Abstract
Relatively few studies have documented the impacts of afforestation, particularly production forestry, on belowground carbon dioxide (CO2) effluxes to the atmosphere. We evaluated the changes in the soil CO2 efflux—a proxy for soil respiration (Rs)—for three years following a native grassland conversion to eucalypt plantations in southern Brazil where minimum tillage during site preparation created two distinct soil zones, within planting row (W) and between-row (B). We used root-exclusion and carbon (C)- isotopic approaches to distinguish Rs components (heterotrophic-Rh and autotrophic-Ra respirations), and a CO2 profile tube (1-m deep) to determine the concentration ([CO2]) and isotopic C signature of soil CO2 (δ13[CO2]). The soil CO2 efflux in the afforested site averaged 0.37 g CO2 m−2 h−1, which was 56% lower than the soil CO2 efflux in the grassland. The δ13CO2 in the afforested site ranged from − 14.1‰ to − 29.4‰, indicating a greater contribution of eucalypt-derived respiration (both Rh and Ra) over time. Higher soil CO2 efflux and lower [CO2] were observed in W than B, indicating that soil preparation creates two distinct soil functional zones with respect to C cycling. The [CO2] and δ13[CO2] decreased in both zonal positions with eucalypt stand development. Although the equilibrium in C fluxes and pools across multiple rotations is needed to fully account for the feedback of eucalypt planted forests to climate change, we provide quantitative information on soil CO2 dynamics after afforestation and show how soil preparation can leverage the feedback of planted forests to climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land-use change (LUC) accounts for about 20% of human-induced carbon dioxide (CO2) emissions (Tubiello et al. 2015), contributing ~ 1.15 Pg of carbon (C) per year (yr) into the atmosphere over recent decades (Houghton et al. 2012). Globally, LUC has been dominated by deforestation and grassland conversion to crop or pastoral land, which commonly led to a C debt through vegetation removal and increase on soil organic carbon (SOC) via mineralization (Smith et al. 2016). Afforestation, in turn, may induce a positive C balance by increasing the C sink strength and residence time of sequestered C (Powlson et al. 2011; Minasny et al. 2017). Commercial forest plantations are a paramount business in South America (IBÁ 2019). The recent expansion of eucalypt production on native grasslands of the Pampa Biome, notably in the southernmost of Brazil, and the little quantitative information on its impact on C cycling have raised ecological concerns about this LUC (Oliveira et al. 2017). Previous studies have reported increased soil CO2 effluxes after conversion of the Pampa Biome into row-crop agriculture (Pillar et al. 2012), prompting the need for investigating similar conversions of native grassland to plantation forest.
Short-rotation eucalypt plantations create an ephemeral C sink through rapid C accumulation in their biomass (Bouillet et al. 2013). Conversely, the large production of aboveground litter (Gatto et al. 2010) and root allocation to deep soil layers (Laclau et al. 2013) might yield SOC accumulation, and thus promote long-term C sequestration through afforestation. A high soil C input, however, can be counterbalanced by increases in soil CO2 efflux. Soil CO2 efflux is the most common proxy used to measure soil respiration (Rs), i.e., the flux of microbially (Rh) and plant-respired (Ra) CO2 from the soil to the atmosphere (Maier et al. 2011). Thus, to foresee the feedback of planted forests to human-induced CO2 emissions it is imperative to consider and better understand Rs and its components. Despite the vast area occupied by eucalypt plantations in Brazil (IBÁ 2019), few studies directly address Rs (e.g. Binkley et al. 2006; Nouvellon et al. 2012b; Ryan et al. 2010). Most of the available data report Rs measurements at discrete time periods, mainly at the end of rotation. Although site preparation can be a major driver of the soil CO2 efflux dynamics, the early-impact of eucalypt afforestation on soil CO2 efflux has rarely been investigated (La Scala et al. 2006; Fialho et al. 2018; Teixeira et al. 2020). The majority of eucalypt stands in Brazil are row-cropped established following minimum cultivation practices, in which soil preparation (e.g., subsoiling, ripping, ridge-tillage) and fertilizations are restricted to within the planting row (W) (Gonçalves et al. 2013). These practices might create distinct functional zones within a stand (W and between-row (B)) that likely influence patterns of Rs and its components (Liebig et al. 1995), but it has been neglected in most forest studies.
Abiotic factors such as soil temperature and moisture are strong drivers of Rs seasonality (Davidson and Janssens 2006; Yuste et al. 2007; Hu et al. 2018). Rs is commonly reported to increase exponentially with soil temperature (Raich and Tufekciogul 2000; Yu et al. 2017), and to be linearly correlated with soil water content (Cook and Orchard 2008; Moyano et al. 2012). However, Rs components (Rh and Ra) respond differently to changes in soil temperature and moisture according to geographic location, vegetation type and developmental stage, and other soil properties (Tang and Baldocchi 2005; Heinemeyer et al. 2012; Hinko-Najera et al. 2015). Thus, environment-induced changes on Rs might not be as clearly predicted. For instance, in two studies under temperate climate, soil temperature was a good predictor of Rh, but not of Ra in a eucalypt site (Hinko-Najera et al. 2015), while it could explain both Ra and Rh in grassland sites (Heinemeyer et al. 2012). In the same studies, there was no correlation between Rh and soil moisture in the eucalypt site (Hinko-Najera et al. 2015), but Rh was inversely proportional to soil moisture in the grassland site (Heinemeyer et al. 2012). Additionally, Rs variability may be attributed to variations in the consumption and transport of CO2 from and within the soil profile to the atmosphere (Cook et al. 2007; Cook and Orchard 2008; Maier et al. 2012), requiring better knowledge of subsurface controls on Rs. Soil preparation practices can alter soil physical properties, which is expected to influence subsurface CO2 dynamics (Blagodatsky and Smith 2012); however, to our knowledge, this has been rarely documented in short-rotation forests (but see Teixeira et al. 2020).
Afforestation of grasslands alters belowground C allocation (Anderson-Teixeira et al. 2013), soil microbial community structure (Lupatini et al. 2013), and substrate availability (Don et al. 2011); consequently, afforestation may potentially alter the soil-to-atmosphere C flux by altering both Rh and Ra. The flux of readily oxidizable C and SOC mineralization drive Rh, while gross primary productivity and C allocation regulate the majority of Ra (Sey et al. 2010; Hopkins et al. 2013). A more dynamic and rapid influence on Rs components is expected to occur in young forest plantations, since afforestation will likely create distinct soil environmental conditions from those occurring in grassland soils through changes in above- and belowground litter input and turnover. Large belowground C allocation, enhanced Ra, and faster C cycling have been reported to result in greater Rs in grassland soils than in tropical or temperate forests (Raich and Tufekciogul 2000; Kellman et al. 2007). The partitioning of Rs following LUC can help to understand major Rs drivers and improve our ability to predict future changes in plant-soil C cycling, such as whether the afforested soils will potentially become net sources or sinks for atmospheric C, and define management practices to increase the sustainability of the new land use.
Here, we evaluate soil CO2 concentration [CO2], efflux, and partitioning for ~ 3 yr following the conversion of a grassland to a commercial eucalypt plantation in southern Brazil. We hypothesized that (1) eucalypt afforestation would result in progressively lower soil CO2 efflux following soil preparation; and (2) minimum cultivation practices result in two distinct soil functional zones (W and B) that differ regarding soil CO2 efflux rate and [CO2] dynamics over forest development. To test these hypotheses, we combined isotopic and root-exclusion approaches to distinguish Rs components and study how eucalypt development might change Ra, Rh, and Rs dynamics in a recently afforested site.
