Abstract
Aims
Organic manure (OM) is an effective amelioration measure for acidic soils. Acid (ACP) and alkaline phosphatases (ALP) encoded by bacterial phoC and phoD genes, respectively, are responsible for organic phosphorus (P) mineralization. However, the short-term influence of OM application on phosphatase activity and organic P-mineralizing bacterial communities of bulk and rhizosphere soils in acidic soils is less known.
Methods
Maize was grown in acidic soil (pH 4.40) supplied with 0, 1, 5, 10, 20 and 50 g OM kg− 1 dry soil for six weeks. Maize biomasses and nutrients, soil physicochemical properties and phosphatase activities, and P-mineralizing bacterial communities were observed.
Results
Rhizosphere showed higher ACP and ALP activities than bulk soils, and the rhizosphere effects were stronger than OM application. The Shannon index of phoC- and phoD-harboring bacteria responded differently to both rhizosphere effect and OM application, with a stronger influence from maize rhizosphere. The rhizosphere effect significantly affected both phoC- and phoD-harboring bacterial community structures, but OM application only influenced phoD-harboring bacterial community structure. Co-occurrence network of the phoD-harboring bacteria had higher average degree and more nodes and edges than phoC-harboring bacteria. PLS-PM results suggested that the rhizosphere effect exhibited greatest contribution to soil ACP and ALP activities than OM treatment.
Conclusion
Compared with short-term OM application, maize rhizosphere effect showed stronger influences on soil phosphatase activities and P-mineralizing bacterial communities in acidic soils. The phoD-harboring bacteria showed the more sensitive response to the rhizosphere effect and OM application, while phoC-harboring bacteria was only influenced by rhizosphere effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acidic soils (pH < 5.5) make up approximately 2.18 million km2 in China and are widely distributed in southern China (Zhao 2002). Phosphorus (P) deficiency in acidic soils severely limits crop production (Kochian et al. 2004). Among the amelioration measures used with acidic soils, the application of organic fertilizer, especially animal manure, has been widely accepted as a sustainable management practice (Liu et al. 2017). Animal manure has become a main source of organic material used to amend acidic soils in China (Yang et al. 2022). Unlike alkaline substances used for neutralizing the soil pH, animal manure can not only increase soil pH, but also effectively improve soil nutrients, especially soil P availability (Pan et al. 2019). Organic P (Po) occupies a large part of the P storage of animal manure and can become P source for plants when inputted into the soil (Wang et al. 2019). Before soil Po is biologically utilized, it must be mineralized into inorganic P. Phosphate-solubilising microorganisms (PSM) are mainly responsible for soil Po mineralization by producing extracellular enzymes including acid phosphatase (ACP) and alkaline phosphatase (ALP) (Wang et al. 2022). It has been well known that the PSM containing the phoC and phoD genes are in charge of the production of ACP and ALP, respectively (Wang et al. 2021a). The bacterial phoC and phoD genes have been frequently used as molecular biomarkers to evaluate the responses of PSM communities to fertilization management practices (Luo et al. 2019; Tan et al. 2013).
Phosphatase activity in soils is reported to be closely correlated with the diversity and composition of PSM communities (Fraser et al. 2017; Luo et al. 2019). The effects of animal manure on soil ACP and ALP activities and P-mineralizing bacterial communities have been investigated (Chen et al. 2019; Luo et al. 2019), and the phoC- and phoD-harboring bacterial communities have shown various responses (Luo et al. 2019; Zheng et al. 2021). For instance, Luo et al. (2019) found that animal manure application improved phoC gene diversity but exhibited none influence on phoD gene diversity, while Liu et al. (2021a) observed that animal manure addition increased phoD gene diversity and ALP activity in an acidic soil. In the study of Chen et al. (2019), animal manure addition did not affect phoD gene diversity, but decreased ALP activity in a brown earth. The inconsistencies in the responses of both phoC- and phoD-harboring bacteria to animal manure may be due to the differences in ecosystem type (Ragot et al. 2017), microbial taxa (Luo et al. 2019; Zheng et al. 2021), as well as type and amount of manure (Diacono and Montemurro 2010).
Numerous studies mostly focused on the long-term effects of manure application on phosphatase activity and P-mineralizing bacterial communities, over periods such as 27 years (Zheng et al. 2021), 30 years (Luo et al. 2019; Liu et al. 2021a), and 39 years (Wan et al. 2020). In these studies, the researchers assumed that changes in soil quality are very slow and soil microbial community structure takes a long time to reach stability (Sun et al. 2015). In fact, animal manure can influence soil microbial activity and community in a short period of time due to the rapid change in soil physical and chemical properties. For example, Urra et al. (2019) found that soils amended with animal manure showed higher microbial biomass and activity after 8 weeks. The mineralization rate of Po in soil with the addition of animal manure was found to be highest within a few weeks in an acidic soil (Yang et al. 2011). Therefore, more attention needs to be paid to the influence of animal manure on PSM community and activity over short timescales.
Manure-related factors, especially application dose, impose a significant impact on soil microbes (Diacono and Montemurro 2010; Yang et al. 2020). The dose of organic manure addition is not always linearly correlated with the improvement of soil microbial function and plant growth. Sun et al. (2014) investigated the changes in the microbial function within loam soil following different applications of manure doses (5%, 10%, 15%, 20%, and 25%), and found that soil with 10% manure application showed the highest biological activity. Even high manure application doses have been reported to cause negative effects on crop yield (Yang et al. 2020). Therefore, the dose of manure must be considered when applying animal manure to ameliorate acidic soils. Understanding the changes in soil phosphatase activities and associated bacterial communities following different doses of animal manure is essential for sustainable agriculture management strategies and for the manipulation of the PSM function during practical amelioration of acidic soils.
