Abstract
Background and Aims
Many microbes are beneficial to plants and are termed as plant growth promoting bacteria (PGPB). This study explores the effect and mechanism of endophytic bacteria on wheat iron stress.
Methods
Bacteria and wheat seedlings were hydroponically co-cultured under different concentrations of iron. Growth parameters were measured and transcriptions of ferritins as well as transporters were quantified by real-time quantitative PCR.
Results
An endophytic Bacillus altitudinis WR10 was isolated from the root of Triticum aestivum L. The strain is resistant to 5 mM iron and it bioleaches more than 80% iron after 24 h of incubation. Meanwhile, WR10 produces as much as 35pM indole 3 acetic acid (IAA) during fermentation but there was no accumulation of cytokinin (zeatin to be precise). Inoculation of WR10 significantly improves the growth of the primary root and main sprout in wheat seedlings in a co-culture model under iron stress after two weeks hydroponic cultivation. The presence of WR10 up-regulates the expression of many genes encoding ferritins in wheat roots under iron stress.
Conclusions
Besides its ability to bioleach iron, IAA producing B. altitudinis WR10 can alleviate iron stress in wheat by up-regulation of ferritin-encoded genes in roots, which is important for maintaining iron homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) is an essential micronutrient for both plants and animals. Though iron is widely found in soil and aquatic ecosystems, it is also known as a trace element due to its trace presence in the environment (Nagajyoti et al. 2010). As an essential element for plants, iron can be absorbed from both soil and water. In plants, iron performs biochemical and physiological functions in the processes of photosynthesis, chloroplast development and chlorophyll biosynthesis (Briat and Gaymard 2007; Briat et al. 2015). Furthermore, iron is a major constituent of cell redox systems (Schützendübel and Polle 2002). However, high concentrations of heavy metals are also widely regarded as environmental pollutants, and their toxicity is a problem of increasing significance for crop production and animal health (Becker and Asch 2005). In crops, on one hand, iron deficiency has been widely studied as a worldwide nutritional problem (Zuo and Zhang 2011). On the other hand, iron excess is a major threat in the humid tropical regions of Asia, Africa, and South America, where soils are frequently flooded or acidified (Khabaz-Saberi et al. 2010; Guo et al. 2010). Rice yields can be reduced by 12–100% depending on the intensity of iron stress and the tolerance of cultivars (Sahrawat 2004; Fofana 2009; Pereira et al. 2014).
Less significant but still prevalent, is that wheat grain yield is also influenced by iron stress and other factors in above areas, like phosphorus fertilizers and pesticides (Khabaz-Saberi et al. 2012). In hexaploid wheat, iron toxicity mainly occurs in acidic soils (Huang et al. 1995). Toxicity of iron in shoot tissues of wheat genotypes after water-logging of an acidic soil has been observed in Western Australia (Khabaz-Saberi et al. 2006, 2010). The toxicity in wheat was further verified using several acidic soils and two wheat varieties: a strong negative correlation (r = −0.86) was found between Fe concentration and dry weight of shoot after water-logging (http://www.aciar.gov.au/project/CIM/1996/025) (Setter et al. 2009). Furthermore, ammonium-based fertilizers are widely used for improving wheat production. However, repeated use of ammonium-based fertilizers leads to significant soil acidification (Johnson et al. 2010). Considering water-logging and the application of ammonium-based fertilizers are common practices in current agriculture, iron toxicity in wheat is increasingly drawing the attention of researchers and farmers.
Plants evolved to resist iron stress by down-regulation of iron transportation or up-regulation of ferritin which sequesters iron. Iron transportation is mediated by a series of transporters, including iron-regulated transporters 1 (IRT1), NAC transcription factor (NAM-B1), and so on. IRT1 is a member of the ZIP (Zink-regulated, Iron-regulated-like Proteins) metal transporter family and can transport multiple divalent metals (Kim and Guerinot 2007). NAM-B1 increases nutrient remobilization and regulates the translocation of iron from vegetative tissues to grain in wheat (Uauy et al. 2006; Waters et al. 2009). Ferritins are multimeric protein complexes that are composed of several different subunits. Ferritins can effectively control iron homeostasis since each molecule of ferritin sequesters up to 4500 atoms of iron (Briat 1996). Besides protection from iron stress, endophytes and rhizobacteria can have complex effects on plant iron resistance (Bar-Ness et al. 1992; Compant et al. 2010; Freitas et al. 2015). Many microorganisms are able to bioleach heavy metals that are widely present in waste-water, especially in industrial waste-water. This method of bioleaching heavy metals from waste-water, which is a major cause of soil contamination, is cheap and eco-friendly (Alluri et al. 2007; Jing et al. 2007). Therefore, several bacteria species, like Bacillus strains have been isolated and used for their inherent abilities to accumulate and absorb metal ions in water (Kim et al. 2007; Wen et al. 2013). Bacillus strains have also been widely isolated as endophytic plant growth promoting bacteria (PGPB), which improve soil fertility and produce plant hormones, such as indole 3 acetic acid (IAA) and cytokinins (CTKs) (Da Mota et al. 2008; Liu et al. 2013). However, using PGPB to alleviate iron stress and prevents iron toxicity in wheat has not been fully studied. Therefore, this study was designed to (1) characterize an endophytic isolate WR10, (2) alleviate the toxicity of excess iron in wheat seedlings using a co-culture model, and (3) explore the impact of endophytic Bacillus altitudinis WR10 on the expression of ferritins and transporters in wheat.
