Abstract
Background and aims
Although a number of different factors influence C and N isotopic fractionation of organic matter, the δ13C and δ15N values of soil organic matter both tend to increase with soil depth, following similar trajectories. This similarity has not been investigated at the global scale. As microbial decomposition increases organic matter δ13C and δ15N values, soil isotopic values are hypothesized to generally increase with depth across local and global scales.
Methods
Soil δ13C and δ15N values for 16 soil depth-profile sites were used for local-scale investigation, and 5447 global single-depth sites were used for global-scale investigation of the correspondence between δ13C and δ15N. Correlative and boosted regression tree analyses were used to determine the main drivers of the variance in soil δ15N globally and also the environmental association of variability in the correlation with depth between δ13C and δ15N at a number of sites.
Results
Strong positive correlations between δ13C and δ15N values through soil profiles were found at a number of sites and were found to be independent of vegetation type. Globally, soil δ13C and δ15N values were also found to be significantly positively correlated across a wide range of climates and biomes.
Conclusion
The global correspondences between δ13C and δ15N values may suggest a mechanistic link between δ13C and δ15N through the process of SOM decomposition and microbial processing and highlight the importance of soil-related processes in determining isotopic signals in soils. The variability in these soil processes should be considered when interpreting soil isotopic values of δ13C and δ15N as indicators of ecosystem sources of soil C and N and inferring vegetation inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil δ15N values tend to decrease with increasing mean annual precipitation (MAP) and decreasing mean annual temperature (MAT) across a broad range of climate and ecosystem types (Amundson et al. 2003). To some extent, this variation in soil δ15N values is associated with vegetation inputs, given that foliar δ15N values range over 35‰ across plants globally (Craine et al. 2009). Soil δ15N values, however, increase with decreasing soil organic C as global soil organic C concentrations also decline with increasing MAT and decreasing MAP (Craine et al. 2015b). As a consequence, the dependence of soil δ15N on MAP and MAT has been ascribed to this association of soil C with environmental variables and the consequences of these for microbial transformation of both C and N. Furthermore, soils with greater clay concentrations often have higher soil δ15N values. The dependence of soil δ15N on soil C and clay is through fractionation associated with decomposition of soil organic matter that might at least partially be due to better water retention by clay, further linking it with environmental variables (SOM; Craine et al. 2015b).
Like soil δ15N, global patterns of soil δ13C values are correlated with MAP and MAT (Lu et al. 2004), but also with soil texture (Sollins et al. 2009). The largest influence on soil δ13C values, however, is the δ13C value of the input of C to the soil organic carbon (SOC) pool, which is either directly or indirectly derived from primary productivity (Kuzyakov and Domanski 2000). As a consequence, soil δ13C has been used as a proxy for historical vegetation shifts in the distribution of C3 and C4 vegetation (Swap et al. 2004; Gillson et al. 2004; Kuzyakov et al. 2006; Gillson 2015). Despite these clear geographic differences, changes in soil δ13C with depth do not necessarily reflect historic changes in the relative inputs of C3 and C4 vegetation. Turnover processes during soil development also contribute to changes in soil δ13C (Cerling 1984; Balesdent et al. 1993; Qiao et al. 2014) with more decomposed SOC having higher δ13C values (Boström et al. 2007). Thus both soil δ15N and δ13C values are, at least partially, determined by soil processes (i.e. decomposition and mineralization via microbial processing of OM), which may link the patterns of fractionation of these isotopes in the soil. If soil N and C isotope patterns are at least partially linked through common soil processes (i.e. decomposition and mineralization), then we may expect coordinated changes in δ15N and δ13C values with depth through a soil profile.
The δ13C values of SOM through soil profiles commonly increase by 1–3‰ as depth increases below 0.2 m relative to that of the surface litter layer (Chen et al. 2005; Boström et al. 2007). The enrichment of 13C with depth has been shown to occur in tropical, temperate and boreal systems (Hobbie and Ouimette 2009). Although atmospheric δ13CO2 has declined by 1.5‰ over the past 100 years, this has been shown to contribute only marginally to the enrichment of soil δ13C with depth (Ehleringer et al. 2000; Esmeijer-Liu et al. 2012). At least four hypotheses have been proposed for C isotope fractionation through soil profiles. Firstly, kinetic discrimination against 13C during respiration may result from microorganisms preferentially respiring CO2 that is 13C–depleted relative to the substrate, resulting in 13C enrichment of the remaining SOC (Ågren et al. 1996). Although some studies show large 13C depletion of the CO2 formed (e.g. Fernandez et al. 2003), others show no or only minor isotopic fractionation (e.g. Ekblad and Högberg 2000). Secondly, microorganisms are 13C–enriched by 2 to 4‰ compared to plant material (Hobbie et al. 1999) and thus influence SOM, resulting in decreasing C:N ratios with soil depth (Wallander et al. 2003), and compound-specific shifts in soil organic matter to higher δ13C values in products of microbial origin (Huang et al. 1996; Ehleringer et al. 2000). Thirdly, variable mobility (e.g. fulvic acids; Heil et al. 2000) and sorption of isotopes of dissolved organic C on soil particulates (especially clay) may contribute to soil δ13C profiles (Craine et al. 2015b), although some authors have questioned the significance of these mechanism (Boström et al. 2007). Finally, although preferential utilization of 13C–depeleted compounds has been suggested (Boström et al. 2007), the more recalcitrant C fractions of plant biomass (e.g. lignin, lipids and cellulose) that accumulate at depth (Rovira and Vallejo 2002) are 13C–depleted relative to the whole plant (Wilson and Grinsted 1977), and thus cannot contribute to increased 13C–enrichment with depth (Wynn et al. 2006). Apart from this, some, or all, of these processes may thus contribute to determining soil δ13C values to variable extents in different ecological contexts.
