Abstract
In the present study, ten morphologically distinct indigenous plant growth-promoting rhizobacteria (PGPR) from wheat roots and rhizosphere were screened for multiple plant growth promoting (PGP) traits and out of ten, two strains (SIR1 and KUR2) possessing maximum PGP traits along with ACC (1-aminocyclopropane-1-carboxylic acid) deaminase activity were evaluated at three water stress regimes in wheat: (i) 80% field capacity (FC), (ii) 60% FC, (iii) 40% FC for 45 days, starting from 15 days after sowing to the maturity. Inoculation of SIR1 strain with wheat at 80% FC, induced a significant increase in plant biomass (root biomass, 66.67%; shoot biomass, 39.09%) together with decreased reactive oxygen species and increased activity of antioxidant enzymes (superoxide dismutase, peroxidase and catalase) over uninoculated control at 80% FC. 16s rDNA analysis of SIR1 strain revealed its lineage to Bacillus subtilis. Present investigations demonstrated the potential of bacterial partner (B. subtilis) in alleviating drought stress in wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main problem hampering the crop growth and productivity is drought, which is the result of global climate change events and is estimated to reduce cereal productivity by 9–10% (Lesk et al. 2016). More than 50% of the cultivable lands by 2050 are going to face devastating drought consequences on plant growth (Vinocur and Altman 2005). Plant and water relationships are affected by drought stress at both cellular and whole plant levels, resulting in various physiological complex processes and phenotypical responses in plant. Oxidative stress is generated within sub cellular compartments due to alleviated levels of reactive oxygen species (ROS). ROS consist of superoxide radical, hydrogen peroxide (H2O2), and the hydroxyl radical, all of these affect building components of the cell (lipids, proteins, carbohydrates, nucleic acids etc.) and cause cell demise (Naseem and Bano 2014; Mittler 2002). Therefore, there is an increased interest among the scientists in finding solutions to water associated problems such as drought and its impacts on food security. Particularly, there is an utmost need to redress sustainable solutions, which will improve drought tolerance in crop plants, so as to satisfy the food requirement with the limited water resources in today’s world (Mancosu et al. 2015).
Crop productivity can be increased by inoculating plants with PGPR (Plant Growth Promoting Rhizobacteria) facing drought stress (Ngumbi and Kloepper 2016). Alleviation of drought stress by PGPR is mediated by alteration of some physiological and biochemical processes (phytohormone levels, antioxidant enzyme activities and organic solutes content). A key trait of PGPR mediated drought stress alleviation is the ability to regulate ethylene formation using the ACC deaminase enzyme, thus acting as a sink of ACC. PGPR hydrolyze the ACC exuded from the roots in the rhizosphere into ammonia and α-ketobutyrate, and stimulate the extrusion of ACC from the roots to the soil. The lowering of ACC concentration in root tissues reduces the formation of endogenous ethylene, thus promoting plant growth. Plant resistance to drought is enhanced by reducing ethylene-mediated inhibitory effects on plant growth (Saleem et al. 2007; Chandra et al. 2018; Kaushal and Wani 2016; Mishra et al. 2017). Biofilms formation is another important mechanism of bacterial drought tolerance and bacteria mediated plant drought tolerance, especially in Bacillus subtilis. Variety of macromolecules (oligo- and polysaccharides) in the form of extracellular matrix, released by bacteria helps in plant growth and development by retaining the moisture, thereby improving water availability in root medium (Timmusk et al. 2014; Yang et al. 2009; Dimkpa et al. 2009).
The current study is focused on isolation, screening and characterization (16s rDNA) of potent ACC deaminase producers from High hills (dry and wet temperate zones) of North Western Himalayas and their effect on drought stress alleviation in wheat (variety HPW- 42).