Materials and methods
Bacterial isolation
Bacterial strains were isolated from the roots of wheat with minor modifications of the protocol used by former researchers (Pereira and Castro 2014). Briefly, healthy winter wheat, Triticum aestivum L. zhoumai 26 grown in the field in Zhoukou city in spring (N33°38′ E114°40′, around 180 days after seeding, namely during grain filling stage) was carefully collected and rinsed in water to clean any attachments. The primary roots, without lesions, were soaked in 70% ethanol for 5 min. Then, it was rinsed again in distilled water. The clean root tissue was cut into 0.5 cm segments. Segments of approximately 0.5 g were ground in 1 mL ddH2O. The fluid was transported into a 1.5 mL tube, and then boiled in water at 100 °C for 5 min. After centrifugation at 2000RCF for 1 min, 0.2 mL of supernatant was removed and plated on the agar of Bacillus megatherium medium (HB8786, Hopebio Com. Ltd., Qingdao) and cultivated at 30 °C for 24 h. The last time rinsed water was collected for plating either Luria-Bertani (LB) or Bacillus megatherium agar. After incubation at 30 °C for 3 days, no visible colony on both control plates confirmed the effectiveness of root sterilization.
Identification of WR10 by staining and phylogenetic analysis
Staining was conducted with Gram staining solutions and Spore Staining Kit (Cat No. G1060 and G1132, Solarbio Com. Ltd., Beijing) according to the instructions and images were acquired with an optical microscope (MV5000, NOVEL) connected to a CCD camera (Mshot, Ningbo). Amplification of 16S rRNA and gyrB gene of strain WR10 was performed with corresponding primers (supplementary Table S1, designed by GenScript Online PCR Primers Designs Tool) in 50 μl-reaction containing100nM dNTPs mix, 3 mM MgCl2, 500 nM each primer in final concentration, 0.5 U of Taq DNA polymerase and 100 ng genomic DNA in 1 × Taq buffer. Genomic DNA was extracted with a TIANamp Bacteria DNA Kit (Tiangen, Cat No. DP302). DNA was quantified by reading Abs.260 nm with a spectrophotometer (Thermo Fisher, NanoDrop 1000). The thermo-cycling parameters are: initial de-naturation at 94 °C for 3 min; 35 amplification cycles of 94 °C de-naturation for 30 s, 55 °C annealing for 30 s, 72 °C elongation for 1.5 min; and a final polymerization step of 72 °C for 5 min with an Eppendorf master thermal cycler. The final PCR product was resolved in 1.0% agarose gel, excised and purified by a Universal DNA Purification Kit (Tiangen, Cat No. DP214). DNA sequencing was performed by ABI 3730 (Genescript, Nanjing) and the sequence was subjected to nucleotide BLAST analysis on the NCBI website (Zhang et al. 2000). The ClustalW software incorporated in the BioEdit was used with the progressive methods for multiple alignments. Phylogenetic trees were calculated by the BioEdit accessory application DNADist using the neighbor-joining method with representative sequences that were extracted from different species of Bacillus spp. (Hall 1999). Twenty 16S rRNA sequences with more than 95% identity and fifteen gyrB sequences with more than 75% identity to WR10 were used for phylogenic trees construction, respectively (details in Table S2 and S3).
IAA and CTK assays
Glycerol stock of B. altitudinis WR10 was sub-cultured in LB broth as described above. Overnight cultivated WR10 was inoculated in 10 mL LB broth with an initial OD600 = 0.01. The tube was incubated at 30 °C, 150 rpm for 24 h. During incubation, samples were collected at different time points. Bacterial production of IAA and cytokinins was assayed by reading absorbance at 450 nm (Abs.450 nm) with a commercial Plant IAA ELISA Kit or Plant CTK ELISA Kit (Bluegene Co. Ltd., Shanghai) using a microplate reader (DNM-9602, CANY Tec, Shanghai).
Iron resistance and bioleaching ability
A single colony of WR10 was picked and propagated in LB broth shaking at 150 rpm, at 30 °C. Growth was monitored by reading OD600. Comparing to growth in LB broth, iron resistance was evaluated by relative growth under different concentrations of FeSO4 (Khabaz-Saberi et al. 2010). The iron concentration in supernatants was determined based on a previous method with minor modification (Dawson and Lyle 1990). Briefly, iron concentration was measured by absorbance of Fe2+ complex at 425 nm at pH 6.0 using sulfosalicylic acid as a reducing agent.
