Abstract
Medium composition and culture conditions for the bleaching stable alkaline protease production by Aspergillus clavatus ES1 were optimized. Two statistical methods were used. Plackett–Burman design was applied to find the key ingredients and conditions for the best yield. Response surface methodology (RSM) including full factorial design was used to determine the optimal concentrations and conditions. Results indicated that Mirabilis jalapa tubers powder (MJTP), culture temperature, and initial medium pH had significant effects on the production. Under the proposed optimized conditions, the protease experimental yield (770.66 U/ml) closely matched the yield predicted by the statistical model (749.94 U/ml) with R 2 = 0.98. The optimum operating conditions obtained from the RSM were MJTP concentration of 10 g/l, pH 8.0, and temperature of 30 °C, Sardinella heads and viscera flour (SHVF) and other salts were used at low level. The medium optimization contributed an about 14.0-fold higher yield than that of the unoptimized medium (starch 5 g/l, yeast extract 2 g/l, temperature 30 °C, and pH 6.0; 56 U/ml). More interestingly, the optimization was carried out with the by-product sources, which may result in cost–effective production of alkaline protease by the strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms are the most important sources for enzymes production. Among these enzymes, proteases account for nearly 60% of the total industrial enzyme market (Rao et al. 1998; Banerjee et al. 1999). A large proportion of commercially available proteases are currently derived from Bacillus strains. Nevertheless, the potential use of fungal proteases is being increasingly realized (Gupta et al. 2002b). In fact, fungal enzymes are commonly used in industries due to various technical reasons, including the feasibility of obtaining enzymes at a high concentration in the fermentation medium (Mitchell and Lonsane 1992). Fungal proteases offer a distinct advantage over the bacterial enzymes in terms of easing the downstream processing (Laxman et al. 2005).
The use of alkaline proteases has been increased remarkably in various industrial processes such as in the production of detergents, in processing food and animal feed, and in the production of X-ray films (Gupta et al. 2002a; Kumar and Takagi 1999).
It is well known that extracellular protease production in microorganisms is greatly influenced by media components, especially carbon and nitrogen sources, and physical factors such as temperature, pH, incubation time, agitation, and inoculum density (Nehete et al. 1985; Kole et al. 1988; Varela et al. 1996; Johnvesly and Naik 2001). Medium composition is one of the most important parameters when enzymes are particularly produced for industrial purposes, because around 30–40% of the production cost was estimated to be accounted for the cost of the growth medium (Kirk et al. 2002). Optimization of media compounds by the traditional “one-variable-at-a-time” strategy involving changing one independent variable is the most frequently used operation in biotechnology (Haaland 1989). This strategy is extremely time-consuming and expensive when a large number of variables are considered and is incapable of detecting the true optimum, due especially to the interactions among the factors.
In recent years, the use of statistical approach involving Plackett–Burman designing and response surface methodology (RSM) has gained a lot of impetus for the medium optimization and for understanding the interactions among various physicochemical parameters using a minimum number of experiments. The Plackett–Burman design allows the screening of main factors from a large number of variables that can be retained in further optimization process. RSM is a collection of statistical techniques for designing experiments, building models, evaluating the effects of factors, and searching optimum conditions of studied factors for desirable responses (De Coninck et al. 2000). RSM has been successfully applied in many areas of biotechnology, such as the α-amylase production (Kunamneni et al. 2005), protease production (Dutta et al. 2004), and neomycin production (Adinarayana et al. 2003).
Aspergillus clavatus ES1 has been recently identified as a producer of an extracellular bleaching stable alkaline protease (Hajji et al. 2007). The enzyme has a molecular weight of 32 kDa, it was identified as a serine protease, and it is active over a wide range of pHs with an optimum between 8.0 and 9.0. The optimum temperature of activity is around 50 °C. More interestingly, the protease is stable in the presence of sodium dodecyl sulfate, Triton X-100, and sodium perborate. Considering these properties, ES1 alkaline protease may find a potential application in laundry detergents.
