Abstract
Aims
There is a trend of increasing woody biomass in tropical savannas. Here we ask what effect this increase may have on soil carbon pools and fluxes.
Methods
Using a field experiment we determine the amount of soil carbon directly under grasses, a juvenile tree among grasses and a juvenile tree with no grasses. We also measure CO2 efflux at the soil surface and use gas wells to extract CO2 from several soil depths.
Results
Our results show that grasses contribute substantially more than trees to both soil carbon and soil respiration. Grasses also make a disproportionate contribution to the δ13C value of SOC in the tree only treatments. The surface gas efflux data show that soil respiration increased with an increase in volumetric soil moisture and temperature and plots with both grasses and trees had higher respiration rates than plots with trees only or with grasses only.
Conclusions
The highest soil respiration is in the top 20 cm of the soil with grasses the primary contributors to both δ13CSOC and δ13CR. Any increase in woody biomass will result in a decline in SOM turnover and nitrogen mineralization rates resulting in higher SOC pools. The associated increases in SOC and above ground biomass will however be associated with negative economic and biodiversity impacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropical savanna is typically composed of a discontinuous layer of C3 trees interspersed among a continuous layer of C4 grasses (Frost et al. 1986). Changes in the relative proportion of these two plant life forms may alter savanna structure considerably from a few scattered trees as in the Kalahari to the well wooded Miombo of Central Africa (Chidumayo 1990; Dean et al. 1999). The combination of C3 trees and C4 grasses can be found across a rainfall gradient from around 280 mm in the southern Kalahari in southern Africa to 2500 mm in the South American Llanos (Lehmann et al. 2014). The defining feature for these ecosystems however is that this rainfall is distinctly seasonal with a 5–8 month warm wet season followed by a cool dry season when soil moisture may drop below wilting point (February and Higgins 2016). The high moisture deficit in the dry season leads both grasses and trees to abandon growth in the dry season (February and Higgins 2016). Although savanna trees may be both deciduous or evergreen, the dry season is always associated with reductions in leaf area and rates of carbon uptake (February and Higgins 2016; Seghieri et al. 1995). As a result, rainfall seasonality in savanna has a very strong influence on productivity with high rates of photosynthesis and soil respiration in the wet season (Bowling et al. 2015; Makhado and Scholes 2011; Richards et al. 2012).
As trees and grasses are the dominant plant life forms in savanna soil organic carbon (SOC) is primarily composed of carbon from these two life forms. While trees may contribute more to above ground biomass grasses contribute substantially more to soil carbon (February et al. 2013; Hudak et al. 2003; Jackson et al. 2002; Wigley et al. 2020). It has also been demonstrated that nitrogen mineralisation rates are higher beneath grasses than beneath trees (Higgins et al. 2015). This higher contribution of carbon with higher mineralization rates suggest higher carbon fluxes under grasses (Higgins et al. 2015). Furthermore, several studies have also demonstrated that SOC and nitrogen pools are higher under the canopy of savanna trees relative to inter-canopy sites (Coetsee et al. 2010; February et al. 2013; February and Higgins 2010; Holdo and Mack 2014). As such, any change towards an increase in tree biomass will also change the soil carbon pools, fluxes and nutrient dynamics (Coetsee et al. 2010; Craine et al. 2008).
Savanna ecosystems contain 10–30% of the global SOC pool and as a result are important carbon reservoirs (Dintwe and Okin 2018; Grace et al. 2006). The content, composition and distribution of this SOC is controlled by plant communities and the dominant plant life forms (trees and grasses) that differ in their litter chemistry, patterns of detrital input and rooting depth (Chen et al. 2005). Several studies have reported an increase in woody plant biomass in savanna and grassland globally (Buitenwerf et al. 2012; Nackley et al. 2017; Wigley et al. 2010). This increase in woody biomass, termed bush encroachment, will result in changes in the interactions between trees and grasses that will impact both soil carbon pools and fluxes.
