Abstract
Aims
Selenium (Se) has been reported to mitigate the harmful effects of salt stress on plants; however, the internal mechanisms remain unknown. Here, the effects of Se supplementation on tomato plants under salt stress were investigated.
Methods
The biomass, relative electrical conductivity (REC), relative water content (RWC),
photosynthetic parameter, inorganic ion contents, malondialdehyde (MDA), soluble sugar and proline contents, as well as the regulation of plant hormones of Se application in tomato plants were investigated after exposure to Se and salt stress treatments.
Results
Exogenous Se application improved photosynthesis and the water use efficiency (WUE) of tomato plants under salt stress, thereby promoting the growth of tomato plants under salt stress. Se supplementation also maintained the K+ and Na+ homeostasis, reduced the REC, decreased MDA, H2O2 and O2•− contents, and mitigated the oxidative damage caused by salt stress. In addition, exogenous Se increased the salicylic acid (SA) content in tomato leaves and roots via up-regulating the PAL or ICS pathways involved in SA biosynthesis. After pre-treatment with the SA inhibitor 1-aminobenzotriazole, the photosynthetic efficiency of tomato plants decreased, plant growth was weakened, and the REC was increased, indicating that the alleviating role of Se on salt stress was abolished.
Conclusions
Our results clarified the roles of Se and its regulation mechanisms in plant salt stress tolerance and the critical involvement of SA in this process. The study of Se in plant abiotic stress tolerance will give a more theoretical foundation for using exogenous Se in agricultural production to enhance crop growth and yield under adversity stresses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity is a major abiotic stress that severely restricts plant growth and crop yield (Soltabayeva et al. 2021; Shrivastava and Kumar 2015), which has been exacerbated by poor irrigation practices, rising population, and industrial pollution (Yu et al. 2020). Currently, 20% of the world’s arable land and 50% of the irrigated areas are significantly affected by extreme salt stress (Wichern et al. 2020). Salt stress leads to ionic toxicity and osmotic and oxidative stress, which affect many plant physiological and biochemical processes, including photosynthesis, energy metabolism, and water and nutrient uptake (Yang et al. 2020). Therefore, plants have developed strategies to survive in highly saline environments, such as ion homeostasis and compartmentalization, biosynthesis of osmoprotectants, activation of antioxidant compounds, and regulation of several stress-responsive genes (Gupta and Huang 2014). However, applying exogenous substances (melatonin, silicon, brassinosteroids, polyamine, and selenium (Se), etc.) may be a promising strategy to improve plant salt tolerance effectively, saving time and effort (Rady et al. 2020).
Se is an essential trace element required by humans and animals (Gupta and Gupta 2017). Adequate daily Se supplementation is essential for many physiological functions in the human body, such as preventing cardiovascular diseases and type 2 diabetes, regulating immunity, and reducing cancer risk (Rayman 2012). Since plants are the primary source of dietary Se, studies on Se compounds in plants are essential (Zhu et al. 2018). Selenate (SeO42−) and selenite (SeO32−) are the main forms of Se available to plants (Gupta and Gupta 2017). Although there is no direct evidence that Se is essential for higher plants, recent studies have shown that low concentrations of Se could influence plant growth and physiological processes, such as enhancing photosynthesis (Borbély et al. 2021) and root and shoot growth (Diao et al. 2014), promoting germination and nitrogen assimilation, increasing starch accumulation, and delaying senescence (Hajiboland et al. 2019). Other studies have reported that Se could mitigate the adverse impacts on plants of different environmental stresses, e.g., salt, drought, high temperature, and heavy metals (Elkelish et al. 2019; Rady et al. 2020). Jiang et al. (2017) reported that Se enhanced the plant growth index of maize under salt stress. Other studies have shown that Se could protect plants against severe stresses by improving the antioxidant capacity, increasing the photosynthetic indices, and promoting secondary metabolism (Elkelish et al. 2019; Zsiros et al. 2019). However, much research remains to be done to explore the impacts of exogenous Se supplementation on plants in response to salinity.
Previous studies have shown that phytohormones played essential roles in Se-enhanced tolerance of abiotic stresses, such as abscisic acid (Zahedi et al. 2019), auxin (Jia et al. 2018), jasmonic acid, and ethylene (Tamaoki et al. 2008). However, it is unknown whether salicylic acid (SA) is involved in the Se-induced salt tolerance of plants. SA is an endogenous plant hormone that is crucial in regulating physiological and developmental processes in plants, including growth, maturity, senescence, photosynthesis, and transpiration (Koo et al. 2020). SA also serves as a signaling molecule, triggering allergic reactions and inducing stress resistance of plants to salt, drought, heat, heat, cold, and heavy metals (La et al. 2019; Wassie et al. 2020). Khalvandi et al. (2021) found that 0.5 mM SA treatment could improve photosynthetic performance, maintain membrane permeability, induce stress-response-related proteins, and increase antioxidant enzyme activity, effectively alleviating the negative impacts caused by drought stress on winter wheat growth. Ardebili et al. (2014) found that a combination of Se and SA mitigated the effects of salt stress in soybean more successfully than either Se or SA alone. Although Se or SA can improve salt stress tolerance, their interactions during salt stress are unknown. Therefore, the underlying mechanisms of Se-enhanced plant tolerance under salt stress, and the impact of plant hormones like SA, should be investigated.
Cultivated tomato (Solanum lycopersicum L.), one of the most popular vegetable fruit crops in the world, has moderate sensitivity to soil salinity (Fan et al. 2021). Sustainable approaches are needed to enhance the growth and production of tomato plants under salinity. Previous studies reported that Se treatment promoted seed germination and plant growth of salt-stressed tomato plants and improved the nutritional quality of tomato fruit (Zhu et al. 2018). However, the precise mechanism through which exogenous Se improves the salt stress resistance of tomatoes remains unclear. This study investigated the Se involvement in plant regulatory mechanisms under salt stress by analyzing the photosynthesis, plant growth indicators, chloroplast antioxidant systems, and plant hormone regulation following Se application. Our findings will clarify the mechanisms underlying Se’s ability to enhance salt resistance in tomato plants and provide theoretical support for using Se fertilizer for agricultural production in high-salinity areas.
Materials and methods
Plant materials and treatment
The experimental materials were tomato (Solanum lycopersicum L.) cultivar "Ailsa Craig”. Plump and uniform seeds were sterilized by soaking in deionized water for 15 min at 55 °C, then germinated for 2 days on wet filter paper in a growth chamber without light at 28℃. The germinated seeds were planted in the medium in the greenhouse with a day/night temperature of 25℃/18℃. At the three-leaf stage, the seedlings were moved to the half-strength modified Hoagland solution (Gou et al. 2020a), which was aerated for 10 min every 3 h. The culture solution pH was adjusted to 6.2 and changed every 3 days. Five days later, the seedlings were subjected to Se or salt stress treatment by adding 25 μM sodium selenite (Na2SeO3) or 150 mM sodium chloride (NaCl) to the nutrient solution. Among the different Se doses tested, supplementation with 25 μM Se had the most noticeable effect in relieving salt stress damage in our early pre-experiment (data not shown). These experiments include four different treatments: (1) CK: no Se and no NaCl; (2) Se: 25 μM Na2SeO3; (3) NaCl: 150 mM NaCl; (4) NaCl + Se: 150 mM NaCl + 25 μM Na2SeO3. After 15 days of salt stress, the fully expanded leaves and roots of tomato seedlings were wrapped in tin foil and preserved at -80℃ after being frozen in liquid nitrogen.
