Abstract
Background and aims
Desert environments are characterized by limited and highly variable rainfall, which is an intermittent source of water critical to the evolution of the structure and functioning of desert ecosystems. The present study was the first to assess the effects of different amounts of rainfall received through discrete rainfall events and of their seasonality on two little-studied desert shrubs, namely Reaumurica soongorica and Nitria sphaerocarpa, in an arid inland river basin in north-western China.
Materials and methods
Pulse rainfall events were simulated at the following magnitudes (amount of water in millimeters per rainfall event): 0, 5, 10, 15, 25, and 40 mm on two days in 2016, 24th June (early growing season) and 4th August (middle growing season), respectively. The rainfall effects were measured in terms of the following parameters: plant uptake of rainwater by plants as assessed by δ18O in the water within plant tissue, pre-dawn plant water potential, and the rates of photosynthesis and stomatal conductance.
Results
N. sphaerocarpa shows faster response to simulated pulse rainfall events than R. soongorica, and the response of both species was quicker in the middle- than in early- growing season. Water uptake increased non-linearly with the amounts of rainfall, reaching a plateau at about 25 mm in R. soongorica and 20 mm in N. sphaerocarpa. The accumulative carbon gain relative to the controlled experiment increased with the amounts of rainfall, the increase being greater in the middle growing season.
Conclusion
Our results clearly show that both the seasonality and the amounts of rainfall in discrete rainfall events took great impacts on response of plant ecophysiological activities and such responses are species dependent. The challenge ahead is to understand the consequences of long-term variability in rainfall for the physiology of desert plants and species dynamics in desert ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a key resource and a driver of plant productivity and ecosystem and dynamics in desert environments (Chesson et al. 2004; Fan et al. 2016; Ivans et al. 2006; Resco et al. 2008; Sala et al. 1988), where rainfall is the primary source of water (Cheng et al. 2006; Huang and Zhang 2016; Yang et al. 2014). Ecosystems in these regions are particularly sensitive to changes in the climate, and many fundamental aspects of their structure and functioning are closely related to spatial and temporal variations in rainfall (Chen et al. 2009; Zhang et al. 2017). A small shift in rainfall pattern can have large impacts on the availability of soil moisture to plants in terms of its season and quantity and on other biogeochemical processes in desert ecosystems (Weltzin et al. 2003). A previous study showed that the precipitation patterns characterized by an increasing rainfall intensity and long dry spells in semi-arid and arid areas of north-western China over the past 40 years (Liu et al. 2005). Earlier studies of arid ecosystems have shown that it is the distribution of rainfall and not so much its total amount that determines species survival and thus the composition, structure, and functioning of plant communities or assemblages (Cheng et al. 2006; Gao et al. 2015; Sher et al. 2004). Our ability to accurately predict and model the dynamics of species distributions in arid ecosystems in response to increasing rainfall variability in the near future depends critically on short-term response of individuals and populations to diverse rainfall events.
Rainfall patterns, which comprise the amount of rainfall, its intensity, and its temporal distribution, strongly affect the dynamics of plant water use during the growing season in arid environments (Cheng et al. 2006; Yang et al. 2014). Different plant species respond differently to such temporal dynamics (Sher et al. 2004). For example, Kulmatiski and Beard (2013) pointed out that without changing the total amount of rainfall, a shift to heavier but fewer rainfall events is likely to favour woody plants, promoting their dominance in grassland ecosystems. Marked differences between the responses of these species in terms of water use to differences in the amount of rain received during single rainfall events are closely linked to the depth of the root systems and the time taken by the roots to respond. Summer rainfall in arid regions of north-western China is characterized by lighter rainfall events and infrequent heavy rainfall events (Yang et al. 2014). The lighter events limit the depth of infiltration, which is too shallow for deep-rooted woody species.
Heavier rainfall events facilitate percolation of moisture to deeper depths, thereby stimulating the physiological processes of woody plants (Fravolini et al. 2005; Lauenroth and Bradford 2009). However, the extent to what soil moisture supplied by heavier rainfall events in summer is used by the woody species ultimately depends on a combination of their water-use strategy, rooting patterns, hydraulic characteristics of soil, and its nutrient status (Fay et al. 2002; Huxman et al. 2004; Potts et al. 2006). Apart from the size of the rainfall events, desert ecosystems also respond strongly to the patterns of water supply—in this case, the seasonality of rainfall events (Ehleringer et al. 1999; Noy-Meir 1973). However, the combined effects of the amount of rainfall per event and the temporal distribution of rainfall events on ecosystem structure and functioning are not well understood so far.
Reaumurica soongorica and Nitria sphaerocarpa are two typical desert shrubs, which are extensively distributed in north-western China. These two species usually co-occur in desert ecosystems, but their composition and structure within the plant community vary considerably, depending on the amounts of rainfall, this variation implies differences in their sensitivity to changes in the pattern of rainfall. Moreover, R. soongorica enjoys a wider distribution than N. sphaerocarpa in north-western China because of the differences in water-use patterns and physiological traits (Zhang et al. 2017). Changes in rainfall pattern can change the species composition of the plant community and may also alter the relationships among members of the community—changes that may, in turn, affect water and carbon exchange in the local ecosystem significantly. An earlier study reported that N. sphaerocarpa showed greater seasonal variation and was more responsive to summer rainfall than R. soongorica was (Zhang et al. 2017). However, limited information on how the water-use strategy of these two coexisting species changes in response to changes in the pattern of rainfall in general, and to the amount of rain received during single rainfall events and their seasonality in particular, in the temperate desert ecosystem of north-western China constrains our understandings of the underlying processes of those ecosystem in responses to shifting rainfall regime.
