Abstract
Aims
To realize the so-called “Second Green Revolution”, it is imperative to study the roots of crop plants, and identify those traits that improve the efficiency of nitrogen (N) acquisition. We aimed to evaluate how the N acquisition efficiency of six hybrid maize lines commonly grown in northern China depends on their root anatomy.
Methods
Maize hybrids classified as having high nitrogen uptake efficiency (HNUE) and low nitrogen uptake efficiency (LNUE) were grown under high-N and low-N conditions in the greenhouse and the field.
Results
Under N stress in the field and the greenhouse, HNUE increased shoot dry weight, root length density, N content and nitrogen use efficiency compared to LNUE. Low N availability increased the percentage of root cortical aerenchyma and the size of cortical cells. Root anatomy, with greater formation of root cortical aerenchyma and larger cortical cell size, was associated with increased specific nitrogen absorption efficiency (SNAE) and shoot biomass under N stress. Under low N availability, the percentage of aerenchyma and their total area had significant positive correlations with the shoot dry weight, total N uptake, SNAE.
Conclusions
The results suggest that plants in limited N availability form more root cortical aerenchyma and have larger cortical cells, which is of benefit to root growth, soil exploration, N acquisition, and shoot biomass. These observations support the hypothesis that root anatomical phenotypes that affect the metabolic and construction costs of producing root length merit consideration as selection criteria for breeding to improve N acquisition in hybrid maize.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The combined effects of stagnating crop yield gains, an increasing human population, and environmental degradation are a global challenge for agriculture (Wiesmeier et al. 2015; Crist et al. 2017; Ramankutty et al. 2018). Nitrogen (N) is a primary macronutrient required for plant growth and yield, and N fertilizer intensification has been widely used to drive improvements in crop production (Hirel et al. 2007). However, while the use of chemical fertilizers in China increased by 51% from 1996 to 2005, the grain yield from Chinese cereals increased by only 10% (Chen et al. 2011). Losses of N from fertilizer applications can either increase linearly or exponentially especially when N fertilizer rates exceed the optimal N fertilizer, due to nitrate leaching, ammonia volatilization, and N2O emission (Jia et al. 2014; Sun et al. 2017; Song et al. 2018; Bizimana et al. 2021). Deploying excessive N inputs gives rise to environmental problems such as eutrophication (Huang et al. 2017), greenhouse gas emission (Xia et al. 2017), and soil acidification (Raza et al. 2020). These problems have become increasingly severe in rapidly developing countries, and have significant environmental consequences on a global scale. If we are to meet the growing demand for food without further compromising the Earth's environmental integrity, it is essential to come up with novel strategies to substantially increase yield trajectories in a sustainable way. This paper studies the root anatomy of maize hybrids in environments with varying N availability as a possible angle of approach to this challenge.
Roots are directly responsible for nutrient and water acquisition and the spatial distribution of the root system throughout the soil profile determines the capacity of a plant to acquire resources with heterogeneous distributions (Yu et al. 2014; Liu et al. 2017; Lynch 2019, Zhang et al. 2020). For example, increased numbers of lateral roots and nodal roots and longer length of root hairs have been shown help crops like maize to acquire nutrients distributed near the soil surface, whereas reduced number of nodal roots, reduced lateral branching density, and steeper root angles permit deeper rooting and improve the capture of resources localized in deeper soil strata (Trachsel et al. 2013; Zhan and Lynch 2015; Saengwilai et al. 2014a; Sun et al. 2018; Lynch 2019).
Lynch (2013) proposed an ideotype for superior N and water acquisition in maize called “Steep, Cheap and Deep (SCD)”, which is comprised of root architectural, anatomical, and physiological phenotypes that could be expected to feature an increased rooting depth and thereby an enhanced ability to capture nitrate. Previous studies on recombinant inbred maize lines have demonstrated that root anatomical traits such as the area of root cortical aerenchyma (Saengwilai et al. 2014b), living cortical area (Galindo-Castañeda et al. 2018), cortical cell size (CCS) (Chimungu et al. 2014a) and cortical cell file number (CFN) (Chimungu et al. 2014b), influence nutrient and water acquisition efficiency under edaphic stress. Although the research on the recombinant inbred maize lines used in these studies contributes to our understanding of how genetics control the root anatomy, and sheds light on the relationships between root phenotypes and their N capture efficiency (Lynch 2015; Saengwilai et al. 2014b), these inbred lines are not representative of the hybrid genotypes commonly used in agricultural settings around the world. It is worth studying genetic variations that control the root anatomy, and therefore the NUE, of hybrid maize lines, because they are essential for potential breeding approaches aimed at developing maize genotypes that produce more grain with less N input.