Co-culture of B. altitudinis WR10 and T. aestivum
Co-culture was conducted in 100 mL half-strength Hoagland’s medium (Orhan 2016) in a beaker for 2 weeks at room temperature (25 °C) in humid condition (80%) under dark or light for 12 h every day. As shown in supplementary Fig. S1, 12 beakers were assigned into four groups (NC, Fe2+ 0 mM WR10 -; NC, Fe2+ 5 mM WR10 -; Treat, Fe2+ 0 mM WR10 +; Treat, Fe2+ 5 mM WR10 +). Briefly, each beaker in treated groups was inoculated with 1 mL pre-cultured B. altitudinis WR10 (about 108 cells), while NC groups were not inoculated with WR10. In Fe2+ 5 mM groups, 1 M FeSO4 was added to each beaker to a final concentration of 5 mM. At last, three pieces of 3 mm filter paper (Cat No. 3030–861, Whatman) were placed on the surface of medium and seeded with 10 well pre-germinated T. aestivum grains. Wheat grains were sterilized by soaking in 0.1% AgNO3 solution for 10 min and, then planted in Hoagland’s medium for 3 days germination (Speakman and Krüger 1983). In this model, well-germinated grains of wheat were grown in half-strength Hoagland’s medium containing 106cfu/mL WR10 that are alive but cannot propagate. To confirm bacterial cells are alive and not propagate, 1 mL suspension was removed at day 1, 7, 14, respectively for plating and counting colony-forming units. Analysis of count numbers showed that there is no significant decrease or increase of live cells during two weeks treatment. After the 2 weeks’ co-cultivation, wheat seedlings were collected for the measuring of lengths of primary root and main sprout.
qPCR quantification of genes encoding ferritins and transporters in T. aestivum
After two weeks co-culture under either 0 or 5 mM iron, all shoots and roots in each group were collected separately for total RNA isolation. Total RNA was extracted using TRIzol (Cat No. 15596026, Invitrogen) and reverse transcribed using a SuperScript® III First Strand cDNA Synthesis Kit (Cat No. 18080051, Invitrogen). qPCR was conducted in 10 μL reaction in 8-tube strips with triplicates in a thermo cycler (CFX96, Biorad, USA) using Sybr Green as dye according to MIQE guidelines (Bustin et al. 2009). Parameters were designed according to a previous study (Finatto et al. 2015). Briefly, de-naturation at 94 °C for 30 s, annealing at 55 °C for 10 s, elongation at 72 °C for 30 s, and cycling for 40 cycles. Nine genes were amplified for real-time quantification, including 5 genes encoding ferritin or its subunits (labeled as G1 to G5: TaFER1-A, TaFER1-B, TaFER1-C, TaFER2, TaFER2-B, corresponding accession number is FJ225137, FJ225141, FJ225144, EU143671, and FJ225149), 3 genes encoding potential transporters (labeled as G6 to G8: TaNAM, ZIP, UPP, corresponding accession number is HM027575, AY864924, and HG670306), and one reference gene GAPDH (EU022331). To confirm GAPDH is a suitable reference gene, we compared the expression of GAPDH in both root and sprout under iron treatment in advance and its expression is relatively constant.
Data analysis
All data is mean +/− standard deviation of three experiments. Statistical analysis was performed using a two-way analysis of variance (ANOVA) with Bonferroni post-test correction for multiple comparisons. The difference between means was compared by Tukey’s test. A p-value <0.05 indicated statistical significance. *** P < 0.01, highly significant; * P < 0.05, significant.
Results
Isolation, staining, and identification of endophytic B. altitudinis WR10
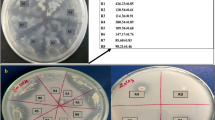
Several Bacillus spp. were isolated from the roots of T. aestivum on a selective agar. Separate colonies with different morphology were randomly picked and propagated in LB broth. One isolate WR10 was used for gram-staining and spore-staining with respective reagents. As shown in Fig. 1a, WR10 cells are short, rod-like, and spores are small, round from a morphological aspect. Furthermore, WR10 seems are gram-positive as cells are stained to grey or blue (data unshown). Further 16S rRNA alignment shows high identity of WR10 to a variety of Bacillus spp. (supplementary Table S2). In particular, the similarity of WR10 to B. altitudinis 41KF2b is 99% with the number of identical 1438 bp/total analyzed 1442 bp (only 4 gaps). Phylogenetic analysis based on both gyrB and 16S rRNA suggests WR10 is closest to B. altitudinis 41KF2b (corresponding sequences are KJ_809604.1 and NR_042337.1 in Fig. 1b and c).
