Abstract
Aims
Vegetation in high-altitude regions is hypothesized to be more responsive to increasing atmospheric CO2 concentrations due to low CO2 partial pressure. However, the underlying mechanisms driving this response at an ecosystem scale are poorly understood. We aimed to explore the plant carbon (C) and nitrogen (N) relationships and biomass allocation in response to elevated CO2 and N addition in a Tibetan meadow.
Methods
A 5-year manipulation experiment was conducted in an alpine meadow (4585 m above sea level) to explore the responses of plant carbon (C), nitrogen (N), and biomass dynamics, as well as their allocation schemes, to elevated CO2 (from 380 ppm to 480 ppm) and N fertilization.
Results
Elevated CO2 alone significantly enhanced aboveground plant biomass by 98%, exhibiting a stronger CO2 fertilization effect than the global average level (20%) for grasslands. Elevated CO2 favored N accumulation in aboveground parts despite the declined concentration. Nitrogen fertilization alleviated the N constraints on CO2 fertilization effects, which strengthened C sequestration capacity for the aboveground plant tissues. Moreover, our results indicate a decoupling between C and N cycles in alpine ecosystems under elevated CO2, especially in the N-enrichment environments.
Conclusions
Overall, this study shows a high sensitivity of aboveground plant biomass and decoupled C-N relationships under elevated CO2 and N fertilization for high-elevation alpine ecosystems, highlighting the need to incorporate altitude effects into Earth System Models in predicting C cycle feedbacks to climate changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From molecular metabolism to ecosystem nutrient cycling, carbon (C) and nitrogen (N) are biologically coupled because of the conserved elemental stoichiometry of plants and microorganisms (Finzi et al. 2011). Carbon and N are crucial to many aspects of plant physiological function and microbial metabolism, through their biochemical controls on primary production, respiration, and decomposition in terrestrial ecosystems (Evans and Burke 2013). However, global changes, such as elevated CO2 and N deposition, have drastically altered the biogeochemical cycles of C and N in Earth’s ecosystems. These disturbances are suggested to decouple C and N cycles (eliminate the interrelationship between them), owing to the different degrees of controls by biological and geochemical processes (Peñuelas et al. 2012; Delgado-Baquerizo et al. 2013).
Human activities have raised CO2 concentrations in the atmosphere more than 40% above their pre-industrial levels, and this rising trend is projected to continue for the coming decades (Terrer et al. 2018). Elevated CO2 can stimulate the rate of CO2 assimilation by plants (Leakey et al. 2009; Franks et al. 2013), and potentially reinforce the C sequestration abilities of terrestrial ecosystems (Ainsworth and Long 2005), which may counterbalance the increasing CO2 in the atmosphere released by human activities (Schimel et al. 2015). This CO2 fertilization effect on the terrestrial C sink is supposed to be largely hampered by N availability (Luo et al. 2004; Reich and Hobbie 2013; Langley and Megonigal 2010). Decades of increased atmospheric N deposition across various ecosystems may partially lessen the N constraints on CO2-induced enhancement of terrestrial productivity (Eastman et al. 2021). This human-induced C and N fertilization may cause the asynchrony in C and N supply and the biogeochemical cycles of C and N could be uncoupled, with subsequent consequence on terrestrial ecosystem C storage and cycling (Asner et al. 1997; Evans and Burke 2013). For example, many studies have reported an increase C:N ratio of plant tissue as a result of elevated CO2, and N deposition generally decreases the C:N ratio (Wang et al. 2021). Both litter decomposition and herbivory are shown to be at least in part a function of tissue C:N ratios in many studies (Knops et al. 2007; Burghardt et al. 2018). Therefore, if an unbalanced C and N supply and demand decouple the C and N cycles, and further affect tissue and litter chemistry, elevated CO2 and N deposition may accelerate or decelerate litter decomposition rates and ecosystem C cycles (Knops et al. 2007; Park et al. 2020).
The altered C and N cycles under environmental disturbance are found to affect plant C, N, and biomass allocation between the above- and belowground portions (Valentine and Mäkelä 2012), which reflects evolutional strategies for resource acquisition and adaptation to environments (Dietze et al. 2014; Dickman et al. 2015). The above- and belowground plant structures are exposed to dramatically different temperatures and moisture conditions, largely determining the decomposition of plant litter and thus the permanence of sequestered C in the ecosystem (Wang and Taub 2010; Schmidt et al. 2011). Consequently, the tradeoff of plant C, N, and biomass between short-lived organs (aboveground parts) and long-lived organs (belowground parts) can produce significant effects on the pathway for ecosystem C reentering the atmosphere (Friedlingstein et al. 1999). Current empirical and theoretical methods have demonstrated distinct strategies of plant C, N and biomass allocation, which varies with environments and plant species (Peichl et al. 2012; Poorter et al. 2012). To optimize growth and survival, plants conventionally respond to global changes by altering the ratio of C, N and biomass allocation to shoots or roots (Poorter et al. 2012; Bachofen et al. 2019; Zhou et al. 2020). For example, some ecosystems increased N and biomass allocation to roots to alleviate CO2-induced N limitation in soils (Suter et al. 2002; Iversen et al. 2008; Leuzinger and Hättenschwiler 2013). Conversely, some forests decrease belowground components in response to elevated CO2, likely because elevated CO2 can mitigate water limitation by reducing plant water consumption (Franks et al. 2013). Nitrogen fertilization increases biomass allocation to aboveground parts more strongly than to belowground parts (Li et al. 2020). Furthermore, a growing body of reports from long-term observations and manipulative experiments have demonstrated that elevated CO2 and N deposition are not occurring separately, and their combined effects will likely be more complex (Reich et al. 2006b; Sillen and Dieleman 2012; Zhao et al. 2020). Despite these advances, we know little about the response of C and N cycles associated with biomass allocation to the interaction of elevated CO2 and N deposition for alpine ecosystems.