Materials and methods
Collection and isolation of potential strains
The rhizospheric soil and roots samples of wheat (Triticum aestivum L.) plants were collected from high hills (dry and wet temperate zones; Fig. 1) of Himachal Pradesh falling in North Western Himalayan region. Standard serial dilution and pour plate techniques were used for isolation of rhizospheric microbes on nutrient agar, Pikovskaya’s and Jensen’s medium. Endophytic microorganisms were isolated by removing the soil particles from wheat roots with tap water, then roots surface was sterilized using sodium hypochlorite (2%) solution. After surface sterilization is complete, wheat roots were crushed in sterile water. The extracts were centrifuged and supernatant was collected. Standard serial dilution and pour plating techniques were used for isolation of endophytic bacteria on nutrient agar, Pikovskaya’s and Jensen’s medium.
Molecular (16s rDNA) Identification of best bacterial strain
Genomic DNA was extracted by the phenol/chloroform extraction method (Sambrook et al. 1989). The bacterial strain SIR1 was grown in nutrient broth at 32 °C and cells were harvested after 72 h of incubation and processed for DNA isolation by Real Genome DNA isolation kit. The PCR amplification was carried out in a final volume of 20 µl. The amplification reaction containing 50 ng of DNA template, 20 p mole each of Universal primers, 0.2 mm dNTPs and 1 U Taq Polymerase in 1X PCR buffer. Reaction was cycled 35 times at 94 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min 30 s followed by final extension at 72 °C for 10 min. Amplified PCR product was separated by gel electrophoresis on 1.2% (w/v) agarose gel. Excision of 1375 bp band from the gel after electrophoresis was done by using gel extraction kit (Real genomics). Segments of purified DNA were sequenced from commercial sequencing facility (Xleris lab). 16s rDNA gene sequence was compared via NCBI databases by using BLAST algorithm. On the Basis of maximum similarity first 10 sequences were selected and aligned using multiple alignment software program CLUSTALW. Distance matrix was generated using RDP database and the phylogenetic tree was constructed using MEGA 6.0.
Plant growth promoting traits
Phosphate solubilization activity of the selected strains was estimated using Pikovskaya (1948) medium. The production of indole-3-acetic acid (IAA) and siderophores were also assessed (Aneja 2010). Ammonia production was also assessed (Aneja 2010). Ability of strains to produce ACC deaminase was assessed by spotting bacterial strains on DF minimal salt medium as amended with 3 mM ACC as sole nitrogen source and micronutrient solution (10 ml) (Dworkin and Foster 1958). The biocontrol potential of the bacterial strains against test fungal pathogens (Fusarium graminearum, Claviceps purpurea and Alternaria triticina) was ascertained by agar streak plate method on potato dextrose agar medium and per cent growth inhibition was determined as described (Vincent 1947). Out of total ten strains only two endophytic strains (SIR1 and KUR2) possessing maximum quantitative and qualitative PGP traits were further selected for net house studies to test the physiological efficacy of wheat under drought stress. All the PGP activities were done in replications (each treatment replicated thrice) and the data recorded was analyzed using completely randomized design (CRD).
Efficacy of bacterial strains on physiological growth parameters, nutrient uptake and antioxidant enzyme activities
Effect of isolated PGPR for physiological efficacy under water stress conditions on growth of wheat variety HPW-42, was studied. The potting mixture was prepared by mixing sand, soil and farm yard manure (FYM) in the ratio of 1:2:1, sterilized by autoclaving the mixture at 80–100 °C for 30 min and then incubated for 24 h. The procedure was repeated for three successive days (also known as Tyndallization). The pH level, electrical conductivity and organic carbon content of the potting mixture were found to be 6.1, 0.39 dS/m and 0.40%, respectively. Available nutrient (N, P and K) contents of experimental soil before the experiment were recorded to be 291.5, 21.4 and 245.9 kg/ha, respectively. The soil used for pot (15 cm diameter and 15 cm deep) experiment belongs to Entisols order as per USDA (United States Department of Agriculture) soil taxonomy. The soil texture is sandy loam (28% silt, 17% clay and 55% sand). The maximum water holding capacity (MWHC) and field