The rhizosphere is a highly complex ecosystem consisting of the narrow zone of nutrient-rich soil that surrounds and is closely influenced by plant roots (Venturi and Keel 2016). The absorption of plant P mainly occur in the rhizosphere (Hinsinger 2001), in which high soil phosphatase activity and reduced diversity of rhizosphere microbial community are generally observed (Fan et al. 2017; Kuzyakov and Blagodatskaya 2015; Liu et al. 2021b). It is known that plants can secrete ACP into the rhizosphere under P-deficiency condition (Nannipieri et al. 2011). Furthermore, root exudates from plants can stimulate PSM to secrete phosphatase due to the input of carbon (C) sources and other nutrients (Richardson et al. 2009). The rhizosphere PSM community is often reported to be distinct from that in bulk soil (Liu et al. 2021a, b; Mendes et al. 2014). The intensity of the rhizosphere effect on soil enzyme activity and microbial community is strongly affected by fertilization through changing soil characteristics and plant growth (Liu et al. 2020). Conversely, the rhizosphere effect can also influence the intensity of fertilization on soil microbes (Ai et al. 2012). Thus, the function and community composition of rhizosphere microbes are collectively affected by the host plant and fertilization regime. Although the effects of animal manure and plant rhizosphere on soil phosphatase activity and P-mineralizing bacterial communities have been separately determined (Chen et al. 2019; Luo et al. 2019; Mendes et al. 2014), their interaction and the strength of relative effect have been rarely reported, especially in acidic soils.
In this study, considering the important role of PSM in mineralizing soil Po, we conducted a short-term experiment with different animal manure doses in acidic soil and determined ACP and ALP activities, and P-mineralizing bacterial communities in both bulk and rhizosphere soils. Our objectives are (1) to determine the relative strength of animal manure addition and rhizosphere effect on soil phosphatase activities and P-mineralizing bacterial populations in acidic soils; (2) to assess the distinct responses of the phoC- and phoD-harboring bacteria to animal manure addition and the rhizosphere effect.
Materials and methods
Experimental setup
The acidic soil used in this study was collected from surficial soil (0–20 cm) of the Ah horizons (humus layer) in a pine forest in April 2019 at the Yingtan Red Soil Ecological Experiment Station (28°14′N, 117°03′E) in Jiangxi Province, China. After roots and forest litter were removed, the acidic soil was homogenized by sieving through a 2-mm mesh. Air-dried pig manure was used as organic manure in this study. The basic properties of soil and pig manure were shown in Table S1.
Maize (Zea mays L.) is a major crop in the acidic soil regions of China, thus maize was chosen as the experimental plant in this study. Maize seeds (cv. Zhengdan 958) were planted in the plastic pots with dimensions of 175 mm (open top) × 125 mm (flat bottom) × 135 mm (height). Organic manure (OM) was applied at different doses of 0 (OM0), 1 (OM1), 5 (OM5), 10 (OM10), 20 (OM20), and 50 (OM50) g kg− 1 dry soil, without the addition of any other fertilizer. Maize was cultivated in a greenhouse with natural lighting condition. During the pot experiment, the temperature in the greenhouse was in the range of 18–32 °C and a relative humidity of 40–80%.
There are eight replicate pots for each dose treatment. Four pots were planted with maize and four were non-planted. For each pot, OM and 2.5 kg of soil were thoroughly mixed and preincubated for 7 days before sowing. The maize seeds were washed three times with distilled water after sterilized using 10% hydrogen peroxide. Five maize seeds were sown into the soil per pot and thinned to three plants after emergence. Soil moisture was adjusted with tap water to 60% field capacity, and maize grew in a natural greenhouse.
Plant and soil sampling
Maize was harvested at jointing stage after six weeks of sowing. The roots were separated from the soils. The rhizosphere soil was defined as the soils which adhered to the roots after gentle shaking. The bulk soil samples were sampled from the non-plant pots. The rhizosphere/bulk soil from an individual pot was mixed into a single sample. Each soil sample was passed through a 2-mm sieve and then divided into three portions. One portion was immediately stored at -20 °C until DNA extraction; the second portion was stored at 4 °C for the determination of soil ACP and ALP activities, ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3–-N); the last portion was air-dried for the determination of the soil pH, soil organic matter (SOM), total N (TN), total P (TP), total K (TK), available P (AP) and available K (AK).
Determination of plant nutrient, soil properties and phosphatase activities
Shoots and roots of maize were washed three times with deionized water and then dried at 85 °C until a constant weight. The oven-dried shoots were ground (< 1.0 mm) and digested with H2SO4-H2O2 to measure the nutrient contents (N, P and K). The Kjeldahl (Lu 1999) and Bray (Bray and Kurtz 1945) methods were used for the determination of N and P in digestions, respectively, and the K content was measured by flame photometry (FP640, Shanghai, China).
Soil pH, SOM, TN, TP, TK, NH4+-N, NO3–-N, AP and AK were determined according to the methods of our previous study (Zheng et al. 2021).
The activities of ACP and ALP were measured using Tabatabai method (1994). Briefly, 0.5 g of fresh soil was incubated with modified universal buffer of pH 6.5 and pH 11.0, respectively, which contained 50 mM p-nitrophenyl phosphate (pNPP, Sigma-Aldrich, USA) at 37 °C for 1 h. The potential enzyme activities were defined as the amount of µg of p-nitrophenol (pNP) produced by per gram soil (dry weight) per 1 h.
High-throughput sequencing and data processing
Each soil DNA was extracted from 0.5 g of fresh bulk or rhizosphere soil sample using the Fast DNA SPIN Kit (MP Biomedicals, CA, USA) and then stored at -20 °C. The universal primers phoc-A-F1 (5′- CGGCTCCTATCCGTCCGG − 3′)/phoc-A-R1 (5′- CAACATCGCTTTGCCAGTG − 3′) (Fraser et al. 2017) and ALPS-F730 (5′- CAGTGGGACGACCACGAGGT − 3′)/ALPS-R1101 (5′- GAGGCCGATCGGCATGTCG − 3′) (Sakurai et al. 2008) were used to amplify the phoC and phoD genes, respectively. The PCR thermal cycling process and reaction system were described in our previous study (Zheng et al. 2021). The 7-bp barcodes were incorporated into the forward primer to identify and sort the reads from different treatments. Triplicate PCR products of each DNA sample were pooled as one single sample. PCR products were quantified and pooled in equimolar concentrations and then paired-end sequenced using Illumina HiSeq PE150 for the phoC gene and Illumina Miseq PE250 for the phoD genes (Shanghai Personal Biotechnology Co., Ltd., China). The sequence data were submitted to NCBI Sequence Read Archive (SRA) with accession number SRP370141 for phoC gene and SRP370156 for phoD gene.
