Abstract
Semiarid areas in the US have realized extensive and persistent exotic plant invasions. Exotics may succeed in arid regions by extracting soil water at different times or from different depths than native plants, but little data is available to test this hypothesis. Using estimates of root mass, gravimetric soil water, soil-water potential, and stable isotope ratios in soil and plant tissues, we determined water-use patterns of exotic and native plant species in exotic- and native-dominated communities in Washington State, USA. Exotic and native communities both extracted 12 ± 2 cm of water from the top 120 cm of soil during the growing season. Exotic communities, however, shifted the timing of water use by extracting surface (0–15 cm) soil water early in the growing season (i.e., April to May) before native plants were active, and by extracting deep (0–120 cm) soil water late in the growing season (i.e., June to July) after natives had undergone seasonal senescence. We found that δ 18O values of water in exotic annuals (e.g., −11.8 ± 0.4 ‰ for Bromus tectorum L.) were similar to δ 18O values of surface soil water (e.g., −13.3 ± 1.4 ‰ at −15 cm) suggesting that transpiration by these species explained early season, surface water use in exotic communities. We also found that δ 18O values of water in taprooted exotics (e.g., −17.4 ± 0.3 ‰ for Centaurea diffusa Lam.) were similar to δ 18O values of deep soil water (e.g., −18.4 ± 0.1 ‰ at −120 cm) suggesting that transpiration by these species explained late season, deep water use. The combination of early-season, shallow water-use by exotic winter-actives and late-season, deep water-use by taprooted perennials potentially explains how exotic communities resist establishment of native species that largely extracted soil water only in the middle of the growing season (i.e., May to June). Early season irrigation or the planting of natives with established root systems may allow native plant restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Much of the semiarid landscapes in western North America have been invaded by exotic plants that appear capable of maintaining site dominance for decades (Kulmatiski In press; Stylinski and Allen 1999). These invasions reduce native species abundance and alter ecosystem function, costing land managers billions of US dollars each year (DiTomaso 2000; Sheley and Petroff 1998). Because soil water limits plant growth in arid and semi-arid regions, it has been suggested that some exotic invaders succeed by altering the timing, location, or extent of soil water use (Dyer and Rice 1999; Holmes and Rice 1996). Differences in soil water use among native plants have provided key insights into species coexistence in native-dominated arid and semi-arid environments (Ludwig et al. 2004; Williams and Ehleringer 2000), but less is known about water-use by exotic plants (Ewe and Sternberg 2002; Holmes and Rice 1996).
Winter-active annual grasses, non-native to western North America, have been suggested to inhibit the establishment of perennial natives by exhausting surface soil resources early in the growing season (Dyer and Rice 1999; Norton et al. 2004; Sheley and Larson 1994). This alone is not a satisfactory explanation for persistence in annual-dominated communities because successional shifts from annual to perennial species are well-documented in many ecosystems (Bonet and Pausas 2004; Foster and Tilman 2000). It has also been suggested that deep-rooted exotics may exercise a ‘taproot advantage’ over native plants, especially native grasses (Marler et al. 1999; Roche and Roche 1998), because they access water that native plants cannot access. This advantage, for example, has been suggested for members of the genus Centaurea, the knapweeds, but rarely tested (Enloe et al. 2004). We hypothesized that exotic communities comprised of annual species that exhaust surface soil resources and taprooted species that exhaust deeper soil resources, prevent the establishment of natives and promote their own persistence.
The temporal and spatial patterns of soil water use by plants can be difficult to monitor, but isotopic analyses of stem and soil water offer a unique means of determining interspecific differences in soil water uptake (Ewe and Sternberg 2002; Williams and Ehleringer 2000). The technique involves comparing the ratio of isotopes of oxygen (O) and/or hydrogen (H) in plant tissue with isotopic ratios in soil water (Dawson and Ehleringer 1991; White et al. 1985). Under field conditions, the technique has been used to differentiate water use by different plant species where two or more discrete source pools (e.g., saturated and unsaturated soils) are present (Ewe and Sternberg 2002; Williams and Ehleringer 2000). Under laboratory conditions, the technique has also been used to differentiate plant water use of surface and deep unsaturated soil water (Yamanaka and Yonetani 1999). In both cases, the isotopic signatures of source pools can differ because light isotopes evaporate more readily than heavy isotopes. As a result, the 18O/16O and the deuterium (D)/H ratios in soil water increase with evaporative water loss (Gazis and Feng 2004; Yamanaka and Yonetani 1999). Thus, in arid and semi-arid regions, evaporation may produce an isotopic profile with soil depth wherein surface soil water is enriched in 18O and D relative to deep soil water.
It may be possible, therefore, to determine the depth of soil water used by a plant by comparing the natural abundance of O and H isotopes (expressed as ∂18O and ∂D) in vascular water from the plant to the natural abundance of O and H isotopes in the soil. These measurements may be susceptible to differences in evaporative losses or transpiration rates among plant species, and to hydraulic redistribution, but should be representative of water source pools used within one to several days prior to sampling (Leffler et al. 2005; Takahashi 1998; Webb and Longstaffe 2003).