Because of the non-uniform environmental stresses worldwide, elevated CO2 and N deposition may have regional, but not global, effects on the plant C and N cycles associated with their allocation (Wang and Taub 2010), emphasizing the high uncertainty in projecting future global C budgets. Alpine ecosystems at high elevation, with an area of approximately 2,847,868 km2, are extremely vulnerable to global changes (Elser et al. 2020). High-elevation plants are supposed to be more responsive to increasing CO2 concentrations in the atmosphere due to their low CO2 partial pressure (Hättenschwiler et al. 2002). Although some experiments in alpine regions have been designed to test the co-regulations of elevated CO2 and N addition on plant C and N dynamics and allocation, inconsistent conclusions from these studies constrain the incorporation of altitude effects of CO2 fertilization into Earth System Models (Schäppi and Körner 1997; Dawes et al. 2011; Inauen et al. 2012). Further field experiments are needed to complement our knowledge in those under-represented ecosystems (Curtis and Wang 1998; Zhang et al. 2014; Bachofen et al. 2019).
As the highest plateau in the world, the Tibetan Plateau hosts the largest alpine grassland ecosystem worldwide (Zhang et al. 2019). It is an ideal region to investigate the responses of plants to global changes. The Tibetan Plateau is currently experiencing significant anthropogenic environmental change, including increased N deposition. Understanding how these two primary global change factors (e.g., rising CO2 and N deposition) affect plant C and N dynamics and the biomass allocation are critical to understanding ecosystem C cycling in facing the accelerated global changes on the plateau. In this study, we explored the responses of plant C and N dynamics, and the biomass allocation to treatments in an elevated CO2 and N deposition study (simulated by N addition) in a Tibetan meadow over five years. We aimed to test the two hypotheses: (i) alpine plants may exhibit stronger responses to elevated CO2 as they grow at lower CO2 partial pressure, and (ii) altered CO2 and N availability may decouple the C:N relationships in plant tissues due to the imbalanced nutrient supply.
Materials and methods
Study site
The study was conducted in a typical alpine meadow ecosystem in the North Tibetan Plateau, China (31°38.513′N, 92°0.921′E, 4585 m a.s.l.). This study constitutes the highest-elevation CO2 enrichment experiment conducted on grassland worldwide (Zhu et al. 2020). The climate is characterized by a mean annual temperature of approximately −1.16 °C and a mean annual precipitation of ~ 430 mm, mainly falling during the summer season from June to September (Zhu et al. 2017). Moreover, the area has gale-force winds lasting nearly a quarter of each year. The vegetation community is dominated by Kobresia pygmaea, with companion species including Potentilla saundersiana, Potentilla cuneata, Youngia simulatrix and Saussurea stoliczkai. The growing season normally starts in mid-May and ends in mid-September.
Experimental design
Due to the gusty winds and low stature vegetation on the Tibetan Plateau, CO2 enrichment could not be implemented using a free-air CO2 enrichment (FACE) design. Instead, eight semi-FACE designed open-top chambers (OTCs) were constructed with steel frames and glass in 2013 (Fig. 1). Each octagonal OTC measures 1.5 m in length for each side and has a height of 2 m, covering a surface area of 10.86 m2. The CO2 concentration within the CO2-enriched chambers (480 ppm) was designed to be 100 ppm higher than the ambient levels (380 ppm) (Fig. 2). From 2014 to 2018, enriched CO2 was injected into the OTCs to artificially increase CO2 concentrations during the growing season of each year.
The experiment follows a full-factorial split-plot design, with elevated CO2 as the primary factor and N addition as the secondary factor. Among them, four octagons were exposed to ambient CO2 concentration (380 ppm), and the other four were exposed to rising CO2 concentrations (480 ppm). Each octagon was divided into four plots with a size of 1.25 m×1.25 m, with one supplemented with N and one without N (the remaining two plots had no N fertilizer and were not considered in the study). For each N deposition treatment (simulated by N fertilization), 5 g N m− 2 year− 1 in the form of urea was dissolved in 200 ml water and sprayed on the plots during June from 2014 to 2018. To prevent possible active N transfer along the soil profile between adjacent plots, a stainless-steel plate was inserted to the depth of 30 cm soil in 2013.