The pairs of reads obtained were merged using FLASH (version 1.2.7) software (Magoc and Salzberg 2011), and then the Quantitative Insights Into Microbial Ecology (QIIME, version 1.8.0) pipeline was employed to process the sequencing reads (Caporaso et al. 2010). Low-quality sequences were eliminated if they did meet the following criteria: (i) the sequences lengths < 130 bp for phoC gene and < 150 bp for phoD gene; and (ii) the sequences containing ambiguous nucleotides and not matching the primer. The chimeric sequences were also removed using USEARCH (version 5.2.236). Using UCLUST (Edgar 2010), we clustered the remaining high-quality sequences at 97% sequence similarity defining the operational taxonomic units (OTUs). The sequences were identified using the closest relative from a BLAST with the GenBank database (http://blast.ncbi.nlm.nih.gov/blast.cgi) with a cutoff E-value of 10− 5. OTU compositions were randomly rarefied to 14,248 reads per sample of the phoC and phoD reads for the downstream analysis.
Network analysis
To allow robustness, core OTUs present in at least 8 samples were considered. The Spearman’s correlation coefficient (r) > 0.6 and a P-value < 0.05 were chosen as statistically robust correlation between OTUs in the network. The P-values were further corrected for multiple comparisons using the method of Benjamini-Hochberg. The correlations between OTUs were calculated in R (version 4.0.2) using the “Hmisc” package (Yang et al. 2022). The network graphs were visualized, and the network topological features were calculated using Gephi (version 0.9.2). The sub-network for each soil sample was extracted from the original co-occurrence network according to the study of Xiao et al. (2017).
Statistical analysis
The α-diversities (richness and Shannon index) were analyzed by QIIME. Significant differences in the maize biomass, nutrient (N, P and K) contents of the maize shoots and the relative abundances of dominant phoC- and phoD-harboring bacterial genera in bulk and rhizosphere soils among treatments were processed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA), based on one-way analysis of variance (ANOVA) with Duncan’s post-hoc test. Two-way ANOVA was performed to analyze the effects of OM application and rhizosphere effect (bulk versus rhizosphere) on soil properties, ACP and ALP activities, α-diversity indices and network topologies. If the differences were significant, one-way ANOVA or t-tests were further carried out to analyze the homogeneity of the variance. The Spearman correlation analysis among OM doses, soil properties, the relative abundance of dominant genera, network topologies and soil ACP and ALP activities were performed using SPSS 20.0.
The principal coordinate analysis (PCoA) based on Bray–Curtis distance was used to visualize the β-diversity of the phoC- and phoD-harboring bacterial communities across all samples. Permutational multivariate analysis of variance (PERMANOVA) was performed to evaluate the influences of OM application and rhizosphere effect on the phoC- and phoD-harboring bacterial community compositions. The dissimilarities of the phoC- and phoD-harboring bacterial community compositions between each OM application treatment and OM0 treatment were assessed using the ADONIS function. PCoA, PERMANOVA and ADONIS were implemented in R (version 4.0.2) using the “vegan” package (Liu et al. 2021a).
Partial least squares path modeling (PLS-PM) was conducted using the “plspm” package in R (Sanchez 2013) to reveal the effects of OM application, rhizosphere effect, soil properties, phoC- and phoD-harboring bacterial α-diversities and community compositions as well as network connectivity on ACP and ALP activities. Bacterial α-diversity was indicated by the richness and Shannon index, and community composition was indicated by the first principal coordinate (PCoA1). The network connectivity was represented by the clustering coefficient of each sub-network, according to the study of Xiao et al. (2017). All codes used in this study were shown in the Supplementary Material.
Results
OM application improves maize growth and affects soil properties and phosphatase activities
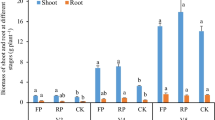
Maize biomass (shoot and root) and plant nutrient contents (N, P and K) improved with increased doses of OM (Table 1). The shoot and root biomass were significantly (p < 0.05) higher in OM5, OM10, OM20 and OM50 than those in OM0 and OM1, but root biomass between OM20 and OM50 did not show significant difference (Table 1). Compared to OM0, both OM20 and OM50 significantly (p < 0.05) increased the N contents of maize shoot, and OM10, OM20 and OM50 significantly (p < 0.05) increased the P contents of maize shoot (Table 1). Except for OM1, all OM applications sharply (p < 0.05) improved the K contents of maize shoot relative to OM0 (Table 1).
Two-way ANOVA showed that both OM application and rhizosphere effect significantly (p < 0.05) affected soil pH and the contents of soil TN, NH4+-N, NO3−-N, TK, SOM, AP and AK (Table S2). OM20 and OM50 increased (p < 0.05) soil pH and the contents of soil TN, SOM, AP and AK, compared with OM0 (Table S2). The rhizosphere soils had higher (p < 0.05) SOM contents than bulk soils (Table S2). The contents of NH4+-N and NO3−-N in the rhizosphere soils were lower than those in the bulk soils under all treatments, except for NH4+-N in the OM1 treatment (Table S2).
Both OM application and rhizosphere effect significantly (p < 0.05) affected soil ACP activities, and only rhizosphere effect influenced (p < 0.05) soil ALP activities (Fig. 1). Besides, rhizosphere effects on both ACP and ALP activities were more obvious than OM application. The rhizosphere soils showed higher (p < 0.05) ACP and ALP activities than bulk soils under all treatments, except for the ALP activity in OM10 (Fig. 1). However, compared to OM0, all treatments of OM application significantly (p < 0.05) decreased rhizosphere ACP activities, while only OM50 significantly (p < 0.05) reduced rhizosphere ALP activity (Fig. 1). OM50 showed lowest rhizosphere ACP and ALP activities (Fig. 1). The highest ACP activity in the bulk soils was observed under the OM10 treatment, but there was not statistically different with OM0 (Fig. 1A). In contrast, OM10 showed highest ALP activity in the bulk soils and significantly (p < 0.05) higher than OM0 (Fig. 1B).