In the context of globally observed increases in woody biomass and limited knowledge on soil respiration in savanna, we ask whether we can estimate the relative contributions of grasses and trees to soil CO2 fluxes. We hypothesise that due to higher root activity and lower root longevity grasses contribute more to soil carbon pools and fluxes than trees do and as a result, the carbon flux in experimental treatments without grass will be much lower than in treatments with grasses (Fig. 1). We hypothesise further that since the majority of the roots for both trees and grasses are in the upper 20 cm of the soil the highest contribution to soil respiration fluxes will be from grass roots in the upper layers of the soil and despite some tree roots going deeper down there is a larger contribution to carbon pools and smaller contribution to carbon fluxes from trees in these deeper layers of the soil (Fig. 1) (February and Higgins 2010; Wigley et al. 2020).
We explore this hypothesis in an experiment where we not only measure CO2 efflux at the soil surface but also use gas wells to extract CO2 from several depths through the soil profile directly under grasses, under a juvenile tree among grasses and under a juvenile tree with no grasses (Bowling et al. 2015). For ease of experimental manipulation, we concentrate on juvenile trees which are small non reproductive trees, that may exist in the landscape for several decades, regularly resprouting after fire (Bond and van Wilgen 1996). Using the stable carbon isotope ratios of soil CO2 and soil organic matter we show the relative contributions of trees and grasses to soil carbon pools as well as CO2 fluxes at different levels through the soil profile.
Methods
Study site
The study was conducted in a fenced animal exclosure located 6.2 km northwest of Pretoriuskop in the southwest of the Kruger National Park, South Africa (25° 7’45.32"S 31°13’59.98"E). The exclosure is located on deep eutrophic red and yellow coarse apedal sands with plinthic subsoil horizons derived from granite (Venter and Govender 2012). The vegetation is classified as Pretoriuskop Sour Bushveld with dominant tree species Terminalia sericea, Sclerocarya birrea, and Dichrostachys cinerea and dominant grasses Heteropogon contortus, Hyperthelia dissoluta, and Setaria sphacelata (Mucina and Rutherford 2006).
The climate of the region is characterized by a hot wet season and cool dry season. Mean monthly maximum and minimum temperatures at the study site for the duration of the study were 25 and 17.5 °C, respectively. Rainfall at Pretoriuskop (25° 10.5’S 31° 16.1’E) was 754 mm in 2011 and 852 mm in 2012 (SANParks weather data). This rainfall primarily occurs from October to March and consists predominantly of thundershowers from the north and north-east or occasional tropical cyclones from the Indian Ocean to the east.
Study design
The vegetation in the exclosure was burnt at the end of the dry season in August 2011 with our first measurements starting at the end of September 2011 and running for 16 months to the end of December 2012. The fire top killed all juvenile trees but all trees resprouted and the experimental plots were established around resprouting T. sericea trees or/and a mix of the dominant grasses in three treatments: (1) tree, (2) grass, and (3) tree with grass. The plots with grasses had a grass canopy cover of 100% with grass up to the base of the trees. The tree only plots had all grasses physically removed in a separate experiment three years before. All treatment combinations were replicated five times yielding a total of fifteen experimental plots. Each plot had a diameter of 2 m and was isolated from the surrounding soil with a polyvinyl chloride (PVC) sheet inserted to a soil depth of 50 cm (Supp Fig. 1). This depth of 50 cm was based on the results of studies at the same experimental site that showed the majority of fine roots in the top 20 cm of the soil (February and Higgins 2010; Verweij et al. 2011).
Soil CO2 efflux
On all plots a 100 mm diameter, 90 mm high PVC collar was inserted 40 mm into the soil for soil respiration measurements. Soil CO2 efflux was measured on all plots at the end of each month for the duration of our experiment using a closed chamber, portable non-dispersive infrared gas analyser (LI- 8100, Li-Cor Inc., Lincoln, Nebraska, U.S.A.). All soil CO2 efflux measurements were accompanied with a soil temperature measurement to a depth of 5–10 cm (ML2X Theta Probe, Delta_T Devices, Cambridge, England). For determination of gravimetric soil moisture, a soil sample with a diameter of 4 cm was taken to a depth of 10 cm.