As with δ13C, δ15N values usually increases with soil depth, although occasionally maximum δ15N is evident at an intermediate depth possibly as a result of increased volatilization in this soil zone (Hobbie and Ouimette 2009) followed by a subsequent decline at greater depths. The degree of enrichment that δ15N undergoes through a soil profile can have a much broader range than δ13C. In arid and semi-arid systems where soil pH is high, surface δ15N values can be elevated by as much as 7‰ relative to deeper soils (Pataki et al. 2008). There are six potentially important mechanisms that influence δ15N values within soil profiles. Firstly, depletion of 15N by mycorrhizal fungi and transfer of that 15N–depleted N to plants (Hobbie and Ouimette 2009) results in the accumulation of 15N–enriched N derived from mycorrhizal fungi (Hogberg 1997; Hobbie and Ouimette 2009). Secondly, depletion of 15N through enzymatic hydrolysis (Silfer et al. 1992), ammonification, nitrification, or denitrification and the associated fractionation during gaseous loss of 15N–depleted N-containing gas or leaching loss of 15N–depleted NO3 − and the preferential utilization of 14N by plants, drives soil δ15N values up (Handley and Raven 1992; Austin and Vitousek 1998). Thirdly, mixing of soil N among different soil layers through bioturbation (Gabet et al. 2003) and trophic fractionation (i.e. faunal processes; Ponsard and Arditi 2000) could alter soil δ15N profiles. Fourthly, soil texture (i.e. clay) may moderate 14N gaseous loss pathways and/or the differential retention of 15N–enriched SOM (Craine et al. 2015b). Fifthly, preferential microbial utilization of 14N compounds could contribute to accumulation of 15N–enriched compounds deeper in the soil (Boström et al. 2007). Finally, N deposition has been shown to decrease δ15N values of soils because deposited N is typically depleted in 15N, although this effect is relatively small (Liu et al. 2017; Esmeijer-Liu et al. 2012).
SOM decomposition is thus common to both δ13C and δ15N fractionation in soil. At the global scale, climate influences decomposition through both temperature and moisture (Gholz et al. 2000). The SOM composition and nutrient concentrations (especially N) also strongly affect decomposition (Parton et al. 2007). Although most SOM is derived from plants, only a small fraction of the yearly litter and root inputs are incorporated into the stable organic matter pool, most of it after repeated processing by soil microbes (Lerch et al. 2011). SOM transport through soils is generally downward through advection and soil development, and thus the effects of decomposition on soil δ13C and δ15N values are more noticeable deeper in the soil profile. With increasing depth, SOM is more highly processed by microbes (Trumbore 2009) with lower C:N ratios (Marin-Spiotta et al. 2014) and increasing δ13C and δ15N values (Heil et al. 2000; Billings and Richter 2006). This change in δ13C and δ15N is often modelled as “Rayleigh distillation”, which predicts soil δ13C and/or δ15N values based on the soil [C]/[N] in order to account for microbial isotopic enrichment of SOM during decomposition (Mariotti et al. 1981; Baisden et al. 2002; Wynn et al. 2005; Fischer et al. 2008). This enrichment results from the kinetic fractionation during microbial processing (Dijkstra et al. 2006) with subsequent stabilization of products by fine mineral particles in soils (Wynn et al. 2006). This Rayleigh distillation model, however, only pertains to closed systems, potentially ignoring continuous inputs (Fry 2006) that do occur in soils.
Although a number of different factors influence the isotopic fractionation of C and N isotopes, δ13C and δ15N values both increase with soil depth and commonly follow similar trajectories. We hypothesized that changes in soil δ13C and δ15N values are coordinated, possibly through decomposition-related processes, and that the scale of decomposition related changes in δ13C may confound interpretation of soil δ13C as indicative of prior C3 or C4 vegetation. Although the initial isotope composition of the organic matter is indisputably important, subsequent soil fractionation may result in δ13C and δ15N following similar trajectories in space and time. We therefore predict that changes in δ13C and δ15N values correspond with each other both locally through soil depths at a site and globally due to the extent of decomposition and other soil processing. In order to test these predictions, we compiled data from soil depth profiles from sixteen widely distributed sites and also conducted an analysis of global δ13C and δ15N variations in surface soils in order to determine relationships between soil isotopes with climate and soil properties.
Methods
Data sources
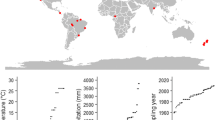
Data for soil δ13C and δ15N values were acquired from literature and by contacting individual researchers known to have collected soil isotope data in the past. Soil depth-profile data included δ13C and δ15N for mineral soils at multiple depths at a single site. A second independent dataset included both mineral soil δ13C and δ15N values at a single depth at a number of geographic locations. For each site, climate data were taken from the original source and also, using the geographic coordinates, from the 50-year climatic means (1950–2000) obtained from www.worldclim.org (accessed Sep 2014) at ca. 1 km2 resolution. Variables included were mean annual temperature (MAT), mean annual precipitation (MAP) and 17 other derived climatic variables (Supp. Table 1).
Potential evaporation (PET) was obtained from Trabucco and Zomer (CGIAR Consortium for Spatial Information, 2009. Accessed: http://www.csi.cgiar.org) in which PET was modelled using the method of Hargreaves et al. (1985) with data from Hijmans et al. (2015) and verified by comparison with separate data sources. From the climatic data, the monthly PET was subtracted from monthly precipitation to obtain an index of water availability (P–PET) and averaged to obtain the annual average. Normalized difference vegetation index (NDVI) data was obtained from eMODIS TERRA (US Geological Survey Earth Resources Observation and Science Center), which is corrected for molecular scattering, ozone absorption and aerosols. The NDVI data spanned between 19/12/2009 to 18/12/2012 and was at a spatial resolution of 250 m. The data was averaged to obtain monthly and annual average values using the “raster” (Hijmans et al. 2015) and “RCurl” (Lang and Lang 2016) packages in R.