Soil ACP (A) and ALP activities (B) in the bulk and rhizosphere soil samples of maize after six weeks of cultivation with different manure doses. Values are presented as the mean ± SD of four pot replicates. All data were first subjected to two-way ANOVA. OM: organic manure addition; R: rhizosphere effect. Lower case and capital letters indicate differences of ACP and ALP activities in bulk and rhizosphere among treatments, respectively (p < 0.05). Asterisk indicates significant difference (* p < 0.05 or ** p < 0.01) between bulk and rhizosphere soil samples
Compositions and diversities of the phoC- and phoD-harboring bacterial communities differently respond to rhizosphere effect and OM application
The dominant phoC-harboring genera (average relative abundance above 1%) contained Cupriavidus, Klebsiella, Stenotrophomonas and Xanthomonas (Fig. 2A). The dominant phoD-harboring genera (average relative abundance above 1%) contained Collimonas, Pleomorphomonas, Bradyrhizobium, Streptomyces, Pseudomonas, Gemmatimonas and Cupriavidus (Fig. 2B). For the phoD-harboring bacteria, OM10, OM20 and OM50 significantly (p < 0.05) reduced the relative abundances of Collimonas and Streptomyces in the bulk soils, but improved (p < 0.05) the relative abundance of Pleomorphomonas relative to OM0 (Table S3). Additionally, OM20 and OM50 significantly (p < 0.05) increased the relative abundance of Pseudomonas in the rhizosphere soils, but decreased (p < 0.05) the relative abundance of Collimonas (Table S3). The Spearman correlation analysis revealed that ALP activity was positively (p < 0.01) related to the relative abundance of the phoD-harboring genus Collimonas, and negatively (p < 0.01) correlated with the relative abundances of Pleomorphomonas, Bradyrhizobium, Streptomyces, Pseudomonas and Cupriavidus (Fig. 2C). However, ACP activity did not show the correlation with the dominant phoC-harboring genera (Fig. 2C).
The relative abundances of the dominant (average relative abundance above 1%) phoC-(A) and phoD-(B) harboring bacterial genera in the bulk and rhizosphere soil samples of maize after six weeks of cultivation with different organic manure doses. The correlations between the OM addition, soil variables, ACP, ALP and phoC- and phoD-harboring dominant genera were determined by Spearman test (C). * indicates significant correlation at 0.05 level; ** indicates significant correlation at 0.01 level
The rhizosphere effect significantly influenced (p < 0.05) the richness and Shannon index of the phoC-harboring bacteria (Fig. 3A and C). Both OM application and rhizosphere effect affected (p < 0.01) the richness and Shannon index of the phoD-harboring bacteria (Fig. 3B and D). The richness of the phoC- and phoD-harboring bacteria were higher in the rhizosphere soils than those in the bulk soils under the OM20 and OM50 treatments (Fig. 3A and B). In addition, compared to bulk soils, the rhizosphere soil showed the higher (p < 0.05) richness of phoD-harboring bacteria under OM10 treatment (Fig. 3B). The rhizosphere soils exhibited lower (p < 0.01) Shannon index of the phoD-harboring bacteria than bulk soils under the OM0, OM1, OM5 and OM10 treatments (Fig. 3D).
The richness and Shannon index of the phoC- (A, C) and phoD-harboring (B, D) bacterial communities in the bulk and rhizosphere soil samples of maize after six weeks of cultivation with different organic manure doses. Values are presented as the mean ± SD of four pot replicates. All data were first subjected to two-way ANOVA. OM: organic manure addition; R: rhizosphere effect. Lower case and capital letters indicate differences among treatments in the bulk and rhizosphere soils, respectively (p < 0.05). Asterisk indicates significant difference (* p < 0.05 or ** p < 0.01) between bulk and rhizosphere soil samples
phoD-harboring bacterial community structure is more sensitive to rhizosphere effect and OM application than phoC-harboring bacteria
The PCoA results illustrate the community structures of the phoC- and phoD-harboring bacteria (Fig. 4A and B). PERMANOVA results revealed that both phoC- and phoD-harboring bacterial community structures were significantly (p < 0.01) influenced by the rhizosphere effect, and the phoD-harboring bacterial community structure rather than the phoC-harboring bacterial community were affected (p < 0.01) by OM application (Fig. 4C). Moreover, the rhizosphere effect showed the higher influence on the phoD-harboring bacterial community structure (F = 47.498, p = 0.001) than the phoC-harboring bacterial community (F = 4.506, p = 0.001). ADONIS analysis further indicated that compared with OM0, the phoD-harboring bacterial community structures of OM20 and OM50 were significantly (p < 0.05) altered, while the phoC-harboring bacterial community structure of each OM application treatment was not significantly different from OM0 (Table S4).
The principal coordinate analysis of the phoC-(A) and phoD-(B) harboring bacterial communities, and permutational multivariate analysis of variance (PERMANOVA) (C) among treatments. Circle and triangle dots represent bulk and rhizosphere soil samples, respectively. Different colors indicated different treatments. OM: organic manure addition; R: rhizosphere effect
Network topological features of the phoC- and phoD-harboring bacteria differently respond to rhizosphere effect and OM application
The co-occurrence networks of phoC- and phoD-harboring bacteria were established based on the correlations among OTUs (Fig. 5A and B). Compared with the phoD-harboring bacterial network, the phoC-harboring bacterial network showed the higher average clustering coefficient, density and modularity, but lower diameter, average degree, average path length and the numbers of nodes and edges (Fig. 5C). Both networks had high percentages of positive correlations of edges (Fig. 5C).
For the phoC-harboring bacterial network, both rhizosphere effect and OM application significantly influenced (p < 0.05) the numbers of node and edge, average path length and clustering coefficient, and rhizosphere soils showed lower values of degree than bulk soils (Table 2). The rhizosphere soils in the treatments of OM application significantly (p < 0.05) decreased the edge numbers of the phoC-harboring bacterial network compared with bulk soils (Table 2). Both rhizosphere effect and OM application significantly (p < 0.05) affected the numbers of node and edge, degree and clustering coefficient of the phoD-harboring bacterial network (Table 3). Rhizosphere soils under all treatments showed higher edge numbers, degree and clustering coefficient of the phoD-harboring bacterial network compared to bulk soils, except for the degree under OM50 treatment (Table 3).
Rhizosphere effect contributes mostly to the variations of PSM communities and phosphatase activities
The Spearman correlation analysis showed that soil TN, NH4+-N, NO3−N, TK and SOM contents were significantly (p < 0.05) related to both ACP and ALP activities, and soil pH was positively (p < 0.05) correlated with ALP activity (Fig. S1). Additionally, ACP activity was positively (p < 0.05) related to positive edge number and average path length and negatively correlated (p < 0.05) with the numbers of total edge and negative edge, degree and clustering coefficient of the phoC-harboring bacterial network (Fig. 6). ALP activity was positively (p < 0.05) related to the numbers of node, total edge and negative edge, degree and clustering coefficient and negatively correlated (p < 0.05) with the number of positive edges of the phoD-harboring bacterial network (Fig. 6). Most of the soil variables measured were positively or negatively (p < 0.05) correlated with the topological features of the two networks (Fig. 6).