In the present study, an effort was done to maximize the alkaline protease production of A. clavatus ES1 by using a low-cost fermentation medium. The optimization steps were performed as follows: selecting carbon and nitrogen sources by one-variable-at-a-time approach, screening the main factors influencing protease production using the Plackett–Burman design, and assessing the optimal region of the significant variables using the RSM (full factorial design).
Material and methods
Chemicals
All microbiological media components were from Bio-Rad, France. Casein and casein peptone were purchased from Sigma Chemicals Co. (St Louis, MO, USA). Mirabilis jalapa tuber powder (MJTP) and sardinelle (Sardinella aurita) heads and viscera flour (SHVF) were prepared in our laboratory. All other chemicals used were of analytical grade.
To obtain SHVF, heads and viscera were cooked until boiling, pressed to remove water and fat, minced, and then dried (Ellouz et al. 2001). To obtain MJTP, raw material was peeled, grinded, and then dried at 80 °C for at least 5 h. The dried preparation was minced again to obtain a fine powder and then stored in glass bottles at room temperature. The MJTP contained 32.6 ± 2% starch, 17.3 ± 3% proteins, 20 ± 2% ash, and low content of lipids.
Microorganism
A. clavatus ES1 producing a bleach-stable alkaline protease was isolated from the wastewater. It was identified on the basis of 860 bp of the 18S rRNA analysis (Hajji et al. 2007). The strain was propagated on the potato dextrose agar plates at 30 °C, and the inocula of spores were prepared from 7-day-old colonies by flooding with 10 ml of sterile distilled water and scraping off the agar plates. The strain was stored at −80 °C.
Selection of the best carbon and nitrogen sources
Initial screening of the most significant carbon and nitrogen sources allowing the maximum protease production was performed by one-variable-at-a-time approach. Seven different nitrogen sources (2 g/l) include casein peptone, urea, casein, sodium nitrate, yeast extract, SHVF, and (NH4)2SO4, and the seven simple and complex carbon sources (5 g/l) include sucrose, glucose, maltose, potato starch, hulled grain of wheat, inulin, and MJTP. Initial M1 medium consists of (g/l): starch 5.0, yeast extract 2.0, CaCl2.7H2O 0.4, KH2PO4 1.0, Na2HPO4 0.8, MgSO4.7H2O 0.5, ZnSO4 0.1, and NaCl 0.3. The above different nitrogen and carbon sources are used instead of the yeast extract and starch, respectively. Media were autoclaved at 120 °C for 20 min. Cultures were inoculated with 107 spores/ml in the 300-ml Erlenmeyer flasks with a working volume of 50 ml and incubated on a rotatory shaker (200 rpm) for 72 h. Cultures were centrifuged at 8,000×g for 15 min to remove the fungi mycelia and the supernatants were used for estimation of the proteolytic activity. All experiments were carried out in duplicate and repeated at least twice.
Determination of mycelium dry weight
The fungal mycelia were harvested by centrifugation at 8,000×g for 15 min. The pellet was washed with autoclaved bidistilled water and the dry weight was determined after heating at 105 °C to constant weight (Chi and Zhao 2003).
Protease assay
Protease activity was measured by the method of Kembhavi et al. (1993) using casein as a substrate. Half milliliter of the enzyme, suitably diluted, was mixed with 0.5 ml of 100 mM Tris-HCl (pH 8.5) containing 1% (w/v) casein and incubated for 10 min at 50 °C. The reaction was stopped by adding 0.5 ml trichloroacetic acid (20%; w/v). The mixture was allowed to stand at room temperature for 15 min and then centrifuged at 10,000×g for 15 min to remove the precipitate. The soluble fraction was estimated at 280 nm. A standard curve was generated using the solutions of 0–50 mg/l tyrosine. One unit of protease activity was defined as the amount of enzyme required to liberate 1 μg of tyrosine per minute under the experimental conditions used.