In OTCs with CO2 enrichment, polyvinyl chloride (PVC) pipes had 1 mm pinholes drilled every 0.1 m along the pipe and were framed 0.3 m aboveground, to transport pressured CO2 from tanks to chambers. The CO2 flux from the tank was controlled by a flow meter carrying the Vaisala GMP222 sensor (Vaisala, Helsinki, Finland), which monitored CO2 concentrations in the middle of chambers every 15 min. To account for shading effects, the same pipes were installed for the ambient CO2 chambers. Rainfall shielded by the OTCs was supplemented by spraying equal amounts of water in the chambers.
Plant and soil sampling
In the middle of each growing season (late August) from 2015 to 2018, a 10 cm×10 cm quadrat was randomly selected in each plot, and all aboveground plants within the selected quadrat were harvested. After collecting the aboveground biomass, a cylinder auger (7 cm in diameter) was used to take a soil core to a depth of 30 cm. Collected soil cores for each treatment were passed through a 2-mm sieve to remove roots, organic debris, and rocks. Root samples were then collected from soil cores, which were cleaned and dried for belowground biomass measurements. To reduce the influence of soil sampling on plot, the cores were filled with local soil each time after sampling. All above- and belowground plant samples were oven-dried at 65 ℃ for 48 h to a constant weight. Then, soil and plant samples were ground and analyzed for C and N concentration using the elemental analyzer (Vario MAX CN analyzer, Germany). Above- and belowground plant C and N storage were calculated as the product of biomass and corresponding tissue C and N concentrations.
Statistical analysis
In assessing responses of plant biomass and C:N stoichiometry to elevated CO2 and N fertilization in different tissues, the “relative change” index was applied and calculated as:
Xa represents the average value of a specific variable in the control group, and Xe represents the average values of a specific variable in each experimental group.
Repeated measures analysis of variance was applied to evaluate the effect of elevated CO2, N deposition, and year on plant biomass from 2015 to 2018. A split-plot analysis of variance was used to assess the effects of elevated CO2 and N fertilization on plant and soil C and N concentrations using statistical software 20.0 (SPSS Inc., Chicago, IL, USA). Regression analysis and Pearson’s correlation were used to evaluate the relationship between C and N concentrations as well as the relationship of the biomass allocation and the C:N ratio. All the differences and effects were considered statistically significant at P < 0.05.
Results
Biomass, C and N allocation in plant above- and belowground components
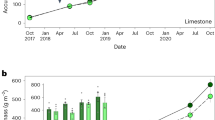
For this alpine meadow, the above- and belowground biomass range during 2015–2018 was 51–410 g m2, and 6649–13,340 g m2, respectively (Fig. 3). In general, plant belowground biomass accounted for 94–99% of the community total. Elevated CO2 significantly increased aboveground biomass (P < 0.05) but showed no significant effects on belowground parts (Fig. 3; P > 0.05). Nitrogen fertilization alone significantly increased aboveground biomass by 36–166% (P = 0.018), with no significant effect on belowground biomass (P = 0.310). Combined elevated CO2 and N fertilization significantly enhanced aboveground plant biomass by 84–340% (P < 0.05).
Effects of elevated CO2 and N addition on above- (A) and belowground (B) plant biomass for 4 years, from 2015 to 2018. Error bars indicate standard error. The inserted text is the results of the repeated measures ANOVA analysis. Control, control treatment; CO2, elevated CO2; N, nitrogen addition; AGB, aboveground biomass; BGB, belowground biomass
Plant above- and belowground biomass presented a significant relationship under control treatment, but the correlations were nonsignificant under elevated CO2 or N addition treatment (Fig. 4). The ratios of belowground biomass (BGB) to aboveground biomass (AGB) varied between 31 and 74. Elevated CO2 alone altered the biomass allocation between above- and belowground plant components (P = 0.001), causing a significant decrease (40%) in the ratio of BGB to AGB. N fertilization alone significantly lowered the ratio of BGB to AGB by 35% (P = 0.006). Combined elevated CO2 and N fertilization significantly decreased the ratio of BGB to AGB by 58% (P < 0.05), while there were no significant interaction effects of elevated CO2 and N fertilization on the biomass allocation (P = 0.337). For the biomass allocation strategy (Fig. 4), the relative changes in the ratios of BGB to AGB were caused more by the changes in AGB (percentage changes between −0.47% and 3.90%) rather than the changes in BGB (percentage between −0.47% and 0.69%).