To better understand the relative contributions of rhizosphere effect and PSM community to the changes of ACP and ALP activities, we constructed the partial least squares path modelling (PLS-PM) (Fig. 7). Rhizosphere effects exhibited stronger influences on both ACP (path coefficient = 0.788) and ALP activities (path coefficient = 1.061) than the α-diversity, community composition and network clustering coefficient of phoC- and phoD-harboring bacterial communities. OM application positively influenced soil properties which were indicated by soil pH, TN, TK, SOM, NH4+-N and NO3–-N, and soil properties showed positive effects on diversities and community structures of both phoC- and phoD-harboring bacteria. However, soil properties exhibited negative influences on the clustering coefficient of phoC- and phoD-harboring bacterial network, and there was stronger effect on phoD-harboring bacterial network (path coefficient = -0.526). The diversity (path coefficient = 0.021) and composition (path coefficient = -0.007) of the phoC-harboring bacterial community showed little contribution to ACP activity, while the clustering coefficient (path coefficient = -0.260) of the phoC-harboring bacterial network exhibited a negative effect on ACP activity. The community composition (path coefficient = 0.566) and clustering coefficient (path coefficient = 0.379) of phoD-harboring bacteria had high positive contributions to ALP activity, while the diversity (path coefficient = -0.126) showed a negative contribution.
Partial least squares path models (PLS-PM) of the drivers of ACP and ALP activities. For ACP activity, soil properties included soil TN, TK, SOM, NH4+-N, NO3–-N; for ALP activity, soil properties included soil pH, TN, TK, SOM, NH4+-N, NO3–-N. The α-diversity of phoC-/phoD-bacterial was indicated by the richness and Shannon index; the community composition of phoC-/phoD-bacterial is represented by the first principal coordinates (PCoA1). Each oblong box represents a latent variable, which was chosen according to the correlations among these indicators. Path coefficients were calculated after 1000 bootstraps. The red and blue lines represent positive and negative effects, respectively. The full and dashed lines indicated the significant correlations (p < 0.05) and no correlations (p > 0.05), respectively
Discussion
Rhizosphere effect showed the stronger influences on soil phosphatase activities and P-mineralizing bacterial communities than short-term manure application
In acidic soils, the promoting effect of organic manure application on crop growth is mainly attributable to the improvements of soil nutrients and pH (Waldrip et al. 2011). Soil pH is an important factor influencing plant grow and the uptake of plant nutrients (Tandzi et al. 2018). In this study, OM5 significantly increased maize shoot and root biomass, but did not significantly improve total P and N contents of maize shoot (Table 1). This suggested that the low dose of OM application improved maize growth mainly through increasing soil pH rather than nutrients. Obviously, maize growth was contributed by both increased soil pH and nutrients at high dose of OM addition (> 10 g kg− 1) in this short-term experiment.
Considering the important role of PSM, the long-term effects of animal manure application on soil phosphatase activities and associated PSM communities have received much attention (Liu et al. 2021a; Luo et al. 2019; Wan et al. 2020), but the rhizosphere effect in plant-soil systems, especially on phoC-harboring bacteria, has not been extensively examined (Luo et al. 2019; Zheng et al. 2021). In a study with short-term pot experiment, the diversity, composition and function of soil PSM communities that are modulated by the plant rhizosphere are of great significance to soil P availability and plant biomass (Guo et al. 2022). On the basis of results from our present short-term experiment, the rhizosphere effect of maize growing in acidic soil had a stronger influence on soil ACP and ALP activities, and α-diversities and compositions of both phoC- and phoD-harboring bacterial communities, than animal manure application (Figs. 1, 2 and 3). Thus, although fertilization affected the soil phosphatase activities and P-mineralizing bacterial community, the rhizosphere effect should be carefully considered in the short-term amended acidic soil using organic manure.
The strong effect of plant rhizosphere on soil microbes has been reported (Guo et al. 2022; Wang et al. 2020a). Plant rhizosphere is a highly heterogeneous microenvironment and nutrient-dense region (Broeckling et al. 2008; Wang et al. 2020b), which is strongly influenced by the energy and nutrition derived from root exudates as well as the symbiotic relationships between plants and microbes (Kuzyakov and Blagodatskaya 2015; Wang et al. 2020b). The rich nutrient source in the rhizosphere supports a diverse population of PSM (Shrivastava et al. 2010), and the variations of the rhizosphere PSM community and function largely depend on the type and amount of the root exudates (Chaparro et al. 2014). Especially in acidic soils with P deficiency and aluminum (Al) toxicity, plants will increase the secretion of organic acids (Liao et al. 2006; Chen and Liao 2016), which can be effectively utilized as a C source by soil microbes (Jones et al. 2003). Additionally, plant roots are able to secrete more ACP into the rhizosphere under P-deficiency condition (Nannipieri et al. 2011). Therefore, the rhizosphere effect on soil phosphatase activities and P-mineralizing bacterial communities may be more intense in acidic soils (Ren et al. 2020), even though the amelioration of acidic soil with animal manure dramatically improves the soil environment (Urra et al. 2019).
In the soil-plant system, plant roots and soil microbes simultaneously contribute to the soil phosphatase activity, and the interaction of plant-microbes can further affect microbial-derived phosphatase activity (Spohn and Kuzyakov 2013). In this study, compared to PSM, the rhizosphere effect showed stronger influence and provided a main contribution to soil phosphatase activities (Figs. 1 and 7). More obviously, the variation of ACP activity was mainly influenced by plant rhizosphere, and the attributes of the phoC-harboring bacterial community showed little contribution (Fig. 7). This suggested that the increased ACP activity in the rhizosphere was mainly produced by plant roots rather than by phoC-harboring bacteria in the current experiment. Plant-derived ACP is an important mechanism for the improvement of mineralization of soil Po and the absorption of plant P (Nannipieri et al. 2011). Plants in response to P-deficient conditions can improve the secretion of ACP to increase soil P availability (Nannipieri et al. 2011). These plant-derived ACP may inhibit ACP production by phoC-harboring bacteria (Fraser et al. 2017). Besides, low soil pH in acidic soil depressed soil microbial activity (Cha et al. 2021), which might limit the function of phoC-harboring bacteria, even though the rhizosphere environment was improved.