The fraction of the vegetation with C4 photosynthesis was obtained from Berry et al. (2009) in which the percentage of vegetation within each one degree by one degree grid cell of the land surface which possesses the C4 photosynthetic pathway was determined using ‘C4 climate map’ from Collatz et al. (1998), ‘Continuous fields of vegetation characteristics’ from DeFries et al. (2000) as well as ‘Cropland fraction distribution’ from Ramankutty and Foley (1998). Where necessary, the component fields were re-sampled to bring them to a common one degree by one-degree spatial resolution.
The “SoilGrids1km” global soil data product (Hengl et al. 2014), which has mean soil information at 1 km resolution for six soil depths to 1.5 m deep (ISRIC – World Soil Information 2013), was averaged across the full depth by depth weighted-averaging. The environmental data included in the models is shown in Supp. Table 1.
Soil depth data
Data for 9 sites, which include 4 sites in Africa (Paulshoek, Pretoriuskop, Satara, Hluhluwe) and sites in Alaska, France, Sweden, New South Wales and the Amazon in Brazil were compiled from a number of publications (Table 1). These made up a total of 16 different sampling groups within distinct vegetation types and included data for 79 soil profiles at multiple depths. As most sites were represented by repeated sampling of different vegetation types, the average value of the N and C isotopes at each depth for each vegetation type, as well as the confidence intervals, were determined for each site. As the ranges of δ13C and δ15N values through soil profiles were different in magnitude, the actual measured values were scaled using the “scale” function in R (z-transformation). This allowed both the N and C isotope patterns through the soil profiles to be plotted on the same set of axes for comparison using the ‘ggplot2’ package (Wickham 2009) in R. A Pearson correlation test was then performed on the scaled data. This correlation was then treated as a derived variable. As one of the locations, Hluhluwe, consisted of a number of different vegetation types, each vegetation type at the site was plotted separately rather than averaging across the site.
Global analysis of surface soil
In order to determine the main global correlates of soil δ15N values, the dataset from Craine et al. (2015b), which included soil and climatic data for sites around the globe, was re-analysed. Records that did not include a depth or mineral soil components were removed leaving a total of 5447 sites for the analysis. As the δ13C and δ15N values were from single depths only, the dataset was used to determine the global correlation of soil δ13C and other variables with δ15N.
Boosted regression tree analyses
Boosted regression tree models were used to determine how differences in soil and environmental conditions influence the correlation between δ13C and δ15N values for soil depth-profiles, as well as the main drivers of δ15N at the global scale. Boosted regression tree analysis is a form of non-linear modelling that uses machine learning (Elith et al. 2008). The modelling entails decision trees splitting the data into two homogenous groups, a process repeated many times (boosting) so as to improve the prediction of the response variable. Models are parameterized by adjusting their learning rates, tree complexity and bag fraction (Elith et al. 2008). We used a cross-validation procedure to identify the optimal number of trees and tree size for the model, and to guard against over-fitting (Hastie et al. 2001). Initially, the data set was randomly divided into 10 mutually exclusive subsets of equal size, 9 of which were used as a training set to create the boosted tree while the remainder was used as a test set to determine the predictive accuracy of the model. The data in the training sets were fitted using trees of different sizes (range = 2 to 10) by incrementally adding trees in sets of 50. For each combination of tree size and number of trees, the predictive accuracy of the model was determined by comparing values in the test set with those predicted by the model. This procedure was repeated 10 times so that all groups were used as cross-validation groups, and the mean predictive error calculated across all subsets for each level of complexity. The combination of tree size and tree number that produced the lowest predictive error was chosen for all subsequent analyses. Performance was evaluated by expressing the predictive deviance of 10-fold cross validation as a percentage of the null deviance.
Two different models were used, either to explain the correlation of δ13C and δ15N values at the local scale across soil depths (BRTlocal), or to explain the value of δ15N at the global scale for a single soil depth (BRTglobal). The climatic and soil variables listed in Supp. Table 1 were used as the predictor variables. The ‘select07’ function (Dormann et al. 2013) in R, was used to identify collinear predictors. In cases where the predictor variables were found to be strongly collinear with each other, the variable with either the strongest correlation with the response variable, or the most biologically relevant, was retained. Following an initial run (learning rate = 0.01, tree complexity = 5, bagging fraction = 0.5), a simplification procedure was implemented (Elith et al. 2008) to eliminate variables with low influence (such as NDVI and PET). Both models were run ten times using the libraries ‘gbm’ (Ridgeway et al. 2013) and ‘dismo’ (Hijmans and van Etten 2014) packages in R. Model outputs were used to ascertain the relative influence and relationship of each predictor with the correlation between δ13C and δ15N at the local scale or δ15N at the global scale.
To account for C3 and C4 vegetation input into the SOM pool, global soil δ13C values were analyzed for bimodality using libraries ‘diptest’ (Maechler 2015) in R and cutoffs were calculated using the ‘mixtools’ (Benaglia et al. 2009). δ13C for C3 and C4 were then treated as separate sets of data on which BRT modeling for global δ15N values were independently reanalyzed.
Results
Isotopic variation with soil depth
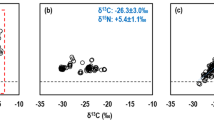
For 11 out of 16 sampling groups analyzed, the variation in average soil δ13C and δ15N values with depth were significantly positively correlated with each other (Fig. 1, Table 1). For many of these sites, both δ13C and δ15N values increased with depth, with the majority of the increase occurring in the upper 10–20 cm of the profile. The range of variability for both isotopes was ca. 2–8‰ through the soil profiles and this range was independent of the average δ13C and δ15N signature for the sites (Table 1). Within the relatively small geographic area of the Hluhluwe Nature reserve, the significant positive correlations between δ13C and δ15N values were independent of vegetation types comprising forest, grassland, savanna and thicket sites. Across all of these distinct vegetation types, δ13C and δ15N values increased similarly with depth (Fig. 2, Table 1). For these sites the range of variability for both isotopes was also ca. 2–8‰ with the majority of the increase in δ13C and δ15N values occurring within the upper ca. 20 cm of the soil. Although most sites had significant positive correlations between δ13C and δ15N, for 5 of the 16 sampling groups, changes in average soil δ13C and δ15N values through the soil profiles were either not significantly associated or negatively correlated with each other (Fig. 3, Table 1). For these sites δ13C and δ15N values also increased with depth, with the exception of the Paulshoek site in which δ15N initially increased before subsequently decreasing below ca. 10 cm. These sites also had a wider range of δ13C and δ15N values than those for which there were significant correlations between δ13C and δ15N (Figs. 1, and 2).