Effects of elevated CO2 and N addition on relationships between above- and belowground biomass, and biomass allocation (right). For the right figure, solid line in the box marks the median of each dataset, with the upper and lower ends of boxes representing the 0.25 and 0.75 percentiles, respectively. The upper and lower whisker caps denote the maximum and minimum values, respectively. The inserted text is the results of the two-way ANOVA analysis. Different lowercase letters in the right figure indicate a significant difference of C, N and C:N among treatments according to Turkey’s b test at P < 0.05. Control, control treatment; CO2, elevated CO2; N, nitrogen addition; AGB, aboveground biomass; BGB, belowground biomass
The plant C and N storage range varied from 2819 g m− 2 to 3479 g m− 2, and from 66 g m− 2 to 80 g m− 2, respectively (Fig. 5). Overall, elevated CO2 and N fertilization caused no significant effects on belowground plant C and N storage (P > 0.05). On the contrary, elevated CO2 and N fertilization significantly increased aboveground plant C storage by 100% and 70%, respectively. Combined CO2 and N fertilization significantly enhanced aboveground plant C storage by 180% (P < 0.05). Meanwhile, elevated CO2 and N fertilization significantly increased aboveground plant N storage by 87% and 102% (P < 0.05), respectively. For plant C and N allocation, both elevated CO2 and N fertilization, as well as their combined effects, significantly lowered the ratio of BGB to AGB for C and N storage (P < 0.05).
Responses of plant carbon and nitrogen storage to elevated CO2 and nitrogen addition. Error bars indicate standard error. Solid line in the box marks the median of each dataset, with the upper and lower ends of boxes representing the 0.25 and 0.75 percentiles, respectively. The upper and lower whisker caps denote the maximum and minimum values, respectively. The inserted text is the results of the two-way ANOVA analysis. Different lowercase letters in the figure indicate a significant difference of C, N and C:N among treatments according to Turkey’s b test at P < 0.05. Cont, control treatment; CO2, elevated CO2; N, nitrogen fertilization; AGB, aboveground biomass; BGB, belowground biomass
Dynamics of C:N stoichiometry in the alpine ecosystem
The total soil C concentrations varied from 42.1 mg g− 1 to 51.0 mg g− 1, and the total soil N concentrations varied from 3.8 mg g− 1 to 4.2 mg g− 1 (Table 1). Elevated CO2 alone significantly increased soil C concentrations from 42.1 mg g− 1 to 51.0 mg g− 1 (P < 0.05) and increased soil C:N ratio from 11.16 to 12.07 (P < 0.05). However, nitrogen fertilization had no significant effects on soil total C and N, and the associated C:N ratio (P > 0.05; Table 1). For plants, elevated CO2 significantly increased C concentration (P = 0.013) and the associated C:N ratio (P < 0.01) in the aboveground plant portions, and significantly decreased the N concentration in aboveground tissues (P = 0.002; Table 2). Nitrogen fertilization alone significantly increased N concentrations and decreased C:N of aboveground tissues but had no significant effects on C concentrations. Combined elevated CO2 and N fertilization significantly increased C concentrations in aboveground tissues from 421.6 mg g− 1 to 432.8 mg g− 1 but had no significant effect on N concentration and C:N ratio (P > 0.05). Elevated CO2 and N fertilization showed no significant effects on C and N concentrations, and the C:N ratio of the belowground tissues (P > 0.05).
Response of plant C and N relationships to elevated CO2 and N fertilization
Under natural conditions, C and N concentrations in both above- and belowground plant tissues were significantly and positively correlated (P < 0.01) with R2 values of 0.49 and 0.46, respectively (Fig. 6). However, elevated CO2 decoupled this relationship in aboveground tissues (P = 0.20) and lowered the C and N correlation coefficients from 0.49 to 0.27. Under CO2 enrichment environments, N fertilization further weakened the C and N correlation coefficients from 0.27 to 0.24 and from 0.12 to 0.07 for above- and belowground tissues, respectively (Fig. 6).
The relationships between carbon and nitrogen contents of different plant tissues under elevated CO2 and N treatments. Error bars indicate standard error. The inserted text is the results of the two-way ANOVA analysis. Different lowercase letters in the figure indicate a significant difference of C, N and C:N among treatments according to Turkey’s b test at P < 0.05. aCO2, ambient CO2; eCO2, elevated CO2
Given that the biomass allocation is largely regulated by signals involving plant C and N dynamics, the relationship between the C:N ratio and plant biomass allocation were further analyzed. In general, Ln (C/N) in belowground tissues showed no significant relationship with Ln (BGB/AGB) (P > 0.05; Fig. 7). Under the control treatment, Ln (C/N) significantly correlated with Ln (BGB/AGB), generating an R2 value of 0.335 (P < 0.05) for aboveground tissues. The R2 of these relationships decreased under elevated CO2 and N fertilization from 0.335 to 0.216 and 0.260, respectively. The combined effects of elevated CO2 and N fertilization further decoupled the correlation between the C:N ratio and BGB/AGB ratio, generating a nonsignificant R2 value of 0.029 (P = 0.54).