Plackett–Burman design
The Plackett–Burman design is an efficient way to screen the main physicochemical parameters, required for the elevated protease production, among a large number of process variables (Plackett and Burman 1946). The carbon and nitrogen sources, which had been screened earlier, were added to the main culture medium for optimization. The Plackett–Burman method allows the evaluation of N variable in the N +1 experiments; each variable was examined at two levels: −1 for a low level and +1 for a high level. Table 1 illustrated the factors under investigation as well as the levels of each factor used in the experimental design, whereas Table 2 represents the design matrix. ‘Design Expert® 7.0’ Stat-Ease, Inc., Minneapolis, MN, USA, was used to analyze the experimental Plackett–Burman design.
Experimental design
Based on the results of the one-variable-at-a-time experiments and the Plackett–Burman design, the effect of the three factors, MJTP concentration, temperature, and pH, was studied using the response surface methodology. A full factorial experimental design involving 27 experiments with the three variables at three levels (low, middle, and high) was employed to optimize the variable levels for the increase of alkaline protease production. Table 3 shows the coded levels and the corresponding actual values employed.
The experimental results of RSM were fitted via the response surface regression procedure, using the following second-order polynomial equation:
where Y is the predicted response; α 0 is the intercept term; α i is the linear coefficient; αii is the quadratic coefficient; α ij is the interaction coefficients. X i and X j are the levels of the independent variables.
Statistical significance of the coefficients and predicted protease production in the above equation were evaluated by using a linear regression analysis employing the Statistical Package for the Social Sciences Software (SPSS 11). Retention of the significant coefficient terms was based on p values (<0.05). Three-dimensional surface plots were obtained using the MATLAB software to illustrate the main and interactive effects of the independent and combined variables.
Validation of the experimental model
The statistical model was validated with respect to all of the three variables within the design space. A random set of seven experimental combinations were used to study the protease production under the experimental conditions described above.
Results
Selection of carbon and nitrogen sources
A series of experiments were first carried out to study the effects of various simple and complex carbon and nitrogen sources on the protease production by A. clavatus ES1. Cultures were first conducted in medium M1 containing the different carbon sources, each added at a concentration of 5 g/l. Protease activity was produced at a high level in the presence of complex organic carbon sources. The maximum levels were carried out with MJTP (203 U/ml) followed by a hulled grain of wheat (148 U/ml). MJTP was tested as a carbon source since it contained a high starch content (32.6 ± 2%). The easily assimilated simple carbon sources like sucrose, glucose, maltose, and starch resulted in a weak alkaline protease production, 77, 63, 125, and 56 U/ml, respectively.
In general, both organic and inorganic nitrogen sources were used efficiently for the protease production. In the present study, different organic (yeast extract, casein peptone, casein, and SHVF) and inorganic (ammonium sulfate, sodium nitrate) nitrogen sources, at a concentration of 2 g/l, were tested in the M1 medium containing MJTP at 5 g/l as carbon source. Higher protease production level was obtained with SHVF (298 U/ml) as the nitrogen source compared to casein peptone, yeast extract, and casein (237, 210, 186 U/ml, respectively). Nevertheless, SHVF could also be used as carbon source by the strain. Ammonium sulfate and sodium nitrate, as inorganic nitrogen sources, showed weak alkaline protease production levels, 106 and 130 U/ml, respectively.
Among the various carbon and nitrogen sources tested, MJTP and SHVF were found to be the most suitable substrates for the production of alkaline protease. Thus, these substrates were selected for further optimization steps.