Materials and methods
Site description
This study was conducted in two nearby areas located at São Gabriel, Rio Grande do Sul, Brazil: a grassland (30°29′ S; 54°32′ W) and a eucalypt site (30°26′ S; 54°31′ W). The grassland site represents a native vegetation from the Pampa biome, which holds a diversity of soils and a complexity of plants with the prevalence of C4-grass species (Overbeck et al. 2007) and spans ~ 700,000 km2 in South America within Brazil, Argentina, Uruguay, and Paraguay (Di Giacomo and Krapovickas 2005). The Brazilian Pampa covers 63% of the Rio Grande do Sul state and has experienced the expansion of eucalypt plantations over the last decade. Currently, eucalypt plantations cover ~ 200,000 ha of the Brazilian Pampa (C.H. Prass, FEPAM, personal communication). The eucalypt site is a commercial plantation representative from the sites where the eucalypt expansion is occurring, in which standard silviculture practices have been applied (see details below). Several studies have been recently performed in adjacent stands aiming at understanding the interactions of climate, spacing, and genetics on eucalypt production (Binkley et al. 2017), as well as developing the best fertilization regime for the region (Ferreira et al. 2018).
The sites are in a humid subtropical climate with no defined dry season and hot summers (Cfa, Köppen’s classification). Monthly average temperature and precipitation ranged from 13.1 to 26.9 °C and 22 to 255 mm, respectively, during experimental period (November 2012–July 2015; Fig. 1). The soil in both sites was classified as an Oxyaquic Hapludalf (Soil Survey Staff 2014) with silty clay texture and low degree of soil weathering (Table 1). Markedly in the eucalypt site, the soil showed distinct red and gray mottles in deeper layers (below 60 cm depth), indicating some fluctuation in the water table depth over the year. Initial SOC and total nitrogen (TN) concentrations at 1 m depth were higher in eucalypt than in grassland site, while no pronounced differences on C/N ratio, soil porosity or soil density between sites were observed (Table 1).
The native grasslands used in our study had historically been grazed; however, grazing was stopped during our study and the only anthropogenic disturbance was occasional grass mowing to allow CO2 sampling (see Sect. 2.3). In the afforested site, grasses were eliminated through a single glyphosate application. Then, 2 Mg ha−1 of lime was broadcasted before soil preparation. Soil was prepared following minimum cultivation practices by subsoiling (up to 40 cm depth) and soil displacement only within the planting row (i.e., W), creating a ridge of ~ 20 × 80 cm (height × width). Simultaneously, mineral-phosphate (200 kg ha−1) was incorporated into the subsoiled rows using a tube attached behind the subsoiler shank. Eucalyptus dunnii Maiden L. seedlings were planted in August-2012 (end of winter season), spaced 3.3 m between-row (B) and 2.2 m along the W position. Soil was amended with 06:30:06 NPK fertilizer (150 g plant−1) at planting, and with 12 kg ha−1 of N as side-dress fertilization, 1-yr after planting.
Experimental design and soil air samplers
In each site, the experiment was set up as a randomized block design with four replicates three months after eucalypt planting, i.e., in the very early stage of the LUC. In the eucalypt stand, each block consisted of seven rows with 20 trees each totaling ~ 1000 m2. In the grassland site, an area of ~ 2500 m2 was fenced and equally split into four blocks.
Soil CO2 efflux was measured using two different approaches that captured bulk soil Rs and Rh, and thereby allowed Ra to be mathematically determined. We used chambers constructed from polyvinyl chloride (PVC) tubes with 40 cm diameter and 15 cm length buried 10 cm into the soil to assess Rs in both eucalypt and grassland sites. A longer PVC chamber (1.30 m length, buried 1.20 m into the soil) with the same diameter (40 cm) was used to preclude root development, and thus distinguish Rh from Ra. To install the Rh chamber, the soil was vertically excavated to a depth of 1.20 m around a 40 cm diameter section, resulting in an exposed intact vertical soil section. Then, the Rh chamber was sunk into the exposed soil and the excavated soil was disposed around the Rh chamber. In the eucalypt site, we installed two Rs and two Rh chambers in each block to account for each zonal tillage position (W and B). In the grassland site, one Rs chamber was installed per block, totaling four chambers. All chambers were installed around one month prior to the first measurement to reduce the effects of soil disturbance over the “settling period” (Li and Kelliher 2005). All chambers were kept in place throughout the experiment (2.91 yr).
We constructed a [CO2] tube to sample the [CO2] to 1 m soil depth (Fig. 2). The [CO2] profile tube was constructed from PVC (6 cm diameter × 1.10 m length), which was divided into five different sections (to measure the [CO2] of each specific soil layer) using nylon rods of 6 cm diameter × 2 cm length. Sections contained perforations (1 cm diameter) in their tube wall to balance the [CO2] inside the tube and the surrounding soil. Each nylon rod contained a specific 1 mm hose to allow the CO2 sampling from that section specifically. Similarly to Rs and Rh chambers, [CO2] tubes were installed in both W and B positions in the eucalypt site around 1 month before initial measurements and maintained in place throughout the experimental period. To install the [CO2] tubes, we excavated the soil with a stainless-steel core of 6 cm diameter × 1 m length. Afterward, the [CO2] tubes were vertically inserted into the excavated section, while the upper 10 cm section of the tube was left accessible above the soil surface. Therefore, the five tube sections matched with the respective soil depths: 0–10, 10–20, 20–40, 40–60, and 60–100 cm. The [CO2] tube remained capped between samplings to prevent it from filling with rainwater.
Soil CO2 sampling
Soil CO2 measurements were carried out across the first three years of stand development, which were divided into eight measurements (0.25, 0.50, 1.00, 1.25, 1.50, 1.91, 2.33, 2.91 yr after afforestation) distributed across different seasons (Fig. 1). All measurement campaigns occurred simultaneously during the daytime in the eucalypt and grassland sites. The litter overlying the soil inside the chambers in the eucalypt site was not removed for CO2 sampling. On the grassland site, the vegetation inside the chamber was clipped to allow capping. To determine CO2 efflux, the chambers were capped for 40 min and the air in the Rh and Rs chambers headspace was sampled at 0, 10, 20, and 40 min after capping. The internal air was accessed through a rubber septum inserted on the top of each lid. For the [CO2] tube, we did not use a CO2 increment time sequence, i.e., the air within each hose (each layer) was sampled only once after being homogenized with each section atmosphere by repeated syringe flushing. We used 50 mL syringes connected with a three-way stop for all samplings, which were then placed in cooler-containers and transported to the laboratory. The concentration [CO2] and isotopic C signature (δ13C) of CO2 were quantified in a cavity ring-down spectrometer (CRDS G2131-i Analyzer, Picarro, Sunnyvale, USA). Soil moisture and temperature at 10 cm soil depth were determined using probe sensors (EC-5, Decagon Devices, Pullman, USA) simultaneously with air sampling.