Measures of the isotopic signatures of soil and stem water can be used to determine the location and timing of soil water used by individual plants but cannot easily be used to determine the relative volumes of soil water used by plants or plant communities. Relative soil water-use by different plant communities can be estimated from changes in gravimetric soil water storage over time. Soil samples removed to determine gravimetric soil water can also be used to determine root mass and soil water tension.
In this study, we determined if soil water use by exotic plants could potentially explain their success relative to native plants. More specifically, we determined: 1) the timing, depth, and amount of water loss from soils in exotic and native communities using measures of gravimetric soil moisture, 2) if soil water stable isotope ratios changed with depth, and if so, 3) could differences in these ratios be used to distinguish differences in the depth and timing of water-use by the dominant native and exotic species? A study area was chosen that contains adjacent exotic and native communities that are distinct and have been persistent over decadal timescales (Kulmatiski, In press).
Materials and methods
Study site and species
Research was conducted in a shrub-steppe ecosystem, Methow Valley, Washington (WA) (48° 37′N, 107° 10′W) on the Newbon soil series (coarse–loamy, mixed mesic Typic Haploxerolls). Mean annual precipitation was 36 cm between 1909 and 2001. Precipitation is seasonal with nearly 70% falling mostly as snow from October to March (Fig. 1). The growing season typically begins at the end of April and continues until snowfall in mid- to late November, though most native grasses and forbs are seasonally dormant from late June until late April. Several exotic species (i.e., Bromus tectorum L., Centaurea diffusa Lam., Poa bulbosa L., Sissymbrium altissimum L., and S. loeselii L.), that are able to germinate in the fall or early spring, have invaded the study area (Kulmatiski pers. obs.). Precipitation was negligible during the study periods, April through August 2002 and 2003 (Fig. 1). Calculated and observed rates of pan evaporation derived from several meteorological stations in Washington State with similar climate ranged between 104 and 132 cm year−1 (Western Regional Climate Center). The water table is between 30 and 130 m below the soil surface, as suggested by the depth at which water is pumped in active residential wells near the study sites.
There were eight sites used in the study, each consisted of paired tilled and never-tilled fields with similar slope, aspect, and soil series. Tilled fields had been abandoned from agricultural production of alfalfa and wheat between 1955 and 1998. Sites were separated by 5 to 25 km and located between 680 and 880 m in elevation. All abandoned, tilled fields (henceforth, exotic) were dominated by persistent, exotic plant communities and all never-tilled fields (henceforth, native) were dominated by persistent, native-dominated plant communities (Kulmatiski, In press). In June 2002, plant cover by species was determined in 60, 1-m2 quadrats in each of the eight sites. Thirty quadrats were placed in each exotic field and 30 quadrats were placed in each native field. Half of the 30 quadrats in each field type were in transects that were 5 m and half were in transects that were 50 m from the tillage boundary. Quadrats were placed every 5 m in each transect. Plant cover was measured as percent ground cover determined from visual estimation. Visual estimates of percent cover were verified with estimates of plant cover determined from the proportion of times a plant species was observed to intersect 81 points regularly spaced within each of 100 randomly selected quadrats. There was a strong correlation between estimates of species cover determined from the visual estimation and 81-point grid techniques (R 2 = 0.95, P < 0.0001). Data from visual estimates are reported.
Soil and plant isotope analyses
The dominant exotic and native species (C. diffusa and Pseudoroegneria spicata Pursh, respectively) were selected as focal species for the determination of species-level water use. Five of the eight sites were selected that demonstrated a sufficient number of P. spicata in exotic fields and C. diffusa in native fields to allow repeated, destructive sampling of native and exotic individuals that were presumed to be within rooting distance of one another (i.e., < 1 m). Sampling locations within both field types were located where at least three vigorous C. diffusa individuals and one vigorous P. spicata individual could be found within a 1-m2 quadrat. This sampling requirement was chosen because C. diffusa individuals were smaller and more abundant within sampling areas than P. spicata individuals. For both species, three to 10 subsamples of non-transpiring tissues from either one or multiple individual plants were collected from within 3 cm of the soil surface and composited into a single sample vial. In June 2003, non-transpiring tissues were similarly collected from other common species found within the 1-m2 sampling area. For all species, shallow, non-lateral roots and shoot materials that were encased in dead leaf sheaths or other non-photosynthetic materials were collected. For annuals we were more careful to collect from at or below the soil surface than we were from larger, perennial species (i.e., shrubs). It was not possible to produce a balanced sampling design for non-focal species, because all species were not present in all quadrats.