Discussion
Elevated CO2 increases C allocation to plant aboveground parts
The aboveground plant biomass in this alpine ecosystem showed a higher sensitivity to five years of CO2 enrichment (increased by 98%; Fig. 8) in comparison with other experiments worldwide (increased by 20% on average; Sillen and Dieleman 2012). Meanwhile, elevated CO2-induced high C concentrations in aboveground tissues suggest a reinforced capability of C sequestration for plants besides the increased aboveground biomass. These support our first hypothesis that high-elevation plants can exhibit particular response to elevated CO2. Although CO2 concentrations in the atmosphere remain constant with rising elevation, CO2 partial pressure and density drop dramatically in alpine regions (Körner and Diemer 1987). Given that atmospheric pressure decreases by roughly 11% per km of elevation in the atmosphere, the CO2 partial pressure at this alpine meadow (approximately 50% of the pressure at sea level) is considerably less than that at lowland grassland. Since the concentration of CO2 in the liquid phase is proportional to its partial pressure in the surrounding gas phase, the concentration of CO2 in chloroplasts of alpine plants would be considerably lower than that in lowland plants (Terashima et al. 1995). Alpine plants are observed to enhance CO2 utilization efficiencies (Körner 2003), to compensate for the negative influence of declined CO2 partial pressures on photosynthesis. Therefore, alpine plants with increased photosynthetic CO2 use efficiency may respond more strongly to elevated CO2 than comparable lowland plants.
The tradeoff of biomass, C and N stoichiometry between the above- and belowground plant parts under elevated CO2 and N fertilization. Red and green arrows in the figure represent increasing and decreasing trend, respectively. CO2, elevated CO2; N, nitrogen addition; AGB, aboveground biomass; BGB, belowground biomass
According to optimal partitioning models, the plant would allocate more biomass to plant belowground components to optimize nitrogen acquisition, thereby alleviating CO2-induced N limitation (Leuzinger and Hättenschwiler 2013; Avila et al. 2020; Noyce et al. 2019; Frew et al. 2021). Inconsistent with the optimal partitioning predictions, elevated CO2 causes more biomass distribution to aboveground components but not to belowground parts in the current experiment. Two reasons may account for this phenomenon. First, plants under elevated CO2 may increase C allocation to soils as root exudates instead of increasing belowground biomass, and thus enhance N uptake through intensified soil organic matter decomposition rates (e.g., “priming effect”). Second, the unique changes in the C and biomass allocation patterns of the alpine ecosystem may be related to the special high-altitude and cold environments. Plants in alpine ecosystems have evolved better-developed root systems than shoots in adapting to the environmental conditions of the low fertility soils and cold temperatures (Yang et al. 2009). Under these harsh environments plants are prone to possessing vast biomass in long-lived organs, such as roots (Hermans et al. 2006), to strengthen nutrient uptake from the soil and improve resistance to cold stress. Therefore, a high root/shoot ratio (from 31.2 to 73.6 in this study) for alpine plants may lead to the unresponsiveness of root systems to elevated CO2.
Regulation of N availability on plant C dynamics under elevated CO2
Our results demonstrate that N availability largely regulates the plant C dynamics and allocation under elevated CO2. Elevated CO2 alone increased plant C concentrations and C:N ratio but decreased plant N concentrations, consistent with previous reports (Knops et al. 2007; Leakey et al. 2009). This consistency indicates that CO2 fertilization-induced N-limitations are reflected in the changes in tissue quality. It is worth noting that no significant effect of N addition on plant and soil C concentrations were observed, while the interacted N addition and CO2 enrichment significantly increased the C concentrations in aboveground plant tissues and soils. That is, the C concentration of plants grown in N-enriched soils would be more responsive to elevated CO2 than those grown in N-poor soils.
Enriched CO2 and N influence not only the amount of plant tissue quality but also the plant biomass produced. A combination of N fertilization with CO2 enrichment simulates the aboveground biomass, indicating an alleviated CO2-induced N limitation on plant growth and boosted C fixation capacity for the alpine ecosystem with future nitrogen deposition scenarios. We also found that the CO2 fertilization effect had weakened with increasing treatment duration independent of N treatment, although the aboveground plant biomass under combined CO2 and N fertilization could sustain at a high level in contrast to that under elevated CO2 alone. This phenomenon suggests that besides the N availability (Reich et al. 2006a), the CO2 fertilization effect on biomass may also be modulated by other factors, possibly water or phosphorus limitation (Reich et al. 2014; Farrior et al. 2015; Terrer et al. 2019).
Elevated CO2 and N fertilization decouple plant C and N cycles
In natural conditions, C and N cycles are biologically coupled from molecular to global scales due to the required balance in basic elements proportion and conserved elemental stoichiometry in organisms (Finzi et al. 2011; Delgado-Baquerizo et al. 2013). Here, we observed a significant positive correlation between plant aboveground C and N concentration and a positive relationship between above- and belowground biomass without CO2 and N treatments. In addition, a significant correlation between the C:N ratio in aboveground tissues and the ratio of BGB/AGB exists, even under elevated CO2 or N additions. That is, aboveground tissue C and N concentrations are primarily related to the relative changes in biomass allocation. As the C and N status has been suggested as an important indicator of plant source and sink balance (Wang et al. 2021), these CO2-induced variations in chemometrics are therefore tightly correlated with the biomass allocation (Hilbert 1990; Sugiura and Tateno 2011). This phenomenon agrees with a previous hypothesis (McCarthy and Enquist 2007) that the biomass allocation is largely modulated by signals involving plant C and N balance and the C:N ratio would be an appropriate indicator to predict plant allocation strategies.