To address these knowledge gaps, we conducted a field experiment to evaluate the time-course dynamics of water uptake and ecophysiological processes in response to simulated rainfall events that differed in the amount of rainfall and timing in respect to plant growing phase. More specifically, the study sought answers to the following three questions: (1) How do the amount of rainfall received in a single rain event and its date of occurrence affect water uptake by plants? (2) How do those two variables affect other physiological processes in plants? (3) Do the two desert shrubs differ in their responses to rainfall events? Previous studies have shown that plants uptake more irrigation water and performed faster in photosynthesis in summer than in spring (Gebauer and Ehleringer 2000). Therefore, we hypothesize that plants will respond faster to the rainfall pulse and take up more pulse water in the middle growing season (MGS) than that in the early growing season (EGS); the rate of photosynthesis and stomatal conductance will respond more sensitively to plant water status in MGS than in EGS.
Materials and methods
Study site and environmental conditions
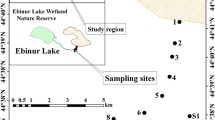
The experiment was conducted in Linze county, which lies in the middle reaches of the Heihe river basin in north-western China (39.4°N, 100.12° E; elevation 1442 m) (Fig. 1a). The study site has a temperate continental desert climate, with an average annual rainfall of 110.3 mm (1965–2011), over 80% of which is received during the growing season (May–September) (Fig. 2a). Most rainfall events measure less than 5 mm, accounting for 42.07% of the total rainfall and 86% of the total number of rainfall events. Events with 5–10 mm contribute 24.83% to the total annual rainfall and account for 8.79% of the number of rainfall events, whereas rain events greater than 15 mm are rare (Fig. 2b). Similar patterns occur in quite different arid regions across the globe (Schwinning et al. 2004; Yang et al. 2014). The mean annual potential evaporation at the site is over 2400 mm; the mean monthly air temperature varies from −11.5 °C in January to 18.5 °C in July; and the mean annual temperature is 7.6 °C. The soil is composed of sand (79.4 ± 0.1%), silt (18.1 ± 0.08%), and clay (2.42 ± 0.04%). Soil organic matter within the top 1 m of soil layer was 42.8 g kg−1and total nitrogen 1.4 g kg−1 (Zhang et al. 2017). This study site is a typical desert region dominated by R. soongorica and N. sphaerocarpa (Fig. S1), the main morphological characteristics of which are given in Table 1.
Distribution of average monthly rainfall: 1961–2011 (a). Error bars represent standard errors of means. Distribution characteristics of various rainfall amounts, 1965–2011(b), rainfall data taken from Yang et al. (2014)
Experimental design and treatments
One week before the treatments, two large plots (50 × 50 m) were selected at random at the study site, containing both R. soongorica and N. sphaerocarpa. Within each large plot, 24 subplots (12 each for R. soongorica and N. sphaerocarpa) were selected and labeled, thus giving a total of 48 subplots, 24 for each species. Each of the 48 subplots was randomly assigned to one of the six treatments (4 subplots for each treatment for each species). The six treatments were as follows: a control (CK), receiving no simulated rain, and five levels of a single simulated rainfall event, namely 5 mm, 10 mm, 15 mm, 25 mm, and 40 mm. To avoid any possible influence of the treatment in the adjacent subplots, each was separated from those next to it by a 3 m gap.
The treatments, namely simulated rainfall in varying amounts, were applied twice during the summer season of 2016: on 24 June to represent an early growing season (EGS) rainfall event and 4 August to represent a middle growing season (MGS) rainfall event. Water for the treatments was taken from a nearby well. We took advantage of the fact that the δ18O value of the well water was quite different from that of any other source of water (soil and natural rainfall) for the plants (the average δ18O value of well water, natural precipitation and soil water was −8.68 ± 0.67‰, −4.7 ± 2.9‰ and 2.83 ± 3.8‰, respectively). Furthermore, an earlier study had shown that neither of the two species at the study site could tap groundwater as a source of water (Zhang et al. 2017). Water for the treatments was pumped from the well into a tank and then applied using a device to simulate rainfall (Fig. 1c). The device is a needle-type artificial rainfall simulator, 1 m × 1 m and 1.5 m deep and made of iron, the bottom of which consists of 760 evenly distributed tiny openings (rainfall systems). The openings are formed by criss-crossing wires, 19 by 20 in one half and 20 by 19 in the other (differing in their orientation), and deliver water droplets uniformly over the treatment area. Water was applied at the rate of less than 1 mm min−1 (slow enough to prevent any run-off from a 1 m2 area around the main stem of each plant in the experiment). The amount of water applied to the target plants was determined based on a combination of the duration and the rate of delivery and confirmed using two standard rain gauges before starting the experiment. To minimize evaporation from soil and to allow full infiltration, the experiment was conducted at night. Leaf gas exchange, Ψpd, and soil water content (SWC) were measured one day before the treatments and on 1, 2, 3, 5, 7, and 11 days thereafter. Plant samples for isotope analysis were collected only on the day prior to the treatment and on 1, 2, and 3 days after it because the isotopic value of the applied water in the soil would have been affected by evaporation over time, leading to that fraction of water uptake that formed part of the simulated rainfall being underestimated.