This paper studies the roots of hybrid maize genotypes grown under high-N and low-N conditions in the greenhouse and in the field, with the aim to evaluate the relationship between their anatomical traits and their ability to acquire N. Based on our previous screening work, we selected six hybrid maize genotypes featuring contrasting nitrogen uptake efficiency (NUE) (high nitrogen uptake efficiency and low nitrogen uptake efficiency: HNUE and LNUE, respectively). We hypothesized that hybrid genotypes with the HNUE phenotype had root anatomical traits that served to reduce the metabolic costs of soil exploration (such as increased root cortical aerenchyma, reduced CFN and large CCS), leading to increased capacity of N uptake and a better performance under N limitation.
Materials and methods
Maize hybrids included in the study
This study utilized six maize (Zea mays L.) genotypes: Zhengdan 958 (ZD), Nongda 108 (ND), Zhongnong 99 (ZN), Xianyu335 (XY), Xiuqing 73–1 (XQ) and Lainong14 (LN). These genotypes are single cross hybrids that are commonly cultivated in the summer maize production area of northern China. These varieties were selected based on a study conducted in the field from 11 June to 8 October 2013, which included 13 varieties of common maize hybrids that were evaluated after having been grown in two different environments; HN, with a sufficient N supply of (260 kg N ha−1), and LN, with a deficient N supply of (80 kg N ha−1). For this experiment, shoots were sampled 108 d after planting, and the 13 varieties were grouped into four categories based on the shoots' biomass (Fig. S1) with the following result: ZD, ND and ZN were classified as genotypes of high nitrogen use efficiency (HNUE), XY, XQ and LN were classified as genotypes of low nitrogen use efficiency (LNUE) under low nitrogen conditions.
Design of the greenhouse experiments
The greenhouse experiments utilized a randomized complete block design, in the greenhouse at the Maize Technological Innovation Center, located at Shandong Agriculture University (36°09′N, 117°09′E, 128 m above sea level) in Tai’an. The greenhouse experiments were started in June 2014. We grew all six maize hybrids (ZD, ZN, ND, XY, XQ, and LN) and applied HN and LN treatments to each of them, for a total of 12 experiments. Maize plants were grown in PVC cylinders that were 15.2 cm in diameter and 100 cm in height, and contained 18 L of growth medium composed of 45% sized commercial grade sand, 45% vermiculite, 5% perlite, 5% topsoil (containing 0.85 g N per kilogram soil). The low N soil incorporated into the growth medium was collected from the Maize Technological Innovation Center in Tai’an (sandy loam, Typic Hapli-Udic Argosol, pH = 6.85). Before planting, a polymer-coated urea fertilizer containing 42% N, (Kingenta Ecological Engineering Co. Ltd., Shandong, China) was uniformly mixed into the growth medium from 0–15 cm depth. The High-N (HN) treatment received 4.2 g N per plant, and the Low-N (LN) treatment received 1.5 g N per plant. Each block was replicated four times. Seeds of the six genotypes were surface-sterilized in 0.05% v/v NaOCl in water for 30 min and hydrated for 24 h in 0.5 mM CaSO4, then placed in darkness at 28 ± 1 °C in a germination chamber for 2 d. Two days before planting, each cylinder was irrigated with 3.0 L of deionized water. Three seedlings were transferred into each cylinder and then thinned to one plant per cylinder 5 d after transplanting. Following seedling establishment, 200 ml of water was applied in the HN and LN treatments every other day. Irrigation volume and frequency increased as plant development proceeded.