The isotopic composition of soil and plant materials was determined in May and June in both 2002 and 2003. During vegetation sampling a new soil pit was dug in each field on each sampling date. Samples were removed only from freshly excavated soils to minimize fractionation. Soil samples were removed from a range of depths that were expected to include soil water that was plant unavailable (e.g., soil water tension [Ψ] < −1.5 MPa) and plant available (e.g., Ψ > −1.5 MPa). Samples were taken from depths of 15, 30, and 60 cm in May 2002; 10, 20, 30, 60, and 105 cm in May 2003; 15, 30, 60, 120, and 130 cm in June 2002; and 15, 30, 45, 60, 120, and 130 in June 2003. In August, 2003, one field was sampled to 220 cm to estimate the isotopic signature of soil water below the rooting zone. Snow samples were collected from the three accessible sites in April 2003 to estimate the isotopic signature of source water. Rainwater samples were collected from leaves during a small rain event in May 2003.
Samples to be analyzed for δ 18O and δD were immediately placed in 13 × 100 mm borosilicate glass tubes, sealed with a rubber stopper, and wrapped with Parafilm®. Sealed samples were placed in a cooler with dry ice and delivered to a freezer within 5 h. Samples remained frozen until the sampled water was extracted by cryogenic distillation from the sampled medium (i.e., plant tissue or soil). Samples that did not maintain vacuum pressure throughout the extraction procedure were discarded. Water extracts were equilibrated with CO2 and Cr reduction for the analysis of stable O and H isotopes, respectively. Equilibrated samples were analyzed at the University of Arizona Laboratory of Isotope Geochemistry using a Finnigan Delta S mass spectrometer. All isotope values are expressed in delta notation (δ) as the 18O/16O and D/H ratio relative to a standard (Vienna standard mean ocean water). For clarification, δ = [(R sa/R std) −1] x 1000 expressed as “parts per mil” or “‰”, where R = the ratio of heavy to light isotope, sa = sample, and std = standard. Analytical precision (2σ) was 0.08 ‰ for δ 18O and 0.9 ‰ for δD.
Fine-root biomass, gravimetric water, and soil-water potential
Community-level patterns of water use were determined using measures of fine-root biomass, gravimetric water, and soil-water potential. Sampling for these variables did not require the existence of paired exotic and native individuals in each field and, therefore, allowed sampling in all eight sites during the first week in May, June, and July 2003. During each sampling period, at each site, soil pits were dug to 120 cm in the exotic- and native-dominated fields. Bulk soil samples (200–400 g) were collected by hand at 15-cm depth increments from each pit. A soil corer was used to collect four additional samples from the 0–15 cm layer at cardinal points 1 m from each pit. Core samples were collected only in May because soils in the 0–15 cm depths were extremely dry (i.e., Ψ < −15 MPa) in June and July. In August 2003, an additional soil pit was dug and sampled to 220 cm in exotic and native fields at one site.
Each soil sample was immediately sealed in a plastic bag. Within 10 h, samples were weighed and placed in an oven to be dried to a constant weight at 105°C. Oven-dried soils were re-weighed and passed through a 2-mm mesh sieve. Rock (> 2 mm) mass was subtracted from wet and dry soil mass and percent moisture was determined as total water mass loss/dry mass of fine-soil material. Fine-roots were also removed using the 2-mm mesh sieve. Fine-root biomass concentration in the soil was determined as the mass of oven-dried fine roots divided by the oven-dried mass of fine-soil material. Particle size analysis was then performed on sieved 40 g soil sub-samples using the Bouyoucos hydrometer method (Gee and Bauder 1986). Gravimetric moisture and particle size distributions from each sample were used to calculate the water potential for each soil sample using the equation: Ψ = A Θ B, where Ψ = water potential in kiloPascals (kPa), A = exp[a + b(% clay) + c(% sand) 2 + d(% sand)2(% clay)] × 100, B = e + f(% clay)2 + g(% sand)2(% clay), Θ = volumetric soil water content (m3/m3), and where a = −4.396, b = −0.0715, c = −4.880 × 10−4, d = −4.285 × 10−5, e = −3.140, f = −2.22 × 10−3, and g = 3.484 × 10−5 (Saxton et al. 1986).
Gravimetric water contents were converted to volumetric water contents by adjusting for the bulk density of the fine materials. The bulk density of fine materials in 100 soil cores (4 cm diameter and 15 cm length) had previously been determined in exotic and native fields to be 1.18 Mg m−3 (Kulmatiski, unpubl. data). Because soil compaction appeared to increase greatly with depth and because a dense till layer and the C horizon occur between 45 and 60 cm (Lenfesty 1980), the bulk density of fine materials was assumed to increase with depth to 1.23 Mg m−3 in the 15–60 cm strata and 1.28 Mg m−3 in the 60+ cm strata (Heuscher et al. 2005). Thus, estimates of soil-water tension were not determined directly; however, they provide unbiased estimates of water tension for the comparison of exotic and native fields.