Variation with soil depth of δ13C and δ15N values for sites in which δ13C and δ15N are significantly correlated with each other (Table 1). The data was averaged for each depth and the confidence interval is represented by the coloured bands. The δ13C and δ15N data were independently centred on 0 so as to allow comparison of the variation of these within a site and thus the range of the data corresponds to that of the original data. Sites designated OC and UC are from open-canopy and under-canopy, respectively
Variation with soil depth of δ13C and δ15N values for sites in which the dominant vegetation types differ. The data was averaged for each depth and the confidence interval represented by the coloured bands. The δ13C and δ15N data were independently centred on 0 so as to allow comparison of the variation of these within a site and thus the range of the data corresponds to the original data
Variation with soil depth of δ13C and δ15N values for sites in which δ13C and δ15N are poorly correlated with each other. The data was averaged for each depth and the confidence interval represented by the coloured bands calculated from the standard error. The δ13C and δ15N data were independently centred on 0 so as to allow comparison of the variation of theses within a site. The range of the data corresponds to the original data
BRT analysis of the correlation between δ13C and δ15N values ranked CEC, mean diurnal temperature range, bulk density, MAT, clay and MAP as the top predictors (Fig. 4a), explaining 38% of the variance in the correlation between δ13C and δ15N. Partial dependency plots, which show the effect of a variable on the response after accounting for the average effects of all other variables in the model, of the BRT analysis of the soil profile correlations between δ13C and δ15N values (Fig. 5), showed that this was strongest at sites with CEC < 20 cmol kg−1 and a mean diurnal temperature range < 13°C. Sites with bulk density above 1400 kg m3 had strong correlation between soil δ13C and δ15N values. The influence of clay concentration on the correlation between δ13C and δ15N values was generally high. A number of sites with clay concentrations between 30 and 35%, however, had a relative low influence of clay on the correlation. These sites were arid, receiving <500 mm mean annual precipitation and had a relatively poor correlation compared to mesic sites (i.e. between 500 and 1000 mm) with a moderate influence in hydric sites (>1000 mm). The correlation between δ13C and δ15N values was stronger at sites with MAT >19°C (Supp. Fig. 4f).
Relative influence of variables in determining the correlation between global soil δ13C and δ15N as determined by BRT analysis (a) as well as the relative influence of variables in determining the global soil δ15N as determined by BRT analysis (b). Values are the mean ± SE of 10 runs of each model. Error bars represent standard error
Global geographic variation
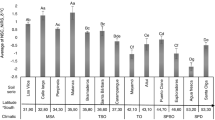
Globally, soil δ15N values of surface soils were significantly positively correlated with δ13C, MAT and the prevalence of C4 photosynthetic vegetation and negatively correlated with CEC and diurnal T range (Table 2). Geospatial variation in global δ13C and δ15N values that were spatially averaged over 0.1° corresponded relatively well with each other at high latitudes (> 50°) where both δ13C and δ15N values were more negative compared to sites located nearer the equator (Fig. 5). Sites in which δ13C values were relatively high (Fig. 5) were from more arid regions such as Southern Africa, Australia and North America and in which C4 grass communities exist (Fig. 6).
BRT analysis of global soil δ15N values (BRTglobal) ranked MAT, δ13C, CEC, C4, diurnal range and MAP as the top predictors of soil δ15N (Fig. 4b), which explained 62% of the variation in δ15N values. The partial dependency plots for the BRTglobal (Supp. Fig. 5) showed that as MAT increased, δ15N values also increased. Sites with δ13C values below ca. -30‰ had low δ15N values, which increased rapidly with increased δ13C values up until ca. -20‰, above which changes in δ15N values were relatively small. Therefore, much of the change in δ15N values associated with δ13C values occurred in a range of δ13C values considered to be characteristic of C3 dominated sites (Supp. Fig 2). Sites with CEC values >10 cmol kg−1 had relatively low soil δ15N values. δ15N values were also low for sites with <75% C4 vegetation. Soil δ15N values were reduced with increases in mean diurnal temperature range and generally with increased MAP (Supp. Fig. 5f).
Global δ15N values predicted from the full BRTglobal model, including both C3 and C4 sites, were strongly correlated with observed global δ15N values (Supp. Fig. 1). There was, however, a degree of under-prediction of δ15N values at low observed δ15N values and over-prediction at high observed δ15N values. Global soil δ13C values were bimodal with two ranges of δ13C values having peaks at −26.36‰ and −17.58‰, indicating that there were a number of sites dominated by either predominantly C3 or C4 plants (Supp. Fig. 2). BRT’s predicting global soil δ15N based on a subset of sites that were predominantly C3 dominated ranked MAT, δ13C, CEC, bulk density, diurnal T range and MAP as top predictors (Supp. Table 2). The BRT developed for C4 dominated sites ranked MAT, CEC, bulk density, MAP, diurnal range and δ13C as top predictors. Although soil δ13C was found to be a strong predictor of δ15N for C3 sites, it was a weak predictor in C4 dominated sites.
Discussion
This study suggests that either common or coordinated processes contribute to fractionation of soil C and N isotopes. The link between soil δ13C and δ15N values may inform understanding of these processes due to this coordination of soil processes determining both C and N isotope fractionation. Our results suggest that although the initial isotope composition of the organic matter is indisputably important, subsequent fractionation via soil processes, such as decomposition and related processes, may result in correlations between δ13C and δ15N values in geographic space and commonly following similar trajectories with soil depth. More positive δ13C and δ15N values with soil depth (Fig. 1) must result from increasing fractionation or more prolonged fractionation in deeper soils relative to shallower soils.