Soil sampling
Soil samples were taken from the eucalypt site at 0.25, 1.50, and 2.91 yr of afforestation. In each block, five soil samples were collected within each zonal tillage position (W and B) and depth (0–10, 10–20, 20–40, 40–60, and 60–100 cm), and composited for a single sample per position and depth. Samples were air-dried and ground (< 2 mm) to prepare for determination of δ13C-SOC using an elemental analyzer interfaced with an isotope ratio mass spectrometer (EA-IRMS ANCA-GSL 20–20 system, Sercon, Crewe, UK).
Soil respiration calculations
Soil CO2 efflux, a proxy for Rs (Maier et al. 2011), and Rh were calculated using the formula:
where dC/dt is the rate of CO2 change during 40 min, estimated by the slope from the linear regression between soil CO2 concentration and sampling time; P is the atmospheric pressure inside the chamber; V is the chamber volume after capping; R is the ideal gas constant; T is the air temperature (K) during air sampling; M is the molecular weight of CO2; and A is the chamber area.
The Rs in the afforested site was measured in both W and B positions. To compare the Rs in the eucalypt site with that in the grassland, we averaged Rs at W and B positions by considering their proportional area within the stand, i.e., 35 and 65% respectively.
We used Miller and Tans (2003) approach to calculate the δ13C value of Rs (δ13CO2). Briefly, the δ13CO2 value was multiplied by the respective CO2 concentration at each sampling time (0, 10, 20, and 40 min) and plotted against the CO2 concentration. The slope of the linear regression was considered equivalent to the δ13CO2.
The contribution of eucalypt-derived CO2 to soil CO2 efflux (C3-derived CO2) over the experiment was calculated as:
where δ13CO2 is the isotopic C signature of soil CO2 efflux in each sampling time (x) and at the beginning of the experiment (i), and δ13CO2EUC is the δ13CO2 from eucalypt roots respiration (− 32‰) which was obtained through root incubation.
The relationship between Rs and soil temperature or moisture was performed by nonlinear (Eq. 3) and linear regressions (Eq. 4), respectively, as follows:
where T is the air temperature (°C), m is the soil water content (m3 m−3) and α, β, a, and b are the estimated parameters. The Q10 of Rs was calculated as the factor by which Rs is multiplied when temperature increases by 10 °C (Davidson and Janssens 2006):
Data analysis
Data were checked for normality (Shapiro–Wilk test) and power-transformed to meet normality assumptions when needed. The influence of land use and time on the soil CO2 efflux rate and δ13CO2 was tested using a two-way repeated measures ANOVA. Within the eucalypt stand, a two-way repeated measures ANOVA (position × sampling time) evaluated the effects of zonal tillage position and time after afforestation on soil CO2 efflux and Rh, while a three-way repeated measures ANOVA was used to compare [CO2] and δ13[CO2] across the zonal tillage position, time after afforestation, and soil depth. When interactions were significant (p < 0.05), means were compared using Fisher’s LSD post hoc test (p < 0.05). All ANOVA’s were carried out using SISVAR software (Ferreira 2011). We evaluated the influence of δ13C-SOC on δ13[CO2] by comparing the slopes of lines relating the two variables at each soil depth using the Statgraphics Centurion XVI software package (StatPoint Technologies, Inc., Virginia, USA).
Results
Soil CO2 efflux in the grassland and eucalypt sites
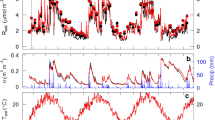
Soil CO2 efflux exhibited pronounced variation under both land uses, but a larger seasonal variation was observed in the grassland site (Fig. 3a). The CO2 efflux rate in the grassland ranged from 0.21 to 1.34 g CO2 m−2 h−1 during our observation period (Fig. 3a). The highest CO2 efflux rate in the grassland soil occurred during the first six months of our experimental study, while lower CO2 efflux rates were observed during winter seasons. In the eucalypt site, the average soil CO2 efflux was 0.37 ± 0.20 g CO2 m−2 h−1 (Fig. 3a). The highest CO2 efflux rate (0.76 ± 0.07 g CO2 m−2 h−1) coincided with the beginning of the experiment and was 2.4 times higher than the soil CO2 efflux after 2.91 yr of afforestation. Soil CO2 efflux rates were lower in the eucalypt than in the grassland throughout the experiment, except at 1.00 yr and 2.91 yr after afforestation, when soil CO2 efflux was similar in both sites (Fig. 3a).
Soil CO2 efflux rate (a) and its 13C isotopic signature (δ13CO2; b) in the grassland site and in the eucalypt plantation; and the contribution of eucalypt-derived CO2 (C3-derived CO2) to soil CO2 efflux over 2.91 yr of afforestation (c). Errors bars represent standard errors (n = 4). n.s. means no significant (p > 0.05) difference between grassland and the eucalypt afforested site at that specific period. p-values for the two-way repeated measures ANOVA are presented in Online Resource 1
The δ13CO2 exhibited lower variation in the grassland site, averaging − 15.6 ± 1.6‰ throughout the experiment (Fig. 3b). In the eucalypt site, δ13CO2 exhibited a downward trend, with extremes being observed at the beginning of the experiment (− 14.1‰) and after 2.91 yr of afforestation (− 29.4‰). Peaks on δ13CO2 were observed at 1.50 and 2.33 yr of afforestation, which coincided with lower rainfall period and higher temperature (Fig. 1). The δ13CO2 in the eucalypt site was generally lower (more negative) than in the grassland, except at 0.50 yr, when no difference between the land uses was observed.
There was an increasing contribution of C3-derived CO2 in soil CO2 efflux through time in the eucalypt stand (Fig. 3c), except at 1.50 and 2.33 yr of afforestation when C3-derived CO2 reduced. The C3-derived CO2 ranged from 3% at the beginning of the experiment to 85% after 2.91 yr of afforestation.
Soil CO2 efflux in the grassland was inversely proportional to soil moisture (p < 0.05; Fig. 4a). Fitting an exponential function (Eq. 3) for the soil CO2 efflux rate in the grassland against soil temperature resulted in a Q10= 1.49 (Fig. 4b). In the eucalypt site, neither soil moisture nor soil temperature could be modeled through linear or exponential functions, respectively, to explain soil CO2 efflux variability (p > 0.05; Fig. 4a, b).
Relationship between soil CO2 efflux rate (Rs) in the grassland and in the eucalypt site with soil moisture (m, a) and soil temperature (T, b) measured during 2.91 yr of afforestation. Regressions were fitted with the 36 paired observations, but values are represented (symbols) by the average (n = 4) of each sampling time. Regression line is shown only for grassland since equation coefficients were not significant for explaining Rs in the eucalypt site
Influence of silvicultural practices on soil CO2 efflux, Rs partitioning, and [CO2] to 1 m soil depth in the eucalypt site
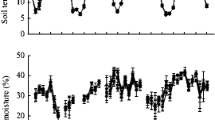
Averaged across the entire observation period, soil CO2 efflux was 20% higher in W (0.42 g CO2 m−2 h−1) than in B (0.34 g CO2 m−2 h−1) (Fig. 5a). Greater soil CO2 efflux in W than in B occurred at 1.25, 1.91 and 2.91 yr of afforestation. The soil CO2 efflux at 0.25 yr was 3.6- and 1.3-fold greater than that after 2.91 yr of afforestation in B and W, respectively. Distinct pattern of Rh over the experimental period was observed between B and W (Fig. 5b). High Rh proportion in W occurred at 1.50 and 2.33 yr of afforestation. Conversely, lower Rh proportions in W were observed at 1.25 yr after afforestation and in the winter seasons (1.91 and 2.91 yr).