The biomass, root morphological traits, relative electrical conductivity (REC), and relative water content (RWC)
A straightedge and a vernier caliper were used to measure the plant height and stem diameter of six randomly picked plants. The tomato seedlings were divided into above-ground and roots, and their fresh weight (FW) was assessed. Then the plants were dried at 105 °C for 15 min, stayed at 75 °C for 1 d, and weighed to determine the plant dry weight (DW). Total root length, root surface area, root volume and average root diameter were investigated by a dual lens system (V700. EPSON, Japan).
The REC of tomato leaves was measured as follows: the fully expanded third leaves were taken and cut into pieces. Weigh 0.1 g sample and put it into a centrifuge tube containing 20 ml of deionized water, shaken for 2 h and its electrical conductivity was measured (EC1). The sample was then boiled for 10 min, cooled and measured for electrical conductivity (EC2). The REC was calculated with the formula: REC (%) = EC1⁄EC2*100. The RWC of leaves was measured as described by Fan et al. (2022).
Measurement of photosynthetic pigments
The contents of chlorophyll and carotenoid were determined in fresh weight (FW) of leaves by sample homogenization with 95% (v/v) ethanol. The absorbance was measured at 665 nm for chlorophyll a, 649 nm for chlorophyll b, and 470 nm for carotenoids using an ultraviolet spectrophotometer (UV-2450, Shimadzu, Japan). Chlorophyll and carotenoid contents were calculated as described by Arnon (1949).
Measurement of photosynthetic parameters and chlorophyll fluorescence parameters
The second fully expanded leaves of tomato plants were used to determine the photosynthetic parameters using the Li-6800 portable photosynthesis system (LI-COR, Lincoln, USA). The formula: WUE = Pn/Tr was used to calculate the instantaneous water use efficiency (WUE). The efficiency of PSII (Fv/Fm) and quantum yield of PSII (ΦPSII) were determined in fully expanded intact leaves using a PAM chlorophyll fluorometer (Heinz Walz, GmbH, Effeltrich, Germany).
Determination of Se, K, Na, and Cl contents
Plant tissues were dried for 3 days under 80 °C in an oven and ground into powder. 0.2 g sample was weighted and placed in a digestion tube containing 5 mL extracting solution (nitric acid: perchloric acid = 4:1). After incubation overnight, the solution was first digested at 120 °C in the metal bath for 2 h, and then warmed up to 170 °C. The solution was heated until the remaining volume is about 2 mL, cooled, and diluted to 30 mL. 5 mL of the solution was taken out, added the same volume of 6 mol/L hydrochloric acid, reaction at 100 °C for 30 min. After making the blank control and Se standard curve, an atomic fluorescence spectrometer (LC-AFS-8530, China) was applied to determine the Se content. To measure the content of K+ and Na+, the oven-dried plant shoots and roots were digested in 5 mL HNO3–H2O2 solution and heated at 250 °C. The concentration of K+ and Na+ was determined by flame spectrophotometry (M410, Sherwood, Britain). The Cl− content was determined with silver nitrate titration as described by Zhang et al. (2011).
The malondialdehyde (MDA), soluble sugar and proline content
The MDA content was measured using thiobarbituric acid reaction according to Gou et al. (2020a). According to Fan et al. (2022), the soluble sugars were determined with the sulphate ketone colorimetry method.
The proline was measured as described by Shi et al. (2016). 0.1 g of sample was added with 3 mL of 3% sulfosalicylic acid, incubated for 10 min in a boiling water bath, then cooled down and centrifuged at 6,000 g for 10 min. After that, 1 ml of the supernatant was collected and added with 4 mL extraction buffer (distilled water: glacial acetic acid: acidic ninhydrin = 1:1:2). The mixture solution was boiled for 1 h and cooled down. After that, 2 mL of toluene was added and stratification was carried out for 2 h. After full extraction, the red toluene phase was taken for colorimetric analysis at 520 nm. The proline content was calculated based on a standard curve.
Quantification and visualization of reactive oxygen species (ROS)
The hydrogen peroxide (H2O2) content was assayed with a fluorescent dye 2,7-dichlorofluorescin diacetate as described by Han et al. (2020). The superoxide anion (O2•−) content was determined using the sulphanilic acid and α -naphthylamine solution as described by Shi et al. (2014). The visualization of H2O2 and O2•− accumulation was performed with diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) solution as reported by Zhu et al. (2020).
Antioxidant enzymes activity assays
The weight of 0.3 g of frozen material was ground into homogenate with 4 mL ice-cold sodium phosphate buffer (100 mM, pH 6.8). The homogenate was centrifuged under 12,000 g at 4 °C for 20 min, and the supernatant was collected to determine the antioxidant enzymes activity (Gou et al. 2020b).
Plant hormone content
The weight of the 0.1 g of frozen material was ground into powder with liquid nitrogen, then mixed thoroughly with 2 mL of the extraction buffer (isopropanol: sterile water: HCl = 2:1:0.002) and incubated at 4℃ overnight. The supernatant was obtained by centrifuging the solution at 7000 g for 12 min at 4 °C. The supernatant was collected, added with 2 mL of dichloromethane and shaked in an oscillation incubator at 4 °C for 1 h, and centrifuged at 7000 rpm for 10 min at 4 °C. Then, 2 mL of the lower fraction was taken, blew dry with nitrogen, added with 100 μL methanol and filtered through a 0.22 μm microporous membrane. The liquid chromatography (QTRAP5500, AB, USA) was applied to determine the content of various phytohormones.
Analysis of gene expression
Total RNA was isolated and purified using Trizol Reagent (Omega, Norcross, USA), and first-strand cDNA was generated with HiScript® II Q RT SuperMix for qPCR kit (Vazyme, Nanjing, China). The gene expression analysis was performed on a QuantStudio®5 Real-Time PCR System (ABI, Carlsbad, CA) using SYBR Premix Ex TaqTM kit (Takara). The specific primers were listed in Table S1. The GAPDH (GenBank: NM_001321306) gene was selected as internal control.
SA biosynthesis inhibitor treatment
Two days in advance, the four-leaf stage seedings were pre-sprayed with a salicylic acid biosynthesis inhibitor ABT (1-aminobenzotriazole, Yang et al. 2018), followed by the addition of Se and NaCl. The experiment included four treatments: (1) CK; (2) CK + 100 μM ABT; (3) 150 mM NaCl; (4) 150 mM NaCl + 100 μM ABT; (5) 150 mM NaCl + 25 μM Se; and (6) 150 mM NaCl + 25 μM Se + 100 μM ABT. The PH of culture solution was adjusted to 6.2 and changed every 3 days.