Isotopic composition and source partitioning of SOC
We obtained soil samples for our study site from an earlier study where soil was excavated from five replicate pits under and away from the canopy of a tree (February and Higgins 2010). At each pit, soil was extracted from a 5 cm deep 20 × 20 cm section every 5 cm for the first 20 cm and then every 20 cm to bedrock (150 cm). All roots were removed from the soil by dry sieving through an 850 µm sieve. We determined 13C/12C ratios of the carbon in the SOM (δ13CSOC) using a Thermo Finnigan Delta plus XP mass spectrometer coupled with a conflo III device to a Thermo Finnigan Flash EA1112 Elemental Analyser with automatic sampler (Thermo Electron, Bremen, Germany). We calibrated these results relative to Pee-Dee Belemnite as well as to correct for drift in our reference gas. Deviation from the standard is denoted by the term δ, and the results expressed as parts per thousand (‰) with precision of duplicate analysis 0.1‰ (February et al. 2011). Using a simple mixing model based on the δ13C values of the soil and end member (mean) δ13C values of the grasses (-13.17‰) and trees (-27.61‰) at our study site we determined the relative proportion of C3 (trees and forbs) and C4 (grasses) derived carbon in the soil with depth (February et al. 2013; Mordelet et al. 1997). This mixing model was only applied to the 0–20 cm soil horizon (surface soils)because of unrelated fractionation processes at 50 cm depth causing enrichment of soil δ13C unrelated to the inputs from C3 or C4 derived carbon (Balesdent and Mariotti 1996; Nel et al. 2018).
Isotopic composition of CO2 with depth
Gas wells installed at five depths 5, 10, 20, 40 and 80 cm (15 plots X 5 wells = 75 wells) were used to extract pore gas for stable carbon isotope analysis with as little soil disturbance as possible (Bowling et al. 2015). These wells consisted of a 6 mm OD stainless steel tube fitted with a rubber septum (Microsep F-138, CRS, Louisville, Kentucky, U.S.A.) inside a Swagelok straight fitting (Swagelok, Solon, Ohio, U.S.A). The tube was inserted to the appropriate depth angled into the centre of the plot through a 50 mm X 25 mm wooden block with appropriately drilled guide holes (Supp Fig. 1). The tubes were inserted using a hammer, with a metal rod inside the tube to prevent clogging the tube with soil. The metal rod was removed when the tube was at the required depth. At the end of each month the gas in the tube was extracted using a gastight syringe (Hamilton, Nevada U.S.A.). The gas was then transferred into a pre-evacuated 12 ml borosilicate vial (standard Labco Exetaine, Labco Ltd, Lampeter, Wales, United Kingdom). The isotopic composition of the CO2 in the gas (δ13CR) was analysed using a Thermo Finnigan Model II gasbench attached to a Delta Plus XP mass spectrometer (Thermo electron, Bremen, Germany). All isotope results are reported in δ13C notation as per mil (‰) relative to the Pee Dee Belemnite standard using our own internally run standards.
Results
Soil CO2 efflux
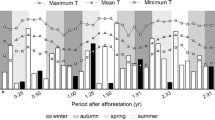
The study included two wet and two dry seasons (Fig. 2a). Rainfall in the first dry season (May – Sept) was 32 mm, followed by a 688 mm wet season (Oct – Apr) and 0 mm and 979 mm in the following dry (May – Aug) and wet (Sept – Apr) seasons. The second wet season started unusually early (September 15). Our results show that gravimetric soil water content increases rapidly from the end of the dry season in September to December-January after which soil moisture declines steadily throughout the dry season (Fig. 2a). CO2 efflux for our three treatments follow soil moisture, declining after January with the lowest rates of efflux between May and August 2012, with a mean of 0.41 µmol m− 2 s− 1 ± 0.3, and ranging between 0.2 and 1.9 µmol m− 2 s− 1. The highest rates of efflux were between October and February 2011–2012, with a mean of 2.4 µmol m− 2 s− 1 ± 0.7, ranging between 0.1 and 3.8 µmol m− 2 s− 1 and again between September and November 2012 (Fig. 2b). Soil efflux follows temperature with the highest temperatures of 28 °C ± 4 in the wet season with a range from 22 to 47 °C. In the dry season temperatures range between 20 °C ± 3 and 28 °C ± 15 (Fig. 2c).
