doi.org/10.1016/j.tree.2015.03.015
Jacobs SR, Timbe E, Weeser B et al (2018) Assessment of hydrological pathways in East African montane catchments under different land use. Hydrol Earth Syst Sci 22:4981–5000. https://doi.org/10.5194/hess-22-4981-2018
Jiménez D, Aguirre N (2017) Generación de indicadores florísticos para el monitoreo de la restauración ecológica en áreas degradadas del bosque siempreverde montano de la cordillera oriental de los andes del sur (BSMN02). Bachelor thesis. Carrera de Ingeniería Forestal, Universidad Nacional de Loja
Kattan GH, Murcia C (2012) Ecological patterns and processes in noncommercial, monospecific tree plantations in the tropical Andes. In: Simonetti JA, Grez AA, Estades CF (eds) Biodiversity conservation in agroforestry landscapes: challenges and opportunities. Editorial Universitaria, Santiago, pp 131–144
Killingbeck KT (1993) Inefficient nitrogen resorption in genets of the actinorhizal nitrogen fixing shrub Comptonia peregrina: physiological ineptitude or evolutionary tradeoff? Oecologia 94:542–549. https://doi.org/10.1007/BF00566970
Kingston HM, Jassie LB (1986) Microwave energy for acid decomposition at elevated temperatures and pressures using biological and botanical samples. Anal Chem 58:2534–2541
Knoke T, Bendix J, Pohle P et al (2014) Afforestation or intense pasturing improve the ecological and economic value of abandoned tropical farmlands. Nat Commun 5:5612. https://doi.org/10.1038/ncomms6612
Knoke T, Paul C, Hildebrandt P et al (2016) Compositional diversity of rehabilitated tropical lands supports multiple ecosystem services and buffers uncertainties. Nat Commun 7:11877. https://doi.org/10.1038/ncomms11877
Kumar A, Singh UM, Manohar M, Gaur VS (2015) Calcium transport from source to sink: understanding the mechanism(s) of acquisition, translocation, and accumulation for crop biofortification. Acta Physiol Plant 37:1722
Lavery JM, Comeau PG, Prescott CE (2004) The influence of red alder patches on light, litterfall, and soil nutrients in adjacent conifer stands. Can J For Res 34:56–64. https://doi.org/10.1139/x03-194
Liu C, Westman CJ, Berg B et al (2004) Variation in litterfall-climate relationships between coniferous and broadleaf forests in Eurasia. Glob Ecol Biogeogr 13:105–114. https://doi.org/10.1111/j.1466-882X.2004.00072.x
Löf M, Madsen P, Metslaid M et al (2019) Restoring forests: regeneration and ecosystem function for the future. New For 50:139–151. https://doi.org/10.1007/s11056-019-09713-0
Mejía E, Pacheco P (2013) Aprovechamiento forestal y mercados de la madera en la Amazonía Ecuatoriana. CIFOR, Bogor
Mellert KH, Göttlein A (2012) Comparison of new foliar nutrient thresholds derived from van den Burg’s literature compilation with established central European references. Eur J For Res 131:1461–1472. https://doi.org/10.1007/s10342-012-0615-8
Middendorp RS, Pérez AJ, Molina A, Lambin EF (2016) The potential to restore native woody plant richness and composition in a reforesting landscape: a modeling approach in the Ecuadorian Andes. Landsc Ecol 31:1581–1599. https://doi.org/10.1007/s10980-016-0340-7
Ministerio del Ambiente de Ecuador (2006) Plan Nacional de Forestación y Reforestación. Subsecretaría de Capital Natural, Dirección Nacional Forestal, Ministerio del Ambiente de Ecuador, Quito
Morales-Hidalgo D, Oswalt SN, Somanathan E (2015) Status and trends in global primary forest, protected areas, and areas designated for conservation of biodiversity from the Global Forest Resources Assessment 2015. For Ecol Manage 352:68–77. https://doi.org/10.1016/j.foreco.2015.06.011