Statistical analysis
The data was analyzed using SPSS 19.0 software (IBM, Armonk, NY). Data are means ± SD (n = 3) calculated with three biological replicate samples. One-way analysis of variance (ANOVA) and Duncan’s multiple range test or t-test in Excel (2010) were performed for comparison of significant differences between treatments (P < 0.05). All the experiments were repeated at least three times.
Results
Effects of Se on plant growth parameters under salt stress
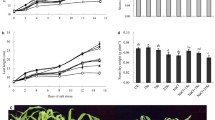
In order to investigate the function of Se on plant growth and development under salt stress, tomato seedlings were applied with 150 mM NaCl and exogenous Se to evaluate the salt resistance of various treatment groups (Fig. 1, Table 1). Under normal conditions, Se supplementation significantly increased plant height, but did not change the stem diameter or the fresh and dry weights of tomato shoots and roots (Table 1). Compared with the controls, salt stress treatment for 15 d significantly reduced plant height and stem diameter. Compared with the seedlings without Se treatment, the plant heights and stem diameters of Se-treated tomato seedlings significantly increased by 17.7 and 23.0%, respectively, and the fresh weight of shoots by 13.9%, under salt stress treatment (Table 1). The shoot dry weights were also higher in the Se-treated tomato seedlings under salt stress, although these findings were not significant (Table 1). Moreover, the tomato seedlings without Se treatment showed obvious wilting and petiole drooping, whereas the Se-treated plants did not exhibit apparent symptoms of salt damage (Fig. 1A). In addition, under normal and salt stress conditions, Se treatment significantly promoted tomato root growth (Fig. 1B, Table 1). Under normal conditions, the total root surface area, total root volume, and average root diameter of Se-treated tomato plants increased by 22.3, 43.5, and 58.6%, respectively, compared to control plants without Se addition (Table 1). Under salt stress, Se treatment increased the total root length, total root volume, and average root diameter of tomato plants by 29.1, 39.0, and 28.6%, respectively, compared to plants without Se treatment (Table 1).
Influence of exogenous Se on tomato plant growth and development under salt stress treatment with/without Se supplementation for 15 d. (A) Plant phenotype observation. (B) The root morphology of tomato seedlings. (C) The relative water content (RWC) of tomato seedlings. (D) The relative electrical conductivity (REC) of tomato seedlings. CK, control; Se, selenium; NaCl: salt treatment; NaCl + Se: salt + selenium treatment. Data are presented as mean ± SD of six biological replicates. Different small letters indicate significant differences between treatment groups at P < 0.05
The RWC of tomato seedlings from different treatment groups was tested (Fig. 1C). We also investigated the REC, which is a key parameter of stress damage (Fig. 1D). Exogenous Se treatment did not change the RWC and REC of tomato plants compared to the control plants under non-stress conditions. However, under salt stress, the RWC of leaves increased by 9.8% and the REC of leaves decreased by 16.2% in Se-treated tomato seedlings compared to seedlings without Se treatment (Fig. 1C, D).
Effects of Se on the photosynthetic pigment contents
Under control conditions, Se treatment increased the contents of chlorophyll b and carotenoids but did not affect the chlorophyll a content (Table 2). Salt stress decreased the contents of all these pigments. However, Se supplementation significantly increased the chlorophyll a, chlorophyll b, and the total chlorophyll contents of tomato leaves by 20.8, 43.8, and 26.4%, respectively (Table 2).
Effects of Se on photosynthesis and chlorophyll fluorescence parameters
We investigated the photosynthetic parameters of various treatment groups, such as the net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) to ascertain the impact of exogenous Se on plant photosynthesis under salt stress (Table 2). Under normal conditions, Se addition did not affect the photosynthetic characteristics of tomato plants compared to the controls. However, salt stress dramatically reduced the Pn, Tr, Gs, and Ci in tomato seedlings, and Se treatment significantly recovered the Pn, Tr, Gs, and Ci by 27.9, 11.0, 73.4, and 10.6%, respectively, compared to the seedlings without Se treatment (Table 2). Under salt stress conditions, Se treatment also increased the WUE of tomato leaves by 14.6%. Chlorophyll fluorescence is a non-intrusive and accurate indication of photosynthetic performance (Murchie and Lawson 2013). Under non-saline conditions, exogenous Se did not change the maximum quantum yield of photosystem II (Fv/Fm) and the actual photochemical efficiency of photosystem II (ΦPSII) in tomato seedlings (Table 2). However, under salt stress conditions, Se application significantly increased the Fv/Fm and ΦPSII values by 5.3% and 8.5%, respectively (Table 2).
Se contents in different parts of tomato plants
We measured the Se content in tomato shoots and roots with exogenous Se addition under normal and salt stress (Table 3). Applying Se to the culture solution increased the Se content in tomato tissues under non-stress and salt stress conditions (Table 3). This demonstrates that plants absorb Se via their roots and is an effective strategy to enhance Se content in different tomato tissues.
Effects of Se on inorganic ions concentrations under salt stress
Under normal conditions, there were no significant differences in the Na+ contents in the shoots and roots of Se-treated plants compared with control plants (Table 3). However, Se treatment resulted in a decrease in K+ content and an increase in Cl− content in tomato shoots. Under salt stress, the K+ in the shoots and roots decreased significantly, but Se treatment increased the K+ content in tomato shoots by 38.6% compared to plants without Se (Table 3). The Na+ and Cl− contents were markedly increased by NaCl addition compared to control plants. The addition of Se decreased the Na+ and Cl− contents in tomato shoots by 16.5 and 14.8%, respectively (Table 3). However, the presence of Se did not significantly affect the Na+ content in tomato roots under salt stress.
Effects of Se on lipid peroxidation and osmoprotectants under salt stress
Under non-saline conditions, the malondialdehyde (MDA), soluble sugar, and proline contents of tomato leaves or roots were not changed by Se treatment (Fig. 2). However, their contents were significantly induced after 15 days of salt stress. Se treatment decreased the MDA and soluble sugar contents of leaves under salt stress by 30.1 and 37.6%, respectively, compared to the seedlings under salt stress without Se treatment (Fig. 2A, B). Se treatment also reduced the MDA and soluble sugar contents in tomato roots in response to salt stress by 44.7 and 29.8%, respectively (Fig. 2A, B). The addition of Se also slightly decreased the proline content in leaves but did not change its content in roots under salt stress (Fig. 2C).
Influence of exogenous Se on lipid peroxidation and osmoprotectants content of tomato seedlings under salt stress treatment with/without Se supplementation for 15 d. (A) The malondialdehyde (MDA) content. (B) The soluble sugar content. (C) The proline content. Data are presented as mean ± SD of three biological replicates, each of which contains three seedlings. Different small letters indicate significant differences between treatment groups at P < 0.05
H2O2 and O2 •− accumulation and antioxidant defense
Histochemical staining showed that Se application did not affect H2O2 and O2•− accumulation in tomato leaves under non-stress conditions (Fig. 3A). Salt stress increased the reactive oxygen species (ROS) accumulation in tomato leaves, which was significantly decreased by Se application (Fig. 3A, upper). On the other hand, Se supplementation decreased the H2O2 and O2•− contents in tomato leaves under salt stress by 22.2 and 47.2%, respectively (Fig. 3A, lower).