Collections of plant and soil samples
Plant samples for determining the water sources were collected early in the morning and before carrying out the leaf gas exchange measurements. Pieces of non-photosynthetic and lignified twigs were collected from the lower part of the canopy of both the species. Samples were collected from three plants each time, and these served as three replicates. Immediately after collection, the samples were placed into 15-mL screw-topped glass vials, which were sealed with Parafilm® and stored in a refrigerator (below −18 °C) until required for the isotope analysis. Soil cores were taken from points around the target plant at 10-cm intervals to a depth of 40 cm. Three to four soil cores were taken from each treatment from randomly chosen plants and then pooled before the analysis. Gravimetric water content of well-mixed subsamples was determined by the conventional oven-drying and weighing method and expressed as a percentage by weight: (g water/g soil) × 100.
Measurements of pre-dawn water potential and gas exchange
Pre-dawn water potential (Ψpd) was measured before sunrise (between 0400 and 0600) using a pressure chamber (1515D pressure chamber instrument, PMS Instrument Company, Albany, OR, USA). The measurements were taken immediately after the twigs had been cut off from the plants to minimize any changes in Ψpd due to water loss. Terminal branches with leaves were used for determining the water potential. For each set of measurements, three branches, one from each plant of similar canopy size, were taken for each species.
Leaf gas exchange rates were measured with a portable infrared gas analyzer (LI-6400, LI-COR, Lincoln, NE, USA) under natural field conditions between 0800 and 1000 from three or four plants of each species using mature and healthy leaves or assimilative organs. The assimilative organs or leaves of the two species were labeled with paper tags before the treatments and used repeatedly throughout. At the end of each experiment, leaves with labelled tags were collected for the estimation of leaf area. Then, leaf area was measured with a leaf area meter (LI-3000, LI-COR, Lincoln, NE, USA). Photosynthetically active radiation was set at 1800 μmol m−2 s−1, and relative humidity and carbon dioxide (CO2) concentration in the chamber remained close to their ambient values during the measurements. Simultaneously, we calculated the instantaneous water use efficiency (WUE, μmol mmol−1), define as the ratio of photosynthesis to transpiration, the expression as follows:
where An (μmol m−2 s−1) is the rate of net photosynthesis; E (mmol m−2 s−1) is the rate of transpiration.
Analysis on composition of stable isotopes
Plant water was extracted using the cryogenic vacuum distillation method (Ehleringer et al. 2000; West et al. 2006) and allowing enough time to ensure that water from the xylem was completely extracted. The stable hydrogen and oxygen isotope composition of xylem water and of that from the simulated rainfall was determined by isotopic ratio infrared spectroscopy (IRIS) system (DLT-100 liquid water isotope analyzer, Los Gatos Research, Mountain View, CA, USA). A hydrogen-isotopes fraction is usually observed in halophytes or xerophytes, whereas that of oxygen isotopes is negligible during water uptake (Ellsworth and Williams 2007; Lin et al. 1993), which is why we used only oxygen isotopes to determine the proportion of water from the simulated rain in the total water uptake by plants. The oxygen isotopic composition can be expressed as follows:
where Rsample and Rstandard are the molar isotopic value (18O/16O) of the sample and standard (Standard Mean Ocean Water, or SMOW), respectively. The long-term analytical uncertainty (one standard deviation) was determined as 0.1‰ for δ18O.
To eliminate the effect of contamination with methanol and ethanol, which may be produced during the extraction (West et al. 2010), on the δ18O values of plant water, all results were checked for spectral interference with a Spectral Contamination Identifier (LWIA-SCI) post-processing software package (Los Gatos Research) and corrected by using a standard curve (Schultz et al. 2011). For details of correction procedures and standard curves, see Wu et al. (2014) and Wu et al. (2016). A separate experiment was carried out to test the accuracy of the corrections by comparing the δ18O values from IRIS analysis with corresponding data obtained from an isotope ratio mass spectrometer (Delta V Advantage, IRMS, Thermo Fisher Scientific, MA, USA) before the correction procedures. A total of 21 samples were chosen for the comparison. The mean difference in δ18O between IRIS and IRMS data was 3.13‰ (σ = 2.27) for uncorrected data; after the correction, the mean difference was reduced to 0.14‰ (σ = 0.16). Overall, the comparison showed that the correction had effectively eliminated the influence of organic contamination (methanol and ethanol) on the δ18O value of plant water.