The importance of the vegetation characteristics in determining C isotopic composition is apparent from the bimodal distribution of soil δ13C values associated with C3 (−22‰ to −32‰; Troughton 1979) and C4 (−9.2‰ to −19.3‰; Hattersley 1982) vegetation (Fig. S2) whereas the variation in δ13C within the C3 and C4 groupings is caused by climatic and geographical factors (Damesin et al. 1997). Likewise, global variation in soil δ15N values (Fig. 5) is associated with variation in foliar δ15N that varies with MAP, MAT, N availability, foliar N concentration, species composition and with the degree of N2 fixation (Craine et al. 2009). Organic matter enters soils in a diversity of ways and this influences the initial isotopic signature of soil C and N (Eissfeller et al. 2013). The majority of SOM, however, enters the soil as plant-derived detritus, where it is utilized by soil microbes (Berg and McClaugherty 2008) and decomposer fauna (Hättenschwiler and Gasser 2005). Consequently, the isotopic values of the dominant vegetation and the variation in δ13C and δ15N values, both between and within species (Damesin et al. 1997; Craine et al. 2015a), strongly influence SOM isotopic composition.
Unlike for C, however, there are also strong ecosystem feedbacks between soil and vegetation N in determining ecosystem δ15N values, because soil δ15N also partially determines plant δ15N. Despite this dependence of SOM isotopic composition on that of OM and vegetation, the variations in δ13C (range: −27.8 to −12.4‰) and δ15N (range: −0.1 to 10.1‰) with depth in soil profiles were often strongly correlated with each other (Table 1). Likewise, geospatial variation in global δ13C and δ15N values also corresponded relatively well across a wide range of climates and biomes (Fig. 5). For example, C3 and C4 dominated sites showed similar patterns of δ13C and δ15N enrichment through soil profiles (Fig. 2), although the range of values was smaller with C4 vegetation.
The correspondence between the increases of δ13C and δ15N values with depth is probably through processing of SOM, which is further supported by the most influential predictors in the BRT model for the correlation between δ13C and δ15N values through soil profiles (Fig. 4a), which themselves are related to microbial activity. Furthermore, soil δ13C values were also strong determinants of δ15N globally (regardless of soil and ecosystem type) while the remaining top predictors of δ13C could be related to SOM decomposition (Fig. 4b). Processing of SOM is determined by characteristics of the SOM, such as the C and N composition (Fernandez et al. 2003), as well as by environmental factors including soil temperature, moisture and aeration (Gholz et al. 2000; Zhang et al. 2008). The reason for the positive correlation between MAT and both δ15N and δ13C values could therefore be due to microbial activity increasing with increasing temperature. Mean diurnal temperature range (e.g. Li et al. 2011), CEC and soil fertility (Sikora 2013) may also be linked to SOM decomposition through soil microbial processes. Although favorable moisture conditions stimulate decomposer communities (Cotrufo et al. 2013), MAP was not significantly correlated with either δ13C or δ15N values at the global scale (Fig. 4b, Table 2). This is likely because many ecosystem properties depend on MAP obscuring clear relationships. For example, Craine et al. (2015a) related variation in global soil δ15N to variation in clay concentrations. Further, there is the possibility that the limited range in MAP at the regional scale can obscure relationships between soil δ15N and MAP as the increase in soil δ15N with increasing MAP at the regional scale often breaks down at broader scales (Amundson et al. 2003; Austin and Vitousek 1998).
Despite strong global geographic correspondence between δ13C and δ15N and correspondence over soil depth (11 of 16 sites), some sites had non-significant (New South Wales, France, Sweden) or negative (Paulshoek) correlations between δ13C and δ15N (Fig. 3, Table 1). These sites indicate the complexity to the relationship between soil δ13C and δ15N, and dependence on other factors. For example, the New South Wales sites had a large proportion of N2-fixing microbes in the surface soil (Macdonald et al. 2015) resulting in δ15N being close to 0‰. The non-significant Swedish and French sites were both associated with plantations (Boström et al. 2007; Zeller et al. 2007), whereas a corresponding natural site in France showed a significant relationship (Fig. 1). Paulshoek exhibited a maximum soil δ15N value at intermediated depths, which is indicative of N-loss during nitrification and denitrification (Hobbie and Ouimette 2009). This is not surprising as Paulshoek is arid with high soil temperatures and sporadic rainfall (Table 1) and these conditions increase nitrification/denitrification rates (Craine et al. 2015b). Thus despite the general global relationship between δ13C and δ15N, this correspondence does vary depending on local biotic, disturbance and environmental influences.
As a consequence of a link between soil δ13C and δ15N, interpretation of soil δ13C values as indicators of historical vegetation assemblages is complicated by the role of soil processes in determining soil δ13C values, as also shown by Wynn et al. 2005. The ranges of δ13C values with depth are commonly large (up to 11.0 ‰, Supp. Fig. 3) which overlaps the range of values commonly associated with vegetation change. For example, δ13C values between −16 and −20‰ have been used to indicate mixed C3 and C4 vegetation and > −16% to indicate C4 dominance (Gillson 2015). From our study, however, whilst the minimum δ13C values of soils with C3 and C4 vegetation reflect the isotopic signature of the vegetation inputs, the maximum δ13C values are indistinguishable. Since the maximum δ13C values of soils supporting C3 vegetation overlap with the minimum δ13C values of C4 vegetation, interpretation of intermediate δ13C values (i.e. < ca. -15 ‰) as indicating historical vegetation characteristics should be approached with caution. Furthermore, in order to demonstrate that ancient δ13C SOC values are indeed representative of ancient vegetation assemblages in samples of deep SOC, one must establish that the fraction of SOC remaining in the sample is very close to the original maximum concentration during soil formation and that fractionation has not been great (Wynn et al. 2006). This is because Rayleigh distillation and mixing processes vary with environmental and soil properties, with particularly strong effects associated with fine mineral particles (i.e. clay) in fine grained soils (Krull and Skjemstad 2003; Wynn et al. 2005) and should not be assumed to be constant everywhere.