Mosandl R, Günter S, Stimm B, Weber M (2008) Ecuador suffers the highest deforestation rate in South America. In: Beck E, Bendix J, Kottke I et al (eds) Gradients in a tropical mountain ecosystem of Ecuador SE-4. Springer, Berlin, pp 37–40
Murcia C (1997) Evaluation of Andean alder as a catalyst for the recovery of tropical cloud forests in Colombia. For Ecol Manage 99:163–170. https://doi.org/10.1016/S0378-1127(97)00202-8
Ochoa-Cueva P, Fries A, Montesinos P et al (2013) Spatial estimation of soil erosion risk by land-cover change in the andes of southern Ecuador. Land Degrad Dev. https://doi.org/10.1002/ldr.2219
Oñate-Valdivieso F, Fries A, Mendoza K et al (2018) Temporal and spatial analysis of precipitation patterns in an Andean region of southern Ecuador using LAWR weather radar. Meteorol Atmos Phys 130:473–484. https://doi.org/10.1007/s00703-017-0535-8
Palomeque X, Günter S, Siddons D et al (2017) Natural or assisted succession as approach of forest recovery on abandoned lands with different land use history in the Andes of Southern Ecuador. New For 48:643–662. https://doi.org/10.1007/s11056-017-9590-8
Parsons SA, Valdez-Ramirez V, Congdon RA, Williams SE (2014) Contrasting patterns of litterfall seasonality and seasonal changes in litter decomposability in a tropical rainforest region. Biogeosciences 11:5047–5056. https://doi.org/10.5194/bg-11-5047-2014
Potthast K, Hamer U, Makeschin F (2010) Impact of litter quality on mineralization processes in managed and abandoned pasture soils in Southern Ecuador. Soil Biol Biochem 42:56–64. https://doi.org/10.1016/j.soilbio.2009.09.025
Potthast K, Hamer U, Makeschin F (2012) In an Ecuadorian pasture soil the growth of Setaria sphacelata, but not of soil microorganisms, is co-limited by N and P. Appl Soil Ecol 62:103–114. https://doi.org/10.1016/j.apsoil.2012.08.003
Proctor J (1983) Tropical forest litterfall. I. Problems of data comparison. In: Sutton SL, Whitmore TC, Chadwick AC (eds) Tropical rain forest: ecology and management. Blackwell Scientific Publications, Oxford, pp 267–273
Quesada CA, Lloyd J, Schwarz M et al (2010) Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences 7:1515–1541. https://doi.org/10.5194/bg-7-1515-2010
Quichimbo P, Tenorio G, Borja P et al (2012) Efectos sobre las propiedades físicas y químicas de los suelos por el cambio de la cobertura vegetal y uso del suelo: páramo de Quimsacocha al sur del Ecuador. Suelos Ecuat 42:138–153
Quichimbo P, Veintimilla D, Carrión Y, Jiménez L (2016) Litterfall production under pine plantations in the southern Andes region of Ecuador. Enfoque UTE 7:14–25
Quichimbo P, Jiménez L, Veintimilla D et al (2017) Forest site classification in the southern Andean region of Ecuador: a case study of pine plantations to collect a base of soil attributes. Forests 8:473. https://doi.org/10.3390/f8120473
R Core Team (2018) R: a language and environment for statistical computing. R version 3.4.4. 51
Radwan MA, Harrington CA, Kraft JM (1984) Litterfall and nutrient returns in red alder stands in western Washington. Plant Soil 79:343–351. https://doi.org/10.1007/BF02184327
Ramírez JA, León-Peláez JD, Craven D et al (2014) Effects on nutrient cycling of conifer restoration in a degraded tropical montane forest. Plant Soil 378:215–226. https://doi.org/10.1007/s11104-014-2024-x
Scholes MC, Nowicki TE (1998) Effects of pines on soil properties and processes. In: Richardson DM (ed) Ecology and biogeography of Pinus. Cambridge University Press, Cambridge, pp 341–353