The proportion (%) of pulse water controbution (PWC) in a plant is derived by the following linear mixing model (Cheng et al. 2006; Fravolini et al. 2005):
where δ18Op, δ18Oxi, and δ18Oxcon are the ratios of stable oxygen isotopes in the water used for the treatment, in the water from the xylem of the treated plants, and in the water from the xylem of untreated (CK) plants, respectively, sampled on the same day. Solving this equation for PWC gives:
Data analysis
To evaluate the effects of the amount of rainwater from the treatment on carbon gain and water loss over the course of the observations, the differences between the plants from the treatments and those from the CK in terms of the rates of photosynthesis (An) and stomatal conductance (gs) were determined and then collapsed into a single average of the values from 6 days of observation. Multivariate analysis for repeated measures (MANOVA) was performed to test the significance of the effect of (1) the amount of rainwater applied (the treatments), (2) the species, (3) the season, and (4) the interactions by using An, gs, Ψpd, and PWC as the response variables. Multiple measurements on a given plant species after the treatment represented the repeated variables. A one-way ANOVA followed by post hoc Tukey’s least significant difference (LSD) test at 0.05% level of probability was used for testing whether the differences in SWC, An, gs, Ψpd, and PWC among the different treatments were significant. To test whether An, and gs were correlated to Ψpd (P < 0.05), Pearson’s correlation was performed. All the statistical tests were undertaken using SPSS ver. 17.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Variations in soil moisture
The SWC increased significantly after the simulated rainfall events (Fig. S3). In the EGS rainfall, the mean SWC of the 0–10 cm soil layer increased with increasing amounts of rainfall, and that following the 10–40 mm treatments was significantly higher than that in the control (P < 0.05; Table 2). The mean SWC of the 10–20 cm layer was also significantly higher than that in the control when the amount of rainfall was 15 mm or greater, but the difference was not significant when it was less than 15 mm. The mean SWC of the 30–40 cm layer was significantly higher (P <0.005) than that in the rest of the treatments when the simulated rainfall was 25 mm and 40 mm, indicating that at those amounts, infiltration can reach to a depth 40 cm (Table 2). In the case of the EGS rainfall, the SWC followed a similar pattern, although the mean values were markedly higher.
Response of pre-dawn water potential to simulated rainfall
We evaluated the effect of water treatments on plant water status by comparing Ψpd between controls and water treatments. The pre-dawn water potential (Ψpd) did not increase significantly (P > 0.05) in either of the species when the rainfall was 5 mm but when the amount of rainfall was increased, both the species responded significantly, although the time and magnitude of the response varied with the species and the season (Fig. 3a–d; Table 3). When the rainfall event occurred in EGS, the Ψpd values following 10–40 mm of rainfall peaked on the third day following the event in R. soongorica but on the second day in N. sphaerocarpa (Fig. 3a and b). When the event occurred in MGS, the Ψpd values peaked on the second day in R. soongorica and on the first day in N. sphaerocarpa (Fig. 3c and d).
Changes in pre-dawn water potential Ψpd (MPa) of two desert species in early growing season (a, b) and middle growing season (c, d) under varying amounts (mm) of simulated rainfall. Day 0 is 24th June 2016 in early growing season and is 4th August 2016 in middle growing season. Negative days mean the days before water treatments. Error bars indicate ±1standard deviation
Compared to the control, the extent of increase in Ψpd was greater in N. sphaerocarpa than in R. soongorica following 10 mm and 15 mm of rainfall in EGS and in MGS (P < 0.05). On all sampling days, the average Ψpd in both species was significantly higher at 25 mm and 40 mm of rainfall than that at any of the other treatments (Table 3).
Uptake of simulated pulse water by both species
The uptake of simulated rainwater was influenced by the season, the amount of rain, and the interaction of those two factors, and the two species responded differently to the varying amounts of rainfall depending on the season (Table 4; Fig. 4). In both species, the uptake increased with the amount of rain and then leveled off when the amount exceeded a threshold, although the threshold for R. soongorica (25 mm; Fig. 4a and c) was higher than that for N. sphaerocarpa (20 mm; Fig. 4b and d). The average uptake in both species was significantly higher at 25 mm and 40 mm than that at any of the other treatments and significantly lower in EGS than that in MGS (P < 0.05) (Table 3), suggesting that plants use rainwater more efficiently when it rains in MGS.
The effect of the amount of rain on the uptake of rainwater was similar to that observed in the case of Ψpd, with N. sphaerocarpa responding faster than R. soongorica (Fig. 4a-b), and the response of both species being faster in MGS than that in EGS. Following the rainfall event in EGS, the uptake of rainwater by N. sphaerocarpa at 10–40 mm of rain peaked on the second day and that by R. soongorica peaked on the third day (Fig. 4a and b). However, in MGS, the uptake was maximum on the first day in N. sphaerocarpa and on the second day in R. soongorica (Fig. 4c and d). In both species, PWC and Ψpd were positively correlated, although the slope of the fitted line for the MGS event was steeper than that for the EGS event (Fig. 5a and b).
Pulse effects on photosynthesis
The pattern of the response of An to simulated rainfall more or less follows that of Ψpd, with N. sphaerocarpa responding to rainfall faster than R. soongorica (Fig. 6a–d). In addition, both species respond to rainfall faster in the MGS than in the EGS. The cumulative carbon gain relative to the control increased markedly as the amount of rainfall increased (Fig. 7a and b), and the increase was greater in MGS than in EGS for both species. The increase in N. sphaerocarpa was slighter greater than in R. soongorica (Fig. 7a and b).