Plants were harvested 40 d after planting (V6 stage). Shoots were excised at the soil surface, oven-dried at 80 °C for 72 h, and weighed and ground for nutrient analysis. N content was analyzed with a Rapid N analyzer (Elementar, Germany). Roots were separated from the soil by vigorous rinsing with water at low pressure, and after which they were stored at 4 °C until further analysis. The vitality of apical root segments was estimated according to the triphenyl-tetrazolium chloride (TTC) reduction method (Li, 2000). For the root vitality analysis, about 0.5 g of fresh root samples originating from the root section at 0–2 cm from the root tip were cut into 1–2 cm long fragments and put into 25 ml vials containing 5 ml TTC solution (0.4% (w/v) TTC) and 5 ml pH 8.4 Tris–HCL buffer solution. Then, the samples were degassed for 15 min under vacuum, and incubated for 20 h at 30 ℃ (Clemensson-Lindell 1994) and subjected to dehydrogenase extraction for 15 min at 80 ℃ using 95% (v/v) ethanol. Finally, the samples were analyzed through measuring the absorption at 485 nm, and calculating the TTC reduction per unit mass. The root length was determined by scanning the roots at a resolution of 600 dpi (Epson, Perfection V700 Photo, Epson America, Inc. USA), and analyzing the scans using the WinRhizo software (2016 WinRhizo Pro, Régent Instruments, Québec, Canada), which provides values for the root length density (cm root length per cm3 soil). The specific N absorption efficiency (SNAE) of the shoots was calculated from their total N content per unit root dry weight (g g−1).
To analyze the anatomical traits of the plant roots, a 4 cm root segment was excised from each plant at 5 cm from the base of a third nodal crown root and stored in 75% ethanol at 4 °C prior to sectioning. Root cross sections with a thickness of 30—50 μm were obtained using Teflon-coated double-edged stainless-steel blades and examined using a DM21-J1200 light microscope. The microscope was fit with a black and white XC-77 CCD Video Camera Module (SonyCorporation, Tokyo, Japan). Images were captured and saved using the Scope Image 9.0 software and subsequently analyzed with the RootScan program to characterize the root anatomical phenotypes (Burton et al. 2012). Root anatomy traits measured included root cross-section area (RXSA), total cortex cross-section area (TCA), total stele cross-section area (TSA), total aerenchyma area (AA), cortical cell area (CCA), total metaxylem vessel area (MXVA), median cortical cell size (CCS), median single metaxylem vessel area (MXA), percentage of aerenchyma area in the cortex (AAP), number of cortical cell files (CFN), number of metaxylem vessels (MXN), percentage of total metaxylem vessel area in the stele (MXP) (Table 1).
Design of the field experiments
Field experiments were carried out at the Maize Technological Innovation Center, located at Shandong Agriculture University in Tai’an (36°09′N, 117°09′E, 128 m above sea level), from 14 June 2014 to 10 October 2014 and from 16 June 2015 to 11 October 2015. The soil at the field is sandy loam (Typic Hapli-udic Argosols). All assays were conducted at the State Key Laboratory of Crop Biology located at the Shandong Agriculture University. Initial soil status was: total N, 0.53 g kg−1; Alkali-N, 57.79 mg kg−1; Olsen-P, 14.2 mg kg−1; organic matter, 11.3 g kg−1; pH, 6.9.
The field experiment was arranged in randomized complete block design with two N treatments (HN and LN) for each of the six hybrids. All treatments were replicated three times in separate plots. Each plot was 6 × 10 m, and was arranged with 25 cm between plants within a row and 60 cm between rows. A thin polymer-coated urea containing 42% N was applied at the seeding and V12 stage, for a total N input of 300 kg N ha−1 for the HN treatment and a total N input of 150 kg N ha−1 for the LN treatment. At seeding, P2O5 and K2O were applied at a rate of 300 kg ha−1. Irrigation was carried out as needed.
Shoots were sampled at 108 d after planting. Three adjacent plants were randomly selected for shoot dry weight, and dried at 70 °C. A block of soil (60 cm length × 25 cm width × 60 cm depth) was extracted from the center of the region surrounding the plant. To determine the grain yield at the maturity stage, all ears of the plants in 5 m from the middle three rows in each plot were harvested and analyzed. The excavated roots were cleaned by vigorous rinsing with water at low pressure, after which the total root length was determined by scanning the roots at a resolution of 600 dpi (Epson, Perfection V700 Photo, Epson America, Inc. USA), and analyzing the scans using the WinRhizo software (2016 WinRhizo Pro, Régent Instruments, Québec, Canada). After analysis, the scanned roots were oven-dried at 80 °C for 72 h and weighed to determine the root dry weight.