Identification of WR10. (a) Staining of WR10 cells was conducted with a spore-staining kit according to instruction. The image was acquired by a light microscope using a 5.0 MP CMOS camera (MD50). The ClustalW software was used with the algorithm of progressive methods for multiple alignments. After alignment, all gaps were deleted. Phylogenetic trees were constructed by BioEdit using the Neighbor-Joining method for gene gyrB (b) and 16S rRNA (c). There were 992 positions in gyrB, and 1409 positions in 16S rRNA in the final dataset. The bootstraps value is 1000. Italic letters highlight genes from WR10. The detail bacterial names in Fig 1c can be found in supplementary Table S2
IAA and CTK production in B. altitudinis
Plant hormones play leading role in regulation of growth and productivity. It has been reported that many PGPB can produce different kinds of hormones, like IAA and CTK. In this study, production of IAA and CTK in WR10 was assessed using supernatant collected from different time points after inoculation, namely 0, 4, 8, 12, 16, and 24 h. Accumulation of IAA in the supernatants of WR10 shows similar pattern to growth curve (Fig. 2b v.s. Fig. 2a). According to a standard curve, WR10 produces as much as 35pM IAA perking at 12 h that is the late exponential growth phase. In contrast, there is low production of CTK as there is no obvious accumulation during growth (Fig. 2c).
Bacteria growth and hormone production. (a) Growth curve of WR10. Bacteria were grown in 5 mL LB broth shanking at 150 rpm, 30 °C incubator. b Production of IAA in WR10 by ELISA assay. c Production of CTK in WR10 by ELISA assay. A 0.5 mL culture fluid was collected at 0, 4, 8, 12, 16, and 24 h respectively, and supernantants were used for ELISA assay. Values are mean +/− standard deviation of three independent experiments with three technical replicates
Iron resistance and bioleaching ability
Resistance of WR10 to iron was evaluated by a growth inhibition experiment. As shown in Fig. 3a, 0.1-1 mM iron (supplemented as FeSO4) has little impact on the growth of WR10, whereas 5 mM iron clearly inhibits the growth. After 24 h of incubation, WR10 can grow by around 70% compared to its counterpart without iron stress, even when as much as 5 mM iron is applied. To evaluate the iron bioleaching ability of WR10, the remaining iron in the cell free supernatants was assayed. At the beginning, 5 mM iron was added to the medium before inoculation (Fig. 3b, 0 h). After 12 h of incubation, more than 70% of the iron was absent from the medium. The value increased to more than 80% after 24 h of incubation indicating the strong iron bioleaching ability of WR10.
Iron resistance and bioleaching ability. a Assay of iron resistance. Fresh pre-cultures of WR10 were inoculated in triplicate into 5 mL LB broth supplemented with 0, 0.1, 1, 5 mM (final concentration) FeSO4. Growth was measured by reading OD600 at 12 h and 24 h, respectively. Data are mean of percentage relative to samples without adding FeSO4. b Assay of bioleaching ability of WR10. Iron concentration in supernatants from tubes supplemented with 5 mM FeSO4 was determined by reading Abs.425 nm using a spectrophotometer. Data are mean of percentage relative to samples without adding WR10
Co-culture of B. altitudinis WR10 and T. aestivum
A co-culture model was used for probing the potential effects of WR10 on T. aestivum growth under iron stress. As shown in Fig. 4, adding WR10 does not obviously affect wheat seedling growth in the absense of iron (Fig. 4a, Fe2+ 0 mM, WR10 +). While, in groups that were supplemented with 5 mM iron, adding WR10 improves growth (Fig. 4a, Fe2+ 5 mM, WR10 +). Furthermore, there is no significant difference in the mean number of lateral roots among groups co-cultivated with WR 10 and the NC group without iron stress. Co-culture with WR10 increases the mean number of lateral roots from roughly 1 to 4 under 5 mM iron that is detrimental to wheat seedlings (Fig. 4a and supplementary Table S4). Statistical analysis of the lengths of the primary root (Fig. 4b) and main sprout (Fig. 4c) of all seedlings in each group suggests adding WR10 can highly significantly decrease the toxicity of iron on rooting (P < 0.01), and significantly decrease the toxicity of iron on sprouting (P < 0.05).