Statistical analyses
To compare root mass, δ 18O and δD in soil water, and gravimetric and volumetric soil water, we considered the effects of plant community type (exotic or native), soil depth, and their interactions in May, June, and July (where applicable). The effects of plant community type and depth were assessed using an analysis of variance (ANOVA). The plant community type factor was evaluated in a randomized block design in which each site was a block for two soil pits, one located in an exotic field and the second in a native field. The depth factor was evaluated as a repeated measure; the covariance structure among depth measurements within a pit was modeled using a first-order autoregressive structure. Site and interactions with site (site, site × depth, and site × depth × plant community type) were used as an error term resulting in 105 degrees of freedom: 7 from site, 49 from the site × depth interaction, and 49 from the site × depth × plant community type interactions. Tukey post-hoc pairwise comparisons (t-tests) were used to determine mean differences. Differences in gravimetric moisture between exotic and native fields were also determined using data from four replicate core samples taken from around each pit using a one-way ANOVA. Gravimetric soil moisture and the concentration of root mass in the soil were arcsine transformed prior to analyses to meet model assumptions.
A two-way factorial ANOVA of a split-plot design with whole plots in blocks was used to test for differences in δ 18O and δD values of plant species by plant community type. A one-way ANOVA was used to test for differences in δ 18O and δD values of stem water among plant species. Computations were made using Proc MIXED in SAS/STAT Release 9.1.2. (SAS Institute, Cary, North Carolina).
Results
Vegetation cover
Total ground cover of vegetation in eight exotic fields was 67 ± 5% (mean ± SE). The five most abundant plant species in exotic-dominated fields in descending order of abundance were C. diffusa, a winter-active taprooted biennial forb; P. bulbosa, a winter-active perennial grass; B. tectorum, a winter-active annual grass; Cardaria draba [L.] Desv., a rhizomatous perennial forb; and S. altissimum and S. loeselii, winter-active taprooted annual/biennial forbs (Table 1). The five most abundant species covered 38 ± 4% of the ground in exotic fields. Annual species accounted for 13 ± 3% ground cover and perennials accounted for 53 ± 5% ground cover. Several exotic perennial species began growth in the fall and early spring at least one week prior to the dominant native plant, P. spicata (Kulmatiski pers. obs.). Early-season species, including winter-active perennials and annuals, covered 42 ± 6% of the ground.
Total ground cover in eight native fields was 74 ± 2%. The five most abundant plant species in native fields were P. spicata, a perennial bunchgrass; Balsamorhiza sagittata Pursh., a taprooted perennial forb; Purshia tridentata Pursh., a shrub; Lupinus arbustus Dougl. ex Lindl., L. aridis Dougl., and L. caudatus Kellogg, a taprooted perennial forb; and Artemisia tridentata Nutt., a shrub (Table 1). These five species covered 56 ± 3% of the ground in native fields. Annual species accounted for 1 ± 0% ground cover and perennials accounted for 71 ± 2% ground cover. Early-season species, including winter-active perennials and annuals, covered 7 ± 2% of the ground. Community composition has been reported elsewhere for fields throughout the Methow Valley (Kulmatiski, In press).
Stable isotopes
Isotopic signatures of soil water differed among depths, between exotic and native fields, and between May and June. With the exception of δD in June 2002 (Fig. 2G), soil water was isotopically enriched in δ 18O and δD near the surface relative to deeper soils at every sampling period (Fig. 2). Soil water was also enriched in native fields relative to exotic fields in May 2003 (F1,19 = 6.88, P = 0.017; F1,19 = 5.73, P = 0.027 for δ 18O and δD, respectively; Fig. 2B and F). The same pattern was present, but not significant in May 2002 (F1,14 = 0.55, P = 0.47; F1,14 = 1.48, P = 0.24 for δ 18O and δD, respectively; Fig. 2A and E). In 2003, soil water became depleted of heavy isotopes (i.e., δ values were more negative) between May and June (Fig. 2B versus D and F versus H)(F1,74 = 8.49, P = 0.0047, F1,74 = 39.75, P < 0.001, for δ 18O and δD, respectively).
δ18O (A–D) and δD (E–H) values (± SE) of soil and plant water sampled from 1-m2 plots in exotic and native fields in each of five sites in May and June 2002 and 2003 (N = 5 for each date). The upper portion of each panel shows the isotopic composition of soil water by soil depth in exotic fields (solid lines) and native fields (dashed lines). The lower portion of each panel shows the isotopic composition (± SE) of plant water for P. spicata (PSSP) or C. diffusa (CEDI) in exotic or native fields (N = 5 for each plant in each field type). Different lower case letters in each depth strata or for each plant species/field type combination indicate significant differences at the 0.05 level in an ANOVA

Stem water extracted from the dominant invasive plant, C. diffusa, was depleted in δ 18O relative to stem water from the dominant native plant, P. spicata, in every month sampled: May 2002 (F1,8 = 9.84, P = 0.014; Fig. 2A), June 2002, (F1,8 = 30.96, P < 0.001: Fig. 2C), May 2003 (F1,8 = 35.85, P < 0.001; Fig. 2B), and June 2003 (F1,8 = 25.03, P = 0.001; Fig. 2D). The δD in stem water demonstrated a similar pattern as δ 18O, but these differences were only significant in May and June 2003 (F1,8 = 14.97, P = 0.0047, Fig. 2F; F1,8 = 7.84, P = 0.023, Fig. 2H). Stable isotope ratios of stem water did not differ between exotic and native fields (F1,4 = 0.49, P = 0.52, F1,4 = 0.62, P = 0.47; for C. diffusa and P. spicata, respectively; Fig. 2).