Plackett–Burman design
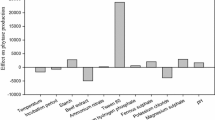
Plackett–Burman design was used to screen the importance of 11 factors, mentioned in Tables 1 and 2, on the alkaline protease production by A. clavatus ES1. Statistical analysis of the responses were performed and represented in Table 4. Factors having a confidence greater than 95% (Prob > F ≤ 0.05) were considered to have a significant effect on the response (protease production). From the experimental data analysis, incubation temperature and MJTP with Prob > F ≤ 0.0001 were found to be the most influencing factors in the medium followed by the initial pH (Prob > F= 0.0001). MgSO4.7H2O with Prob > F = 0.043 affected slightly the alkaline protease production. Three of the four significant variables identified, MJTP, pH, and MgSO4.7H2O had a maximum response at level −1, while the temperature exerted positive influence on the enzyme production as indicated by the effect estimated (Table 4).
Optimization by response surface methodology
Based on the Plackett–Burman design, three variables temperature, MJTP, and pH, which significantly influenced the alkaline protease production, were selected for the design of RSM. A total of 27 experiments with different combinations of the selected parameters were performed. The full factorial experimental designs, employed to determine the optimum levels of the three screened factors, are presented in Table 3. Coefficients of the model were evaluated and tested for their significance by linear regression analysis using SPSS 11. The p values are used as a tool to check the significance of each coefficient, which also indicate the interaction strength between each independent variable; the smaller the p values, the bigger the significance of the corresponding coefficient. The non-significant coefficients from Eq. 1 (see “Material and Methods”) were eliminated on the basis of their p values (>0.05). The final response function to predict the protease activity after eliminating the non-significant terms was as follows:
The regression coefficients and the analysis of the variance presented in Tables 5 and 6 indicate the high significance of the model. The highest R 2 value (0.98) showed also the good agreement between the experimental results and the theoretical values predicted by the model (Weisberg 1985). Results revealed that linear and quadratic terms of the temperature and MJTP had a significant effect on the protease production (p < 0.05). However, the linear effect of pH and interactive effect of temperature and pH were less significant than the other factors with p < 0.034 and p < 0.040, respectively.
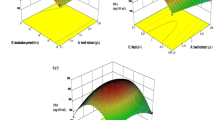
The three-dimensional (3-D) response surfaces were plotted on the basis of the model equation to investigate the interaction among variables and to determine the optimum concentration of each factor for maximum protease production by ES1 strain. The response surfaces shown in Fig. 1 were based on the final model, holding one variable constant at its optimum level, while the other two within their experimental range. The effects of varying the pH and one of the other variables are shown in Fig. 1a,b, which demonstrate that the response surfaces for the two combinations were similar to each other. Protease production varied significantly upon changing the initial MJTP concentration and the temperature. The three-dimensional plots (Fig. 1) show that the increase in concentration of MJTP or temperature cause an increase in the protease production to optimum values, whereas, further increase leads to the decrease of enzyme production.
The 3-D response surface corresponding to the combined effect of temperature and MJTP (Fig. 1c) had an elliptical contour suggesting that not only there were well-defined optimum operating conditions but also the interaction effect between the two factors was significant (Dutta et al. 2004).
Model validation
The model was verified for the three variables within the design space. A random set of seven production combinations were prepared and tested for the protease production (Table 7). The experimentally determined production values were found to be in good agreement with the statistically predicted ones (R 2 = 0.97), confirming the model’s authenticity. In addition, the average error (difference between observed and predicted value) is close to zero, indicating the absence of bias in the model’s predictions.
Time course of protease production by A. clavatus ES1
The time courses of protease activity and the growth of A. clavatus ES1 for both the optimized and unoptimized media are shown in Fig. 2. The results show that the biosynthesis of the enzyme was associated with the cell growth. In optimized medium, protease activity increased during the exponential growth phase and reached a maximum activity of 788 U/ml at 72 h. This value was almost near to the actual predicted value (749.9 U/ml). The production of protease decreased slightly at the beginning of the stationary phase, which may be attributed to an autodegradation mechanism (Hoffman and Breuil 2002). However, the maximum protease activity under unoptimized conditions was only 56 U/ml. By optimizing the medium composition and the culture conditions, the production of alkaline bleaching protease was enhanced from 56 to 788 U/ml.