Gravimetric soil water content (a) in the top 10 cm of the soil, as well as average gas efflux (b) and temperature (c) at the soil surface on each of our three treatments (♦ Tree + no grass, ● Grass + no tree and ▲Tree + Grass) from September 2011 to December 2012. Standard errors not shown for ease of visual display
We performed a linear mixed model analysis of the gas efflux data of the form;
Respiration rate ~ Soil temperature + Soil moisture + Treatment + 1| (Plot + Sample-date) The parameters for this model were estimated using Bayesian methods with all parameters assumed to have normal, uninformed priors. The variance of these priors was also assumed to be from uninformed uniform distributions. JAGS (Plummer 2003) was used to estimate the parameters using MCMC sampling. The output from JAGS was analysed in R (R_Development_Core_Team 2019) using the coda package (Plummer et al. 2006). The parameter estimates from this regression model indicated that the soil respiration rate increased by 0.10 µmol m− 2 s− 1 per percent increase in volumetric soil moisture content (95% credible intervals 0.101–0.102) and by 0.039 µmol m− 2 s− 1 per degree Celsius increase in soil temperature (95% credible intervals 0.0385–0.0395). Plots with grasses only or trees only had similar respiration rates when averaged over the time series (grasses 0.0013 higher, credible intervals 0.0019–0.025). Plots with grasses and trees had higher respiration rates: 0.099 higher than grass plots and 0.11 higher than tree plots (respective credible intervals 0.087–0.11; 0.10–0.12).
Isotopic composition and source partitioning of SOC
δ13CSOC become increasingly enriched with depth (from − 18.7‰ at 5 cm to -14.5‰ at 50 cm) after which values again become more depleted (-15.2‰ at 80 cm, Fig. 3). The percentage of soil carbon under the canopy of trees is higher (2.1% ± 0.9) than away from the canopy (1.0% ± 0.3) in the upper 5 cm of the soil after which values decline rapidly down to 20 cm (0.7% ±0.2 under, 0.6% ±0.09 away) (Fig. 4a). The mixing model results show that grasses contribute between 60 and 85% of the carbon in the SOC in the surface layers of the soil down to 20 cm under the canopy of trees and 87–95% away from the canopy of trees (Fig. 4b).
Isotopic composition of CO2 with depth
The disproportionate contribution of grass to δ13CSOC values are also evident in the gas well estimates of δ13CR (Fig. 3). Plots with trees only had wet season δ13CR values in the upper 20 cm of the soil that are depleted (-16.8 and − 17.7‰ in the wet season) relative to the grass only plots but these δ13CR values are still closer to that of grasses (-13.2‰) than trees (-27.6‰) (Fig. 3). There are strong seasonal differences in δ13CR values in the upper layers of the soil in the tree only plots. Under grasses (grass only, grass + tree) there are no significant treatment or seasonal differences in δ13CR values in the upper layers of the soil. These values do however become more depleted with depth, especially so in the dry season (Fig. 5). In the upper layers of the soil tree only δ13CR values are more depleted (closer to the tree end member value of -27.6‰) in the wet season while grasses become marginally more enriched (closer to the grass end member value of -13.2‰). In the tree only plots wet and dry season δ13CR values converge to ⁓ -17‰ from and below 40 cm depth while the grass plots (grass only, grass + tree) are ± 1‰ depleted in the dry season (Fig. 5).
Discussion
Soil organic matter is primarily composed of organic material produced by the roots as well as some detritus filtering down from the surface. Our results show that, in the top 20 cm of the soil, between 60–95% of the SOC at our study site is composed of grass organic matter. With microbial decomposition, the δ13C values of the CO2 released from this SOC is indistinguishable from that of grass root respiration. We were therefore unable to use the differences in δ13C values of trees, grasses and SOC to determine the relative contributions of the individual sources (roots and microbes) to total soil respiration (Rochette et al. 1999).
Our results do however show that in this tropical savanna where rainfall is distinctly seasonal the process rate of soil efflux primarily responds to soil moisture rather than temperature (Bowling et al. 2015; Makhado and Scholes 2011). Relative to the wet season, CO2 efflux at the soil surface decreased with decreasing soil moisture during the dry season regardless of treatment. Monthly gas efflux at the soil surface responded strongly to the onset of the rain at the beginning of the wet season in October 2011. Similarly, the early onset of the rains in September 2012 resulted in a strong increase in soil water content and soil CO2 efflux at the soil surface.