Common plant species growing within 1 m of the focal species were sampled in June 2003. Stem water from these species differed in their δ 18O, but not δD values (Table 1). The native shrub, Artemisia tridentata, demonstrated the least enriched δ 18O values, while the exotic, winter-active perennial grass, P. bulbosa, demonstrated the most enriched δ 18O values. Because this sampling was performed in June, it was difficult to locate annual and winter-active species that had not senesced. As a result, though common on the landscape, only two P. bulbosa and three B. tectorum individuals were sampled.
Mean isotopic composition of the three snow water samples was −15.8 ± 0.1‰ for δ 18O and −124.6 ± 0.7‰ for δD. The three rain water samples were enriched (−9.43 ± 0.13‰ for δ 18O and −108.33 ± 0.88‰ for δD) compared to the snow samples and most soil water suggesting that significant evaporation occurred during the storm event. Local meteoric water lines reported for western Washington and Montana (data not shown), suggest that average source water for the sites is comprised of δ 18O values between −21 and −28, and δD values between −160 and −194 (Corbin et al. 2005; Kendall and Coplen 2001).
Fine-root mass, gravimetric water, water use, and soil-water potential
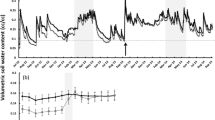
Fine-root mass was lower in exotic than in native fields (F1,5 = 11.01, P = 0.021; Fig. 3) and decreased with depth in both field types (F7,188 = 11.77, P < 0.001). Gravimetric soil moisture was greater in May than June or July (F2,322 = 384.65, P < 0.001; Fig. 4), greater with depth (F7,322 = 52.57, P < 0.001), and greater in exotic than native fields (F1,7 = 5.52, P = 0.051). An interaction between field type and month was found (F2,322 = 9.92, P < 0.001). To isolate the source of this interaction, we tested for differences between field types within each sampling month. There was no effect of field type on gravimetric soil moisture determined from pit samples in May (F1,7 = 0.42, P = 0.54); however, estimates of gravimetric soil moisture determined from replicate core samples (0–15 cm) taken in May revealed that surface soils in exotic fields were drier (0.14 ± 0.01 g g−1) than surface soils in native fields (0.17 ± 0.02 g g−1)(F1,7 = 7.09, P = 0.045). In June, there was more total soil moisture in exotic than native fields (F1,7 = 8.58, P = 0.022), but, by July, this difference had disappeared (F1,7 = 0.63, P = 0.45).
Percent soil moisture (± SE) and soil water potential (± SE) estimated for different soil depths (cm) in (A) May, (B) June, (C) July, and (D) August 2003, Methow Valley, WA, USA. Measurements were taken in eight sites in May, June, and July, and in one site in August (N = 8 for each date × depth combination)
Estimates of gravimetric water and soil particle-size distributions were used to determine the water potential in soils in exotic and native fields for each sampling period (Fig. 4). Across depths, soils in exotic fields contained 73.74 ± 3.02% sand and 10.35 ± 2.71% clay; soils in native fields contained 70.56 ± 3.02% sand and 11.40 ± 2.95% clay. There was no significant difference in the sand or clay content between soils in exotic and native fields (F1,7 = 2.84, P = 0.14; F1,7 = 3.11, P = 0.12, for sand and clay, respectively). Soil-water potentials did not differ between exotic and native fields throughout the growing season (F1,7 = 4.47, P = 0.072), but an interaction between month and field type suggested that there was a difference between field types in at least one of the months sampled (F2,322 = 7.66, P < 0.001). Water potentials did not differ between exotic and native fields in May (F1,7 = 1.91, P = 0.21) or July (F1,7 = 1.68, P = 0.24), but there was a weak difference in June when water potentials were greater in exotic than native soils (F1,7 = 4.91, P = 0.062). Soil-water potentials were also greater with depth (F7,354 = 25.92, P < 0.001). By June, natives had reduced soil moisture to greater depth than exotics. For example, soil-water potential was < −1.5 MPa at depths < −50 cm in native soils but at depths < −40 cm in exotic soils (Fig. 4).