Changes in the rate of photosynthetic (An, μmol m−2 s−1) of two desert species in early growing season (a, b) and middle growing season (c, d) under varying amounts (mm) of simulated rainfall. Day 0 is 24th June 2016 in early growing season and is 4th August 2016 in middle growing season. Negative days mean the days before water treatments. Error bars indicate ±1standard deviation
The pre-dawn water potential showed a significant, positive, and linear relationship to An and gs, and the dynamics of these relationships differed with the season and the species (Fig. 8a–d). An in R. soongorica was more responsive to Ψpd in MGS than in EGS (Fig. 8a), whereas gs showed no such difference (Fig. 8c). In N. sphaerocarpa, the rates of all the three parameters were more sensitive to Ψpd in MGS than in EGS (Fig. 8a–d), WUE in R. soongorica showed no significant difference between EGS and MGS (P > 0.05; Fig. 8e) whereas that in N. sphaerocarpa was significantly lower in MGS than in EGS (Fig. 8f). Also, An and gs were more responsive to Ψpd in N. sphaerocarpa than in R. soongorica irrespective of the season; as can be seen in Fig. 8a–d, the slope of the fitted line for N. sphaerocarpa is steeper than that for R. soongorica.
Pre-dawn plant water potential (Ψpd) versus photosynthetic rate (a, b), stomatal conductance (c, d) and water-use efficiency (e, f) of two desert shrubs in early growing season and middle growing season following varying quantities (mm) of simulated rainfall. Data from six treatments. *, P < 0.05; **, P < 0.01;***, P < 0.001
Discussion
The two species showed clear differences in the way their ecophysiology and water uptake were affected by the simulated rainfall in a desert ecosystem in the Heihe river basin. N. sphaerocarpa was more sensitive and responded faster than R. soongorica. Our results also showed that both timing of rainfall and its amount strongly affect water uptake and ecophysiological acitivies, and plants are more sensitive to these two variables and respond to them faster in MGS than in EGS. These responses to the varying amounts of rainfall are discussed below.
Rainfall pulse effects on plant water status
Infiltration of water into soil following a rainfall event changes the level of soil moisture (Reynolds et al. 2004) and leads to distinct physiological responses by plants. Generally, heavier rainfall means that water percolates deeper, whereas light rainfall may be able to wet only the top few centimeters of soil, and most of that moisture is soon returned to the atmosphere through direct evaporation. In the present study, 5 mm of rain led to only a temporary increase in soil moisture in the top (0–10 cm) layer (Fig. S3), and most of that moisture must have been soon lost through evaporation. In other words, this small amount of rain failed to improve the status of soil moisture and had little effect on photosynthesis in both species. This lack of effect suggests a lack of physiologically active fine roots in the upper 10 cm, which limits the plant’s ability to take up rainwater before it evaporates (Flanagan et al. 1992; Mitra et al. 2016). Ψpd reflects the amount of moisture available in the rhizosphere and thus is a reliable indicator of water stress and of the plant’s response to irrigation (Resco et al. 2008). Our results are consistent with those reported earlier, showing that less than 5 mm of rain is too meager to elicit a physiological response, especially for deep-rooted woody shrubs in water-limited environments (Schwinning and Sala 2004; Schwinning et al. 2003). However, as little as 1 mm of rain can increased sap flow significantly in N. sphaerocarpa growing in desert regions (Zhao and Liu 2010). The delayed response of Ψpd may be due to the time it takes for rainwater to reach the root system and the time it takes for the roots to respond. Soil texture is critical to the rate and depth of infiltration following rainfall: water percolates faster in coarse soils than in fine-textured soils (Fravolini et al. 2005). The distribution of roots is also critical in determining the time for the plant to respond (Golluscio et al. 1998; Noy-Meir 1973). In this study, the soil texture is almost the same, and the relative root distribution between N. sphaerocarpa and R. soongorica is also very similar, with subtle differences in absolute depth and mass (Fig. S4). Thus, the difference in the time lag and in the rainfall threshold between the two species is most likely due to the difference in their active fine root distribution. This lagged response of plants to rainfall pulse has also been found in many other ecosystems (Reynolds 2004; Loik 2007; Burgess 2006), and the lag could range from a few days to a few weeks. For example, Artemisia tridentata and Purshia tridentata in the Great Basin Desert responded to summer rainfall pulses or events of different magnitudes within about 2 days (Loik 2007), whereas the hemi-parasitic species Nuytsia floribunda in southern Australia required over 2 weeks to respond fully (Burgess 2006).
Difference in pulse water uptake between the species and seasons
Our results showed that uptake of rainwater for both species increased non-linearly with the increase in the amount of rainfall, and Cheng et al. (2006) reported a similar response pattern in Cynanchum komarovii, in which the uptake remained constant when rainfall exceeded 14 mm in a semi-arid steppe ecosystem. The explanation for this response lies in the two-tier root system of most plant species in dry environments: a network of branched near-surface roots to absorb water from the surface layers and a deeper tap root system for the deeper layers (Kulmatiski et al. 2006; Nie et al. 2011). This enables plants to keep drawing water from the deeper layers even if water is plentiful in the upper layers (Lin et al. 1996). In the present experiment, the simulated rainfall pulse of 40 mm limited the percolation to 70 cm, whereas 20%–40% of xylem water was reported to be derived from depths greater than 80 cm (Zhang et al. 2017).