Statistical analysis
Experimental data were statistically analyzed according to a one-way, two-way and three-way ANOVA analysis using the SPSS software (SPSS 20.0, IBM Corp., Armonk, NY, USA). Tukey’s Honest Significant Difference method (α = 0.05) was used for multiple comparisons. Linear regression analysis and Pearson correlation coefficients were calculated by OriginLab (OriginLabPro2020b, OriginLab Corp., Northampton, Massachusetts, USA). Redundancy analysis (“vegan” package) and random Forest (“RRF” package based on 100 trees) were employed to present the variable importance of root anatomy in R language of version 4.0.2 (R Core Team 2020).
Results
Shoot and root biomass
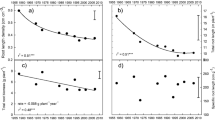
Neither the field nor the greenhouse experiments, revealed any significant differences in shoot biomass between plants of the included genotypes that had been subjected to the HN treatment (Fig. 1A and G; Fig. 2A). As expected, plants subjected to the LN treatment exhibited a lower shoot and root biomass than those that received the HN treatment, but we also observed significant differences between plants from different genotypes in the field (Table 2 and 3). For maize grown in the field experiments in both 2014 and 2015, we found that the shoot biomass of plants of HNUE genotypes subjected to the LN treatment was on average 18.89% lower than that of plants subjected to the HN treatment, while that of LNUE genotypes was 39.52% lower (Fig. 1A and G). In the field, HNUE genotypes had 21.43% and 43.57% significant greater root dry weight than LNUE genotypes in 2014 and 2015 (Fig. 1B and H). With respect to the greenhouse experiments, the shoot biomass of plants of HNUE genotypes that were subjected to the LN treatment was on average 25.61% lower than that of plants subjected to the HN treatment, and for plants of LNUE genotypes it was 56.86% lower (Fig. 2A).
Shoot dry weight (A, G), root dry weight (B, H), root length density (C, I), grain yield (D, J), nitrogen content (E, K), specific nitrogen absorption efficiency (SNAE) (F, L) at maturity stage in 2014 and 2015 in the field under high-N and low-N conditions. The gray bar and pink bar represent the HNUE and LNUE group, respectively
Analysis of the roots contained in the excavated blocks of soil from the field experiments showed that the root dry weight of plants from the LNUE group was significantly lower than that of plants from the HNUE group, under both HN and LN conditions (Fig. 1B and H). The root dry weight of plants from the LNUE group subjected to the LN treatment was 28.39% and 48.96% lower than that of plants from the HNUE group in 2014 and 2015, respectively (Fig. 1B and H). In the greenhouse, low N availability significantly decreased the root dry weight (Table 3), but plants of HNUE genotypes that were subjected to the LN treatment had a significantly greater root dry weight than those of LNUE genotypes (Fig. 2B). Root dry weight was reduced by 12.53% and 32.77% under the low N conditions for HNUE and LNUE genotypes, respectively, compared with that under high N conditions in the greenhouse (Fig. 2B).
Root length density and TTC reduction
In the field and greenhouse, root length density (RLD) was significantly influenced by both N treatment and the NUE phenotype (Table 2 and 3). Analyzing the roots contained in the excavated blocks of soil from the field experiment showed that low N availability gave rise to a reduced RLD (Fig. 1C and I). Under low N availability, the RLD of plants of genotypes from the HNUE group was 47.30% and 110.17% higher than that of plants from the LNUE group in 2014 and 2015, respectively (Fig. 1C and I). Under low N conditions, measurements of the RLD showed a clear suppression of maize root growth (Fig. 2C). Additionally, differences in RLD of the NUE groups was observed where the RLD of the LNUE group averaged 2.40 mm cm−3, while that of the HNUE group averaged 5.18 mm cm−3 under low N conditions in the greenhouse (Fig. 2C). Furthermore, compared with the HN treatment, RLD was reduced by 25.51% and 63.49% of the HNUE and LNUE groups, respectively under low N conditions in the greenhouse (Fig. 2C).
The ability of root cells to reduce TTC to triphenyl formazan is a measure of cell vitality. The level of TTC reduction in root tips was significantly affected by both N level and NUE phenotype (Table 3). Low N availability reduced the level of TTC reduction by 28.75% relative to the level measured under high N availability. When subjected to the LN treatment, the level of TTC reduction in the cells from the root tips of plants from the HNUE group subject to the LN treatment was 57.68% higher than that in the cells from the root tips of plants from the LNUE groups, which indicates an enhanced vitality (Fig. 2D).