Discussion
The improvement of microbial protease production is the purpose of several investigations. In general, no defined medium has been carried out for the production of alkaline proteases from the different microorganisms. Each strain has its specific requirement in special conditions for maximizing the enzyme production (Gupta et al. 2002b). The effect of environmental conditions on the production of extracellular proteolytic enzymes could play an important role in the induction or repression of the enzyme by using specific compounds (Wang et al. 2007). Alkaline protease production is dependent on the availability of both carbon and nitrogen sources within the medium. Both exert regulatory effects on enzyme synthesis (Chu et al. 1992).
Aspergillus species use a wide variety of substrates for growth and can switch between several different biochemical pathways for the assimilation of these various substrates (Hintz et al. 1995). In this study, the ES1 strain showed the high protease production on MJTP and SHVF, used as carbon and nitrogen sources, respectively. Both organic substrates, prepared in our laboratory, are inexpensive and readily available. In Tunisia, sardinelle (Sardinella aurita) catches were about 13,300 tons in 2002. During processing, solid wastes including heads and viscera constitute 30% of the original material. For M. jalapa, production of tubers was estimated to be 60 tons/ha. Thus, the use of these substrates may result in a cost–effective process. The absence of free sugars in MJTP (data not shown) could be beneficial by avoiding catabolic repression, which is often observed in the extracellular proteases production (Calik and Ozdamar 2001). The obtained results confirm in the earlier works reported that complex carbon and nitrogen sources constitute better substrates for protease production than simple organic substrates such as glucose, which induced catabolic repression (Hanlon et al. 1982; Kole et al. 1988).
During fermentation conditions, the temperature and pH also influence the alkaline protease production. These parameters are well studied with bacteria from the genus Bacillus but with fungi, few studies have been reported. The ES1 strain produced a maximum alkaline protease level at temperature values lower than 30 °C. Most of the fungi showed an optimum temperature for protease production ranging from 28 °C to 30 °C (Chakraborty et al. 1995; Malathi and Chakraborty 1991; Germano et al. 2003). However, A. oryzae NCIM 649, A. fumigatus TKU003, and A. fumigatus Fresenius seem to tolerate the higher temperatures where maximum protease production was achieved at 36 °C, 37 °C, and 42 °C, respectively (Agrawal et al. 2005; Wang et al. 2005; Santos et al. 1996). Furthermore, it has been reported that the enzyme production by fungal strains occurred at medium pH slightly lower than neutrality (pH 4.5 to 6.5; Gupta et al. 2002b). Higher level production of the alkaline protease by A. clavatus ES1 was observed at medium pH 8.0 which is a very important propriety of the strain.
Nowadays, there is a growing acceptance for the use of statistical experimental designs in biotechnology to optimize culture medium components and conditions. Few works were reported on the optimization of culture media, for the production of fungal proteases, using statistical approaches. RSM was used for the optimization of alkaline protease production by A. clavatus ES1. The methodology finds out the optimal conditions in any given system by a set of independent variables over a specific region of interest by establishing the relationship between more than one variable and a given response (Haaland 1989). Among the three significant variables selected by the Plackett–Burman design, MJTP concentration and temperature were found to have the greatest effect on the production of the alkaline protease. For A. clavatus ES1, an overall 14.0-fold increase in protease production was obtained compared to the observed before optimization medium (starch 5 g/l, yeast extract 2 g/l, 30 °C, and pH 6.0). A high degree of similarity was observed between the predicted and experimental values that reflected the applicability of RSM to optimize the process of enzyme production. Maximum protease production was achieved at temperature 30 °C, pH 8.0, 10 g/l MJTP, 2 g/l SHVF, and other salts which were taken at low level as shown in the Plackett–Burman design. By deriving Eq. (2), we can obtain the analytical optimal level of the three factors. We find that these optimal values had the temperature 30.2 °C, 9.4 g/l MJTP, and pH 7.6, which are very close to the rate of the optimal combination as determined experimentally.