Effect of exogenous Se on ROS accumulation and activities of antioxidant enzymes in tomato seedlings under salt stress for 15 d. (A) H2O2 and O2•− observation in tomato leaves (upper) and H2O2 and O2•− contents in tomato leaves (lower). (B) The activity of SOD, POD and CAT in tomato leaves and roots. Data are presented as mean ± SD of three biological replicates. Different small letters indicate significant differences between treatment groups at P < 0.05
We investigated the activities of the antioxidant enzymes superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) to ascertain the effect of Se treatment on antioxidant defense in tomato plants in response to salt stress (Fig. 3B). Under non-saline conditions, Se treatment did not affect SOD and POD activity in leaves or roots or CAT activity in roots, but did reduce CAT activity in leaves (Fig. 3B). Salt stress increased SOD, POD, and CAT activities, and Se treatment decreased their activities by 19.2, 34.1, and 39.6% in leaves and 21.7, 15.1, and 34.1% in roots compared to seedlings without Se treatment (Fig. 3B).
Phytohormones contents
To investigate the influence of Se addition on the contents of phytohormones under salt stress treatment, we measured the level of several phytohormones in different tomato tissues after 5 days of salt stress with or without Se addition, including SA, abscisic acid (ABA), melatonin (MT), and jasmonic acid (JA) (Fig. 4). Se treatment increased the SA content in tomato leaves and roots under non-stress and salt stress environments (Fig. 4A). Under salt stress conditions, the SA contents in the leaves and roots of the Se-treated tomatoes were 80.7 and 35.2% higher, respectively, than tomatoes without Se. The ABA content of leaves did not differ significantly between treatment groups, but it was lower in Se-treated tomato roots than control plants (Fig. 4B). Se addition did not significantly affect the MT contents in the tomato tissues under non-stress or stress conditions (Fig. 4C). Both under non-stress and stress conditions, Se application did not change the JA content in tomato leaves. However, the root JA content was decreased by Se treatment under non-stress and stress conditions (Fig. 4D).
Influence of exogenous Se on phytohormone contents and the expression of some genes involved in salicylic acid (SA) biosynthesis in tomato tissues under salt stress for 5 d. (A) SA concentration. (B) Abscisic acid (ABA) concentration. (C) Melatonin (MT) concentration. (D) Jasmonic acid (JA) concentration. (E) The relative expression level (REL) of several genes involved in biosynthesis and transformation. Data are presented as mean ± SD of three biological replicates, each of which contains three seedlings. Different small letters denote significant differences between various treatments at P < 0.05
Expression of genes associated with SA biosynthesis and transformation
The qRT-PCR was performed to analyze the transcripts of genes involved in SA biosynthesis and transformation (Fig. 4E). The transcripts of phenylalanine ammonia-lyase 1 (PAL1) were upregulated in tomato leaves in response to Se application under normal and salt stress conditions for 5 days. However, in tomato roots, the addition of Se induced the expression of PAL1 under salt stress conditions but did not change its expression under normal conditions. Under normal condition, Se application did not change the transcripts of isochorismate synthase (ICS) in tomato leaves, but it downregulated its expression in the roots. In addition, Se treatment increased ICS gene expression in tomato leaves in response to salt stress (Fig. 4E). Se treatment did not affect salicylate carboxymethyltransferase (SAMT) gene expression in tomato leaves under normal and salt stress conditions. However, Se treatment upregulated SAMT expression in tomato roots under normal conditions but not under salt stress (Fig. 4E). Finally, Se treatment downregulated salicylic acid-binding protein 2 (SABP2) gene expression in different tomato tissues under normal conditions, but it had no apparent impact under salt stress (Fig. 4E).
Roles of SA in Se-induced salt stress tolerance in tomato seedlings
As shown in Fig. 5A, under normal conditions, pre-treatment with 100 μM ABT (a SA biosynthesis inhibitor) reduced the plant height but had no apparent effect on the stem diameter, fresh weight, or dry weight of tomato seedlings, indicating that ABT had no apparent inhibitory effect on plant growth under non-stress conditions. In contrast, under salt stress, ABT treatment decreased the plant height and biomass of tomato plants compared to the plants grown without ABT. Se treatment improved the plant height, stem diameter, and biomass of tomato seedlings impacted by salt stress (Fig. 5A); however, pre-spraying with ABT weakened these beneficial effects of Se (Fig. 5B). In addition, we detected the SA content of tomato leaves in each treatment group. Figure 5C showed that ABT treatment did significantly reduce the SA content under normal and salt stress treatments. Se treatment significantly increased the SA content under salt stress, however, the induction effect of Se on SA content was inhibited by ABT treatment (Fig. 5C).
The roles of a SA inhibitor (1-aminobenzotriazole, ABT) in Se-enhanced salt resistance in tomato plants. (A) Plant height, stem diameter, fresh and dry weight. (B) Left: The relative electrical conductivity (REC); Right: the relative water content (RWC). (C) The SA content in various treatment groups. (D) Histological staining of H2O2 (upper) and O2•− (lower). CK, control plants; CK + ABT, control + ABT treatment; NaCl: salt treatment; NaCl + ABT, salt treatment + ABT; NaCl + Se: salt + selenium treatment; NaCl + Se + ABT: salt + selenium + ABT treatment. Data are presented as mean ± SD of three biological replicates, each of which contains three seedlings Different small letters denote significant differences between various treatments at P < 0.05. The asterisks (*) in Fig. 5A means there is a significant difference between “NaCl” and “NaCl + ABT” treatment at P < 0.05 according to t-test
Then the effects of ABT on plant photosynthesis after Se application in response to salt stress were studied (Table 4). Pre-treatment with ABT reduced the Tr and Gs of plants under normal conditions but inhibited the Fv/Fm of plants under salt stress. Pn was significantly reduced under salt stress, which was significantly improved by Se treatment (Table 4). Meanwhile, ABT-pre-treated plants had 53% lower Pn compared to those treated only with Se under salt stress. ABT also reduced the promoting effects of Se on Tr, Gs, and WUE under salt stress, although these findings were not statistically significant when comparing them within groups (Table 4). Under salt stress, ABT pre-treatment raised the REC by 52.3% and reduced the RWC by 12.7% compared to plants with Se treatment alone (Fig. 5B). Finally, histochemical staining of H2O2 and O2•− in plant leaves revealed that ABT pre-treatment abolished the Se-mediated decrease in H2O2 and O2•− accumulation under salt stress (Fig. 5D).