Soil CO2 efflux rate in the eucalypt site measured within planting row (W) and between-row (B) positions (a), and the proportion of heterotrophic respiration (Rh) to the soil CO2 efflux in both positions (b). Values are mean ± standard error. n.s. means no significant difference in the soil CO2 efflux between zonal tillage positions. p-values for the three-way repeated measures ANOVA are presented in Online Resource 2
Zonal tillage management influenced the [CO2] in the soil profile and the δ13[CO2] (Fig. 6; Online Resource 2). Overall, higher [CO2] occurred in the soil profile of B than W, except after 2.91 yr of afforestation, in which [CO2] was similar in B and W (5102 μmol mol−1) (Fig. 6a, b). Differences in [CO2] between B and W were more pronounced below 10 cm depth. In both positions, [CO2] increased with soil depth, but differences among depths were larger in B than W, and less pronounced through time in both positions (Fig. 6a, b). The greatest [CO2] in W was measured after 0.25 yr of afforestation (11,743, 13,636, 18,557, 20,183 and 17,996 μmol mol−1 in 0–10, 10–20, 20–40, 40–60 and 60–100 cm soil layers, respectively, Fig. 6a). The [CO2] concentration in all soil layers of W at the end of the experiment (2065, 4081, 4665, 6566 and 9444 μmol mol−1 in 0–10, 10–20, 20–40, 40–60 and 60–100 cm soil layers, respectively) differed from all other sampling times, but 1.50 and 2.33 yr.
Soil CO2 concentration (a, b) and isotopic signature of CO2 (δ13[CO2]; c, d) in the soil profile (0–100 cm depth) as influenced by zonal tillage management [within planting row and between-row] over 2.91 yr of afforestation. Errors bars represent standard errors. p-value bars represent the least significant difference (p < 0.05) of the three-way ANOVA. p-values for the three-way repeated measures ANOVA are presented in Online Resource 2
The [CO2] at the 0–10 and 10–20 cm soil depths in B followed similar patterns: compared with the initial [CO2] (3014 and 5554 μmol mol−1 in 0–10 and 10–20 cm, respectively), there was a significant increase (~ 3.5-fold) until 1.00 yr of afforestation and then a subsequent [CO2] decrease until 2.91 yr of afforestation (2303 μmol mol−1 and 3794 μmol mol−1 at the 0–10 and 10–20 cm depths, respectively; Fig. 6b). However, [CO2] in both depths at 2.91 yr did not differ from that at 0.25 yr of afforestation. In deeper soil layers, [CO2] increments were observed until 1.25 yr (19,241, 23,772 and 27,729 μmol mol−1 at the 20–40, 40–60 and 60–100 cm depth), and then [CO2] decreased until the end of the experiment. For deeper depths, the [CO2] at 2.91 yr of afforestation (4346, 5378 and 8375 μmol mol−1 at the 20–40, 40–60 and 60–100 cm depth) was lower than those at the beginning.
Overall, a more negative δ13[CO2] occurred in W than in B, except at 0.25 and 1.91 yr (Fig. 6c, d). Differences in δ13[CO2] between W and B were greater below 20 cm depth. The δ13[CO2] decreased with depth mainly in W (Fig. 6c), and it became more negative as afforestation aged in both soil zones. Although, the decreasing δ13[CO2] trend was not observed at 1.50 and 2.33 yr, i.e. at this sampling times the δ13[CO2] become less negative (higher) than the previous measurement.
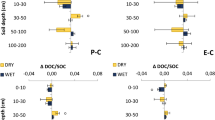
Coefficient of determination (R2) of δ13C-SOC × δ13[CO2] regressions ranged from 0.01 to 0.55 (Fig. 7). Higher R2 was observed in W than B across all soil layers but 40–60 cm. There was a positive correlation between δ13[CO2] and δ13C-SOC in all soil layers of W (Fig. 7a). δ13C-SOC had a greater explanation power on δ13[CO2] at 10–20 and 20–40 cm depths of W (R2 = 0.55 for both depths), in which higher slopes of the linear regressions were observed. A positive correlation between δ13[CO2] and δ13C-SOC was also observed in B, except at 0–10 and 20–40 cm depth; the highest slope was observed at 10–20 cm depth (Fig. 7b). Regression slopes were higher in W than in B across all soil layers.
Discussion
Our study provides quantitative information about the potential feedback of soil CO2 emissions following the afforestation of subtropical grassland with eucalypt. Soil CO2 efflux in the afforested site was lower than in the grassland. The eucalypt-derived CO2 contribution (C3-derived CO2) to soil CO2 efflux increased through eucalypt stand development, while the [CO2] in the soil profile and the isotopic C signature of CO2, either δ13CO2 or δ13[CO2], decreased over time. Site preparation created two distinct soil C cycling functional zones within the eucalypt stand, in which [CO2] across the soil profile was overall greater in B, while soil CO2 efflux rate was higher in W. We observed that Rh exerted a lower control on the magnitude of Rs at the end than at the beginning of the experiment, but the contribution of Rh to soil CO2 efflux varied spatially and temporally, i.e., between tillage zones and through forest development.
Land use and its influence on soil CO2 efflux
The soil CO2 efflux in the grassland site averaged 0.85 g CO2 m−2 h−1 (Fig. 3a), which is in a higher range than the rates reported for either a non-degraded (0.27 g CO2 m−2 h−1) or degraded (0.10 g CO2 m−2 h−1) temperate grasslands of South Africa (Abdalla et al. 2018), and also higher than the global average of tropical grasslands (0.25 g CO2 m−2 h−1) (Wang and Fang 2009), but quite similar to a managed grassland under temperate conditions (0.82 g CO2 m−2 h−1) (Heinemeyer et al. 2012). The Rs is a complex process regulated by the interactions of several biotic (e.g., vegetation type, net primary productivity) and abiotic drivers (e.g., clay and SOC content, climatic conditions) resulting in a highly variable Rs global database (Balogh et al. 2011; Moyano et al. 2012; Zimmermann et al. 2015). The Pampa biome has unique edaphoclimatic conditions, such as the combination of relatively fertile soils with high mean annual temperature (~ 18 °C) and total precipitation (~ 1500 mm), that should support high gross primary production, and thus increased Rs when compared to other grassland sites (Yang et al. 2008; Wang and Fang 2009). Additionally, the intensity of grassland management could also impact Rs. For instance, grazing results in larger belowground C allocation, higher soil microbial biomass and belowground turnover rate than ungrazed areas (López-Mársico et al. 2015; Wilson et al. 2018), which is expected to promote Rs. In our study, the influence of grazing might have reflected in the higher soil CO2 efflux rates observed in the first two samplings which were 22.5% greater than those measured in the same seasons afterwards (e.g., 1.25, 1.50 and 2.33 yr), since grazing was withheld after our observations began.