In this study, we found that elevated CO2 decoupled C and N cycles in plant tissues and this uncoupled relationship further aggravated under an N-enriched environment. This may be the result of imbalanced N supply and plant demand under CO2 and N fertilization. Similarly, the tight linkage between plant above- and belowground biomass become uncoupled under CO2 and N fertilization, with C shifting away from belowground components and towards aboveground biomass production, indicating that perturbations from human activities can alter the natural linkages between C and N cycles associated with C reallocation. This imbalance observed in the C and N with elevated CO2 and N fertilization may have important consequences on the alpine ecosystem, as the decoupling may cause asynchronous nutrient supply and demand, which can aggravate the nutrient loss and create new biogeochemical feedbacks (Delgado-Baquerizo et al. 2013).
Conclusions
This study provides novel insights into how alpine ecosystems respond to global changes (elevated CO2 and N deposition) in terms of plant C and N relationships, and the biomass allocation in an alpine meadow. Our results illustrate that aboveground plant tissues are more responsive to elevated CO2 in contrast to belowground parts, with more biomass being allocated to the aboveground plant parts. We deduce that plants in high elevation alpine ecosystems may be more responsive to low CO2 partial pressure there (approximately 50% of the pressure at sea level), and are inclined to shift the distribution of photosynthates to the aboveground compartment to strengthen the photosynthetic potentials. Nitrogen fertilization alleviates the N limitations on the plant growth induced by elevated CO2 and further amplifies the capacity of plant C fixation simultaneously by increasing biomass and C concentrations in aboveground tissues. Overall, elevated CO2 and N fertilization decouples plant C and N cycling and these effects may alter the impacts and feedbacks of plants on biogeochemical cycles, and either slow down or accelerate climate changes.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Asner GP, Seastedt TR, Townsend AR (1997) The decoupling of terrestrial carbon and nitrogen cycles. Bioscience 47:226–234. https://doi.org/10.2307/1313076
Avila RT, de Almeida WL, Costa LC et al (2020) Elevated air [CO2] improves photosynthetic performance and alters biomass accumulation and partitioning in drought-stressed coffee plants. Environ Exp Bot 177:104137. https://doi.org/10.1016/j.envexpbot.2020.104137
Bachofen C, Wohlgemuth T, Moser B (2019) Biomass partitioning in a future dry and CO2 enriched climate: Shading aggravates drought effects in Scots pine but not European black pine seedlings. J Appl Ecol 56:866–879. https://doi.org/10.1111/1365-2664.13325
Burghardt KT, Bradford MA, Schmitz OJ (2018) Acceleration or deceleration of litter decomposition by herbivory depends on nutrient availability through intraspecific differences in induced plant resistance traits. J Ecol 106:2380–2394
Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313. https://doi.org/10.2307/4221855
Dawes MA, Hagedorn F, Zumbrunn T, Handa IT, Hättenschwiler S, Wipf S, Rixen C (2011) Growth and community responses of alpine dwarf shrubs to in situ CO2 enrichment and soil warming. New Phytol 191:806–818. https://doi.org/10.1111/j.1469-8137.2011.03722
Delgado-Baquerizo M, Maestre FT, Gallardo A et al (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502:672–676. https://doi.org/10.1038/nature12670
Dickman LT, McDowell NG, Sevanto S, Pangle RE, Pockman WT (2015) Carbohydrate dynamics and mortality in a piñon-juniper woodland under three future precipitation scenarios. Plant Cell Environ 38:729–739. https://doi.org/10.1111/pce.12441
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65:667–687. https://doi.org/10.1146/annurev-arplant-050213-040054
Eastman BA, Adams MB, Brzostek ER et al (2021) Altered plant carbon partitioning enhanced forest ecosystem carbon storage after 25 years of nitrogen additions. New Phytol 230:1435–1448. https://doi.org/10.1111/nph.17256
Elser JJ, Wu C, González AL et al (2020) Key rules of life and the fading cryosphere: Impacts in alpine lakes and streams. Global Change Biol 26:6644–6656. https://doi.org/10.1111/gcb.15362
Evans SE, Burke IC (2013) Carbon and nitrogen decoupling under an 11-year drought in the shortgrass steppe. Ecosystems 16:704–705. https://doi.org/10.1007/s10021-012-9593-4
Farrior CE, Rodriguez-Iturbe I, Dybzinski R, Levin SA, Pacala SW (2015) Decreased water limitation under elevated CO2 amplifies potential for forest carbon sinks. P Natl Acad Sci USA 112:7213–7218. https://doi.org/10.1073/pnas.1506262112