Discussions
Exogenous Se promoted plant photosynthesis and growth under salt stress
Excess salinity stress leads to osmotic stress and ion toxicity, which increases the Na+ content in plant tissue, aggravates the cell membrane peroxidation, causes oxidant damage, and suppresses plant photosynthesis, thus leading to nutrient deficiency, retarded plant growth, and reduced biomass (Gupta and Huang 2014). In our study, we analyzed the impact of Se application in enhancing tomato plant growth through its involvement in the regulation of key physiological and biochemical parameters (Table 1). Under normal conditions, exogenous Se application raised the plant height of tomato seedlings, since Se had been found to play important roles in the production and signaling of some phytohormones involved in plant internode elongation, such as cytokinin and gibberellin (Jiang et al. 2019; Shan et al. 2021). Moreover, Se supplementation boosted plant development under salt stress, as evidenced by an increase in plant height, stem diameter, and biomass. Se also relieved the wilting state of tomato plants caused by salt stress (Table 1).
Studies have shown that Se could improve growth and development in many plants exposed to various abiotic stresses, partly attributed to the promotion of plants’ photosynthetic efficiency (Diao et al. 2014; Fan et al. 2022), which was also supported by our results. The reduction in photosynthesis under salinity was probably due to the lower Gs and Pn rates caused by salt stress (Jiang et al. 2017). In this study, adding Se increased plant Pn and Gs under salt stress, protecting the photochemical efficiency and increasing Tr, and further enhanced photosynthesis and promoted plant growth compared to tomato seedlings without Se (Fig. 1, Table 1).
Under saline conditions, Gs rapidly decreases to protect the water supply (Lotfi et al. 2020). However, exogenous Se increased the plants’ Gs under salt stress. This could be related to the high SA content induced by Se supplementation (Fig. 4), since SA reportedly increased the Gs of several plant species in response to various abiotic stresses (Stevens et al. 2006; Khan et al. 2014; Ma et al. 2017; Lotfi et al. 2020; Khalvandi et al. 2021). In addition, K+ deficiency may induce stomatal closure, so the increased K+ concentration (Table 3) under Se treatment might also have contributed to the increase in Gs under salt stress with Se supplementation (Zhang et al. 2020). Interestingly, Benlloch-Gonzalez et al. (2010) found that K+ accumulation in leaves after SA treatment was due to the inhibition of ethylene formation, which regulated Gs. Therefore, we expect an interplay among SA, ethylene, and K+ in regulating stomatal movement and photosynthesis. Restoration of the photosynthetic capacity in salt-treated tomato plants by Se application may also be related to the increased chlorophyll and carotenoid contents under salt stress (Jiang et al. 2017).
Exogenous Se impacted osmotic regulation in tomato plants under salt stress
Plants produce and regulate a variety of organic (soluble sugars, soluble proteins, proline, etc.) and inorganic (Na+, Cl−, K+, Ca2+, etc.) osmolytes or osmoprotectants under salt stress, which have biological functions in the plant response to salt stress (Riffat and Ahmad 2018). Na+ and Cl− are the most toxic ions in plants produced under salt stress, and their excessive accumulation in plants eventually leads to arrested plant growth (Khare et al. 2015). Salt stress often leads to excessive Na+ and Cl− and increased K+ leakage from cells due to membrane damage, resulting in increased Na+ and Cl−, and decreased K+, contents in plant tissues, and a decreased K+/Na+ ratio (Gupta and Huang 2014). In this study, Se application increased the K+ content and reduced the Na+ and Cl− contents in shoots compared to tomato plants without Se treatment under salt stress, which was consistent with the findings of Jiang et al. (2017) in maize. Se application maintained K+ and Na+ homeostasis under salt stress, which is essential for cell osmoregulation and to reduce ion toxicity caused by salt stress. Se application increased intracellular K+ levels, which are essential to maintain the membrane potential, osmoticum production, and the action of numerous cytosolic enzymes (Zhu 2003). Moreover, Se significantly reduced the Na+ content in tomato shoots under salt stress but did not change its content in roots (Table 3), indicating that Se application might reduce the transfer of Na+ from tomato roots to shoots. It was reported by Nemat Alla et al. (2020) that exogenous Se could up-regulate the expression of SOS1 and NHX1 genes under salt stress, while inhibiting the accumulation of Na+. These results indicated that Se application could improve the expression of H+-ATPase and Na+/H+ antitransporter in the vacuolar membrane of root cells under salt stress, thereby limiting the distribution of Na+ in the upper tissue and alleviating its toxicity.
Soluble sugars (sucrose, glucose and fructose), a kind of important osmoprotectants, play crucial roles in regulating osmotic adjustment, maintaining the water balance of plants, scavenging excess ROS, and protecting cell membranes under various adverse environmental stresses (Dien et al. 2019). In addition, soluble sugars act as a kind of chelating agent to entrap Na+ within starch granules, helping to neutralize Na+ toxicity in plants under salt stress (Kanai et al. 2007). Proline is a vital amino acid that benefits plants in response to salinity (El Moukhtari et al. 2020). It not only acts as an osmoprotectant to regulate the water balance of plant cells, but also plays important roles in ROS scavenging and membranes stabilization to prevent electrolyte leakage (Shamsul et al. 2012; Naliwajski and Skłodowska 2021). Several studies reported that the soluble sugar and proline contents were enriched when plants were subjected to salt stress, potentially contributing to plants’ osmotic and salt stress tolerance (Boriboonkaset et al. 2013; El-Katony et al. 2021). However, other studies reported opposite or inconsistent results. For example, Watanabe et al. (2000) reported that the total soluble sugar content increased in young Populus euphratica leaves but decreased in the mature leaves after salt stress treatment. Xu et al. (2015) reported that the soluble sugar content in stress-resistant rice decreased in response to drought stress. Naliwajski and Skłodowska (2021) reported that acclimated cucumber plants accumulated lower proline levels under salt stress than non-acclimated plants under normal and stress conditions. Therefore, changes in plants’ soluble sugar and proline contents in response to salt stress vary among plant species, varieties, and growth stages (Xu et al. 2015; Naliwajski and Skłodowska 2021). Furthermore, in our study, the content of soluble sugars and proline of Se-treated tomato seedlings under salt treatment for 15 d was lower compared to the tomato plants without Se supplementation (Fig. 2B, C), which suggested that the duration of Se application in plants under salt stress might also be one of the factors affecting the accumulation of soluble sugars and proline, since the toxicity and damage to plants caused by salt stress were basically mitigated by Se addition for 15 d (Fig. 1A) and it was not necessary for plants to produce continual high level of osmotic protective substance, which indirectly indicated the promoting effect of Se on plant salt stress tolerance.