The global soil CO2 efflux in grassland is reported higher (~ 20%; Raich and Tufekciogul 2000) compared to forests growing under similar conditions, with differences increasing in temperate regions (+41%; Kellman et al. 2007). Greater availability of photosynthate allocated belowground, increased root respiration and/or microbial respiration may contribute to an elevated soil CO2 rate in grasslands (Raich and Tufekciogul 2000; Kellman et al. 2007). In our study, the soil CO2 efflux in the eucalypt site averaged 56% lower than in the grassland. In afforested sites, a large fraction of root-respiration, i.e. Ra, can remain within the tree system rather than diffusing into the soil (Bloemen et al. 2016). The CO2 moving internally from root-to-shoot-to-atmosphere via xylem varies during eucalypt tree growth (Cerasoli et al. 2009), and in a 4-yr-old eucalypt tree it can divert about one-fourth of the root respiration (Grossiord et al. 2012). Aubrey and Teskey (2009) observed that the CO2 moving internally via xylem in forest plantations can be even greater, reaching an equivalent amount of the CO2 diffusing directly from the soil to the atmosphere. Therefore, to fully account Rs in forested landscapes, the internal CO2 flux should have been considered. If we consider that 25% of Ra was transported via xylem (Grossiord et al. 2012), Rs in the eucalypt site would average 0.40 g CO2 m−2 h−1 over the experiment, which is less than 50% of grassland Rs rate. Moreover, if the soil CO2 efflux in the afforested site represented only half of the total Rs in the eucalypt plantations (Aubrey and Teskey 2009), Rs would still average 35% less than grassland Rs rates. The eucalypt and grassland sites were relatively nearby, under the same soil class, and shared similar land use history, but the afforested site had higher SOC at the start of the experiment (Table 1). While these differences may undermine appropriate comparisons, the lower soil CO2 efflux even with higher SOC in the eucalypt site demonstrates that the conversion from grassland to eucalypt plantations in southern Brazil is not likely to contribute to increased soil CO2 efflux rates.
The influence of soil temperature and soil moisture on CO2 efflux were evident in the grassland but not in the eucalypt site. The absence of a consistent relationship of Rs in forest systems with temperature or soil moisture has also been observed in other studies (Livesley et al. 2009; Wei et al. 2010). Specifically, due to rapid eucalypt growth, phenological variations could overshadow and decouple Rs from temperature and moisture response patterns (Curiel Yuste et al. 2004; Davidson et al. 2006; Machmuller et al. 2018). Additionally, we posit that the LUC effects on the initial availability and quality of the fresh C input to soil microbial community (Lupatini et al. 2013; Veen et al. 2015), and the reduced Ra rates when root biomass was still incipient, might also have helped decouple Rs from temperature or moisture changes.
Soil CO2 dynamics in a eucalypt afforested site
During the first few years of afforestation, alterations in SOC equilibrium induced by the increase in C outflows with soil preparation (Fialho et al. 2018), and the decrease in C inflows due to the lower litter input, might change soil CO2 efflux dynamics (Nouvellon et al. 2008, 2012a). We thus extensively compiled studies that have evaluated Rs in situ (100% of these studies used the soil CO2 efflux to assess Rs) across eucalypt stands in Brazil to obtain a broader perspective of this new land use (Table 2). The average soil CO2 efflux observed over 2.91 yr of afforestation (0.37 g CO2 m−2 h−1) is within the range reported for multiple age short-rotation eucalypt plantations in the country (0.30–0.55 g CO2 m−2 h−1), but lower when longer (27–29-yr) rotations are evaluated (D’Andréa et al. 2011; Vicentini et al. 2019; Table 2). The highest soil CO2 efflux observed in our study (0.76 g CO2 m−2 h−1) could be considered slightly above the nationwide average, but it was measured at the earliest stage of LUC (0.25 yr). Only one of the 13 compiled studies evaluated early stand development (Teixeira et al. 2020—Table 2) like our study, and the numbers are surprisingly similar considering the differences in geographic locations and environmental conditions. At this stage, the forest is expected to experience higher daily soil temperatures due to reduced canopy and greater soil exposure, which might promote microbial activity (Li et al. 2017) and soil CO2 efflux (Gomes et al. 2016). Moreover, tillage operations at planting can enhance soil CO2 efflux (Fialho et al. 2018). Despite the broad range of environmental conditions, biome domain, soil type, number of eucalypt rotations, or stand development stage, the overall agreement of the Rs range across eucalypt plantations in Brazil is intriguing. Likewise, the Rs range reported across eucalypt plantations in Brazil agrees with those in eucalypt stands worldwide (Giardina and Ryan 2002; Livesley et al. 2009; Fest et al. 2009; Grossiord et al. 2012). Collectively, these findings suggest a strong vegetation-type feedback to Rs (Metcalfe et al. 2011).
The isotopic signature of Rs in the eucalypt site become more 13C-depleted (more negative) over time (Fig. 3b), reflecting an increase in C3-derived CO2 (Fig. 3c) either from decomposition of eucalypt-derived C (Rh) or roots respiration (Ra), and the diminished contribution of grass biomass (C4-C) through time. Interestingly, C3-derived CO2 showed a sharp decrease during warmer and drier periods of afforestation (1.50 and 2.33 yr), which resulted in less negative δ13CO2 (Fig. 3b) and δ13[CO2] down the soil profile (Fig. 6c, d). Likewise, during the same periods there was a reduction in soil CO2 efflux and Ra/Rh ratio of the W position (Fig. 5). Collectively, these findings indicate a likely reduction in tree photosynthetic activity under drought conditions (Nouvellon et al. 2008; Ruehr and Buchmann 2010). When the amount of eucalypt-derived organic matter is still small, an incremental stimulation of SOC decomposition under higher soil temperature (Thurgood et al. 2014) is likely to result in less 13C-depleted CO2, which may explain differences on δ13CO2 and δ13[CO2] between 1.50 yr and 2.33 yr of afforestation.
Zonal tillage management created spatial heterogeneity of soil CO2 efflux and [CO2] in the soil profile of the eucalypt stand. The soil CO2 efflux in W was ~ 20% greater than that in B (Fig. 5a). Conversely, B had 25% more [CO2] within 1-m depth and large [CO2] differences between soil depths when compared with W (Fig. 6a, b). Using row-only soil preparation might have impacted the soil CO2 efflux and [CO2] as a consequence of changing soil physical properties related with gas transportation (Liebig et al. 1995; Blagodatsky and Smith 2012) and modifying biological processes, such as the SOC decomposition and root density and exploration (Williams et al. 2017) in W. Indeed, W had lower soil bulk density (1.06 vs. 1.21 for the 0–100 cm soil layer; see Online Resource 3), and higher fine-root biomass and more homogeneous fine-root distribution (see Ferreira et al. 2018). On one hand, the incremental C3 contribution to SOC as afforestation progressed led to more negative δ13[CO2] in W, mainly in the depths 10–20 and 20–40 cm (Fig. 7a), which concentrate higher fine root biomass within 1-m soil depth (Ferreira et al. 2018; Santos et al. 2020). Such evidence indicates that fine-root activity may be a primary driver of [CO2] in W position. On the other hand, the absent influence of δ13C-SOC on δ13[CO2] at 0–10 and 20–40 cm of B, despite the more 13C-depleted [CO2] in these depths over time, suggests that the C input from eucalypt tree is being rapidly respired and not transferred to soil organic matter pools. Thus, we posit that zonal tillage management in eucalypt plantations imposes distinct drivers of Ra and Rh through stand development and should be considered in studies evaluating C cycling dynamics.