Different from ACP, ALP is mainly secreted by soil microbes (Fraser et al. 2017), thus PSM-derived ALP is responsible for increased ALP activity. In this study, the increased rhizosphere ALP activity was mainly attributed to the regulation of plant rhizosphere on phoD-harboring bacteria, reflected by the community composition and connectivity, represented by the clustering coefficient of the network (Fig. 7). Similar finding was reported by Luo et al. (2019). The changes of the relative abundance of dominant genera are the main reflection of community composition variation (Iannucci et al. 2021). Among the dominant phoD-harboring genus, the Collimonas was the key contributor to ALP activity as indicated by the positive correlation between Collimonas and ALP activity (Fig. 2C). The Collimonas belongs to the order Burkholderiale which exhibit high tolerance to low soil pH and Al toxicity (Leveau et al. 2010), thus they can better adapt to acidic soil by secreting phosphatases.
The quality of the connections among species was reflected by the clustering coefficient of the microbial network (Xiao et al. 2017), and the PSM community with a highly connected network has been associated with greater phosphatase activity (Zheng et al. 2021). Thus, the combined influences of OM application and rhizosphere effect should increase the synergic relationships among the phoD-harboring bacterial species, which are conducive to the improvement of ALP activity. Moreover, root exudates can also enhance ALP activity through stimulating PSM functional gene expression at the RNA level (Ragot et al. 2016), but this process cannot be fully reflected in the community composition. For instance, Ragot et al. (2016) found that fertilization up-regulated the expression of functional gene of phoD-harboring Xanthomonadales at the RNA level but showed less influence on phoD-harboring bacterial community at the DNA level.
The strength of rhizosphere effect on soil phosphatase activities was highly dependent on the dose of OM, since the rhizosphere ACP and ALP activities decreased with increased OM doses (Fig. 1). Similarly, Ai et al. (2012) found that organic manure addition reduced the increased degree of phosphatase activity in the wheat rhizosphere. On the one hand, the high soil AP contents caused by OM application can inhibit soil phosphatase activity, because soil phosphatase is a functional induced enzyme and very sensitive to soil AP (Ye et al. 2017). On the other hand, increased root biomass with increased OM doses expands the root absorption area for soil P, which may reduce plant demand for PSM function. Root enlargement can be another important mechanism that improves the absorption of soil P by plants (Ramaekers et al. 2010). Thus, plant biomass and rhizosphere phosphatase activities exhibited opposite response patterns to OM application in an acidic soil. This may indicate a balancing mechanism exists for soil P utilization between plant and soil microbes.
Various responses of the phoC- and phoD-harboring bacterial communities to rhizosphere effect and animal manure
In the present study, the attributes of the phoC- and phoD-harboring bacterial communities, including diversity (Fig. 3), community structure (Fig. 4) and network topological features (Tables 2 and 3), exhibited various responses to both rhizosphere effect and OM application. Different from phoC-harboring bacteria, the rhizosphere effect reduced the Shannon index of phoD-harboring bacteria under low doses of OM application (OM0, OM1, OM5 and OM10). The similar result was reported by Liu et al. (2021b). The decreased Shannon index of the phoD-harboring bacteria should be attributed to the low evenness values, as there was no influence on richness (Fig. 3B). In response to the environmental gradient, the excessive growth of some taxa in the community can reduce total evenness, finally decreasing the Shannon index (Hartmann et al. 2015). However, in this study, high doses of OM application (OM20 and OM50) increased the Shannon index and richness of rhizosphere phoD-harboring bacteria as well as the richness of rhizosphere phoC-harboring bacteria (Fig. 3). Improved maize growth under high OM application may supply more root exudates to phoC- and phoD-harboring bacteria and increase bacterial diversity in the rhizosphere (Kuzyakov and Blagodatskaya 2015).
Short-term fertilizer application changed phoC- and phoD-harboring bacterial community compositions to varying degrees, but exhibited different even opposite effects on their diversities (Guo et al. 2022). For example, Guo et al. (2022) found that short-term application of chemical P fertilizer decreased Shannon of phoD-harboring bacteria in rhizosphere soil, but increased Shannon of rhizosphere phoC-harboring bacteria. In this study, the influence of both OM application and rhizosphere effect on the phoD-harboring bacterial community structure was stronger than on the phoC-harboring bacteria (Fig. 4C), which supported the finding that the response of soil PSM community to fertilization is taxa-dependent (Zheng et al. 2021). On the one hand, ACP produced by plants roots can weaken the response of the phoC-harboring bacterial community to environmental change (Fraser et al. 2017). On the other hand, the phoC- and phoD-harboring bacteria have the different preferences for soil nutrient (Zheng et al. 2021). The majority of phoC-harboring bacterial genus belong to oligotrophs with low nutrient demands (Starke et al. 2016), and thus they can maintain viability and stability when exposed to environmental gradients (Fierer et al. 2007). Contrary to this, the phoD-harboring bacterial species are relatively sensitive to environmental factors, because of significant correlations between ALP activity and most soil variables (Fig. 2C). In the present study, the OM application and rhizosphere effect changed most of the soil variables, including soil pH, SOM, AP and TP which were frequently reported to influence the phoD-harboring bacterial community structure under manure fertilization (Chen et al. 2017; Hu et al. 2018).
Ecological network analysis can visualize the interactions of microbial communities and reveal the co-occurrence relationships of species in microhabitats (Mendes et al. 2018). In the current study, the positive correlations between species dominated both the phoC- and phoD-harboring bacterial networks (Tables 2 and 3), implying that mutual cooperation played an important role in the P-mineralizing bacterial community in manure-amended acidic soils. The strong cooperation of microbial communities can contribute to more active microbial function (Faust and Raes 2012). Nevertheless, there were differences between phoC- and phoD-harboring bacterial networks. Relative to the phoC-harboring bacteria, the phoD-harboring bacterial network exhibited higher average path length but slightly lower modularity, density and average clustering coefficient (Fig. 5C), indicating higher competition for resource between the phoD-harboring bacteria (Mendes et al. 2018). Resource competition can be enhanced in highly abundant and more diverse microbial communities (Mendes et al. 2018). One possible explanation could be that the increased competition for resources can promote interactions between different microbial species (Giovannoni et al. 2014), further affecting phoD-harboring bacterial community structure and increasing ALP activity. This is consistent with more complex interactions in low-fertility acidic soils (Wang et al. 2021b). Moreover, the low modularity of the phoD-harboring bacterial network indicated an unstable community (Xun et al. 2021) and higher sensitivity of the community to environmental gradients (Wang et al. 2016). This was identified by stronger correlations between the OM addition, soil variables and phoD-harboring dominant genera (Fig. 2C), which resulted in the variation of community composition and ALP activity.