Exogenous Se alleviated the oxidative damage caused by salt stress
Salt stress is often accompanied with the secondary oxidative damage for plants, since high salinity leads to the accumulation of excessive ROS, such as H2O2, O2−, and O•−, which potentially causes cell membrane lipid peroxidation and even cell death (Zhang et al. 2016). Moreover, MDA levels and relative electrolyte leakage in plants can represent the integrity of their cellular membranes in response to salt stress (Gou et al. 2020b). Plants have evolved an efficient antioxidant system, including enzymes such as SOD, POD and CAT to scavenge ROS to avoid oxidative damage caused by various environmental stresses (Gill and Tuteja 2010). It was reported that Se treatment could regulate the antioxidant defense system by up-regulating the activities of several antioxidant enzymes, reflected by decreased H2O2 and MDA contents in the cells of different plant species under various environmental stresses (Kaur and Nayyar 2015; Ashraf et al. 2018). In this study, Se treatment mitigated the oxidative damage caused by salt stress (Fig. 3A), whereas the activity of various antioxidant enzymes was lower in Se-treated tomato seedlings under salt stress than in tomato plants in the absence of Se addition (Fig. 3B). This was probably due a decrease of the oxidative damage induced by the salt stress treatment following Se supplementation for 15 days, and the reduced need for antioxidant enzymes to eliminate ROS. Furthermore, Se is an integral component of enzymes like glutathione peroxidase and other seleno-compounds, which have vital roles in reducing cell peroxidation and oxidative damage (Puccinelli et al., 2017), suggesting that Se might regulate other antioxidant mechanisms under salt stress.
SA was involved in Se-induced salt tolerance in tomato plants
Previous studies reported that SA could regulate various plant metabolic processes, and hence regulate the plant stress resistance, including regulating the stomatal and water potential, enhancing plant nutrient uptake and photosynthesis, and promoting plant growth and development (Khan et al. 2015). PAL and ICS encode phenylalanine ammonia-lyase and isochorismate synthase respectively, which are the two key enzymes of the two SA biosynthesis pathways (Ding and Ding 2020). It has been observed that the transcripts of PAL1 were remarkedly upregulated by Se treatment in tomato leaves and roots under salt stress whereas the ICS expression was only induced by Se addition in tomato leaves (Fig. 4E). Moreover, the SA content was obviously induced by Se application in different tomato tissues under salt stress conditions (Fig. 4A). These results suggest that the accumulation of SA in tomato leaves under salt stress is probably attributed to the upregulation of both PAL and ICS pathways following Se application, while its accumulation in roots might only be related to the up-regulation of PAL pathway. As described by Park et al. (2007), SAMT1 catalyzes SA to become methylated SA (MeSA), which is hydrolysed by SABP2 and re-converted into SA again. Under salt stress conditions, the expression of SAMT1 and SABP2 was not changed by exogenous Se treatment, indicating Se did not regulate the mutual transformation between SA and MeSA in response to salt stress (Fig. 4E). Moreover, Se treatment increased the SA content but decreased the JA content in tomato roots under control and salt stress conditions, which was probably because SA was an important antagonist of the JA response (Koornneef et al. 2008). SA can suppress JA signaling through the downregulation of JA-responsive gene expression in various plant species (Van der Does et al. 2013; Wang et al. 2022). Previous studies have revealed the characteristics of the trade-off between JA and SA signaling (Leon-Reyes et al. 2010).
ABT inhibits the SA synthesis by inhibiting the activity of benzoic acid 2-hydroxylase (BA2H), a key enzyme in the formation of SA via the PAL pathway (Leon et al. 1995). Under non-stress conditions, ABT did not affect plant growth, but it had an apparent inhibitory effect on plant growth under salt stress (Fig. 5A), confirming the critical role of SA in plant salt stress tolerance (Wassie et al. 2020; Ahmed et al. 2020). Interestingly, being pre-sprayed with ABT reduced the stimulating effect of Se on plant growth and photosynthesis, increased the REC, worsened the damage caused by oxidative stress, and eventually abolished the beneficial effect of Se application in plant salt resistance (Fig. 5, Table 4). Meanwhile, we detected the content of SA of tomato plants in each treatment groups, which was consistent with the resistance of different tomato plants to salt stress (Fig. 5C). These results further proved the conclusion that Se application played an important role in enhancing salt stress resistance of plants, which was partly due to the induction of SA content by Se addition. Wang et al. (2018) reported that exogenous treatment with SeO32− induced a constitutively higher expression of genes involved in SA synthesis in the Se hyperaccumulator Stanleya pinnata compared to its related non-hyperaccumulator, Stanleya elata. Furthermore, SA played a central role in the high Se tolerance in S. pinnata (Freeman et al. 2010). These results suggest complex interactions between Se and SA in plants, which might depend on the concentration and duration of Se treatment, as well as the plant species and different ability of Se accumulation.
Conclusions
In conclusion, this study demonstrated that low level of exogenous Se (25 μM) generally stimulated plant growth and enhanced the salt stress tolerance of tomato seedlings, which might be due to several mechanisms: (a) exogenous Se application increased the accumulation of photosynthetic pigments, improved the Pn, thereby enhanced the photosynthesis and plants growth under salt stress; (b) Se application maintained K+ and Na+ homeostasis under salt stress, which was essential for cellular osmoregulation and the reduction of ion toxicity caused by salt stress; (c) Se treatment also reduced the REC and decreased the H2O2, O2•−, and MDA contents, and mitigated the oxidative damage caused by salt stress. (d) Exogenous Se increased SA content in tomato leaves and roots via up-regulating the PAL pathway of SA biosynthesis, which played vital roles in plant salt stress resistance. Our results clarify the roles and regulatory mechanisms of Se in plant salt stress tolerance and highlight the importance of SA in this process. These findings help support the application of Se in agricultural production to enhance crop growth and yield under salt stress.