Finzi AC, Austin AT, Cleland EE, Frey SD, Houlton BZ, Wallenstein MD (2011) Responses and feedbacks of coupled biogeochemical cycles to climate change: examples from terrestrial ecosystems. Front Ecol Environ 9:61–67. https://doi.org/10.1890/100001
Franks PJ, Adams MA, Amthor JS et al (2013) Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytol 197:1077–1094. https://doi.org/10.1111/nph.12104
Frew A, Price JN, Oja J, Vasar M, Öpik M (2021) Impacts of elevated atmospheric CO2 on arbuscular mycorrhizal fungi and their role in moderating plant allometric partitioning. Mycorrhiza 31:1–8. https://doi.org/10.1007/s00572-021-01025-6
Friedlingstein P, Joel G, Field CB, Fung IY (1999) Toward an allocation scheme for global terrestrial carbon models. Global Change Biol 5:755–770. https://doi.org/10.1046/j.1365-2486.1999.00269.x
Hättenschwiler S, Handa IT, Egli L, Asshoff R, Ammann W, Körner C (2002) Atmospheric CO2 enrichment of alpine treeline conifers. New Phytol 156:363–375. https://doi.org/10.1046/j.1469-8137.2002.00537.x
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation?. Trends Plant Sci 11:610–617. https://doi.org/10.1016/j.tplants.2006.10.007
Hilbert DW (1990) Optimization of plant root: shoot ratios and internal nitrogen concentration. Ann Bot 66:91–99. https://doi.org/10.1093/oxfordjournals.aob.a088005
Inauen N, Körner C, Hiltbrunner E (2012) No growth stimulation by CO2 enrichment in alpine glacier forefield plants. Global Change Biol 18:985–999. https://doi.org/10.1111/j.1365-2486.2011.02584.x
Iversen CM, Ledford J, Norby RJ (2008) CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytol 179:837–847. https://doi.org/10.1111/j.1469-8137.2008.02516.x
Knops JMH, Naeem S, Reich PB (2007) The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Global Change Biol 13:1960–1971. https://doi.org/10.1111/j.1365-2486.2007.01405.x
Körner C, Diemer M (1987) In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct Ecol 1:179–194. https://doi.org/10.2307/2389420
Körner C (2003) Carbon limitation in trees. J Ecol 91(1):4–17. https://doi.org/10.1046/j.1365-2745.2003.00742.x
Langley JA, Megonigal JP (2010) Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature 466:96–99. https://doi.org/10.1038/nature09176
Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60:2859–2876. https://doi.org/10.1093/jxb/erp096
Leuzinger S, Hättenschwiler S (2013) Beyond global change: lessons from 25 years of CO2 research. Oecologia 171:639–651. https://doi.org/10.1007/s00442-012-2584-5
Li W, Zhang H, Huang G, Liu R, Wu H, Zhao C, McDowell NG (2020) Effects of nitrogen enrichment on tree carbon allocation: A global synthesis. Global Ecol Biogeogr 29:573–589. https://doi.org/10.1111/geb.13042
Luo Y, Su BO, Currie WS, Dukes JS, Finzi A, Hartwig U, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739. https://doi.org/10.1641/0006-3568(2004)054[0731:pnloer]2.0.co;2
McCarthy MC, Enquist BJ (2007) Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol 21:713–720. https://doi.org/10.1111/j.1365-2435.2007.01276.x
Noyce GL, Kirwan ML, Rich RL, Megonigal JP (2019) Asynchronous nitrogen supply and demand produce nonlinear plant allocation responses to warming and elevated CO2. P Natl Acad Sci USA 116:21623–21628. https://doi.org/10.1073/pnas.1904990116
Park HJ, Lim SS, Yang HI et al (2020) Nitrogen effects on quantity, chemistry, and decomposability of Pinus densiflora and Quercus variabilis litters under elevated CO2 and warming. For Ecol Manag 473:118315. https://doi.org/10.1016/j.foreco.2020.118315
Peichl M, Leava NA, Kiely G (2012) Above-and belowground ecosystem biomass, carbon and nitrogen allocation in recently afforested grassland and adjacent intensively managed grassland. Plant Soil 350:281–296. https://doi.org/10.1007/s11104-011-0905-9
Peñuelas J, Sardans J, Rivas-ubach A, Janssens IA (2012) The human‐induced imbalance between C, N and P in Earth’s life system. Global Change Biol 18:3–6. https://doi.org/10.1111/j.1365-2486.2011.02568.x
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
Reich PB, Hobbie SE, Lee TD (2014) Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat Geosci 7:920–924. https://doi.org/10.1038/ngeo2284
Reich PB, Hobbie SE, Lee T, David SE, Jason BW, David T, Johannes MHK, Shahid N, Jared T (2006a) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925. https://doi.org/10.1038/nature04486
Reich PB, Hobbie SE (2013) Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat Clim Change 3:278–282. https://doi.org/10.1038/NCLIMATE1694
Reich PB, Hungate BA, Luo Y (2006b) Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annu Rev Ecol Evol S 37:611–636. https://doi.org/10.2307/30033845