Conclusion
Although short-term OM application clearly amended acidic soil, the rhizosphere effect showed stronger influences on soil ACP and ALP activities and P-mineralizing bacterial communities in the present pot experiment. Notably, the phosphatase activity in the rhizosphere was negatively correlated with the OM application. Rhizosphere ACP activity was mainly derived from plant root rather than phoC-harboring bacteria, while rhizosphere ALP activity was attributed to community composition and species interactions of phoD-harboring bacteria that were induced by OM application and rhizosphere effect. Due to the differences between microbial groups, both phoC- and phoD-harboring bacteria showed different sensitivity to rhizosphere effect and OM application, with stronger influences on phoD-harboring bacteria. Thus, although the short-term organic manure application increased crop growth and changed soil PSM community, the influence of rhizosphere effect on PSM community and function need more attention in the amended acidic soils.
References
Ai C, Liang G, Sun J, Wang X, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 173–174:330–338
Bray RH, Kurtz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Broeckling CD, Manter DK, Paschke MW, Vivanco JM (2008) Rhizosphere ecology. Encycl Ecol 3:574–578
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Tumbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Cha S, Kim YS, Lee AL, Lee DH, Koo N (2021) Liming alters the soil microbial community and extracellular enzymatic activities in temperate coniferous forests. Forests 12:190
Chaparro JM, Badri DV, Vivanco JM (2014) Rhizosphere microbiome assemblage is affected by plant development. ISME J 8:790–803
Chen Z, Liao H (2016) Organic acid anions: An effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J Genet Genomics 43:631–638
Chen X, Jiang N, Chen Z, Tian J, Sun N, Xu M, Chen L (2017) Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials. Appl Soil Ecol 119:197–204
Chen X, Jiang N, Condron LM, Dunfiel KZ, Chen Z, Wang J, Chen L (2019) Soil alkaline phosphatase activity and bacterial phoD gene abundance and diversity under long-term nitrogen and manure inputs. Geoderma 349:36–44
Diacono M, Montemurro F (2010) Long-term effects of organic amendments on soil fertility. A review. Agron Sustain Dev 30:401–422
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Fan K, Cardona C, Li Y, Shi Y, Xiang X, Shen C, Wang H, Gilbert JA, Chu H (2017) Rhizosphere-associated bacterial network structure and spatial distribution differ significantly from bulk soil in wheat crop fields. Soil Biol Biochem 113:275–284
Faust K, Raes J (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Fraser TD, Lynch DH, Gaiero J, Khosla K, Dunfield KE (2017) Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl Soil Ecol 111:48–56
Giovannoni SJ, Thrash JC, Temperton B (2014) Implications of streamlining theory for microbial ecology. ISME J 8:1553–1565
Guo L, Wang C, Shen R (2022) Stronger effects of maize rhizosphere than phosphorus fertilization on phosphatase activity and phosphorus-mineralizing-related bacteria in acidic soils. Rhizosphere 23:100555
Hartmann M, Frey B, Mayer J, Maeder P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9:1177–1194
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hu Y, Xia Y, Sun Q, Liu K, Chen X, Ge T, Zhu B, Zhu Z, Zhang Z, Su Y (2018) Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci Total Environ 628–629:53–63
Iannucci A, Canfora L, Nigro F, De Vita P, Beleggia R (2021) Relationships between root morphology, root exudate compounds and rhizosphere microbial community in durum wheat. Appl Soil Ecol 158:103781
Jones DL, Dennis PG, Owen AG, van Hees PAW (2003) Organic acid behavior in soils - misconceptions and knowledge gaps. Plant Soil 248:31–41
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? - Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493
Kuzyakov Y, Blagodatskaya E (2015) Microbial hotspots and hot moments in soil: concept & review. Soil Biol Biochem 83:184–199
Leveau JHJ, Uroz S, de Boer W (2010) The bacterial genus Collimonas: mycophagy, weathering and other adaptive solutions to life in oligotrophic soil environments. Environ Microbiol 12:281–292
Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV (2006) Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance, exudation of specific organic acids from different regions of the intact root system. Plant Physiol 141:674–684
Liu S, Razavi BS, Su X, Maharjan M, Zarebanadkouki M, Blagodatskaya E, Kuzyakov Y (2017) Spatio-temporal patterns of enzyme activities after manure application reflect mechanisms of niche differentiation between plants and microorganisms. Soil Biol Biochem 112:100–109
Liu J, Ma Q, Hui X, Ran J, Ma Q, Wang X, Wang Z (2020) Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available-P deficiency. Soil Biol Biochem 149:107918
Liu S, Zhang X, Dungait JAJ, Quine TA, Razavi BS (2021a) Rare microbial taxa rather than phoD gene abundance determine hotspots of alkaline phosphomonoesterase activity in the karst rhizosphere soil. Biol Fertil Soils 57:257–268
Liu W, Ling N, Luo G, Guo J, Zhu C, Xu Q, Liu M, Shen Q, Guo S (2021b) Active phoD-harboring bacteria are enriched by long-term organic fertilization. Soil Biol Biochem 152:108071
Lu R (1999) Soil and agricultural chemical analysis methods. Chinese Agriculture and Sciences Press, Beijing
Luo G, Sun B, Li L, Li M, Liu M, Zhu Y, Guo S, Ling N, Shen Q (2019) Understanding how long-term organic amendments increase soil phosphatase activities: insight into phoD- and phoC-harboring functional microbial populations. Soil Biol Biochem 139:107632
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014) Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8:1577–1587
Mendes LW, Raaijmakers JM, de Hollander M, Mendes R, Tsai SM (2018) Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J 12:212–224
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. Springer, Berlin Heidelberg
Pan X, Li J, Deng K, Xu K, Shen R (2019) Four-year effects of soil acidity amelioration on the yields of canola seeds and sweet potato and N fertilizer efficiency in an ultisol. Field Crop Res 237:1–11