Measures of soil moisture were converted to volumetric estimates of water storage (Table 2). Volumetric estimates of water storage were then used to calculate net soil water loss between months. Soils under native plant communities lost more water between May and June than soils under exotic communities (F1,119 = 13.23, P < 0.001; Table 2). The opposite pattern emerged between June and July: soils under exotic communities lost more water than soils under native communities (F1,119 = 17.33, P < 0.001). By the end of the growing season (May through July), net water loss did not differ between field types (F1,117 = 0.22, P = 0.64).
Discussion
Winter-active plants, such as the annuals, B. tectorum and Lappula echinata Gilib., and the perennial P. bulbosa, covered 42 ± 2 % of the ground in exotic fields, but only 7 ± 1 % in native fields. We predicted that this greater relative abundance of winter-active plants in exotic fields would result in faster transpiration rates and less shallow soil water compared to native fields early in the growing season (i.e., April to May). Although gravimetric soil water determined from pit samples taken the first week of May indicated that winter precipitation equally recharged soil water in exotic and native fields (Fig. 4A), gravimetric soil water determined from a more intensive sampling of shallow soils (0–15 cm), using soil cores, revealed less soil water in exotic (2.48 ± 0.18 cm) than native (3.01 ± 0.36 cm) fields. Drier surface soils in exotic fields early in the growing season supported our prediction that winter-active species common to exotic fields would transpire soil water before plants in native fields. Differences in heat loading, precipitation, or snowmelt between exotic and native fields could explain the presence of dry soils in exotic fields, but we attempted to minimize this effect by selecting fields that were adjacent and had similar slopes and aspects.
Soil moisture data from the core samples, therefore, showed that shallow soils in exotic fields were drier earlier in the growing season than shallow soils in native fields. We used measures of the stable isotopes in plants and soils to identify the plant species that used shallow soil water and the plant species that used deep soil water. Several winter-active species, common to exotic fields, (e.g., Bromus tectorum, L. echinata, and P. bulbosa) had the most enriched δ 18O values compared to all other plants (Table 1) suggesting that these species used a larger proportion of the more enriched shallow soil water than other species (Stratton et al. 2000). Although these winter-active species were sampled only at the beginning of June, it is likely that species that use shallow soil water in June will also use shallow soil water in April and May. Species that demonstrated enriched δ 18O values also demonstrated enriched δD values, however, greater variance in δD values decreased the power of statistical tests to distinguish differences in δD values among plant species.
Additional evidence further supports the conclusion that species with enriched isotopic values were active earlier in the growing season than species with depleted isotopic values. Plants with enriched δ 18O values in stem water are likely to access water early in the growing season because enriched soil water did not appear to be readily plant available by the June or July sampling dates (e.g., water potentials were < −1.5 MPa; Fig. 4). Furthermore, although total root mass was lower in exotic than native communities (Fig. 3), the proportion of fine roots in the top 15 cm of soil was greater in exotic fields. Specifically, 53 ± 3 % of fine-root biomass was located in the top 15 cm of soil in exotic fields, whereas 38 ± 4 % of fine-root biomass was located in the top 15 cm of soil in native fields, showing that exotics dedicate a larger proportion of root mass to resource extraction closer to the soil surface than from deeper soils, as has been observed in other studies (Holmes and Rice 1996).
It is possible that evaporation from growing tissues was greater in winter-active species than in other species (i.e., heavily suberized perennials). Greater evaporation in winter-active species could explain isotopic enrichment of stem water in these species; however, we attempted to address this by sampling only non-photosynthetic, non-transpiring tissues. Furthermore, species composition, phenology, soil moisture, stem water isotopic values, and fine-root distributions all suggested that winter-active plants common to exotic fields transpired shallow soil water before plants in native fields.
However, not all exotics were winter-active species with isotopically enriched stem water. Stem water in the dominant exotic, C. diffusa, was consistently isotopically depleted relative to the dominant native, P. spicata (Fig. 2). In May 2003, for example, δ 18O and δD values of C. diffusa stem water were similar to the values of soil water at −25 cm. At the same time, δ 18O and δD values of P. spicata stem water were similar to the values of soil water at −10 cm. Because differences in δ 18O and δD values between shallow and deeper soils were insufficiently large to be used in two or three pool mixing models (Phillips and Gregg 2003), the results can only be interpreted to suggest that C. diffusa was accessing soil water across a range of soil depths, the average isotopic composition of which was similar to soil water at −25 cm. However, the results clearly suggest that the range of depths from which C. diffusa accessed soil water was comprised of a higher proportion of deeper soil water than P. spicata. The rooting morphologies of these species are consistent with these uptake patterns. Centaurea diffusa, a taprooted forb, demonstrated isotopically depleted values relative to P. spicata, a bunchgrass.