Sharma G (2002) Performance of an age series of Alnus-Cardamom plantations in the Sikkim Himalaya: nutrient dynamics. Ann Bot 89:273–282. https://doi.org/10.1093/aob/mcf036
Sharma E, Ambasht RS (1987) Litterfall, decomposition and nutrient release in an age sequence of Alnus nepalensis plantation stands in the eastern Himalaya. J Ecol 75:997–1010
Silva LN, Freer-Smith P, Madsen P (2019) Production, restoration, mitigation: a new generation of plantations. New For 50:153–168. https://doi.org/10.1007/s11056-018-9644-6
Tapia-Armijos MF, Homeier J, Espinosa CI et al (2015) Deforestation and forest fragmentation in south Ecuador since the 1970s—losing a hotspot of biodiversity. PLoS ONE. https://doi.org/10.1371/journal.pone.0133701
Tischer A, Potthast K, Hamer U (2014) Land-use and soil depth affect resource and microbial stoichiometry in a tropical mountain rainforest region of southern Ecuador. Oecologia 175:375–393. https://doi.org/10.1007/s00442-014-2894-x
Tischer A, Blagodatskaya E, Hamer U (2015) Microbial community structure and resource availability drive the catalytic efficiency of soil enzymes under land-use change conditions. Soil Biol Biochem 89:226–237. https://doi.org/10.1016/j.soilbio.2015.07.011
Tripathi O, Pandey H, Tripathi R (2009) Litter production, decomposition and physico-chemical properties of soil in 3 developed agroforestry systems of Meghalaya, Northeast India. Afr J Plant Sci 3:160–167
van Voss O, Aguirre N, Hofstede R (2001) Sistemas forestales integrales para la sierra del Ecuador. ABYA-YALA, Quito
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Vergutz L, Manzoni S, Porporato A et al (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220. https://doi.org/10.1890/11-0416.1
Wang Q, Wang S, Fan B, Yu X (2007) Litter production, leaf litter decomposition and nutrient return in Cunninghamia lanceolata plantations in south China: effect of planting conifers with broadleaved species. Plant Soil 297:201–211. https://doi.org/10.1007/s11104-007-9333-2
Weber M, Stimm B, Mosandl R (2011) Review plantations for protective purposes and rehabilitation. In: Günter S, Weber M, Stimm B, Mosandl R (eds) Silviculture in the tropics SE-30. Springer, Berlin, pp 475–490
Weng C, Bush MB, Chepstow-Lusty AJ (2004) Holocene changes of Andean alder (Alnus acuminata) in highland Ecuador and Peru. J Quat Sci 19:685–691. https://doi.org/10.1002/jqs.882
Wilcke W, Yasin S, Abramowski U et al (2002) Nutrient storage and turnover in organic layers under tropical montane rain forest in Ecuador. Eur J Soil Sci 53:15–27. https://doi.org/10.1046/j.1365-2389.2002.00411.x
Zhang H, Yuan W, Dong W, Liu S (2014) Seasonal patterns of litterfall in forest ecosystem worldwide. Ecol Complex 20:240–247. https://doi.org/10.1016/j.ecocom.2014.01.003
Zhu X, Liu W, Chen H et al (2019) Effects of forest transition on litterfall, standing litter and related nutrient returns: implications for forest management in tropical China. Geoderma 333:123–134. https://doi.org/10.1016/j.geoderma.2018.07.023
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (Project DFG RU 816/2-T1, HA 4597/4-1) for financial support. Partial funding was given by the UTPL, SENESCYT and Universidad de Cuenca.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Quichimbo, P., Jiménez, L., Veintimilla, D. et al. Nutrient dynamics in an Andean forest region: a case study of exotic and native species plantations in southern Ecuador. New Forests 51, 313–334 (2020). https://doi.org/10.1007/s11056-019-09734-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-019-09734-9