References
Ågren GI, Bosatta E, Balesdent J (1996) Isotope discrimination during decomposition of organic matter: a theoretical analysis. Soil Sci Soc Am J 60:1121–1127. https://doi.org/10.2136/sssaj1996.03615995006000040023
Amundson R, Austin AT, Schuur EA, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Glob Biogeochem Cycles. https://doi.org/10.1029/2002GB001903
Austin AT, Vitousek PM (1998) Nutrient dynamics on a precipitation gradient in Hawai'i. Oecologia 113(4):519–529
Baisden WT, Amundson R, Brenner DL et al (2002) A multiisotope C and N modeling analysis of soil organic matter turnover and transport as a function of soil depth in a California annual grassland soil chronosequence. Glob Biogeochem Cycles 16:82–1–82–26. https://doi.org/10.1029/2001GB001823
Balesdent J, Girardin C, Mariotti A (1993) Site-related (13) C of tree leaves and soil organic matter in a temperate Forest. Ecology. https://doi.org/10.2307/1941808?ref=search-gateway:4f6368673c42b4f42edf719325fc510e
Benaglia T, Chauveau D, Hunter DR, Young DS (2009) Mixtools: an R package for analyzing finite mixture models. J Stat Softw 32:1–29
Berg B, McClaugherty C (2008) Plant litter. Decomposition, humus formation, carbon sequestration, 2nd Ed. Springer, 2008. Berry JA, Collatz GJ, DeFries RS (2009) ISLSCP II C4 vegetation percentage
Berry JA, Collatz GJ, DeFries RS (2009) ISLSCP II C4 vegetation percentage
Billings SA, Richter DD (2006) Changes in stable isotopic values of soil nitrogen and carbon during 40 years of forest development. Oecologia 148:325–333. https://doi.org/10.1007/s00442-006-0366-7
Boström B, Comstedt D, Ekblad A (2007) Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 153:89–98. https://doi.org/10.1007/s00442-007-0700-8
Cerling TE (1984) The stable isotopic composition of modern soil carbonate and its relationship to climate. Earth Planet Sci Lett 71(2):229–240
Chen Q, Shen C, Sun Y et al (2005) Spatial and temporal distribution of carbon isotopes in soil organic matter at the Dinghushan biosphere reserve, South China. Plant Soil 273:115–128. https://doi.org/10.2307/24125204?ref=no-x-route:bf8f8a632d5dc84e9c9ef56d558d465a
Collatz GJ, Berry JA, Clark JS (1998) Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: present, past, and future. Oecologia 114(4):441–454
Cotrufo MF, Wallenstein MD, Boot CM et al (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995. https://doi.org/10.1111/gcb.12113
Craine JM, Elmore AJ, Aidar MPM et al (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992. https://doi.org/10.1111/j.1469-8137.2009.02917.x
Craine JM, Brookshire ENJ, Cramer MD, Hasselquist NJ, Koba K, Marin-Spiotta E, Wang L (2015a) Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396(1-2):1-26
Craine JM, Elmore AJ, WANG L et al (2015b) Convergence of soil nitrogen isotopes across global climate gradients. Sci Rep 5:8280–8288. https://doi.org/10.1038/srep08280
Damesin C, Rambal S, Joffre R (1997) Between-tree variations in leaf delta C-13 of Quercus pubescens and Quercus ilex among Mediterranean habitats with different water availability. Oecologia 111:26–35
DeFries RS, Hansen MC, Townshend JR, Janetos AC, Loveland TR (2000) A new global 1-km dataset of percentage tree cover derived from remote sensing. Glob Chang Biol 6(2):247–254
Dijkstra P, Ishizu A, Doucett R et al (2006) 13C and 15N natural abundance of the soil microbial biomass. Soil Biol Biochem 38:3257–3266. https://doi.org/10.1016/j.soilbio.2006.04.005
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JR, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36(1):27–46
Ehleringer JR, Buchmann N, Flanagan LB (2000) Carbon isotope ratios in belowground carbon cycle processes. Ecol Appl 10:412–422
Eissfeller V, Beyer F, Valtanen K et al (2013) Incorporation of plant carbon and microbial nitrogen into the rhizosphere food web of beech and ash. Soil Biol Biochem 62:76–81. https://doi.org/10.1016/j.soilbio.2013.03.002
Ekblad A, Högberg P (2000) Analysis of δ13C of CO2 distinguishes between microbial respiration of added C4-sucrose and other soil respiration in a C3-ecosystem. Plant Soil 219:197–209. https://doi.org/10.1023/A:1004732430929
Elith J, Leathwick JR, Hastie T (2008) A working guide to boosted regression trees. J Anim Ecology 77:802–813. https://doi.org/10.1111/j.1365-2656.2008.01390.x
Esmeijer-Liu AJ, Kürschner WM, Lotter AF, Verhoeven JT, Goslar T (2012) Stable carbon and nitrogen isotopes in a peat profile are influenced by early stage diagenesis and changes in atmospheric CO2 and N deposition. Water Air Soil Pollut 223(5):2007–2022
February EC, Higgins SI (2010) The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. S Afr J Bot 76(3):517–523
Fernandez I, Mahieu N, Cadisch G (2003) Carbon isotopic fractionation during decomposition of plant materials of different quality. Global Biogeochem Cycles 17:n/a–n/a. https://doi.org/10.1029/2001GB001834
Fischer V, Joseph C, Tieszen LL, Schimel DS (2008) Climate controls on C3 vs. C4 productivity in North American grasslands from carbon isotope composition of soil organic matter. Glob Chang Biol 14(5):1141–1155
Fry, B. (2006). Stable isotope ecology (Vol 521). New York: Springer
Gabet EJ, Reichman OJ, Seabloom EW (2003) The effects of bioturbation on soil processes and sediment transport. Annu Rev Earth Planet Sci 31(1):249–273
Gholz HL, Wedin DA, Smitherman SM et al (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Biol 6:751–765. https://doi.org/10.1046/j.1365-2486.2000.00349.x
Gillson L (2015) Evidence of a tipping point in a southern African savanna? Ecol Complex 21:78–86. https://doi.org/10.1016/j.ecocom.2014.12.005
Gillson L, Waldron S, Willis KJ (2004) Interpretation of soil delta(13)C as an indicator of vegetation change in African savannas. J Veg Sci 15:339–350
Grey EF (2011) Some consequences of woody plant encroachment in a mesic South African savanna. Unpublished master’s thesis, University of Cape Town, South Africa