WR10 alleviates iron toxicity to T. aestivum seedlings. Wheat seedlings were seeded on the surface of filter paper in 100 mL half-strength Hoagland’s medium supplemented with 0 or 5 mM FeSO4. Meanwhile, two groups were inoculated with 1 mL WR10 cells to a final cell density of 106cfu/mL. After two weeks, seedlings were collected and imaged (a). Lengths of the primary roots (b) and main sprouts (c) of wheat seedlings among different groups were compared. Data are mean +/− standard deviation of 10 seedlings. Fe2+ 0 mM, supplemented with 0 mM FeSO4; Fe2+ 5 mM, supplemented with 5 mM FeSO4; WR10 -, without adding WR10; Wr10 +, group inoculated with WR10. Statistical analysis was performed using two-way ANOVA with Bonferroni post-test correction for comparisons. *** Highly significant; * Significant
qPCR quantification of genes encoding ferritins and transporters in T. aestivum
The expression of eight genes that encoding either ferritins (G1-G5) or transporters (G6-G8) in T. aestivum was quantified by qPCR analysis under different conditions. Firstly, without inoculation of WR10, iron has little impact on all tested genes, as their relative expressions are approximately 1–2 in roots (Fig. 5a, in blank columns, for detail data seen supplementary Table S5). In contrast, 5 mM iron up-regulates the expression of all tested genes encoded for ferritins and transporters in wheat sprouts in the absence of WR10 (Fig. 5b, in blank columns). Secondly, under 5 mM iron stress, WR10 up-regulates the expression of all genes encoding ferritins except G5 (FER2-B), with G3 (FER1-C) as high as 15.7-fold in roots. Impacts of WR10 on transporters in roots are limited, as only the expression of G7 (ZIP) gene was down-regulated more than 2-fold (Fig. 5a, in grey columns). In the sprouts, WR10 significantly down-regulates the expression of all genes (Fig. 5b, in grey columns). Totally, WR10 antagonizes the up-regulation effect of iron, which induces the expression of all tested genes in sprout. Furthermore, WR10 mainly enhances the expression of genes encoding ferritins in roots of wheat seedlings.
Effects of WR10 on the expression of genes encoding ferritins and transporters in roots (a) and shoots (b) of wheat under 5 mM iron stress. WR10 -, groups without inoculation of WR10; WR10 +, groups inoculated with WR10. G1, TaFER1-A; G2, TaFER1-B; G3, TaFER1-C; G4, TaFER2; G5, TaFER2-B; G6, TaNAM; G7, ZIP; G8, UPP, unnamed protein product; GAPDH, glyceraldehydes-3-phosphate dehydogenase, which is used as a reference gene. Values are mean +/− standard deviation of three technical replicates and results of one representative of three independent experiments were shown
Discussion
Although iron is abundant in most mineral soils, iron toxicity symptoms only frequently occur under flooded conditions (Becker and Asch 2005). Iron toxicity in plants is related to high Fe2+ uptake by roots and its transportation to the leaves. Excess Fe2+ impairs cellular structure irreversibly and damages membranes, DNA and proteins (de Dorlodot et al. 2005). In tobacco and soybean, iron toxicity is accompanied by reduction of plant photosynthesis and yield (Sinha et al. 1997). In rice, which needs irrigation, iron toxicity is an important problem leading to significant reduction of yields and increasing sensitivity to pathogens (Finatto et al. 2015). In sandy regions, such as Western Australia and South China, water logging is common. In these regions, large portions of agricultural land is given over to seeding wheat, and water-logging often results in increased iron concentration in soil that affects the growth of the wheat (Khabaz-Saberi et al. 2010; Li et al. 2012). In this study, excessive iron toxicity on wheat was confirmed by hydroponic cultivation under different concentrations of FeSO4.
PGPB are beneficial microbes that can enhance plant nutrition via improved nitrogen fixation, phosphate solubilization, or phytosiderophore production (Richardson et al. 2009). PGPB can also protect plants from pathogens, such as inhibition of phytoparasites, and/or induce systemic resistance (Couillerot et al. 2009; Lugtenberg and Kamilova 2009). Furthermore, some PGPB help plants withstand abiotic stresses including heavy metals and other pollutants; some are even able to increase the capacity of plants to sequester heavy metals (Jing et al. 2007; Vacheron et al. 2013). Therefore, utilizing PGPB is a promising approach to facilitate phytoremediation of contaminated soils (Zhuang et al. 2007; Shukla et al. 2011). As they are rarely influenced by environmental factors, such as the condition of soils and nutrients, endophytic isolates are sometimes preferred to rhizobacteria because they might have higher colonization ability and are already adapted to the inner tissues of plants (Franken 2012). Therefore, endophytic strains were isolated from the roots of wheat. Sequencing results of 16S rRNA and gyrB genes verified the strain WR10 as B. altitudinis. Other strains in this species were also isolated recently from wheat rhizosphere by other researchers (Verma et al. 2016). The isolate WR10 characterized in this study possesses strong bioleaching ability to both copper (unpublished results) and iron (as shown in Fig. 3b). To precisely quantify the impact of PGPB on roots and the whole plant remains challenging (Compant et al. 2010). An in vitro co-culture model inoculated roots with PGPB and then monitored the resulting effects on the plant during cultivation. It has been shown that many PGPB increase the number and/or length of lateral roots (Combes-Meynet et al. 2011; Chamam et al. 2013), but may reduce the growth rate of the primary root (Dobbelaere et al. 1999). In line with previous reports, WR10 increases the number and length of lateral roots in the study (Fig. 4a). It is perhaps more interesting that WR10 increases the lengths of both primary root and main sprout under iron stress (Fig. 4b and c). This is in contrast to some previous reports, while it agrees with others that have same phenotypes in PGPB-inoculated plants growing in soil (El Zemrany et al. 2007; Veresoglou and Menexes 2010; Walker et al. 2012).