The change in soil-water storage between the first week in May and the first week in June suggested that more water was evapotranspired from native (9.6 ± 1 cm) than from exotic (6.2 ± 1 cm) during that four-week period (Table 2). Long-lived perennials are less common in exotic than native plant communities in this (Kulmatiski, In press) and other ecosystems (Corbin and D’Antonio 2004; Sheley and Petroff 1998). Perennials, which can utilize extensive, lignified, and deep root systems (Fig. 3), could be expected to rapidly extract large volumes of soil water from throughout the soil profile and therefore explain soil water loss in native fields. The δ 18O and δD values could not be used to determine the volume of water extracted by different plant species but did suggest that rapid water use in native communities resulted from a combination of relatively shallow soil water use by P. spicata and relatively deep soil water use by other native species, such as B. sagittata (Table 1). It is important to note, however, that although P. spicata appeared to rely on shallow water relative to other species in native communities, it appeared to rely on deeper soil water relative to the winter-active species common to exotic fields (Table 1). Species composition, phenology, soil moisture, stem-water isotopic values, and fine-root distributions, therefore, suggest that, between the May and June sampling periods, the assemblage of plant species common to native fields transpired soil water from throughout the soil profile before plants in exotic fields.
By July, most forbs and grasses had senesced and annuals had completed their life cycles (Kulmatiski pers. obs.). Only C. diffusa in exotic fields and some shrub species (e.g., A. tridentata) in native fields appeared to be photosynthetically active. It has been suggested that late season growth of C. diffusa and related species could be explained by a ‘taproot advantage’ (i.e., by accessing deep soil water reserves that may not be available to native species) (Enloe et al. 2004; Sheley et al. 1998). The δ 18O values suggested that C. diffusa accessed a higher proportion of deep soil water than the dominant bunchgrass, P. spicata, but not other common native perennials (e.g., B. sagittata). More importantly, measures of gravimetric moisture revealed that deep soil water was more abundant in exotic fields. The success of C. diffusa, therefore, appeared to derive from the ability to grow in exotic communities where shallow soil water is rapidly evapotranspired early in the growing season, but where deep soil water is relatively abundant later in the growing season. Centaurea diffusa may exercise a ‘taproot advantage’, but this advantage appeared to derive from the ability of C. diffusa to grow among winter-active species and not its ability to access deep soil water that is unavailable to native species.
Plant phenologies and water-use patterns provide potential explanations for the presence of exotic- and native-dominated communities on the landscape. More specifically, we suggest that winter-active species in exotic fields inhibit the establishment of native species because exotic species begin to exhaust shallow soil water reserves several weeks before native plants germinate and become photosynthetically active (Kulmatiski pers. obs.). We hypothesize that the combination of shallow, early-season water-use by winter-active species and deep, later-season water-use by taprooted species (i.e., C. diffusa) can explain poor establishment and growth, respectively, of native species in exotic fields. In native fields, which were dominated by perennial species, plants rapidly (Fig. 4) and efficiently (Table 2) used soil water from throughout the soil profile in May (i.e., prior to the June sampling). We hypothesize that this rapid and efficient water-use helps explain the failure of most exotic plants to invade native fields. In support of this hypothesis, Kulmatiski (In press) found that only the winter-active species P. bulbosa invaded native fields over decadal timescales: taprooted exotics were largely unable to invade native fields.
It should be possible to test whether the combination of phenologies found in exotic fields explains the success of exotic species by selectively removing winter-active species or taprooted species. Alternatively, if exotic success is dependent on early-season water-use by winter-active species, a watering treatment could be used to remove this advantage. Similarly, we would predict that native grass establishment would improve in years with abundant spring moisture. If access to soil water is critical for plant success in this semi-arid system, then our data suggest that it should be possible to restore native plants to exotic fields by encouraging native plant root access to soil water that is located below the rooting zone of winter-active species. This is because, once established, native plants appear to be superior competitors for the soil water resource (Table 1). Native plant restoration, however, will require large management efforts, either by transplanting natives with well-established root systems, by intensively removing short-lived competitors, or by irrigating until native plants establish deep root systems.