At our study site tree species are deciduous during the dry season while grass leaves, although they do not abscise, do die (Higgins et al. 2011). With very little dry season photosynthesis the primary source of rhizosphere respiration is maintenance respiration of roots while microbial activity is also reduced (Coetsee et al. 2010; Higgins et al. 2015; Makhado and Scholes 2011). The influence of tree root respiration at depth is illustrated in our study by the dry season δ13CR values of the soil pore CO2 from the plots with grasses being depleted by ± 1‰ and the convergence around − 17‰ in the tree only plots as values trend toward the − 27.6‰ end member value for trees. During the dry season SOC primarily composed of grass roots accumulates because of moisture limitation and a reduction in microbial activity creating a pool of high quality SOC (Higgins et al. 2015). With the onset of the rains microbes become active and preferentially decompose the more labile non-structural carbohydrates releasing 13C enriched CO2 primarily composed of grass organic matter. In the plots with grass these values are diluted close to the surface by mixing with atmospheric CO2 resulting in δ13CR values more enriched than the − 13.2‰ end member value for grasses.
During the wet season surface (top 20 cm) δ13CR were similar in grass-only and tree-grass plots suggesting minimal contribution of trees to δ13CR. Also, in these surface soil horizons our results show that between 60–80% of the SOC at our study site is comprised of grass organic matter. Taken together, these results show that the roots of grasses are contributing more to both SOC and soil rhizosphere respiration than trees. While our focus is on juvenile trees, our findings agree with several studies in Africa, Australia and South America which show that the highest proportion of fine roots for both trees and grasses are in the upper 20 cm of the soil (De Castro and Kauffman 1998; February et al. 2013; February and Higgins 2010; Riginos 2009; Wigley et al. 2020). These studies also show, as do our results, that δ13Csom become enriched from the surface down to 20 cm. Our results, however, show that both our δ13CR and δ13CSOC values become increasingly more depleted below 20 cm suggesting more of an influence from tree roots in the deeper layers of the soil.
A widespread projection of climate models for African savannas is that precipitation intensity will increase (Fischer et al. 2013; Pendergrass and Hartmann 2014). We show that while both trees and grasses are primarily rooted in the upper layers of the soil trees are also rooted deeper down. The projected increase in precipitation intensity will therefore push soil water into the deeper soils that are beyond the rooting zone of grasses (Kulmatiski and Beard 2013). Several causes for bush encroachment have been evoked including land management, and CO2 fertilization (Bond and Midgley 2000; Ward 2005). The ability for trees to source water deeper than grasses is yet another driver that could work in concert with others to exacerbate bush encroachment (Kulmatiski and Beard 2013). Our results show that grasses contribute substantially more than trees to both soil carbon and soil respiration. The rapid turnover of this carbon in the top 20 cm of the soil is an important determinant of plant available nitrogen (February and Higgins 2016; Higgins et al. 2015). There is, however, a documented decrease in grass biomass and increase in soil carbon with bush encroachment (Coetsee et al. 2013; Hudak et al. 2003). An increase in woody biomass below 20 cm combined with the slower decomposition rates for tree carbon will result in a decrease in carbon turnover and nitrogen mineralisation in the top 20 cm of the soil that will substantially change the ecosystem (Coetsee et al. 2013; February and Higgins 2016; Higgins et al. 2015). Worldwide, savanna is a biodiverse and economically important biome, any changes to the ecosystem will have biodiversity and economic consequences (Coetsee et al. 2013; Ratter et al. 1997).
Conclusions
Our results show that the highest proportion of fine roots are in the upper 20 cm of the soil and that grasses are the primary contributors to both SOC and soil respiration at our study site. Trees do, however, contribute to SOC and soil respiration in the deeper soil horizons. The results of our study also show that when grasses are present CO2 fluxes are larger and that the isotope ratio of soil respiration (δ13CR) is more representative of grasses than trees. Any increase in woody biomass at our study site will result in a slowing of soil carbon cycling as SOC turnover declines with subsequent reductions in soil respiration resulting in higher SOC pools. The biodiversity losses and economic consequences of such changes to the ecosystem far outweigh the arguments for carbon sequestration (Coetsee et al. 2013; Ratter et al. 1997).