We found that the two species used pulse water more efficiently in MGS than in EGS, probably because of more active fine roots that can actually take up pulse water in the MGS in both species. In EGS, root growth or activity may be restricted by low water availability in the upper layer due to lack of rainwater recharge; however, greater soil moisture (Fig. S3) and better plant water status (Fig. 3) before the watering experiments in the MGS, the roots system may be stimulated and/or new fine roots grow in the surface soil layers, thereby enabling it to absorb pulse water more efficiently (Padilla et al. 2013). This result highlights the importance role of antecedent soil water on water uptake dynamics in these desert ecosystems, as it can amplify or diminish the effects of a pulse (a rainfall event) on plant response (Reynolds et al. 2004). These findings agree closely with those of Gebauer and Ehleringer (2000), who irrigated five dominant shrub species in south Utah during different seasons and, based on stable isotope data, concluded that all the species used less than 10% of the water from the simulated rainfall in spring but nearly all of it in late summer. Species occurring in arid areas tend to remain semi-dormant in dry periods, and then maximize water uptake when it is abundant in large pulses (Williams et al. 1998; Gebauer and Ehleringer 2000; Schwinning et al. 2003). This feature may promote the uptake of water and nutrients, thereby improving growth under such intermittent pulses of rain.
Effects of seasonality and amount of rain on photosynthesis
Our results demonstrate that N. sphaerocarpa responds to large rainfall pulses more actively in terms of ecophysiology. The two species differed in their capacity to increase An following rainfall, a difference that can be attributed to the difference in Ψpd because An and Ψpd are highly correlated (Fig. 8a and b). The effects of water stress on An were mainly due to the effects on gs, because stomatal behavior controls the flux of CO2 into leaves and thus determines the rate of photosynthesis (Bai et al. 2015; Farquhar and Sharkey 1982; Miner et al. 2017). The close correlation between An and gs also provides robust evidence to support this idea (Fig. 9a and b). Cumulative daytime carbon gain relative to control was greater in MGS than in EGS, especially in N. sphaerocarpa (Fig. 7a and b), indicating that the species could use rain pulse more efficiently from growing season rains for growth in MGS. This ability is crucial to plant survival and growth in this region because a majority of rainfall events occur at MGS (Fig. S2). In addition, air temperature between EGS and MGS was slighter different, and this may not lead to difference in activity of enzymes in the photosynthetic apparatus, and thus the rate of photosynthesis. Therefore, this effect may contributed little to the difference in the rate of photosynthesis between EGS and MGS. Moreover, the slopes of the fitted line (An v.s gs) for both species were almost same between EGS and MGS, indicating that the difference in carbon gain between EGS and MGS is mainly driven by gs.
Implications of variability in rainfall
Earlier studies predicted that heavier rainfall events would favour deep-rooted species over shallow-rooted species in arid ecosystems although the total rainfall in a given year remains unchanged (Kulmatiski and Beard 2013; Sala and Lauenroth 1982). Our hypothesis predicts that both R. soongorica and N. sphaerocarpa would be favored by such a shift although N. sphaerocarpa would be favored more because the increase in the cumulative daytime carbon gain as the amount of rainfall increased was significantly greater in N. sphaerocarpa than in R. soongorica (Fig. 7a and b). In arid regions, statistical analyses of rainfall patterns showed that the divergence between wet and dry years is marked primarily by the presence or absence of heavier spells of rain (typically greater than 15 mm), rather than by the total number of events (Sala 1992). Thus, a shift to heavier events implies either a longer growing season and greater annual rainfall (which would favour species such as N. sphaerocarpa that can use summer rainfall more efficiently) or greater disparity between the wet years and dry years– both becoming more extreme – as supported by both climate models and observational data (Ehleringer et al. 1991; IPCC 2013; Schwinning et al. 2003). The potential changes in the pattern of rainfall will have great consequence on water and carbon exchange of the desert ecosystems (Fig. 10). However, the potential impact of increased variation in inter-annual rainfall on plant community is difficult to assess based on short-term experiments, because such experiments need to consider many more traits, going far beyond root distribution and adjustments in leaf physiology to include dormancy and reproductive strategies (Schwinning et al. 2003). Therefore, our future work will focus on the effects of long-term rain events on plant performance and the composition of plant communities in desert regions.
Conclusions
The present study clearly showed that the magnitude of ecophysiological responses to varying amounts of summer rainfall depends on the interactions between the amounts and timing of the rainfall, and the species. Rainfall less than 5 mm had no significant effect on water status or photosynthesis in either species, whereas rainfall events greater than 5 mm resulted in larger changes in the amount of soil moisture and its duration and in photosynthesis following rewetting. The time lag between the event and its effects was shorter in N. sphaerocarpa than in R. soongorica and shorter in both species in MGS than in EGS. The uptake of rainwater was much greater following the MGS rainfall than that of the EGS rainfall in both species irrespective of the amount of rainfall. Similarly, photosynthesis and stomatal conductance were more sensitive to plant water status in MGS than in EGS. Water use efficiency was significantly lower in MGS than in EGS in N. sphaerocarpa, but not in R. soongorica. The challenge ahead is to understand the consequences of long-term variability in rainfall for the physiology of desert plants and species dynamics in desert ecosystems.