Data availability
The data generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABT:
-
1-Aminobenzotriazole
- DAB:
-
Diaminobenzidine
- H2O2 :
-
Hydrogen peroxide
- ICS:
-
Isochorismate synthase
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- O2 • − :
-
Superoxide anion
- PAL1:
-
Phenylalanine ammonia-lyase 1
- qRT-PCR:
-
Quantitative real-time PCR
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SABP2:
-
Salicylic acid-binding protein 2
- SAMT:
-
Carboxymethyltransferase
- Se:
-
Selenium
- WUE:
-
Water use efficiency
References
Ahmed W, Imran M, Yaseen M, Haq TU, Jamshaid MU, Rukh S, Ikram RM, Ali M, Ali A, Maqbool M, Arif M, Khan MA (2020) Role of salicylic acid in regulating ethylene and physiological characteristics for alleviating salinity stress on germination, growth and yield of sweet pepper. Peer J 8:e8475. https://doi.org/10.7717/peerj.8475
Ardebili NO, Saadatmand S, Niknam V, Khavari-Nejad RA (2014) The alleviating effects of selenium and salicylic acid in salinity exposed soybean. Acta Physiol Plant 36:3199–3205. https://doi.org/10.1007/s11738-014-1686-6
Arnon DI (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M (2018) Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol Biochem 123:268–280. https://doi.org/10.1016/j.plaphy.2017.12.023
Benlloch-Gonzalez M, Romera J, Cristescu S, Harren F, Fournier JM, Benlloch M (2010) K starvation inhibits water - stress - induced stomatal closure via ethylene synthesis in sunflower plants. J Exp Bot 61:1139–1145. https://doi.org/10.1093/jxb/erp379
Borbély P, Molnár Á, Valyon E, Ördög A, Horváth-Boros K, Csupor D, Fehér A, Kolbert Z (2021) The effect of foliar selenium (Se) treatment on growth, photosynthesis, and oxidative-nitrosative signalling of Stevia rebaudiana leaves. Antioxidants 10:72. https://doi.org/10.3390/antiox10010072
Boriboonkaset T, Theerawitaya C, Yamada N, Pichakum A, Supaibulwatana K, Cha-Um S, Takabe T, Kirdmanee C (2013) Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 250:1157–1167. https://doi.org/10.1007/s00709-013-0496-9
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast. J Plant Growth Regul 33:671–682. https://doi.org/10.1007/s00344-014-9416-2
Dien DC, Mochizuki T, Yamakawa T (2019) Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod Sci 22:530–545. https://doi.org/10.1080/1343943X.2019.1647787
Ding P, Ding Y (2020) Stories of salicylic acid: a plant defense hormone. Trends Plant Sci 25:549–565. https://doi.org/10.1016/j.tplants.2020.01.004
El Moukhtari A, Cabassa-Hourton C, Farissi M, Savouré A (2020) How does proline treatment promote salt stress tolerance during crop plant development? Front Plant Sci 11:1127. https://doi.org/10.3389/fpls.2020.01127
El-Katony TM, Ward FM, Deyab MA, El-Adl MF (2021) Algal amendment improved yield and grain quality of rice with alleviation of the impacts of salt stress and water stress. Heliyon 7:e07911. https://doi.org/10.1016/j.heliyon.2021.e07911
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem 137:144–153. https://doi.org/10.1016/j.plaphy.2019.02.004
Fan S, Han N, Wu H, Jia J, Guo J (2021) Plasma membrane intrinsic protein SlPIP1;7 promotes root growth and enhances drought stress tolerance in transgenic tomato (Solanum lycopersicum) plants. Plant Breeding 140:1102–1114. https://doi.org/10.1111/pbr.12978
Fan S, Wu H, Gong H, Guo J (2022) The salicylic acid mediates selenium-induced tolerance to drought stress in tomato plants. Sci Hortic 300:111092. https://doi.org/10.1016/j.scienta.2022.111092
Freeman JL, Tamaoki M, Stushnoff C, Quinn CF, Cappa JJ, Devonshire J, Fakra SC, Marcus MA, McGrath SP, Van Hoewyk D, Pilon-Smits EA (2010) Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol 153:1630–1652. https://doi.org/10.1104/pp.110.156570
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gou T, Yang L, Hu W, Chen X, Zhu Y, Guo J, Gong H (2020a) Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol Bioch 152:53–61. https://doi.org/10.1016/j.plaphy.2020.04.031
Gou T, Chen X, Han R, Liu J, Zhu Y, Gong H (2020b) Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiol Plant 42:12. https://doi.org/10.1007/s11738-019-3007-6
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 2014:701596. https://doi.org/10.1155/2014/701596
Hajiboland R, Rahmat S, Zeinalzadeh N, Farsad-Akhtar N, Hosseinpour-Feizi MA (2019) Senescence is delayed by selenium in oilseed rape plants. J Trace Elem Med Biol 55:96–106. https://doi.org/10.1016/j.jtemb.2019.06.005
Han N, Fan S, Zhang T, Sun H, Zhu Y, Gong H, Guo J (2020) SlHY5 is a necessary regulator of the cold acclimation response in tomato. Plant Growth Regul 91:1–12. https://doi.org/10.1007/s10725-020-00583-7
Jia H, Song Z, Wu F, Ma M, Li Y, Han D, Yang Y, Zhang S, Cui H (2018) Low selenium increases the auxin concentration and enhances tolerance to low phosphorous stress in tobacco. Environ Exp Bot 153:127–134. https://doi.org/10.1016/j.envexpbot.2018.05.017
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Jiang L, Liu C, Cao H, Chen Z, Yang J, Cao S, Wei Z (2019) The role of cytokinin in selenium stress response in Arabidopsis. Plant Sci 281:122–132. https://doi.org/10.1016/j.plantsci.2019.01.028
Kanai M, Higuchi K, Hagihara T, Konishi T, Ishii T, Fujita N, Nakamura Y, Maeda Y, Yoshiba M, Tadano T (2007) Common reed produces starch granules at the shoot base in response to salt stress. New Phytol 176:572–580. https://doi.org/10.1111/j.1469-8137.2007.02188.x
Kaur S, Nayyar H (2015) Selenium fertilization to salt-stressed mungbean (Vigna radiata L. Wilczek) plants reduces sodium uptake, improves reproductive function, pod set and seed yield. Sci Hortic 197:304–317. https://doi.org/10.1016/j.scienta.2015.09.048
Khalvandi M, Siosemardeh A, Roohi E, Keramati S (2021) Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 7:e05908. https://doi.org/10.1016/j.heliyon.2021.e05908
Khan MI, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74. https://doi.org/10.1016/j.plaphy.2014.03.026
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462. https://doi.org/10.3389/fpls.2015.00462
Khare T, Kumar V, Kishor PB (2015) Na+ and Cl- ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 252:1149–1165. https://doi.org/10.1007/s00709-014-0749-2
Koo YM, Heo AY, Choi HW (2020) Salicylic acid as a safe plant protector and growth regulator. Plant Pathol J 36:1–10. https://doi.org/10.5423/PPJ.RW.12.2019.0295
Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CM (2008) Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol 147:1358–1368. https://doi.org/10.1104/pp.108.121392
La VH, Lee BR, Zhang Q, Park SH, Islam MT, Kim TH (2019) Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Hortic Environ Biotechnol 60:31–40. https://doi.org/10.1007/s13580-018-0099-7
Leon J, Shulaev V, Yalpani N, Lawton MA, Raskin I (1995) Benzoicacid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic-acid biosynthesis. Proc Natl Acad Sci USA 92:10413–10417. https://doi.org/10.1073/pnas.92.22.10413
Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SC, Ritsema T, Pieterse CM (2010) Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 232:1423–1432. https://doi.org/10.1007/s00425-010-1265-z
Lotfi R, Ghassemi-Golezani K, Pessarakli M (2020) Salicylic acid regulates photosynthetic electron transfer and stomatal conductance of mung bean (Vigna radiata L.) under salinity stress. Biocatal Agric Biotechnol 26:101635. https://doi.org/10.1016/j.bcab.2020.101635