References
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In: Boutton TW, Yamasaki S (eds) Mass Spectrometry of Soils. Marcel Dekker Inc, New York
Bond WJ, Midgley GF (2000) A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob Chang Biol 6:865–869
Bond WJ, van Wilgen BW (1996) Fire and Plants. Chapman and Hall, London
Bowling D, Egan J, Hall S, Risk D (2015) Environmental forcing does not induce diel or synoptic variation in the carbon isotope content of forest soil respiration. Biogeosciences 12:5143–5160
Buitenwerf R, Bond W, Stevens N, Trollope W (2012) Increased tree densities in South African savannas:> 50 years of data suggests CO2 as a driver. Glob Change Biol 18:675–684
Chen X, Hutley LB, Eamus D (2005) Soil organic carbon content at a range of north Australian tropical savannas with contrasting site histories. Plant Soil 268:161–171
Chidumayo EN (1990) Aboveground woody biomass structure and productivity in a Zambian woodland. For Ecol Manag 36:33–46
Coetsee C, Bond WJ, February EC (2010) Frequent fire affects soil nitrogen and carbon in an African savanna by changing woody cover. Oecologia 162:1027–1034. https://doi.org/10.1007/s00442-009-1490-y
Coetsee C, Gray EF, Wakeling J, Wigley BJ, Bond WJ (2013) Low gains in ecosystem carbon with woody plant encroachment in a South African savanna. J Trop Ecol 29:49–60
Craine JM, Morrow C, Stock WD (2008) Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol 179:829–836
De Castro EA, Kauffman JB (1998) Ecosystem structure in the Brazilian Cerrado: a vegetation gradient of aboveground biomass, root mass and consumption by fire. J Trop Ecol 14:263–283
Dean W, Milton S, Jeltsch F (1999) Large trees, fertile islands, and birds in arid savanna. J Arid Environ 41:61–78
Dintwe K, Okin GS (2018) Soil organic carbon in savannas decreases with anthropogenic climate change. Geoderma 309:7–16
February EC, Cook GD, Richards AE (2013) Root dynamics influence tree–grass coexistence in an Australian savanna. Austral Ecol 38:66–75. https://doi.org/10.1111/j.1442-9993.2012.02376.x
February EC, Allsopp N, Shabani T, Hattass D (2011) Coexistence of a C4 grass and a leaf succulent shrub in an arid ecosystem. The relationship between rooting depth, water and nitrogen. Plant Soil 349:253–260.
February EC, Higgins SI (2010) The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. South Afr J Bot 76:517–523. https://doi.org/10.1016/j.sajb.2010.04.001
February EC, Higgins SI (2016) Rapid leaf deployment strategies in a deciduous savanna. PLoS One 11
Fischer EM, Beyerle U, Knutti R (2013) Robust spatially aggregated projections of climate extremes. Nat Clim Chang 3:1033–1038
Frost P, Menaut JC, Walker B, Medina E, Solbrigo T (1986) Responses of savannas to stress and disturbance. International Union of Biological Sciences Special Issue 10 Paris, France
Grace J, José JS, Meir P, Miranda HS, Montes RA (2006) Productivity and carbon fluxes of tropical savannas. J Biogeogr 33:387–400
Higgins SI, Delgado-Cartay MD, February EC, Combrink HJ (2011) Is there a temporal niche separation in the leaf phenology of savanna trees and grasses? J Biogeogr 38:2165–2175. https://doi.org/10.1111/j.1365-2699.2011.02549.x
Higgins SI, Keretetse M, February EC (2015) Feedback of trees on nitrogen mineralization to restrict the advance of trees in C4 savannahs. Biol Lett 11:20150572
Holdo RM, Mack MC (2014) Functional attributes of savanna soils: contrasting effects of tree canopies and herbivores on bulk density, nutrients and moisture dynamics. J Ecol 102:1171–1182
Hudak A, Wessman C, Seastedt T (2003) Woody overstorey effects on soil carbon and nitrogen pools in South African savanna. Austral Ecol 28:173–181
Jackson RB, Banner JL, Jobbágy EG, Pockman WT, Wall DH (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–626