References
Bai Y, Zhu G, Su Y, Zhang K, Han T, Ma J, Wang W, Ma T, Feng L (2015) Hysteresis loops between canopy conductance of grapevines and meteorological variables in an oasis ecosystem. Agric For Meteorol 214:319–327
Burgess SSO (2006) Measuring transpiration responses to summer precipitation in a Mediterranean climate: a simple screening tool for identifying plant water-use strategies. Physiol Plant 127:404–412
Chen SP, Lin GH, Huang J, Jenerette GD (2009) Dependence of carbon sequestration on the differential responses of ecosystem photosynthesis and respiration to rain pulses in a semiarid steppe. Glob Chang Biol 15:2450–2461
Cheng X, An S, Li B, Chen J, Lin G, Liu Y, Luo Y, Liu S (2006) Summer rain pulse size and rainwater uptake by three dominant desert plants in a desertified grassland ecosystem in northwestern China. Plant Ecol 184:1–12
Chesson P, Gebauer RL, Schwinning S, Huntly N, Wiegand K, Ernest MS, Sher A, Novoplansky A, Weltzin JF (2004) Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141:236–253
Ehleringer JR, Phillips SL, Schuster WS, Sandquist DR (1991) Differential utilization of summer rains by desert plants. Oecologia 88:430–434
Ehleringer J, Schwinning S, Gebauer R (1999) Water use in arid land ecosystems. In: Press MC, Scholes JD, Barker MG (eds) Physiological plant ecology. Blackwell, Oxford, pp 347–368
Ehleringer JR, Roden J and Dawson TE (2000) Assessing ecosystem-level water relations through stable isotope ratio analyses. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, p 181–198
Ellsworth PZ, Williams DG (2007) Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant Soil 291:93–107
Fan Y, Li X, Wu X, Li L, Li W, Huang Y (2016) Divergent responses of vegetation aboveground net primary productivity to rainfall pulses in the inner Mongolian plateau, China. J Arid Environ 129:1–8
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Fay PA, Carlisle JD, Danner BT, Lett MS, McCarron JK, Stewart C, Knapp AK, Blair JM, Collins SL (2002) Altered rainfall patterns, gas exchange, and growth in grasses and forbs. Int J Plant Sci 163:549–557
Flanagan L, Ehleringer JR, Marshall J (1992) Differential uptake of summer precipitation among co-occurring trees and shrubs in a pinyon-juniper woodland. Plant Cell Environ 15:831–836
Fravolini A, Hultine KR, Brugnoli E, Gazal R, English NB, Williams DG (2005) Precipitation pulse use by an invasive woody legume: the role of soil texture and pulse size. Oecologia 144:618–627
Gao R, Yang X, Liu G, Huang Z, Walck JL (2015) Effects of rainfall pattern on the growth and fecundity of a dominant dune annual in a semi-arid ecosystem. Plant Soil 389:335–347
Gebauer RL, Ehleringer JR (2000) Water and nitrogen uptake patterns following moisture pulses in a cold desert community. Ecology 81:1415–1424
Golluscio R, Sala O, Lauenroth W (1998) Differential use of large summer rainfall events by shrubs and grasses: a manipulative experiment in the Patagonian steppe. Oecologia 115:17–25
Huang L, Zhang Z (2016) Effect of rainfall pulses on plant growth and transpiration of two xerophytic shrubs in a revegetated desert area: Tengger Desert, China. Catena 137:269–276
Huxman TE, Cable JM, Ignace DD, Eilts JA, English NB, Weltzin J, Williams DG (2004) Response of net ecosystem gas exchange to a simulated precipitation pulse in a semi-arid grassland: the role of native versus non-native grasses and soil texture. Oecologia 141:295–305
IPCC (2013) The physical science basis. In: Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, USA
Ivans S, Hipps L, Leffler AJ, Ivans CY (2006) Response of water vapor and CO2 fluxes in semiarid lands to seasonal and intermittent precipitation pulses. J Hydrometeorol 7:995–1010
Kulmatiski A, Beard KH (2013) Woody plant encroachment facilitated by increased precipitation intensity. Nat Clim Chang 3:833–837
Kulmatiski A, Beard KH, Stark JM (2006) Exotic plant communities shift water-use timing in a shrub-steppe ecosystem. Plant Soil 288:271–284
Lauenroth WK, Bradford JB (2009) Ecohydrology of dry regions of the United States: precipitation pulses and intra-seasonal drought. Ecohydrology 2:173–181
Lin G, Sternberg L, Ehleringer J, Hall A, Farquhar G (1993) Hydrogen isotopic fractionation by plant roots during water uptake in coastal wetland plants. In: Ehleringer JR, Hall AE, Farquhar GD (eds) Stable isotopes and plant carbon-water relations. Academic Press Inc., New York, pp 497–510
Lin G, Phillips SL, Ehleringer JR (1996) Monosoonal precipitation responses of shrubs in a cold desert community on the Colorado plateau. Oecologia 106:8–17
Liu B, Xu M, Henderson M and Qi Y (2005) Observed trends of precipitation amount, frequency, and intensity in China, 1960–2000. J Geophys Res 110:D08103
Loik ME (2007) Sensitivity of water relations and photosynthesis to summer precipitation pulses for Artemisia tridentata and Purshia tridentata. Plant Ecol 191:95–108
Miner GL, Bauerle WL, Baldocchi DD (2017) Estimating the sensitivity of stomatal conductance to photosynthesis: a review. Plant Cell Environ 40:1214–1238