Handley LL, Raven JA (1992) The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant, Cell and Environment 15:965–985. https://doi.org/10.1111/j.1365-3040.1992.tb01650.x
Hargreaves GL, Hargreaves GH, Riley JP (1985) Irrigation water requirements for Senegal River Basin. J Irrig Drain Eng -Asce 111:265–275
Hastie T, Friedman J, Tibshirani R (2001) Model assessment and selection. In The elements of statistical learning. Springer, New York, pp 193–224
Hättenschwiler S, Gasser P (2005) Soil animals alter plant litter diversity effects on decomposition. Proc Natl Acad Sci U S A 102(5):1519–1524
Hattersley PW (1982) δ13 values of C4 types in grasses. Funct Plant Biol react-text 479(2):139–154
Heil B, Ludwig B, Flessa H, Beese F (2000) 13C and 15N distributions in three spodic dystric cambisols under beech and spruce. Isot Environ Health Stud 36(1):35–47
Hengl T, de Jesus JM, MacMillan RA et al (2014) SoilGrids1km — global soil information based on automated mapping. PLoS One 9:e105992–e105917. https://doi.org/10.1371/journal.pone.0105992
Hijmans RJ, Van Etten J (2014) Raster: geographic data analysis and modeling. R package version 2.2-31. URL http://CRAN.R-project.org/package=raster. Accessed 15 Mar 2014
Hijmans RJ, van Etten J, Cheng J, Mattiuzzi M, Sumner M, Greenberg JA, Lamigueiro OP, Bevan A, Racine EB, Shortridge A, Hijmans MR (2015) Package ‘raster’. R package
Hobbie EA, Ouimette AP (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95:355–371. https://doi.org/10.1007/s10533-009-9328-6
Hobbie EA, Macko SA, Shugart HH (1999) Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia 118:353–360
Hobbie EA, Macko SA, Williams M (2000) Correlations between foliar δ15N and nitrogen concentrations may indicate plant-mycorrhizal interactions. Oecologia 122(2):273–283
Hogberg P (1997) Tansley review no. 95: 15N natural abundance in soil-plant systems. (erratum: July 1998, v. 139 (3), p. 595.). New Phytol 1–26
Huang Y, Bol R, Harkness DD, Ineson P, Eglinton G (1996) Post-glacial variations in distributions, 13 C and 14 C contents of aliphatic hydrocarbons and bulk organic matter in three types of British acid upland soils. Org Geochem 24(3):273–287
Krull ES, Skjemstad JO (2003) δ 13 C and δ 15 N profiles in 14 C-dated Oxisol and Vertisols as a function of soil chemistry and mineralogy. Geoderma 112(1):1–29
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Rev J Plant Nutr Soil Sci 163(4):421–431
Kuzyakov Y, Mitusov A, Schneckenberger K (2006) Effect of C3–C4 vegetation change on δ13C and δ15N values of soil organic matter fractions separated by thermal stability. Plant Soil 283:229–238. https://doi.org/10.1007/s11104-006-0015-2
Lang DT, Lang M (2016) Package “RCurl”, R package
Lerch TZ, Nunan N, Dignac MF et al (2011) Variations in microbial isotopic fractionation during soil organic matter decomposition. Biogeochemistry 106:5–21. https://doi.org/10.2307/41490503?ref=no-x-route:12af3dd2e718256c638206e697516b1e
Li Z, Wang X, Zhang R et al (2011) Contrasting diurnal variations in soil organic carbon decomposition and root respiration due to a hysteresis effect with soil temperature in a Gossypium s. (cotton) plantation. Plant Soil 343:347–355. https://doi.org/10.1007/s11104-011-0722-1
Liu J, Wang C, Peng B, Xia Z, Jiang P, Bai E (2017) Effect of nitrogen addition on the variations in the natural abundance of nitrogen isotopes of plant and soil components. Plant Soil 412(1–2):453–464
Lu H, Wu N, Gu Z et al (2004) Distribution of carbon isotope composition of modern soils on the Qinghai-Tibetan plateau. Biogeochemistry 70:273–297. https://doi.org/10.2307/4151469?ref=no-x-route:06baab149dad744f1af8e19b479345cc
Macdonald BCT, Warneke S, Maïson E, McLachlan G, Farrell M (2015) Spatial decoupling of soil nitrogen cycling in an arid chenopod pattern ground. Soil Res 53(1):97–104
Maechler M (2015) Diptest: Hartigan’s dip test statistic for Unimodality—corrected. URL http://CRAN.R-project.org/package=diptest. R package version 0.75-7
Marin-Spiotta E, Smith AP, Atkinson EE, Chaopricha NT (2014) Landscape disturbance history and belowground carbon dynamics. In: AGU fall meeting abstracts 2014 Dec, vol. 1. p L04
Mariotti A, Germon JC, Hubert P et al (1981) Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant Soil 62:413–430. https://doi.org/10.2307/42935413?ref=search-gateway:51f8021df01bde84558143257f594a9b
Ometto JPHB, Ehleringer JR, Domingues TF, Berry JA, Ishida FY, Mazzi E, Higuchi N, Flanagan LB, Nardoto GB, Martinelli LA (2006) The stable carbon and nitrogen isotopic composition of vegetation in tropical forests of the Amazon Basin, Brazil. Nitrogen Cycling in the Americas: Natural and Anthropogenic Influences and Controls. Springer, New York, pp 251–274
Parton W, Silver WL, Burke IC et al (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364. https://doi.org/10.1126/science.1134853
Pataki DE, Billings SA, Naumburg E, Goedhart CM (2008) Water sources and nitrogen relations of grasses and shrubs in phreatophytic communities of the Great Basin desert. J Arid Environ 72:1581–1593. https://doi.org/10.1016/j.jaridenv.2008.03.011
Ponsard S, Arditi R (2000) What can stable isotopes (δ15n and δ13c) tell about the food web of soil macro-invertebrates? Ecology 81(3):852–864
Pries CEH, Schuur EA, Crummer KG (2012) Holocene carbon stocks and carbon accumulation rates altered in soils undergoing permafrost thaw. Ecosystems 15(1):162–173
Qiao Y, Miao S, Silva LC, Horwath WR (2014) Understory species regulate litter decomposition and accumulation of C and N in forest soils: a long-term dual-isotope experiment. For Ecol Manag 329:318–327
Ramankutty N, Foley JA (1998) Characterizing patterns of global land use: an analysis of global croplands data. Glob Biogeochem Cycles 12(4):667–685
Ridgeway G, Southworth MH, RUnit S (2013) Package “gbm”, R package.