Ma X, Zheng J, Zhang X, Hu Q, Qian R (2017) Salicylic acid alleviates the adverse effects of salt stress on dianthus superbus (caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front Plant Sci 8:600. https://doi.org/10.3389/fpls.2017.00600
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998. https://doi.org/10.1093/jxb/ert208
Naliwajski M, Skłodowska M (2021) The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress. Cells 10:609. https://doi.org/10.3390/cells10030609
Nemat Alla MM, Badran EG, Mohammed FA, Hassan NM, Abdelhamid MA (2020) Overexpression of Na+-manipulating genes in wheat by selenium is associated with antioxidant enforcement for enhancement of salinity tolerance. Rend Fis Acc Lincei 31:177–187. https://doi.org/10.1007/s12210-019-00868-8
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318:113–116. https://doi.org/10.1126/science.1147113
Puccinelli M, Malorgio F, Pezzarossa B (2017) Selenium enrichment of horticultural crops. Molecules 22:933. https://doi.org/10.3390/molecules22060933
Rady MM, Belal HEE, Gadallah FM, Semida WM (2020) Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci Hortic 266:109290. https://doi.org/10.1016/j.scienta.2020.109290
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Riffat A, Ahmad MSA (2018) Changes in organic and inorganic osmolytes of maize (Zea mays L.) by sulfur application under salt stress conditions. J Agric Sci 10:543–561. https://doi.org/10.5539/jas.v10n12p543
Shamsul H, Qaiser H, Alyemeni MN, Wani AS, Pichtel J, Aqil A (2012) Role of proline under changing environments: A review. Plant Signal Behav 7:1456–1466. https://doi.org/10.4161/psb.21949
Shan F, Zhang R, Zhang J, Wang C, Lyu X, Xin T, Yan C, Dong S, Ma C, Gong Z (2021) Study on the regulatory effects of GA3 on soybean internode elongation. Plants 10:1737. https://doi.org/10.3390/plants10081737
Shi Y, Zhang Yi, Yao H, Jiawen Wu, Sun H, Gong H (2014) Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol Biochem 78:27–36. https://doi.org/10.1016/j.plaphy.2014.02.009
Shi Y, Zhang Y, Han W, Feng R, Hu Y, Guo J, Gong H (2016) Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front Plant Sci 7:196. https://doi.org/10.3389/fpls.2016.00196
Shrivastava P, Kumar R (2015) Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Soltabayeva A, Ongaltay A, Omondi JO, Srivastava S (2021) Morphological, physiological and molecular markers for salt-stressed plants. Plants (Basel) 10:243. https://doi.org/10.3390/plants10020243
Stevens J, Senaratna T, Sivasithamparam K (2006) Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul 49:77–83. https://doi.org/10.1007/s10725-006-0019-1
Tamaoki M, Freeman J, Marqusè L, Pilon-Smits E (2008) New insights into the roles of ethylene and jasmonic acid in the acquisition of selenium resistance in plants. Plant Signal Behav 3:865–867. https://doi.org/10.4161/psb.3.10.6050
Van der Does D, Leon-Reyes A, Koornneef A, Van Verk MC, Rodenburg N, Pauwels L, Goossens A, Körbes AP, Memelink J, Ritsema T, Van Wees SC, Pieterse CM (2013) Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 25:744–761. https://doi.org/10.1105/tpc.112.108548
Wang J, Cappa JJ, Harris JP, Edger PP, Zhou W, Pires JC, Adair M, Unruh SA, Simmons MP, Schiavon M, Pilon-Smits EAH (2018) Transcriptome-wide comparison of selenium hyperaccumulator and nonaccumulator Stanleya species provides new insight into key processes mediating the hyperaccumulation syndrome. Plant Biotechnol J 16:1582–1594. https://doi.org/10.1111/pbi.12897
Wang L, Liu S, Gao M, Wang L, Wang L, Wang Y, Dai L, Zhao J, Liu M, Liu Z (2022) The crosstalk of the salicylic acid and jasmonic acid signaling pathways contributed to different resistance to phytoplasma infection between the two genotypes in Chinese Jujube. Front Microbiol 13:800762. https://doi.org/10.3389/fmicb.2022.800762
Wassie M, Zhang W, Zhang Q, Ji K, Cao L, Chen L (2020) Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol Environ Saf 191:110206. https://doi.org/10.1016/j.ecoenv.2020.110206
Watanabe S, Kojima K, Ide Y, Sasaki S (2000) Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult 63:199. https://doi.org/10.1023/A:1010619503680
Wichern F, Islam MR, Hemkemeyer M, Watson C, Joergensen RG (2020) Organic amendments alleviate salinity effects on soil microorganisms and mineralisation processes in aerobic and anaerobic paddy rice soils. Front Sustain Food Syst 4:30. https://doi.org/10.3389/fsufs.2020.00030
Xu W, Cui K, Xu A, Nie L, Huang J, Peng S (2015) Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant 37:1–11. https://doi.org/10.1007/s11738-014-1760-0
Yang T, Zhu LS, Meng Y, Lv R, Zhou Z, Zhu L, Lin HH, Xi DH (2018) Alpha-momorcharin enhances tobacco mosaic virus resistance in tobacco NN by manipulating jasmonic acid-salicylic acid crosstalk. J Plant Physiol 223:116–126. https://doi.org/10.1016/j.jplph.2017.04.011
Yang Z, Li JL, Liu LN, Xie Q, Sui N (2020) Photosynthetic Regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front Plant Sci 10:1722. https://doi.org/10.3389/fpls.2019.01722
Yu Z, Duan X, Luo L, Dai S, Ding Z, Xia G (2020) How plant hormones mediate salt stress responses. Trends Plant Sci 25:1117–1130. https://doi.org/10.1016/j.tplants.2020.06.008
Zahedi SM, Abdelrahman M, Hosseini MS, Hoveizeh NF, Tran LP (2019) Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut 253:246–258. https://doi.org/10.1016/j.envpol.2019.04.078
Zhang XK, Zhou QH, Cao JH, Yu BJ (2011) Differential Cl−/Salt tolerance and NaCl-induced alternations of tissue and cellular ion fluxes in Glycine max, Glycine soja and their hybrid seedlings. J Agron Crop Sci 197:329–339. https://doi.org/10.1111/j.1439-037X.2011.00467.x
Zhang M, Smith JA, Harberd NP, Jiang C (2016) The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol Biol 91:651–659. https://doi.org/10.1007/s11103-016-0488-1
Zhang L, Li D, Yao Y, Zhang S (2020) H2O2, Ca2+, and K+ in subsidiary cells of maize leaves are involved in regulatory signaling of stomatal movement. Plant Physiol Biochem 152:243–251. https://doi.org/10.1016/j.plaphy.2020.04.045
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445. https://doi.org/10.1016/s1369-5266(03)00085-2
Zhu Z, Zhang Y, Liu J, Chen Y, Zhang X (2018) Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem 252:9–15. https://doi.org/10.1016/j.foodchem.2018.01.064
Zhu Y, Jiang X, Zhang J, He Y, Zhu X, Zhou X, Gong H, Yin J, Liu Y (2020) Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol Biochem 156:209–220. https://doi.org/10.1016/j.plaphy.2020.09.014
Zsiros O, Nagy V, Párducz Á, Nagy G, Ünnep R, El-Ramady H, Prokisch J, Lisztes-Szabó Z, Fári M, Csajbók J, Tóth SZ, Garab G, Domokos-Szabolcsy É (2019) Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynth Res 139:449–460. https://doi.org/10.1007/s11120-018-0599-4
Funding
This work was supported by National Natural Science Foundation of China (32072561).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hong Wu and Shuya Fan. HG analyzed the original data. The first draft of the manuscript was written by Jia Guo.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Juan Barcelo.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, H., Fan, S., Gong, H. et al. Roles of salicylic acid in selenium-enhanced salt tolerance in tomato plants. Plant Soil 484, 569–588 (2023). https://doi.org/10.1007/s11104-022-05819-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05819-1