N uptake and uptake efficiency
In the field and greenhouse, N uptake and N uptake efficiency were significantly influenced by both N treatment and the NUE genotype (Table 2 and 3). In the field, HNUE genotypes had significantly greater N uptake and N uptake efficiency than the LNUE group under low N conditions in 2014 and 2015 (Fig. 1E, F, K and L). Compared with plants grown under high N availability, greenhouse mesocosms with low N availability resulted in 29.57% less N uptake and 24.20% lower SNAE for the HNUE genotypes, and 62.24% less N uptake and 41.19% lower SNAE for the LNUE genotypes (Fig. 2E and F). Under low N conditions in the greenhouse, HNUE genotypes had 73.54% greater N uptake and 30.28% greater N uptake efficiency than the LNUE group (Fig. 2E and F).
Root anatomical traits
In the greenhouse, low N availability significantly affected the RXSA, CCA, CCS, CFN, AA and AAP (Table 3). HNUE genotypes had greater CCA and CCS, and had less AA and AAP than LNUE genotype under high N conditions (Fig. 3). Under low N conditions, analysis of variance indicated that maize variety had a significant effect on AA, AAP, and CCS (Fig. 3; Table S1). HNUE genotypes had an average of 36.27% greater AA, 31.66% higher AAP and 15.28% larger CCS than LNUE genotypes under low N conditions when analyzed by one-way ANOVA for genotypes under just low N conditions (Fig. 3D, G, I; Table S1).
Root anatomy parameter of different NUE maize varieties at 40 days in the greenhouse under high-N and low-N conditions. A, RXSA, root cross-section area; B, TCA, total cortex cross-section area; C, TSA, total stele cross-section area; D, AA, total aerenchyma area; E, CCA, cortical cell area; F, MXVA, total metaxylem vessel area; G, CCS, median cortical cell size; H, MXA, median single metaxylem vessel area; I, AAP, percentage of aerenchyma area in the cortex; J, CFN, number of cortical cell files; K, MXN, number of metaxylem vessels; L, MXP, percentage of total metaxylem vessel area in the stele. The gray bar and pink bar represent the HNUE and LNUE group, respectively
We subsequently performed a redundancy analysis (RDA) to illustrated how the observed root anatomical traits affected the N acquisition parameters; N content (NC) and SNAE under low N conditions (Fig. 4). The RDA showed that anatomical traits of roots significantly affected their capacity for N acquisition, giving rise to a total explained variation of 72.25% (F = 4.69, p = 0.036). The first two principal components (PC 1 and PC 2) explain 97.51% and 2.49% of the variation in response to the NC and SNAE (Fig. 4). Among all the constraining variables, the CCS and the AA had the most significant influence on the N uptake index, and explained 16.21% (p = 0.009) and 39.78% (p = 0.005) of the variance (Table S2). As for the RXSA, TCA, TSA, CCA, MXA MXN, AAP, and CFN: none of these variables had a significant impact on the N uptake index (Table S2). As calculated by the random forest algorithm, the most important variables that significantly affected the NC were the CCS and AA (Fig. S2). And the most important variables that significantly affected the SNAE were the CCS, AA and AAP (Fig. S2).
Redundancy analysis (RDA) of nitrogen acquisition in relation to root anatomical traits under low-N conditions. Note: NC, nitrogen content; SNUE, specific nitrogen absorption efficiency; RXSA, root cross-section area; TCA, total cortex cross-section area; TSA, total stele cross-section area; AA, total aerenchyma area; CCA, cortical cell area; MXVA, total metaxylem vessel area; CCS, median cortical cell size; MXA, median single metaxylem vessel area; AAP, percentage of aerenchyma area in the cortex; CFN, number of cortical cell files; MXN, number of metaxylem vessels; MXP, percentage of total metaxylem vessel area in the stele
Another correlation analysis using Pearson algorithm revealed that under low N availability, AA and AAP had significant positive correlations with shoot biomass, total N uptake, SNAE, root length density; CCS had significant positive correlations with shoot biomass, root length density and root surface area (Fig. 5).