Rovira P, Vallejo VR (2002) Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach. Geoderma 107(1):109–141
Sikora F J, Moore KP (2014) Soil test methods from the southeastern United States. S. Coop. Ser. Bul, (419). Clemson University, Clemson
Silfer JA, Engel MH, Macko SA (1992) Kinetic fractionation of stable carbon and nitrogen isotopes during peptide bond hydrolysis: experimental evidence and geochemical implications. Chem Geol Isot Geosci section 101:211–221. https://doi.org/10.1016/0009-2541(92)90003-N
Sollins P, Kramer MG, Swanston C et al (2009) Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization. Biogeochemistry 96:209–231. https://doi.org/10.1007/s10533-009-9359-z
Swap RJ, Aranibar JN, Dowty PR (2004) Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Global change …. https://doi.org/10.1046/j.1529-8817.2003.00702.x
Troughton JH (1979) δ13C as an indicator of carboxylation reactions. In Photosynthesis II. Springer Berlin, Heidelberg, pp 140–149
Trumbore S (2009) Radiocarbon and soil carbon dynamics. Annu Rev Earth Planet Sci 37:47–66
Wallander H, Nilsson LO, Hagerberg D, Rosengren U (2003) Direct estimates of C:N ratios of ectomycorrhizal mycelia collected from Norway spruce forest soils. Soil Biol Biochem 35:997–999. https://doi.org/10.1016/S0038-0717(03)00121-4
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer Science & Business Media, Berlin
Wilson AT, Grinsted MJ (1977) 12C/13C in cellulose and lignin as palaeothermometers. Nature 265(5590):133–135
Wynn JG, Bird MI, Wong VNL (2005) Rayleigh distillation and the depth profile of 13C/12C ratios of soil organic carbon from soils of disparate texture in iron range National Park, Far North Queensland, Australia. Geochim Cosmochim Acta 69:1961–1973. https://doi.org/10.1016/j.gca.2004.09.003
Wynn JG, Harden JW, Fries TL (2006) Stable carbon isotope depth profiles and soil organic carbon dynamics in the lower Mississippi Basin. Geoderma 131:89–109. https://doi.org/10.1016/j.geoderma.2005.03.005
Zeller B, Brechet C, Maurice JP, Le Tacon F (2007) d^{13}C and d^{15}N isotopic fractionation in trees, soils and fungi in a natural forest stand and a Norway spruce plantation. Ann For Sci 64(4):419–429
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93. https://doi.org/10.1093/jpe/rtn002
Acknowledgements
This work was funded by the National Research Foundation (South Africa). We would like to thank Emma Grey, William Bond and Edmund February for providing data for the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers
Electronic supplementary material
ESM 1
Supp. Fig. 1: Observed δ15N against predicted d15N at the global scale based on the global BRT model. Black line indicates the y=x for the observed δ15N values while the red line represents the fitted function for predicted δ15N (y = -0.5x + 1.1). (DOCX 1 mb)
ESM 2
Supp. Fig 2: Test for bimodality of soil δ13C data. Red ribbon represents normal distribution of C4 sites while the blue ribbon represents normal distribution for C3 sites. (DOCX 63.7 kb)
ESM 3
Supp. Fig. 3: Minimum and maximum soil δ13C and δ15N for dominant photosynthetic pathway for all local soil profile sites used in this study. (DOCX 143 kb)
ESM 4
Supp. Fig. 4: The partial dependence of the correlation between δ13C and δ15N on the six most influential environmental predictors. The fitted function represents the modeled relationship between the correlation coefficient of δ13C and δ15N and an environmental predictor once the average effects of all other predictors were accounted for. the Solid line represents the mean of 10 runs of the final, simplified BRT model. Dashed lines represents 27 the 95% CI. A flattening of the line indicates that an increase in the predictor has no further effect on the fitted function. (DOCX 167 kb)
ESM 5
ESM Supp. Fig 5: The partial dependence of δ15N on the six most influential environmental predictors. The fitted function represents the modeled relationship between δ15N and an environmental predictor once the average effects of all other predictors were accounted for. Solid line represents the mean of 10 runs of the final, simplified BRT model. Dashed lines represents the 95% CI. A flattening of the line indicates that an increase in the predictor has no further effect on the fitted function. (DOCX 2.17 mb)
ESM 6
Supp. Table 1. (DOCX 70.9 kb)
ESM 7
Supp. Table 2. (DOCX 39.6 kb)
Rights and permissions
About this article
Cite this article
Nel, J.A., Craine, J.M. & Cramer, M.D. Correspondence between δ13C and δ15N in soils suggests coordinated fractionation processes for soil C and N. Plant Soil 423, 257–271 (2018). https://doi.org/10.1007/s11104-017-3500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3500-x