PGPB are able to produce a wide range of phytohormones, including auxins and/or cytokinins that can interfere with these hormonal pathways in plants. Indole-3-acetic acid (IAA) is the best-characterized auxin produced by many bacteria (Ali et al. 2009). Assay of IAA in the supernatants of WR10 shows that WR10 is a high auxin producer, contrasting with the widely studied PGPB Phyllobacterium brassicacearum STM196 and B. subtilis GB03 (Contesto et al. 2010; Zhang et al. 2007). It is believed that low concentrations of IAA can stimulate primary root elongation, whereas high levels of IAA stimulate the formation of lateral roots (Patten and Glick 2002; Remans et al. 2008). Cytokinin production, especially zeatin, has been documented in various PGPB as well, including Bacillus spp. (Hussain and Hasnain 2009). However, there is no obvious production of cytokinins in WR10.
Both ferritins and transporters are major regulators for iron homeostasis in plants (Kim and Guerinot 2007). The qPCR quantification of 8 genes encoding either ferritins (G1-G5) or transporters (G6-G8) shows up-regulation of the expression in the majority of genes encoding ferritins in roots under iron stress after inoculation of WR10 (Fig. 5a). More precisely, ferritins TaFER1-A (G1) TaFER1-C (G3) are leading responders for iron storage in roots, which attribute to iron resistance and act as metal detoxicants (Joshi et al. 1989). The expressions of ZIP (G7) and UPP (G8) are down-regulated in both root and sprout for decreased iron transportation. Results obtained in this study suggest a major effect of proteins ZIP, UPP, and TaNAM (G6) as effluxes of iron; even UPP is an uncharacterized protein that is annotated as an iron transporter in the NCBI database (Waters et al. 2009; Barberon et al. 2011). Furthermore, the relative expression level of most of these genes is much lower in the shoot than in the root. In fact, many kinds of PGPB can enhance plant iron resistance through different kinds of mechanisms (Lin et al. 1983; Bar-Ness et al. 1992; Freitas et al. 2015). Furthermore, ferritins are expected to be used as a novel approach for solving iron problems in biology that has multiple applications (Theil et al. 2016).
In summary, we have isolated an endophytic growth promoting strain B. altitudinis WR10 from the root of wheat. Growth experiments showed that WR10 has strong resistance to iron stress. Therefore, we speculate the strain can alleviate iron toxicity in wheat. In the co-culture model, supplementation of WR10 improved lengths of both primary root and main sprout significantly suggesting significant effect on decreasing iron toxicity. To probe the mechanism behind this phenomenon, we tested the production of two kinds of hormones. ELISA results indicate the strain produces IAA that is an important regulator during plant growth. Furthermore, we conducted qPCR experiments to study whether ferritins and iron transporters in wheat can be regulated when co-cultivated with WR10. Interestingly, the expression of genes encoding ferritins is up-regulated in roots and down-regulated in shoots when co-cultivated with WR10 in the presence of high concentrations of iron. Up-regulated expression of ferritin-encoded genes in roots may contribute to improved iron resistance and down-regulated expression of ferritin-encoded genes in shoots may decrease iron toxicity. Considering the results obtained in the study, WR10 may benefit wheat growth in flooded regions by either bioleaching of iron or storage of excess iron by increased ferritins in roots, as well as the production of IAA.
Abbreviations
- B. altitudinis :
-
Bacillus altitudinis
- CTKs:
-
Cytokinins
- ELISA:
-
Enzyme Linked Immuno-Sorbent Assay
- IAA:
-
Indole 3 Acetic Acid
- PGPB:
-
Plant Growth Promoting Bacteria
- T. aestivum :
-
Triticum aestivum L.