Correlations of root anatomy and plant growth index at 40 days in the greenhouse under low-N conditions. Note: SDW, shoot dry weight; NC, nitrogen content; SNAE, specific nitrogen absorption efficiency; RDW, root dry weight; RLD, root length density; TTC, root TTC vitality; RXSA, root cross-section area; TCA, total cortex cross-section area; TSA, total stele cross-section area; AA, total aerenchyma area; CCA, cortical cell area; MXVA, total metaxylem vessel area; CCS, median cortical cell size; MXA, median single metaxylem vessel area; AAP, percentage of aerenchyma area in the cortex; CFN, number of cortical cell files; MXN, number of metaxylem vessels; MXP, percentage of total metaxylem vessel area in the stele. n = 18. * p < = 0.05, ** p < = 0.01, *** p < = 0.001
Discussion
Our study analyzed the relationship between root anatomical phenotypes and their N uptake, using hybrid maize genotypes with contrasting NUE. Our data revealed that, compared to hybrid maize plants of LNUE genotypes, those of HNUE genotypes featured a greater root dry weight, a greater RLD, and an improved vitality of the cells in their root tips (Fig. 2). The formation of root cortical aerenchyma was associated with a reduction of root respiration, thus that reduced the metabolic cost of soil exploration (Saengwilai et al. 2014b). And the large CCS was proposed to increase the percentage of vacuolar to cytoplasmic volume, which could reduce the metabolic cost of living tissue by less root respiration and less carbon cost of nutrient content (Lynch 2013). A redundancy analysis and a Pearson’s correlation analysis revealed that hybrid maize plants exhibit significant correlations between the root anatomical traits associated with reduced metabolic costs (CCS, AA, and AAP) and their efficiency of N uptake (Figs. 3, 4, S3).
In the present study, under low N conditions HNUE genotypes had greater root length per volume of soil than those of LNUE genotypes, and that they increased root length growth more than LNUE varieties (Fig. 1 and 2); a likely factor contributing to the improved N acquisition of these hybrids. The enhanced TTC reduction observed in the cells in the root tips of plants of HNUE hybrids may be related to the fact that older root segments produce more aerenchyma, which facilitates the remobilization of growth-limiting resources to the root apices, thereby stimulating continued root growth (Abiko and Obara 2014). N in lysed root tissue of plants with greater root cortical aerenchyma can be reabsorbed and utilized to support growth of additional root length or shoot biomass, as was suggested in a study by Saengwilai (2014b). Similarly, in the present study with hybrid maize lines, the formation of aerenchyma was positively correlated with shoot biomass, TTC reduction, root length and N content (Fig. 4 and 5). Additional evidence was found by Peng et al (2010), who showed that the N-efficient maize inbred line 478 had a larger root system and longer total root length than that of the N-inefficient inbred line Wu312, indicating a higher relative growth rate of roots and greater capacity for N uptake in the N-efficient line.
In addition to the remobilization of resources, root anatomical traits like large cortical cell size and aerenchyma in older root segments also serve to reduce the metabolic costs of soil exploration per length of root, allowing for greater root length and enhanced exploration of soil volume. This is evidenced by the positive correlations between aerenchyma and root length density, as well as root length density and N uptake (Fig. 5). The metabolic cost of soil exploration by roots is quite substantial and can exceed half of the energy captured daily by photosynthesis (Lambers et al. 2002). Therefore, one of the key aspects involved in a plant's successful adaptation to edaphic stress is the efficiency with which it can utilize metabolic resources for soil exploration (Lynch 2019). In the present study, redundancy analysis revealed the relative importance of variations in a plant's root anatomical traits for its capacity to acquire N, which suggests that increasing the number of root cortical aerenchyma and the cortical cell size could improve N acquisition significantly (Fig. 5). Our results in hybrid maize lines support previous observations in inbred maize lines that, confirming that enhanced root cortical aerenchyma formation is associated with improved N use efficiency (Saengwilai, 2014b). In addition to benefit under N stress, Zhu et al (2010) showed that under water stress maize inbred lines with more root cortical aerenchyma had five times greater biomass and eight times greater yield than inbred lines with lower root cortical aerenchyma. Chimungu et al (2015) similarly showed that expression of root cortical aerenchyma in inbred maize roots was strongly correlated with grain yield under water stress.
Living cortical area refers to the portion of the cortex that remains alive after the formation of aerenchyma (Jaramillo et al. 2013). A key component of living cortical area that can affect the respiratory costs and utilization of growth limiting resources is the size of the cortical cells that compose the remaining cortex. Similar to the formation of aerenchyma, large cortical cell size has been observed to improve drought tolerance by reducing the metabolic cost of soil exploration, which enables deeper soil exploration, increases its capacity to acquire water from deeper soil horizons, and improves its growth and yield under water stress (Chimungu et al. 2014a). In the present study, cortical cell size was positively correlated with shoot biomass and N uptake efficiency under low N availability (Figs. 4, 5). Thus, genotypes with high N acquisition efficiency had the greater root cortical aerenchyma formation and large cortical cell size, which supports the hypothesis that the anatomical elements of the ideotype for optimal nitrate capture conform to the anatomical elements of the “Steep, Cheap and Deep” ideotype suggested by Lynch (Lynch 2013).