Conclusion
The present study is the first contribution towards the use of powder from M. jalapa tubers as a new complex organic substrate for the production of bleach-stable alkaline protease by A. clavatus ES1. In this study, Plackett–Burman design and response surface methodology were employed to optimize the medium and culture conditions for the production of a bleaching stable protease by A. clavatus ES1. The final composition of the optimized medium was as follows: MJTP 10 g/l, SHVF 2 g/l, all salts at low levels in Plackett–Burman matrix, pH 8.0, and temperature of 30 °C. The optimization of the medium resulted in about 14.0-fold higher protease production than that of the unoptimized medium. Moreover, the cost of the medium was decreased sharply since MJTP and SHVF, used as carbon and nitrogen sources respectively, are inexpensive and readily available organic substrates in Tunisia. To our knowledge, this is the first report using the RSM when optimizing the production of a bleaching stable protease by A. clavatus species, which would offer some advantages for the large-scale fermentation.
References
Adinarayana K, Ellaiah P, Srinivasulu B, Bhavani Devi R, Adinarayana G (2003) Response surface methodological approach to optimize the nutritional parameters for neomycin production by Streptomyces marinensis under solid-state fermentation. Process Biochem 38:1565–1572
Agrawal D, Patidar P, Banerjee T, Patil S (2005) Alkaline protease production by a soil isolate of Beauveria felina under SSF condition: parameter optimization and application to soy protein hydrolysis. Process Biochem 40:1131–1136
Banerjee UC, Sani RK, Azmi W, Soni R (1999) Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem 35:213–219
Calik P, Özdamar TH (2001) Carbon sources affect metabolic capacities of Bacillus species for the production of industrial enzymes: theoretical analyses for serine and neutral proteases and a-amylase. Biochem Eng J 8:61–81
Chakraborty R, Srinivasan M, Sarkar SK, Raghavan KV (1995) Production of acid protease by a new Aspergillus niger by solid substrate fermentation. J Microb Biotechnol 10:17–30
Chi Z, Zhao S (2003) Optimization of medium and cultivation conditions for pullulan production by a new pullulan-producing yeast strain. Enzyme Microb Technol 33:206–211
Chu IM, Lee C, Li TS (1992) Production and degradation of alkaline protease in batch cultures of Bacillus subtilis ATCC 14416. Enzyme Microb Technol 14:755–761
de Coninck J, Bouquelet S, Dumortier V, Duyme F, Verdier-Denantes I (2000) Industrial media and fermentation processes for improved growth and protease production by Tetrahymena thermophila. J Ind Microbiol Biotech 24:285–290
Dutta JR, Dutta PK, Banerjee R (2004) Optimization of culture parameters of extracellular protease production from a newly isolated Pseudomonas sp. using response surface and artificial neural network models. Process Biochem 39:2193–2198
Ellouz Y, Bayouth A, Kammoun S, Gharsallah N, Nasri M (2001) Production of protease by Bacillus subtilis grown on sardinelle heads and viscera flour. Bioresour Technol 80:49–51
Germano S, Pandey A, Osaku CA, Rocha SN, Soccol CR (2003) Characterization and stability of proteases from Penicillium sp. produced by solid-state fermentation. Enzyme Microb Technol 32:246–251
Gupta R, Beg QK, Lorenz P (2002a) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Gupta R, Beg QK, Khan S, Chauhan B (2002b) An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol 60:381–395
Haaland PD (1989) Statistical problem solving. In: Haaland PD (ed) Experimental design in biotechnology. Dekker, New York, pp 1–18
Hajji M, Kanoun S, Nasri M, Gharsallah N (2007) Purification and characterization of an alkaline serine-protease produced by a new isolated Aspergillus clavatus ES1. Process Biochem 42:791–797