References
Ali B, Sabri AN, Ljung K, Hasnain S (2009) Auxin production by plant associated bacteria: impact on endogenous IAA content and growth of Triticum aestivum L. Lett Appl Microbiol 48:542–547
Alluri HK, Ronda SR, Settalluri VS, Bondili JS, Suryanarayana V, Venkateshwar P (2007) Biosorption: an eco-friendly alternative for heavy metal removal. Afr J Biotechnol 6(25):2924–2931
Barberon M, Zelazny E, Robert S, Conéjéro G, Curie C, Friml J, Vert G (2011) Monoubiquitin-dependent endocytosis of the IRON-REGULATED TRANSPORTER 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci U S A 108(32):E450–E458
Bar-Ness E, Hadar Y, Chen Y, Römheld V, Marschner H (1992) Shorterm effects of rhizosphere microorganisms on Fe uptake from microbial siderophores by maize and oat. Plant Physiol 100:1–56
Becker M, Asch F (2005) Iron toxicity in rice-conditions and management concepts. J Plant Nutr Soil Sci 168:558–573
Briat JF (1996) Roles of ferritin in plants. J Plant Nutr 19:1331–1342
Briat JF, Gaymard F (2007) Iron nutrition and interactions in plants-preface. Plant Physiol Biochem 45:259
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20(1):33–40
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Chamam A, Sanguin H, Bellvert F, Meiffren G, Comte G, Wisniewski-Dyé F, Bertrand C, Prigent-Combaret C (2013) Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa association. Phytochemistry 87:65–77
Combes-Meynet E, Pothier JF, Mënne-Loccoz Y, Prigent-Combaret C (2011) The Pseudomonas secondary metabolite 2,4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Mol Plant-Microbe Interact 24:271–284
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Contesto C, Milesi S, Mantelin S, Zancarini A, Desbrosses G, Varoquaux F, Bellini C, Kowalczyk M, Touraine B (2010) The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 232:1455–1470
Couillerot O, Prigent-Combaret C, Caballero-Mellado J, Mënne-Loccoz Y (2009) Pseudomonas fluorescens And closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett Appl Microbiol 48:505–512
Da Mota FF, Gomes EA, Seldin L (2008) Auxin production and detection of the gene coding for the auxin efflux carrier (AEC) protein in Paenibacillus polymyxa. J Microbiol 46(3):257–264
Dawson MV, Lyle SJ (1990) Spectrophotometric determination of iron and cobalt with Ferrozine and dithizone. Talanta 37(12):1189–1191
Dobbelaere S, Croonenborghs A, Thys A, Vande Broek A, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:153–162
de Dorlodot S, Lutts S, Bertin P (2005) Effects of ferrous iron toxicity on the growth and mineral composition of an inter specific rice. J Plant Nutr 28:1–20
El Zemrany H, Czarnes S, Hallett PD, Alamercery S, Bally R, Jocteur-Monrozier L (2007) Early changes in root characteristics of maize (Zea mays) following seed inoculation with the PGPR Azospirillum lipoferum CRT1. Plant Soil 291:109–118
Finatto T, de Oliveira AC, Chaparro C, da Maia LC, Farias DR, Woyann LG, Mistura CC, Soares-Bresolin AP, Llauro C, Panaud O, Picault N (2015) Abiotic stress and genome dynamics: specific genes and transposable elements response to iron excess in rice. Rice (N Y) 8:13
Fofana AA (2009) Rice yiel gap due to iron toxicity in West Africa. J Agron Crop Sci 195:66–76
Franken P (2012) The plant strengthening root endophyte Piriformospora indica: potential application and the biology behind. Appl Microbiol Biotechnol 96(6):1455–1464
Freitas MA, Medeiros FH, Carvalho SP, Guilherme LR, Teixeira WD, Zhang H, Paré PW (2015) Augmenting iron accumulation in cassava by the beneficial soil bacterium Bacillus subtilis (GB03). Front Plant Sci 6:596
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KW, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327(5968):1008–1010
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Huang B, Johnson JW, NeSmith DS, Bridges DC (1995) Nutrient accumulation and distribution of wheat genotypes in response to waterlogging and nutrient supply. Plant Soil 173:47–54
Hussain A, Hasnain S (2009) Cytokinin production by some bacteria: its impact on cell division in cucumber cotyledons. Afr J Microbiol Res 3:704–712
Jing YD, He ZL, Yang XE (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8:192–207
Johnson CN, Fisher PR, Huang JS, Vetanovetz RP, Argo WR (2010) Quantifying the acidity of an ammonium-based fertilizer in containerized plant production. Hortscience 45(7):1099–1105
Joshi JG, Sczekan SR, Fleming JT (1989) Ferritin-a general metal detoxicant. Biol Trace Elem Res 21:105–110
Khabaz-Saberi H, Setter TL, Waters I (2006) Waterlogging induces high to toxic concentrations of iron, aluminium and manganese in wheat varieties on acidic soil. J Plant Nutr 29:899–912
Khabaz-Saberi H, Rengel Z, Wilson R, Setter TL (2010) Variation for tolerance to high concentration of ferrous iron (Fe2+) in Australian hexaploid wheat. Euphytica 172:275–283
Khabaz-Saberi H, Barker SJ, Rengel Z (2012) Tolerance to iron toxicities enhances wheat (Triticum aestivum L.) grain yield in waterlogged acidic soils. Plant Soil 354:371–381
Kim SA, Guerinot ML (2007) Mining iron: iron uptake and transport in plants. FEBS Lett 581(12):2273–2280