Ragot SA, Huguenin-Elie O, Kertesz MA, Frossard E, Bunemann EK (2016) Total and active microbial communities and phoD as affected by phosphate depletion and pH in soil. Plant Soil 408:15–30
Ragot SA, Kertesz MA, Meszaros E, Frossard E, Bunemann EK (2017) Soil phoD and phoX alkaline phosphatase gene diversity responds to multiple environmental factors. FEMS Microbiol Ecol 93:1–12
Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J (2010) Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crop Res 117:169–176
Ren Y, Xun W, Yan H, Ma A, Xiong W, Shen Q, Zhang R (2020) Functional compensation dominates the assembly of plant rhizospheric bacterial community. Soil Biol Biochem 150:107968
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Sakurai M, Wasaki J, Tomizawa Y, Shinano T, Osaki M (2008) Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci Plant Nutr 54:62–71
Sanchez G (2013) PLS path modeling with R. Trowchez Editions, Berkeley
Shrivastava M, Rajpurohit YS, Misra HS, D’Souza SF (2010) Survival of phosphate-solubilizing bacteria against DNA damaging agents. Can J Microbiol 56:822–830
Spohn M, Kuzyakov Y (2013) Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocation - Coupling soil zymography with 14 C imaging. Soil Biol Biochem 67:106–113
Starke R, Kermer R, Ullmann-Zeunert L, Baldwin IT, Seifert J, Bastida F, von Bergen M, Jehmlich N (2016) Bacteria dominate the short-term assimilation of plant-derived N in soil. Soil Biol Biochem 96:30–38
Sun J, Zhang Q, Zhou J, Wei Q (2014) Pyrosequencing technology reveals the impact of different manure doses on the bacterial community in apple rhizosphere soil. Appl Soil Ecol 78:28–36
Sun R, Guo X, Wang D, Chu H (2015) Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl Soil Ecol 95:171–178
Tabatabai MA (1994) Soil enzymes. In: Bottomley PS, Angle JS, Weaver RW (eds) Methods of soil analysis: Part 2-microbiological and biochemical properties. Soil Science Society of America, Madison, pp 775–833
Tan H, Barret M, Mooij MJ, Rice O, Morrissey JP, Dobson A, Griffiths B, O’Gara F (2013) Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol Fertil Soils 49:661–672
Tandzi LN, Mutengwa CS, Ngonkeu ELM, Gracen V (2018) Breeding maize for tolerance to acidic soils: a review. Agronomy 8(6):84
Urra J, Alkorta I, Lanzen A, Mijangos I, Garbisu C (2019) The application of fresh and composted horse and chicken manure affects soil quality, microbial composition and antibiotic resistance. Appl Soil Ecol 135:73–84
Venturi V, Keel C (2016) Signaling in the Rhizosphere. Trends Plant Sci 30:401–422
Waldrip HM, He Z, Erich MS (2011) Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biol Fertil Soils 47:407–418
Wan W, Li X, Han S, Wang L, Luo X, Chen W, Huang Q (2020) Soil aggregate fractionation and phosphorus fraction driven by long-term fertilization regimes affect the abundance and composition of P-cycling- related bacteria. Soil Tillage Res 196:104475
Wang Y, Zhang R, Zheng Q, Deng Y, Van Nostrand JD, Zhou J, Jiao N (2016) Bacterioplankton community resilience to ocean acidification: evidence from microbial network analysis. ICES J Mar Sci 73:865–875
Wang B, Li S, Zhang S, Xu H, Xu G, Ren L (2019) Responses of acid phosphatase secreted by watermelon roots to organic manure nutrition. Acta Pedol Sin 56:454–465
Wang C, Zheng M, Shen R (2020a) Diazotrophic communities are more responsive to maize cultivation than phosphorus fertilization in an acidic soil. Plant Soil 452:499–512
Wang X, Whalley WR, Miller AJ, White PJ, Zhang F, Shen J (2020b) Sustainable cropping requires adaptation to a heterogeneous rhizosphere. Trends Plant Sci 25:1194–1202
Wang C, Xue L, Jiao R (2021a) Soil phosphorus fractions, phosphatase activity, and the abundance of phoC and phoD genes vary with planting density in subtropical Chinese fir plantations. Soil Tillage Res 209:104946
Wang X, Bian Q, Jiang Y, Zhu L, Chen Y, Liang Y, Sun B (2021b) Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil Biol Biochem 153:108062
Wang C, Xue L, Jiao R (2022) Stoichiometric imbalances and the dynamics of phosphatase activity and the abundance of phoC and phoD genes with the development of Cunninghamia lanceolata (Lamb.) Hook plantations. Appl Soil Ecol 173:104373
Xiao X, Liang Y, Zhou S, Zhuang Y, Sun B (2017) Fungal community reveals less dispersal limitation and potentially more connected network than that of bacteria in bamboo forest soils. Mol Ecol 27:550–563
Xun W, Liu Y, Li W, Ren Y, Xiong W, Xu Z, Zhang N, Miao Y, Shen Q, Zhang R (2021) Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 9:2–15
Yang R, Li Y, Wei H, Gao R, Shi H, Wu J (2011) Study on the nitrogen and phosphorus mineralization of livestock and poultry manure in red soil. J Plant Nutr Fertilizer 17:600–607
Yang Y, Li X, Liu J, Zhou Z, Zhang T, Wang X (2020) Fungal community structure in relation to manure rate in red soil in southern China. Appl Soil Ecol 147:103442
Yang Y, Li G, Min K, Liu T, Li C, Xu J, Hu F, Li H (2022) The potential role of fertilizer-derived exogenous bacteria on soil bacterial community assemblage and network formation. Chemosphere 287:132338
Ye D, Li T, Zhang X, Zheng Z, Dai W (2017) Rhizosphere P composition, phosphatase and phytase activities of Polygonum hydropiper grown in excess P soils. Biol Fertil Soils 53:823–836
Zhao Q (2002) Red soil material cycle and its regulation. Science Press, Beijing
Zheng M, Wang C, Li W, Guo L, Cai Z, Wang B, Chen J, Shen R (2021) Changes of acid and alkaline phosphatase activities in long-term chemical fertilization are driven by the similar soil properties and associated microbial community composition in acidic soil. Eur J Soil Biol 104:103312
Acknowledgements
This work was supported by the National Key Plan for Research and Development of China (2018YFC1803100), the National Natural Science Foundation of China (42020104004 and 52022028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Tim S. George.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 137 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, L., Wang, C., Feng, T.Y. et al. Short-term application of organic fertilization impacts phosphatase activity and phosphorus-mineralizing bacterial communities of bulk and rhizosphere soils of maize in acidic soil. Plant Soil 484, 95–113 (2023). https://doi.org/10.1007/s11104-022-05775-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05775-w