Differences in the soil CO2 efflux between W and B (observed at 1.25, 1.91 and 2.91 yr; Fig. 5a) were driven by a decreased Rh in the W position (Fig. 5b). The Rh contribution to Rs ranged from 14 to ~ 100% (Fig. 5b). Although a 100% Rh is very unlikely during active stand growth, this value indicates that Ra was negligible during some periods. It could be also a result of an artificially increased Rh because of moister soil from inhibited lateral water flow or root water uptake or a possible increase of dead root biomass within Rh chambers (Subke et al. 2006; Shrestha et al. 2016; Han et al. 2018). Nevertheless, we did not observe a clear seasonal trend in the Ra/Rh quotient, which could help distinguish these processes and possible artifacts. For instance, a greater proportion of Rh was observed at 1.00 yr, differing from the pattern observed in other winter sampling times (1.91 and 2.91 yr), which, although, could be another artifact created by the side-dress fertilization performed specifically during that period. Drastic seasonal variability of Rh had been also reported in other forest ecosystems (Saiz et al. 2006; Brown and Markewitz 2018). Thus, Ra/Rh dynamics might be a result of several co-varying factors, such as temporal variations in root production, exploration and turnover, microbial-C processing, and CO2 transport within the soil profile, that were possibly different between W and B (Liebig et al. 1995; Williams et al. 2016, 2017).
Conclusions
Our findings provide quantitative information to account for the soil CO2 efflux caused by LUC in countries that eucalypt plantations are a growing economic activity. We demonstrate that eucalypt afforestation of grasslands is not likely to contribute to greenhouse gas emissions through increased soil CO2 effluxes. Our data suggest that minimum cultivation practices, which are commonly adopted in eucalypt plantations in Brazil, create two distinguished functional soil zones within the stand (W and B), which vary as afforestation aged regarding Rs rates and sources (Ra and Rh), and [CO2] in the soil profile. Therefore, soil management practices in production forestry can influence soil C dynamics and leverage the role of planted forests to mitigate CO2 emissions, and should be accounted for in C cycling models. Observations across entire and multiple rotations are still needed to capture equilibriums in C fluxes and pools in short-rotation production forestry and infer the feedback of eucalypt planted forests to climate change by addressing it properly through management practices.
References
Abdalla K, Mutema M, Chivenge P et al (2018) Grassland degradation significantly enhances soil CO2 emission. CATENA 167:284–292. https://doi.org/10.1016/j.catena.2018.05.010
Anderson-Teixeira KJ, Masters MD, Black CK et al (2013) Altered belowground carbon cycling following land-use change to perennial bioenergy crops. Ecosystems 16:508–520. https://doi.org/10.1007/s10021-012-9628-x
Aubrey DP, Teskey RO (2009) Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol 184:35–40. https://doi.org/10.1111/j.1469-8137.2009.02971.x
Balogh J, Pintér K, Fóti S et al (2011) Dependence of soil respiration on soil moisture, clay content, soil organic matter, and CO2 uptake in dry grasslands. Soil Biol Biochem 43:1006–1013. https://doi.org/10.1016/j.soilbio.2011.01.017
Binkley D, Stape JL, Takahashi EN, Ryan MG (2006) Tree-girdling to separate root and heterotrophic respiration in two Eucalyptus stands in Brazil. Oecologia 148:447–454. https://doi.org/10.1007/s00442-006-0383-6
Binkley D, Campoe OC, Alvares C et al (2017) The interactions of climate, spacing and genetics on clonal Eucalyptus plantations across Brazil and Uruguay. For Ecol Manag 405:271–283. https://doi.org/10.1016/j.foreco.2017.09.050
Blagodatsky S, Smith P (2012) Soil physics meets soil biology: towards better mechanistic prediction of greenhouse gas emissions from soil. Soil Biol Biochem 47:78–92. https://doi.org/10.1016/j.soilbio.2011.12.015
Bloemen J, Teskey RO, McGuire MA et al (2016) Root xylem CO2 flux: an important but unaccounted-for component of root respiration. Trees 30:343–352. https://doi.org/10.1007/s00468-015-1185-4
Bouillet JP, Laclau JP, de Gonçalves JLM et al (2013) Eucalyptus and Acacia tree growth over entire rotation in single- and mixed-species plantations across five sites in Brazil and Congo. For Ecol Manag 301:89–101. https://doi.org/10.1016/j.foreco.2012.09.019
Brown R, Markewitz D (2018) Soil heterotrophic respiration: measuring and modeling seasonal variation and silvicultural impacts. For Ecol Manag 430:594–608. https://doi.org/10.1016/j.foreco.2018.08.018
Campoe OC, Stape JL, Laclau J-P, Marsden C, Nouvellon Y (2012) Stand-level patterns of carbon fluxes and partitioning in a Eucalyptus grandis plantation across a gradient of productivity, in Sao Paulo State, Brazil. Tree Physiol 32(6):696–706
Cerasoli S, McGuire MA, Faria J et al (2009) CO2 efflux, CO2 concentration and photosynthetic refixation in stems of Eucalyptus globulus (Labill.). J Exp Bot 60:99–105. https://doi.org/10.1093/jxb/ern272
Cook FJ, Orchard VA (2008) Relationships between soil respiration and soil moisture. Soil Biol Biochem 40:1013–1018. https://doi.org/10.1016/j.soilbio.2007.12.012
Cook FJ, Knight JH, Kelliher FM (2007) Oxygen transport in soil and the vertical distribution of roots. Aust J Soil Res 45:101–110. https://doi.org/10.1071/SR06137
Curiel Yuste J, Janssens IA, Carrara A, Ceulemans R (2004) Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Glob Chang Biol 10:161–169. https://doi.org/10.1111/j.1529-8817.2003.00727.x
D’Andréa AF, Silva MLN, Freitas DAF et al (2011) Variabilidade espacial do fluxo de CO2 do solo em povoamento de eucalipto. Floresta 41:407–422
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173. https://doi.org/10.1038/nature04514
Davidson EA, Janssens IA, Lou Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob Chang Biol 12:154–164. https://doi.org/10.1111/j.1365-2486.2005.01065.x
Di Giacomo AS, Krapovickas S (2005) Conserving the grassland important bird areas (IBAs) of Southern South America: Argentina, Uruguay, Paraguay, and Brazil. USDA Forest Service 191:1243–1249
Don A, Schumacher J, Freibauer A (2011) Impact of tropical land-use change on soil organic carbon stocks - a meta-analysis. Glob Chang Biol 17:1658–1670. https://doi.org/10.1111/j.1365-2486.2010.02336.x
Epron D, Laclau J-P, Almeida JCR, Goncalves JLM, Ponton S, Sette CR, Delgado-Rojas JS, Bouillet J-P, Nouvellon Y (2012) Do changes in carbon allocation account for the growth response to potassium and sodium applications in tropical Eucalyptus plantations? Tree Physiol 32(6):667–679
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 35:1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Ferreira GWD, Oliveira FCC, Silva LOG et al (2018) Nitrogen alters initial growth, fine-root biomass and soil organic matter properties of a Eucalyptus dunnii Maiden plantation in a recently afforested grassland in Southern Brazil. Forests 9:62. https://doi.org/10.3390/f9020062