Schäppi B, Körner C (1997) In situ effects of elevated CO2 on the carbon and nitrogen status of alpine plants. Funct Ecol 11:290–299. https://doi.org/10.1046/j.1365-2435.1997.00084.x
Schimel D, Stephens BB, Fisher JB (2015) Effect of increasing CO2 on the terrestrial carbon cycle. P Natl Acad Sci USA 112:436–441. https://doi.org/10.1073/pnas.1407302112
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. https://doi.org/10.1038/nature10386
Sillen WMA, Dieleman WIJ (2012) Effects of elevated CO2 and N fertilization on plant and soil carbon pools of managed grasslands: a meta-analysis. Biogeosciences 9:2247–2258. https://doi.org/10.5194/bg-9-2247-2012
Sugiura D, Tateno M (2011) Optimal leaf-to-root ratio and leaf nitrogen content determined by light and nitrogen availabilities. PLoS One 6:e22236. https://doi.org/10.1371/journal.pone.0022236
Suter D, Frehner M, Fischer BU, Nösberger J, Lüscher A (2002) Elevated CO2 increases carbon allocation to the roots of Lolium perenne under free-air CO2 enrichment but not in a controlled environment. New Phytol 154:65–75. https://doi.org/10.1046/j.1469-8137.2002.00368.x
Terashima I, Masuzawa T, Ohba H, Yokoi Y (1995) Is photosynthesis suppressed at higher elevations due to low CO2 pressure?. Ecology 76:2663–2668
Terrer C, Vicca S, Stocker BD et al (2018) Ecosystem responses to elevated CO2 governed by plant–soil interactions and the cost of nitrogen acquisition. New Phytol 217:507–522. https://doi.org/10.1111/nph.14872
Terrer C, Jackson RB, Prentice IC et al (2019) Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat Clim Change 9:684–689. https://doi.org/10.1038/s41558-019-0545-2
Valentine HT, Mäkelä A (2012) Modeling forest stand dynamics from optimal balances of carbon and nitrogen. New Phytol 194:961–971. https://doi.org/10.1111/j.1469-8137.2012.04123.x
Wang C, Sun Y, Chen HYH, Ruan H (2021) Effects of elevated CO2 on the C: N stoichiometry of plants, soils, and microorganisms in terrestrial ecosystems. CATENA 201:105219. https://doi.org/10.1016/j.catena.2021.105219
Wang X, Taub DR (2010) Interactive effects of elevated carbon dioxide and environmental stresses on root mass fraction in plants: a meta-analytical synthesis using pairwise techniques. Oecologia 163:1–11. https://doi.org/10.1007/s00442-010-1572-x
Yang Y, Fang J, Ji CJ, Han W (2009) Above- and belowground biomass allocation in Tibetan grasslands. J Veg Sci 20:177–184. https://doi.org/10.1111/j.1654-1103.2009.05566.x
Zhang C, Kellomäki S, Zhong Q, Wang K, Gong J, Qiao Y, Zhou X, Gao W (2014) Seasonal biomass allocation in a boreal perennial grass (Phalaris arundinacea L.) under elevated temperature and CO2 with varying water regimes. Plant Growth Regul 74:153–164. https://doi.org/10.1007/s10725-014-9907-y
Zhang Y, Zhu Y, Li J, Chen Y (2019) Current status and future directions of the Tibetan Plateau ecosystem research. Sci Bull 64:428–430. https://doi.org/10.1016/j.scib.2019.03.009
Zhao G, Liang C, Feng X, Liu LL, Zhu JT, Chen N, Chen Y, Wang L, Zhang YJ (2020) Elevated CO2 decreases soil carbon stability in Tibetan Plateau. Environ Res Lett 15:114002. https://doi.org/10.1088/1748-9326/abbb50
Zhou L, Hong Y, Li C et al (2020) Responses of biomass allocation to multi-factor global change: A global synthesis. Agr Ecosyst Environ 304:107115. https://doi.org/10.1016/j.agee.2020.107115
Zhu J, Zhang Y, Jiang L (2017) Experimental warming drives a seasonal shift of ecosystem carbon exchange in Tibetan alpine meadow. Agr For Meteorol 233:242–249. https://doi.org/10.1016/j.agrformet.2016.12.005
Zhu J, Zhang Y, Yang X, Chen N, Jiang L (2020) Synergistic effects of nitrogen and CO2 enrichment on alpine grassland biomass and community structure. New Phytol 228:1283–1294. https://doi.org/10.1111/nph.16767
Acknowledgements
This research was jointly supported by the National Natural Science Foundation of China (Grant No. 41725003), the National Key Research & Development Program of China (2019YFA0607302), the Strategic Priority Program of Chinese Academy of Science (Grant No. XDA20050102), the National Natural Science Foundation of China (Grant No. 41991234; 42101067) and the China Postdoctoral Science Foundation funded projected (Grant No. 2019M660781).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Feike A. Dijkstra.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, G., Chen, Y., Zhang, Y. et al. Decoupling of plant carbon and nitrogen under elevated CO2 and nitrogen addition in a typical alpine ecosystem. Plant Soil 474, 485–498 (2022). https://doi.org/10.1007/s11104-022-05354-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05354-z