Kim SU, Cheong YH, Seo DC, Hur JS, Heo JS, Cho JS (2007) Characterisation of heavy metal tolerance and biosorption capacity of bacterium strain CPB4 (Bacillus spp.) Water Sci Technol J Int Asso Water Pollut Res 55:105–111
Li X, Ma H, Jia P, Wang J, Jia L, Zhang T, Yang Y, Chen H, Wei X (2012) Responses of seedling growth and antioxidant activity to excess iron and copper in Triticum aestivum L. Ecotoxicol Environ Saf 86:47–53
Lin W, Okon Y, Hardy RWF (1983) Enhanced mineral uptake by Zea mays and Sorghum bicolor roots inoculated with Azospirillum brasilense. Appl Environ Microbiol 45:1775–1779
Liu F, Xing S, Ma H, Du Z, Ma B (2013) Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biotechnol 97(20):9155–9164
Lugtenberg BJ, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Orhan F (2016) Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz J Microbiol 47(3):621–627
Patten CL, Glick BR (2002) Role of Pseudomonas putida indole acetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Pereira SI, Castro PM (2014) Diversity and characterization of culturable bacterial endophytes from Zea mays and their potential as plant growth-promoting agents in metal-degraded soils. Environ Sci Pollut Res Int 21(24):14110–14123
Pereira MP, Santos C, Gomes A, Vasconcelos MW (2014) Cultivar variability of iron uptake mechanisms in rice (Oryza sativa L.) Plant Physiol Biochem 85:21–30
Remans R, Beebe S, Blair M, Manrique G, Tovar E, Rao IM (2008) Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.) Plant Soil 302:149–161
Richardson AE, Baréa JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Sahrawat KL (2004) Iron toxicity in wetland rice and the role of other nutrients. J Plant Nutr 27(8):1471–1504
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53(372):1351–1365
Setter TL, Waters I, Sharma SK, Singh KN, Kulshreshtha N, Yaduvanshi NP, Ram PC, Singh BN, Rane J, McDonald G, Khabaz-Saberi H, Biddulph TB, Wilson R, Barclay I, McLean R, Cakir M (2009) Review of wheat improvement for waterlogging tolerance in Australia and India: the importance of anaerobiosis and element toxicities associated with different soils. Ann Bot 103(2):221–235
Shukla KP, Sharma S, Singh NK, Singh V, Tiwari K, Singh S (2011) Nature and role of root exudates: efficacy in bioremediation. Afr J Biotechnol 10:9717–9724
Sinha S, Guptha M, Chandra P (1997) Oxidative stress induced by iron in Hydrilla verticillata (i.f) Royle: response of antioxidants. Ecotoxicol Environ Safe 38:286–291
Speakman JB, Krüger W (1983) A comparison of methods to surface sterilize wheat seeds. Trans Br Mycol Soc 80(2):374–376
Theil EC, Tosha T, Behera RK (2016) Solving biology's iron chemistry problem with ferritin protein nanocages. Acc Chem Res 49(5):784–791
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298–1301
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356
Veresoglou SD, Menexes G (2010) Impact of inoculation with Azospirillum spp. on growth properties and seed yield of wheat: a meta-analysis of studies in the ISI web of science from 1981 to 2008. Plant Soil 337:469–480
Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A (2016) Molecular diversity and multifarious plant growth promoting attributes of bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol 56(1):44–58
Walker V, Couillerot O, Von Felten A, Bellvert F, Jansa J, Maurhofer M, Bally R, Moënne-Loccoz Y, Comte G (2012) Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 356:151–163
Waters BM, Uauy C, Dubcovsky J, Grusak MA (2009) Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J Exp Bot 60(15):4263–4274
Wen YM, Cheng Y, Tang C, Chen ZL (2013) Bioleaching of heavy metals from sewage sludge using indigenous iron-oxidizing microorganisms. J Soil Sed 13:166–175
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7(1–2):203–214
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu CM, Allen R, Melo IS, Paré PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Zhuang XL, Chen J, Shim H, Bai Z (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33:406–413
Zuo Y, Zhang F (2011) Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil 339(339):83–95
Acknowledgements
We acknowledge Dr. Hongzhan Liu from Zhoukou Normal University for sharing both seeds and Triticum aestivum L. zhoumai 26 seedlings. This study was partially funded by the Plan For Scientific Innovation Talent of Henan Province (No. 124100510021) to C. Li. Z. Sun received an international cooperation grant from Henan Province Science and Technology Agency (No. 172102410055). K. Liu received a grant from the key scientific and technological project of Henan province (No. 152102410074). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant.
Electronic supplementary material
ESM 1
(DOCX 1355 kb)
Rights and permissions
About this article
Cite this article
Sun, Z., Liu, K., Zhang, J. et al. IAA producing Bacillus altitudinis alleviates iron stress in Triticum aestivum L. seedling by both bioleaching of iron and up-regulation of genes encoding ferritins. Plant Soil 419, 1–11 (2017). https://doi.org/10.1007/s11104-017-3218-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3218-9