Fest BJ, Livesley SJ, Drösler M et al (2009) Soil–atmosphere greenhouse gas exchange in a cool, temperate Eucalyptus delegatensis forest in south-eastern Australia. Agric For Meteorol 149:393–406. https://doi.org/10.1016/j.agrformet.2008.09.007
Fialho RC, da Silva Teixeira R, Teixeira APM, da Silva IR (2018) Short-term carbon emissions: effect of various tree harvesting, transport, and tillage methods under a eucalyptus plantation. L Degrad Dev 29:3995–4004. https://doi.org/10.1002/ldr.3161
Gatto A, Barros NF, Novais RF et al (2010) Estoques de carbono no solo e na biomassa em plantações de eucalipto. Rev Bras Ciência do Solo 34:1069–1079
Giardina CP, Ryan MG (2002) Total belowground carbon allocation in a fast-growing Eucalyptus plantation estimated using a carbon balance approach. Ecosystems 5:487–499. https://doi.org/10.1007/s10021-002-0130-8
Gomes LC, Cardoso IM, Mendonça ES et al (2016) Trees modify the dynamics of soil CO2 efflux in coffee agroforestry systems. Agric For Meteorol 224:30–39. https://doi.org/10.1016/j.agrformet.2016.05.001
Gonçalves JLM, Alvares CA, Higa AR et al (2013) Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For Ecol Manag 301:6–27. https://doi.org/10.1016/j.foreco.2012.12.030
Grossiord C, Mareschal L, Epron D (2012) Transpiration alters the contribution of autotrophic and heterotrophic components of soil CO2 efflux. New Phytol 194:647–653. https://doi.org/10.1111/j.1469-8137.2012.04102.x
Han M, Shi B, Jin G (2018) Conversion of primary mixed forest into secondary broadleaved forest and coniferous plantations: effects on temporal dynamics of soil CO 2 efflux. CATENA 162:157–165. https://doi.org/10.1016/j.catena.2017.12.004
Heinemeyer A, Tortorella D, Petrovičová B, Gelsomino A (2012) Partitioning of soil CO2 flux components in a temperate grassland ecosystem. Eur J Soil Sci 63:249–260. https://doi.org/10.1111/j.1365-2389.2012.01433.x
Hinko-Najera N, Fest B, Livesley SJ, Arndt SK (2015) Reduced throughfall decreases autotrophic respiration, but not heterotrophic respiration in a dry temperate broadleaved evergreen forest. Agric For Meteorol 200:66–77. https://doi.org/10.1016/j.agrformet.2014.09.013
Hopkins F, Gonzalez-Meler MA, Flower CE et al (2013) Ecosystem-level controls on root-rhizosphere respiration. New Phytol 199:339–351. https://doi.org/10.1111/nph.12271
Houghton RA, House JI, Pongratz J et al (2012) Carbon emissions from land use and land-cover change. Biogeosciences 9:5125–5142. https://doi.org/10.5194/bg-9-5125-2012
Hu S, Li Y, Chang SX et al (2018) Soil autotrophic and heterotrophic respiration respond differently to land-use change and variations in environmental factors. Agric For Meteorol 250–251:290–298. https://doi.org/10.1016/j.agrformet.2018.01.003
Indústria Brasileira de Árvores - IBÁ (2019) Relatório anual. São Paulo, SP, Brazil. p 80
Kellman L, Beltrami H, Risk D (2007) Changes in seasonal soil respiration with pasture conversion to forest in Atlantic Canada. Biogeochemistry 82:101–109. https://doi.org/10.1007/s10533-006-9056-0
La Scala N, Bolonhezi D, Pereira GT (2006) Short-term soil CO2 emission after conventional and reduced tillage of a no-till sugar cane area in southern Brazil. Soil Tillage Res 91:244–248. https://doi.org/10.1016/j.still.2005.11.012
Laclau J-P, da Silva EA, Rodrigues Lambais G et al (2013) Dynamics of soil exploration by fine roots down to a depth of 10 m throughout the entire rotation in Eucalyptus grandis plantations. Front Plant Sci 4:1–12. https://doi.org/10.3389/fpls.2013.00243
Li Z, Kelliher FM (2005) Determining nitrous oxide emissions from subsurface measurements in grazed pasture: a field trial of alternative technology. Aust J Soil Res 43:677. https://doi.org/10.1071/SR04106
Li J, He N, Xu L et al (2017) Asymmetric responses of soil heterotrophic respiration to rising and decreasing temperatures. Soil Biol Biochem 106:18–27. https://doi.org/10.1016/j.soilbio.2016.12.002
Liebig MA, Jones AJ, Doran JW, Mielke LN (1995) Potential soil respiration and relationship to soil properties in ridge tillage. Soil Sci Soc Am J 59:1430–1435. https://doi.org/10.2136/sssaj1995.03615995005900050032x
Livesley SJ, Kiese R, Miehle P et al (2009) Soil-atmosphere exchange of greenhouse gases in a Eucalyptus marginata woodland, a clover-grass pasture, and Pinus radiata and Eucalyptus globulus plantations. Glob Chang Biol 15:425–440. https://doi.org/10.1111/j.1365-2486.2008.01759.x
López-Mársico L, Altesor A, Oyarzabal M et al (2015) Grazing increases below-ground biomass and net primary production in a temperate grassland. Plant Soil 392:155–162. https://doi.org/10.1007/s11104-015-2452-2
Lupatini M, Jacques RJS, Antoniolli ZI et al (2013) Land-use change and soil type are drivers of fungal and archaeal communities in the Pampa biome. World J Microbiol Biotechnol 29:223–233. https://doi.org/10.1007/s11274-012-1174-3
Machmuller MB, Ballantyne F, Markewitz D et al (2018) Temperature sensitivity of soil respiration in a low-latitude forest ecosystem varies by season and habitat but is unaffected by experimental warming. Biogeochemistry 141:63–73. https://doi.org/10.1007/s10533-018-0501-7
Maier M, Schack-Kirchner H, Hildebrand EE, Schindler D (2011) Soil CO2 efflux vs. soil respiration: implications for flux models. Agric For Meteorol 151:1723–1730. https://doi.org/10.1016/j.agrformet.2011.07.006
Maier M, Schack-Kirchner H, Aubinet M et al (2012) Turbulence effect on gas transport in three contrasting forest soils. Soil Sci Soc Am J 76:1518. https://doi.org/10.2136/sssaj2011.0376
Metcalfe DB, Fisher RA, Wardle DA (2011) Plant communities as drivers of soil respiration: pathways, mechanisms, and significance for global change. Biogeosciences 8:2047–2061. https://doi.org/10.1016/j.ausmj.2011.10.011
Miller JB, Tans PP (2003) Calculating isotopic fractionation from atmospheric measurements at various scales. Tellus 55B:207–214. https://doi.org/10.1034/j.1600-0889.2003.00020.x
Minasny B, Malone BP, McBratney AB et al (2017) Soil carbon 4 per mille. Geoderma 292:59–86. https://doi.org/10.1016/j.geoderma.2017.01.002
Moyano FE, Vasilyeva N, Bouckaert L et al (2012) The moisture response of soil heterotrophic respiration: interaction with soil properties. Biogeosciences 9:1173–1182. https://doi.org/10.5194/bg-9-1173-2012
Nouvellon Y, Epron D, Kinana A et al (2008) Soil CO2 effluxes, soil carbon balance, and early tree growth following savannah afforestation in Congo: comparison of two site preparation treatments. For Ecol Manag 255:1926–1936. https://doi.org/10.1016/j.foreco.2007.12.026
Nouvellon Y, Epron D, Marsden C et al (2012a) Age-related changes in litter inputs explain annual trends in soil CO2 effluxes over a full Eucalyptus rotation after afforestation of a tropical savannah. Biogeochemistry 111:515–533. https://doi.org/10.1007/s10533-011-9685-9