Conclusion
In conclusion, we observed substantial phenotypical differences with respect to the growth of and N utilization by hybrid maize plants under varying N availability, and found that these differences were associated with the anatomical traits of their roots. Our results demonstrated that HNUE hybrids had greater formation of root cortical aerenchyma and large cortical cell size than LNUE hybrids. Consequently, we concluded that HNUE hybrids have the capacity to allocate more carbon remobilize N to support new root growth, thereby increasing root length, soil exploration, and N uptake. Root anatomical traits like aerenchyma that reduce the metabolic costs of soil exploration and enhance N acquisition efficiency, are a promising breeding target for improving the N foraging capability of hybrid maize varieties, and might be instrumental to making progress on the path towards agricultural sustainability.
Change history
11 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11104-022-05471-9
References
Abiko T, Obara M (2014) Enhancement of porosity and aerenchyma formation in nitrogen-deficient rice roots. Plant Sci 215:76–83. https://doi.org/10.1016/j.plantsci.2013.10.016
Bizimana F, Timilsina A, Dong W, Uwamungu JY, Li XX, Wang YY, Pandey QSP, Hu CS (2021) Effects of long-term nitrogen fertilization on N2O, N2 and their yield-scaled emissions in a temperate semi-arid agro-ecosystem[J]. J Soils Sediments 21(4):1659–1671. https://doi.org/10.1007/s11368-021-02903-4
Burton AL, Williams M, Lynch JP, Brown KM (2012) RootScan: software for high-throughput analysis of root anatomical traits. Plant Soil 357(1):189–203. https://doi.org/10.1007/s11104-012-1138-2
Chen XP, Cui ZL, Vitousek PM, Cassman KG, Matson PA, Bai JS, Meng QF, Hou P, Yue SC, Romheld V, Zhang FS (2011) Integrated soil-crop system management for food security. Proc Natl Acad Sci 108(16):6399–6404. https://doi.org/10.1073/pnas.1101419108
Chimungu JG, Brown KM, Lynch JP (2014a) Large root cortical cell size improves drought tolerance in maize. Plant Physiol 166(4):2166–2178. https://doi.org/10.1104/pp.114.250449
Chimungu JG, Brown KM, Lynch JP (2014b) Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiol 166(4):1943–1955. https://doi.org/10.1104/pp.114.249037
Chimungu JG, Maliro MF, Nalivata PC, Kanyama-Phiri G, Brown KM, Lynch JP (2015) Utility of root cortical aerenchyma under water limited conditions in tropical maize (Zea mays L.). Field Crops Research 171:86–98. https://doi.org/10.1016/j.fcr.2014.10.009
Clemensson-Lindell A (1994) Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: applications and limitations. Plant Soil 159(2):297–300. https://doi.org/10.1007/BF00009293
Crist E, Mora C, Engelman R (2017) The interaction of human population, food production, and biodiversity protection. Science 356(6335):260–264. https://doi.org/10.1126/science.aal2011
Galindo-Castañeda T, Brown KM, Lynch JP (2018) Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant, Cell Environment 41(7):1579–1592. https://doi.org/10.1111/pce.13197
Hirel B, Le GJ, Ney B, Gallais A (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot 58(9):2369–2387. https://doi.org/10.1093/jxb/erm097
Huang J, Xu CC, Ridoutt BG, Wang XC, Ren PA (2017) Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J Clean Prod 159:171–179. https://doi.org/10.1016/j.jclepro.2017.05.008
Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP (2013) Root cortical burden influences drought tolerance in maize. Ann Bot 112(2):429–437. https://doi.org/10.1093/aob/mct069
Jia XC, Shao LJ, Liu P, Zhao B, Gu LM, Dong ST, Bing SW, Zhang JW, Zhao B (2014) Effect of different nitrogen and irrigation treatments on yield and nitrate leaching of summer maize (Zea mays L.) under lysimeter conditions. Agric Water Manag 137:92–103. https://doi.org/10.1016/j.agwat.2014.02.010
Lambers H, Atkin OK, Millenaar FF (2002) The respiratory patterns in roots in relation to their functioning. In Plant Roots: The Hidden Half (pp. 521–552) Marcel Dekker
Liu Y, Bortier MF, De BHJ, Nijs I (2017) Root distribution responses to three-dimensional soil heterogeneity in experimental mesocosms. Plant Soil 421(1):353–366. https://doi.org/10.1007/s11104-017-3472-x
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112(2):347–357. https://doi.org/10.1093/aob/mcs293
Lynch JP (2015) Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant, Cell Environment 38(9):1775–1784. https://doi.org/10.1111/pce.12451
Lynch JP (2019) Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol 223(2):548–564. https://doi.org/10.1111/nph.15738
Peng Y, Niu J, Peng Z, Zhang F, Li C (2010) Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crop Res 115(1):85–93. https://doi.org/10.1016/j.fcr.2009.10.006
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-roject.org/.