Mitra B, Mackay DS, Ewers BE, Pendall E (2016) Response of sagebrush carbon metabolism to experimental precipitation pulses. J Arid Environ 135:181–194
Nie YP, Chen HS, Wang KL, Tan W, Deng PY, Yang J (2011) Seasonal water use patterns of woody species growing on the continuous dolostone outcrops and nearby thin soils in subtropical China. Plant Soil 341:399–412
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–51
Padilla FM, Aarts BH, Roijendijk YO, de Caluwe H, Mommer L, Visser EJ, de Kroon H (2013) Root plasticity maintains growth of temperate grassland species under pulsed water supply. Plant Soil 369:377–386
Potts D, Huxman T, Scott R, Williams D, Goodrich D (2006) The sensitivity of ecosystem carbon exchange to seasonal precipitation and woody plant encroachment. Oecologia 150:453–463
Resco V, Ignace D, Sun W, Huxman TE, Weltzin JF, Williams DG (2008) Chlorophyll fluorescence, predawn water potential and photosynthesis in precipitation pulse-driven ecosystems–implications for ecological studies. Funct Ecol 22:479–483
Reynolds JF (2004) Plant responses to precipitation in desert ecosystems: integrating functional types, pulses, thresholds, and delays. Oecologia 141:282–294
Reynolds JF, Kemp PR, Ogle K, Fernández RJ (2004) Modifying the ‘pulse–reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141:194–210
Sala OE (1992) Long-term soil water dynamics in the shortgrass steppe. Ecology 73:1175–1181
Sala OE, Lauenroth W (1982) Small rainfall events: an ecological role in semiarid regions. Oecologia 53:301–304
Sala OE, Parton WJ, Joyce L, Lauenroth W (1988) Primary production of the central grassland region of the United States. Ecology 69:40–45
Schultz NM, Griffis TJ, Lee X, Baker JM (2011) Identification and correction of spectral contamination in 2H/1H and 18O/16O measured in leaf, stem, and soil water. Rapid Commun Mass Spectrom 25:3360–3368
Schwinning S, Sala OE (2004) Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141:211–220
Schwinning S, Starr BI, Ehleringer JR (2003) Dominant cold desert plants do not partition warm season precipitation by event size. Oecologia 136:252–260
Schwinning S, Sala OE, Loik ME, Ehleringer JR (2004) Thresholds, memory, and seasonality: understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 141:191–193
Sher AA, Goldberg DE, Novoplansky A (2004) The effect of mean and variance in resource supply on survival of annuals from Mediterranean and desert environments. Oecologia 141:353–362
Weltzin JF, Loik ME, Schwinning S, Williams DG, Fay PA, Haddad BM, Harte J, Huxman TE, Knapp AK, Lin G (2003) Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 53:941–952
West AG, Patrickson SJ, Ehleringer JR (2006) Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun Mass Spectrom 20:1317–1321
West AG, Goldsmith GR, Brooks PD, Dawson TE (2010) Discrepancies between isotope ratio infrared spectroscopy and isotope ratio mass spectrometry for the stable isotope analysis of plant and soil waters. Rapid Commun Mass Spectrom 24:1948–1954
Williams KJ, Wilsey BJ, McNaughton SJ, Banyikwa FF (1998) Temporally variable rainfall does not limit yields of Serengeti grasses. Oikos 81:463–470
Wu Y, Zhou H, Zheng XJ, Li Y, Tang LS (2014) Seasonal changes in the water use strategies of three co-occurring desert shrubs. Hydrol Process 28:6265–6275
Wu H, Li X-Y, Jiang Z, Chen H, Zhang C, Xiao X (2016) Contrasting water use pattern of introduced and native plants in an alpine desert ecosystem, Northeast Qinghai–Tibet plateau, China. Sci Total Environ 542:182–191
Yang Q, Zhao W, Liu B, Liu H (2014) Physiological responses of Haloxylon ammodendron to rainfall pulses in temperate desert regions, northwestern China. Trees 28:709–722
Zhang C, Li X, Wu H, Wang P, Wang Y, Wu X, Li W, Huang Y (2017) Differences in water-use strategies along an aridity gradient between two coexisting desert shrubs (Reaumuria soongorica and Nitraria sphaerocarpa): isotopic approaches with physiological evidence. Plant Soil 419(1–2):169–187
Zhao W, Liu B (2010) The response of sap flow in shrubs to rainfall pulses in the desert region of China. Agric For Meteorol 150:1297–1306
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant NSFC 91425301, 41730854), the Strategic Priority Research Program of Chinese Academy of Sciences (grant number XDA20100102), the PCSIRT (IRT-15R06), and projects supported by the State Key Laboratory of Earth Surface Processes and Resource Ecology and by Ten Thousand Talent Program for leading young scientist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Lucas Silva.
Electronic supplementary material
ESM 1
(DOCX 2623 kb)
Rights and permissions
About this article
Cite this article
Zhang, C., Li, XY., Wang, Y. et al. Responses of two desert shrubs to simulated rainfall pulses in an arid environment, northwestern China. Plant Soil 435, 239–255 (2019). https://doi.org/10.1007/s11104-018-3892-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3892-2