References
Bonet A, Pausas JG (2004) Species richness and cover along a 60-year chronosequence in old-fields of southeastern Spain. Plant Ecol 174:257–270
Corbin JD, D’Antonio CM (2004) Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology 85:1273–1283
Corbin JD, Thomsen MA, Dawson TE, D’Antonio CM (2005) Summer water use by California coastal prairie grasses: fog, drought, and community composition. Oecologia 145:511–521
Dawson TE, Ehleringer JR (1991) Streamside trees that do not use stream water. Nature 350:335–337
DiTomaso JM (2000) Invasive weeds in rangelands: species, impacts, and management. Weed Sci 48:255–265
Dyer AR, Rice KJ (1999) Effects of competition on resource availability and growth of a California bunchgrass. Ecology 80:2697–2710
Enloe SF, DiTomaso JM, Orloff SB, Drake DJ (2004) Soil water dynamics differ among rangeland plant communities dominated by yellow starthistle (Centaurea solstitialis), annual grasses, or perennial grasses. Weed Sci 52:929–935
Ewe SML, Sternberg LDL (2002) Seasonal water-use by the invasive exotic, Schinus terebinthifolius, in native and disturbed communities. Oecologia 133:441–448
Foster BL, Tilman D (2000) Dynamic and static views of succession: testing the descriptive power of the chronosequence approach. Plant Ecol 146:1–10
Gazis C, Feng XH (2004) A stable isotope study of soil water: evidence for mixing and preferential flow paths. Geoderma 119:97–111
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed), Methods of soil analysis, Part 1. Physical and Mineralogical Methods. Soil Science Society of America, Madison, WI, pp383–411
Heuscher SA, Brandt CC, Jardine PM (2005) Using soil physical and chemical properties to estimate bulk density. Soil Sci Soc Am J 69:51–56
Holmes TH, Rice KJ (1996) Patterns of growth and soil-water utilization in some exotic annuals and native perennial bunchgrasses of California. Ann Bot 78:233–243
Kendall C, Coplen TB (2001) Distribution of oxygen−18 and deuterium in river waters across the United States. Hydrol Processes 15:1363–1393
Kulmatiski A (In press) Exotic plants establish persistent communities. Plant Ecol. DOI: 10.10 07/s112 58–006-914 0-5
Leffler AJ, Peek MS, Ryel RJ, Ivans CY, Caldwell MM (2005) Hydraulic redistribution through the root systems of senesced plants. Ecology 86:633–642
Lenfesty CD (1980) Soil survey of Okanogan county area. National Cooperative Soil Survey, Washington
Ludwig F, Dawson TE, Prins HH T, Berendse F, de Kroon H (2004) Below-ground competition between trees and grasses may overwhelm the facilitative effects of hydraulic lift. Ecol Lett 7:623–631
Marler MJ, Zabinski CA, Wojtowicz T, Callaway RM (1999) Mycorrhizae and fine root dynamics of Centaurea maculosa and native bunchgrasses in western Montana. Northwest Sci 73:217–224
Norton JB, Monaco TA, Norton JM, Johnson DA, Jones TA (2004) Soil morphology and organic matter dynamics under cheatgrass and sagebrush-steppe plant communities. J Arid Environ 57:445–466
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Roche BF, Roche CT (1998) Diffuse Knapweed. In: Sheley RL, Petroff JK (eds) Biology and management of noxious rangeland weeds. Oregon State University Press, Corvallis, pp217–230
Saxton KE, Rawls WJ, Romberger JS, Papendick RI (1986) Estimating generalized soil water characteristics from texture. Soil Sci Soc Am J 50:1031–1036
Sheley RL, Jacobs JS, Carpinelli MF (1998) Distribution, biology, and management of diffuse knapweed (Centaurea diffusa) and spotted knapweed (Centaurea maculosa). Weed Technol 12:353–362
Sheley RL, Larson LL (1994) Comparative life-history of cheatgrass and yellow starthistle- observation. J Range Manage 47:450–456
Sheley RL, Petroff JK (1998) Biology and management of noxious rangeland weeds. Oregon State University Press, Corvallis
Stratton LC, Goldstein G, Meinzer FC (2000) Temporal and spatial partitioning of water resources among eight woody species in a Hawaiian dry forest. Oecologia 124:309–317
Stylinski CD, Allen EB (1999) Lack of native species recovery following severe exotic disturbance in southern Californian shrublands. J Appl Ecol 36:544–554
Takahashi K (1998) Oxygen isotope ratios between soil water and stem water of trees in pot experiments. Ecol Res 13:1–5
Webb EA, Longstaffe FJ (2003) The relationship between phytolith- and plant-water delta O-18 values in grasses. Geochim Cosmochim Acta 67:1437–1449
White JWC, Cook ER, Lawrence JR, Broecker WS (1985) The deuterium to hydrogen ratios of sap in trees: implications for water sources and tree ring deuterium to hydrogen ratios. Geochim Cosmochim Acta 49:237–246
Williams DG, Ehleringer JR (2000) Intra- and interspecific variation for summer precipitation use in pinyon-juniper woodlands. Ecol Monogr 70:517–537
Yamanaka T, Yonetani T (1999) Dynamics of the evaporation zone in dry sandy soils. J Hydrol 217:135–148
Acknowledgements
This research was funded by USDA - NRI (# 35320–13473), the Utah State Agricultural Experimental Station, the Switzer Foundation, and Sigma Xi. We thank the WA Department of Wildlife, especially J. Mountjoy, Rainier Seeds Inc., X. Feng, G. P. Kyle for field assistance, and P. Attaphongse, and L. Prichard for laboratory assistance. We also thank S. Durham for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulmatiski, A., Beard, K.H. & Stark, J.M. Exotic plant communities shift water-use timing in a shrub-steppe ecosystem. Plant Soil 288, 271–284 (2006). https://doi.org/10.1007/s11104-006-9115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9115-2