Kulmatiski A, Beard KH (2013) Woody plant encroachment facilitated by increased precipitation intensity. Nat Clim Chang
Lehmann CE, Anderson TM, Sankaran M, Higgins SI, Archibald S, Hoffmann WA, Hanan NP, Williams RJ, Fensham RJ, Felfili J (2014) Savanna vegetation-fire-climate relationships differ among continents. Science 343:548–552
Makhado RA, Scholes RJ (2011) Determinants of soil respiration in a semi-arid savanna ecosystem, Kruger National Park, South Africa. Koedoe 53:00–00
Mordelet P, Menaut JC, Mariotti A (1997) Tree and grass rooting patterns in an African humid savanna. J Veg Sci 8:65–70
Mucina L, Rutherford M (eds) (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria, p 807
Nackley LL, West AG, Skowno AL, Bond WJ (2017) The nebulous ecology of native invasions. Trends Ecol Evol 32:814–824
Nel JA, Craine JM, Cramer MD (2018) Correspondence between δ13C and δ15N in soils suggests coordinated fractionation processes for soil C and N. Plant Soil 423:257–271
Pendergrass AG, Hartmann DL (2014) Changes in the distribution of rain frequency and intensity in response to global warming. J Clim 27:8372–8383
Plummer M (2003) JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003) March
Plummer M, Best N, Cowles K, Vines K (2006) CODA: convergence diagnosis and output analysis for MCMC. R News 6:7–11
R_Development_Core_Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ratter JA, Ribeiro JF, Bridgewater S (1997) The Brazilian cerrado vegetation and threats to its biodiversity. Ann Bot 80:223–230
Richards AE, Dathe J, Cook GD (2012) Fire interacts with season to influence soil respiration in tropical savannas. Soil Biol Biochem 53:90–98
Riginos C (2009) Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology 90:335–340. https://doi.org/10.1890/08-0462.1
Rochette P, Flanagan L, Gregorich E (1999) Separating soil respiration into plant and soil components using analyses of the natural abundance of carbon-13. Soil Sci Soc Am J 63:1207–1213
Seghieri J, Floret C, Pontanier R (1995) Plant phenology in relation to water availability: herbaceous and woody species in the savannas of northern Cameroon. J Trop Ecol 11:237–254
Venter FJ, Govender N (2012) A geomorphic and soil description of the long-term fire experiment in the Kruger National Park, South Africa. Koedoe 54:44–54
Verweij RJT, Higgins SI, Bond WJ, February EC (2011) Water sourcing by trees in a mesic savanna: Responses to severing deep and shallow roots. Environ Exp Bot 74:229–236
Ward D (2005) Do we understand the causes of bush encroachment in African savannas? Afr J Range Forage Sci 22:101–105
Wigley BJ, Augustine DJ, Coetsee C, Ratnam J, Sankaran M (2020) Grasses continue to trump trees at soil carbon sequestration following herbivore exclusion in a semi-arid African savanna. Ecology
Wigley BJ, Bond WJ, Hoffman M (2010) Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob Change Biol 16:964–976
Acknowledgements
The research was funded by the Andrew W. Mellon Foundation (#30600716). We would like to thank SANParks for permission to work in the Kruger National Park. We are grateful to Ben Wigley, Corli Coetsee, Henri Combrinck and Paola Vimercati for help with fieldwork. Thanks to Anna Richards for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
ECF and SH conceived and designed the experiments. ECF performed the experiments. ECF, SH and JP analysed the data. ECF, SH and JP wrote the manuscript.
Corresponding author
Additional information
Responsible Editor: Simon Jeffery
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 145 kb)
Rights and permissions
About this article
Cite this article
February, E., Pausch, J. & Higgins, S.I. Major contribution of grass roots to soil carbon pools and CO2 fluxes in a mesic savanna. Plant Soil 454, 207–215 (2020). https://doi.org/10.1007/s11104-020-04649-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04649-3