Hanlon GW, Hodges NA, Russel AD (1982) The influence of glucose, ammonium and magnesium availability on the production of protease and bacitracin by Bacillus licheniformis. J Gen Microbiol 128:845–851
Hintz WE, Kalsner I, Plawinski E, Guo Z, Lagosky PA (1995) Improved gene expression in Aspergillus nidulans. Can J Bot 73:876–884
Hoffman B, Breuil C (2002) Cloning and genetic analysis of subtilases in sap staining fungi. Curr Genet 41:168–175
Johnvesly B, Naik GR (2001) Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem 37:139–144
Kembhavi AA, Kulkarni A, Pant AA (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No 64. Appl Biochem Biotechnol 38:83–92
Kirk O, Borchert TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13:345–351
Kole MM, Draper I, Gerson DF (1988) Production of protease by Bacillus subtilis using simultaneous control of glucose and ammonium concentrations. J Chem Technol Biotechnol 41:197–206
Kumar CG, Takagi H (1999) Microbial alkaline proteases from a bioindustrial viewpoint. Biotechnol Adv 17:561–594
Kunamneni A, Kumar KS, Singh S (2005) Response surface methodology approach to optimize the nutritional parameters for enhance a-amylase. Afr J Biotechnol 4:708–716
Laxman RS, Sonawane AP, More SV, Rao BS, Rele MV, Jogdand VV, Deshpande VV, Rao MB (2005) Optimization and scale up of production of alkaline protease from Conidiobolus coronatus. Process Biochem 40:3152–3158
Malathi S, Chakraborty R (1991) Production of alkaline protease by a new Aspergillus flavus isolate under solid-substrate fermentation conditions for use as a depilation agent. Appl Environ Microbiol 57:712–716
Mitchell DA, Lonsane BK (1992) Definition, characteristics and potential in solid state cultivation. In: Doelle HW, Mitchell SA, Rolz CE (eds) Applied biotechnology series. Elsevier, Amsterdam, pp 1–16
Nehete PN, Shah VD, Kothari RM (1985) Profiles of alkaline protease production as a function of composition of the slant, age, transfer and isolate number and physiological state of culture. Biotechnol Lett 7:413–418
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Santos RMDB, Firmino AAP, de Sá CM, Felix CR (1996) Keratinolytic activity of Aspergillus fumigatus Fresenius. Curr Microbiol 33:364–370
Varela H, Ferrari MD, Belobradjic L, Weyrauch R, Loperena L (1996) Effect of medium composition on the production by a new Bacillus subtilis isolate of protease with promising unhairing activity. World J Microbiol Biotechnol 12:643–645
Wang SL, Chen YH, Wang CL, Yen YH, Chern MK (2005) Purification and characterization of a serine protease extracellularly produced by Aspergillus fumigatus in a shrimp and crab shell powder medium. Enzyme Microb Technol 36:660–665
Wang Q, Hou Y, Xu Z, Miao J, Li G (2007) Optimization of cold-active protease production by the psychrophilic bacterium Colwellia sp. NJ341 with response surface methodology. Bioresour Technol 99:1926–1931
Weisberg S (1985) Applied linear regression. Wiley, New York, p 217
Acknowledgments
We are grateful to Mme Hanen Ghamgui and Mr. Fakher Frikha for their help in the RSM software analysis. We also thank Mr. Zouhaier Bouallagui and Mme Jaouher Zekri for the help with the English writings. This work was financed by the “Ministere de l’enseignement supérieur, de la recherche scientifique et de la technologie, Tunisia”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajji, M., Rebai, A., Gharsallah, N. et al. Optimization of alkaline protease production by Aspergillus clavatus ES1 in Mirabilis jalapa tuber powder using statistical experimental design. Appl Microbiol Biotechnol 79, 915–923 (2008). https://doi.org/10.1007/s00253-008-1508-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1508-0