Ramankutty N, Mehrabi Z, Waha K, Jarvis L, Kremen C, Herrero M, Rieseberg LH (2018) Trends in global agricultural land use: implications for environmental health and food security. Annu Rev Plant Biol 69:789–815. https://doi.org/10.1016/j.scitotenv.2015.07.064
Raza S, Miao N, Wang P, Ju X, Chen Z, Zhou J, Kuzyakov Y (2020) Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob Change Biol 26(6):3738–3751. https://doi.org/10.1111/gcb.15101
Saengwilai P, Tian X, Lynch JP (2014a) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166(2):581–589. https://doi.org/10.1104/pp.113.232603
Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP (2014b) Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166(2):726–735. https://doi.org/10.1104/pp.114.241711
Song XT, Liu M, Ju XT, Gao B, Su F, Chen XP, Rees RM (2018) Nitrous oxide emissions increase exponentially when optimum nitrogen fertilizer rates are exceeded in the North China Plain[J]. Environ Sci Technol 52(21):12504–12513. https://doi.org/10.1021/acs.est.8b03931
Sun BR, Gao YZ, Lynch JP (2018) Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiol 177(1):90–104. https://doi.org/10.1104/pp.18.00234
Sun Z, Sänger A, Rebensburg P, Lentzsch P, Wirth S, Kaupenjohann M, Meyer-Aurich A (2017) Contrasting effects of biochar on N2O emission and N uptake at different N fertilizer levels on a temperate sandy loam. Sci Total Environ 578:557–565. https://doi.org/10.1016/j.scitotenv.2016.10.230
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2013) Maize root growth angles become steeper under low N conditions. Field Crop Res 140:18–31. https://doi.org/10.1016/j.fcr.2012.09.010
Wiesmeier M, Hübner R, Kögel-Knabner I (2015) Stagnating crop yields: An overlooked risk for the carbon balance of agricultural soils? Sci Total Environ 536:1045–1051. https://doi.org/10.1016/j.scitotenv.2015.07.064
Xia L, Lam SK, Chen D, Wang J, Tang Q, Yan X (2017) Can knowledge-based N management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A Meta-Analysis Global Change Biology 23(5):1917–1925. https://doi.org/10.1111/gcb.13455
Yu P, White PJ, Hochholdinger F, Li C (2014) Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta 240(4):667–678. https://doi.org/10.1007/s00425-014-2150-y
Zhan A, Lynch JP (2015) Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J Exp Bot 66(7):2055–2065. https://doi.org/10.1093/jxb/erv007
Zhang DS, Lyu Y, Li HB, Tang XY, Hu R, Rengel Z, Zhang FS, Whalley WR, Davies WJ, Cahill JJF (2020) Neighbouring plants modify maize root foraging for phosphorus: coupling nutrients and neighbours for improved nutrient-use efficiency. New Phytol 226:244–253. https://doi.org/10.1111/nph.16206
Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell Environment 33(5):740–749. https://doi.org/10.1111/j.1365-3040.2009.02099.x
Acknowledgements
We are grateful for grants from National Natural Science Foundation of China (31771713, 32071959), National Basic Research Program of China (2016YFD0300106), and Shandong Province Key Agricultural Project for Application Technology Innovation (SDAIT02-08).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Jiayin Pang.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jia, X., Wu, G., Strock, C. et al. Root anatomical phenotypes related to growth under low nitrogen availability in maize (Zea mays L.) hybrids. Plant Soil 474, 265–276 (2022). https://doi.org/10.1007/s11104-022-05331-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05331-6