Nouvellon Y, Laclau JP, Epron D et al (2012b) Production and carbon allocation in monocultures and mixed-species plantations of Eucalyptus grandis and Acacia mangium in Brazil. Tree Physiol 32:680–695. https://doi.org/10.1093/treephys/tps041
Oliveira TE, Freitas DS, Gianezini M et al (2017) Agricultural land use change in the Brazilian Pampa Biome: the reduction of natural grasslands. Land Use Policy 63:394–400. https://doi.org/10.1016/j.landusepol.2017.02.010
Overbeck GE, Muller SC, Fidelis A et al (2007) Brazil’s neglected biome: the South Brazilian Campos. Perspect Plant Ecol Evol Syst 9:101–116. https://doi.org/10.1016/j.ppees.2007.07.005
Pillar V, Tornquist C, Bayer C (2012) The southern Brazilian grassland biome: soil carbon stocks, fluxes of greenhouse gases and some options for mitigation. Braz J Biol 72:673–681. https://doi.org/10.1590/S1519-69842012000400006
Powlson DS, Whitmore AP, Goulding KWT (2011) Soil carbon sequestration to mitigate climate change: a critical re-examination to identify the true and the false. Eur J Soil Sci 62:42–55
Raich JW, Tufekciogul A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–90. https://doi.org/10.1023/A:1006112000616
Ruehr NK, Buchmann N (2010) Soil respiration fluxes in a temperate mixed forest: seasonality and temperature sensitivities differ among microbial and root-rhizosphere respiration. Tree Physiol 30:165–176. https://doi.org/10.1093/treephys/tpp106
Ryan MG, Stape JL, Binkley D et al (2010) Factors controlling Eucalyptus productivity: how water availability and stand structure alter production and carbon allocation. For Ecol Manag 259:1695–1703. https://doi.org/10.1016/j.foreco.2010.01.013
Saiz G, Byrne KA, Butterbach-Bahl K et al (2006) Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob Chang Biol 12:1007–1020. https://doi.org/10.1111/j.1365-2486.2006.01145.x
Santos RS, Oliveira FCC, Ferreira GWD et al (2020) Carbon and nitrogen dynamics in soil organic matter fractions following eucalypt afforestation in southern Brazilian grasslands (Pampas). Agric Ecosyst Environ 301:106979. https://doi.org/10.1016/j.agee.2020.106979
Sey BK, Manceur AM, Whalen JK et al (2010) Root-derived respiration and nitrous oxide production as affected by crop phenology and nitrogen fertilization. Plant Soil 326:369–379. https://doi.org/10.1007/s11104-009-0018-x
Shrestha P, Seiler J, Strahm B et al (2016) Soil CO2 efflux and root productivity in a Switchgrass and Loblolly Pine intercropping system. Forests 7:221. https://doi.org/10.3390/f7100221
Smith P, House JI, Bustamante M et al (2016) Global change pressures on soils from land use and management. Glob Chang Biol 22:1008–1028. https://doi.org/10.1111/gcb.13068
Soil Survey Staff (2014) Keys to soil taxonomy. In: 12th ed. Natural resources conservation service, Washington, DC, p 410
Subke J-A, Inglima I, Cotrufo MF (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943. https://doi.org/10.1111/j.1365-2486.2006.01117.x
Tang J, Baldocchi DD (2005) Spatial-temporal variation in soil respiration in an oak-grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 73:183–207. https://doi.org/10.1007/s10533-004-5889-6
Teixeira RS, Fialho RC, Costa DC et al (2020) Land-use change with pasture and short rotation eucalypts impacts the soil C emissions and organic C stocks in the Cerrado biome L. Degrad Dev 31:909–923. https://doi.org/10.1002/ldr.3480
Thurgood A, Singh B, Jones E, Barbour MM (2014) Temperature sensitivity of soil and root respiration in contrasting soils. Plant Soil 382:253–267. https://doi.org/10.1007/s11104-014-2159-9
Tubiello FN, Salvatore M, Ferrara AF et al (2015) The contribution of agriculture, forestry and other land use activities to global warming, 1990–2012. Glob Chang Biol 21:2655–2660. https://doi.org/10.1111/gcb.12865
Veen GFC, Freschet GT, Ordonez A, Wardle DA (2015) Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 124:187–195. https://doi.org/10.1111/oik.01374
Vicentini ME, Pinotti CR, Hirai WY et al (2019) CO2 emission and its relation to soil temperature, moisture, and O2 absorption in the reforested areas of Cerrado biome, Central Brazil. Plant Soil 444:193–211. https://doi.org/10.1007/s11104-019-04262-z
Wang W, Fang J (2009) Soil respiration and human effects on global grasslands. Glob Planet Change 67:20–28. https://doi.org/10.1016/j.gloplacha.2008.12.011
Wei W, Weile C, Shaopeng W (2010) Forest soil respiration and its heterotrophic and autotrophic components: global patterns and responses to temperature and precipitation. Soil Biol Biochem 42:1236–1244. https://doi.org/10.1016/j.soilbio.2010.04.013
Williams A, Kane DA, Ewing PM et al (2016) Soil functional zone management: a vehicle for enhancing production and soil ecosystem services in row-crop agroecosystems. Front Plant Sci 7:1–15. https://doi.org/10.3389/fpls.2016.00065
Williams A, Davis AS, Jilling A et al (2017) Reconciling opposing soil processes in row-crop agroecosystems via soil functional zone management. Agric Ecosyst Environ 236:99–107. https://doi.org/10.1016/j.agee.2016.11.012
Wilson CH, Strickland MS, Hutchings JA et al (2018) Grazing enhances belowground carbon allocation, microbial biomass, and soil carbon in a subtropical grassland. Glob Chang Biol 24:2997–3009. https://doi.org/10.1111/gcb.14070
Yang Y, Fang J, Ma W, Wang W (2008) Relationship between variability in aboveground net primary production and precipitation in global grasslands. Geophys Res Lett 35:2–5. https://doi.org/10.1029/2008GL035408
Yu S, Chen Y, Zhao J et al (2017) Temperature sensitivity of total soil respiration and its heterotrophic and autotrophic components in six vegetation types of subtropical China. Sci Total Environ 607–608:160–167. https://doi.org/10.1016/j.scitotenv.2017.06.194
Yuste JC, Baldocchi DD, Gershenson A et al (2007) Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob Chang Biol 13:2018–2035. https://doi.org/10.1111/j.1365-2486.2007.01415.x
Zimmermann M, Davies K, Peña de Zimmermann VTV, Bird MI (2015) Impact of temperature and moisture on heterotrophic soil respiration along a moist tropical forest gradient in Australia. Soil Res 53:286. https://doi.org/10.1071/SR14217
Acknowledgements
This work was in part supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - financial support to I.R.Silva) and Programa em Nutrição e Solos Florestais (NUTREE). CMPC-Celulose Rio Grandense provided access to experimental area and assistance in the fieldwork. The funders had no role on the study design, data analysis, preparation of the manuscript, or decision to publish. We thank João Milagres, Lucas Guimarães, and Daniela Costa for their technical support with analyses, and two anonymous reviewers for their suggestions on an earlier manuscript version.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oliveira, F.C.C., Ferreira, G.W.D., Santos, R.S. et al. Soil CO2 concentration, efflux, and partitioning in a recently afforested grassland. New Forests 52, 737–757 (2021). https://doi.org/10.1007/s11056-020-09824-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-020-09824-z