capacity (1/3 bar tension) of the soil were determined using Keen–Raczkowski box and pressure plate apparatus, respectively. The MWHC and the field capacity of the soil were recorded as 42 and 23%, respectively. The pots were filled with oven dried (to ensure that initial moisture content is 0%) and sterilized soil mixture weighing about 1500 g/pot. 80%, 60% and 40% of FC were initially maintained by adding 276, 207 and 138 ml of sterilized water on the basis of calculations made for determination of FC. Subsequently, the soil moisture levels were maintained by gravimetric method. The pot surface was covered by sterile glass beads to check evaporation. The study was set up as a Completely Randomized Block Design (CRD) with three replicates for each treatment at three water stress regimes; 80%, 60% and 40% (water depletion of field capacity) and two PGPR strains (SIR1 and KUR2) with controls (without PGPR inoculation). All the pots were kept to the field capacity up to 45 days after planting, starting 15 days after sowing to maturity. Surface sterilized wheat seeds were dipped in individual culture broth (Nutrient broth medium) of selected strains (SIR1 and KUR2) with cell density about 108 cells/ml for 1 h. The control seeds were treated with sterilized nutrient broth. Five plants per pot were maintained under net house conditions by taking the following nine treatments: T1: 80% of field capacity; T2: 80% of field capacity + SIR1; T3: 80% of field capacity + KUR2; T4: 60% of field capacity; T5: 60% of field capacity + SIR1; T6: 60% of field capacity + KUR2; T7: 40% of field capacity; T8: 40% of field capacity + SIR1; T9: 40% of field capacity + KUR2 in Completely Randomized Block Design (CRD) with 3 replications.
The observations on root/shoot length and biomass were recorded following standard methods. The total N contents in plant samples were analyzed by the micro- kjeldhal’s method (Helrich 1990). For P and K contents, leaf samples were digested in a diacid mixture of HNO3: HCLO4 (4:1) and final volume was made to 100 ml (Jackson 1973). Phosphorous percentage in the digested sample was determined by vanado-molybdate yellow colour method (Jackson 1973). Potassium contents in the digest were determined using flame photometer (Jackson 1967). Nutrient uptake (mg/plant) was calculated by multiplying NPK concentrations of the plant with total dry matter content. Total chlorophyll contents and relative leaf water contents were determined (Witham et al. 1971). Total amino acid content was estimated using Folin & ciocalteu’s phenol reagent (Folin and Ciocalteu’s phenol reagent, Hi-LR™, HiMedia Laboratories Pvt. Ltd., 23, Vadhani Ind. Est., LBS Marg, Mumbai, India, Cat. No. RM10822-100 ml) (Lowry et al. 1951).
Antioxidant enzyme assay
Crude enzyme extract was prepared by homogenizing 0.5 g of frozen leaf and roots tissues in extraction buffer consisting of 0.5% Triton X-100 and 1% PVP (polyvinyl pyrrolidone) in 100 mM potassium phosphate buffer (pH 7.0) in a chilled pestle mortar. After homogenization, centrifugation was done at 10,000 rpm for 20 min and supernatant was used for antioxidant enzymes assays as given below:
Superoxide dismutase
Superoxide dismutase activity was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm (Beauchamp and Fridovich 1971). The enzyme activity was expressed in U/g fresh weight.
Catalase activity
Total CAT activity was assayed spectrophotometrically by monitoring decrease in absorbance at 240 nm per minute as a result of decomposition of H2O2 (Chandlee and Scandalios 1984). The enzyme activity was expressed in U/g fresh weight.
Peroxidase activity
Peroxidase activity was determined by measuring the quantity of purpurogallin formed spectrophotometrically at 420 nm (Kumar and Khan 1982). The enzyme activity was expressed in U/g fresh weight.
Statistical analysis
Data obtained from the net house and laboratory experiments was analyzed using completely randomized design (CRD) with three replications. Statistical analyses of data were done by using SPSS version 16 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA) at P = 0.05 level of significance.
Results and discussion
Collection and isolation of potential strains
Marked variations were observed in both endophytic and rhizospheric bacterial count associated with wheat falling under high hills (Fig. 1) of Himachal Pradesh. On purification, out of total 71 bacterial strains only 10 strains (KIR2, SIR1, KIS2, LSR1, KUS3, KUR2, CHR1, CHS2, SHR1 and SHS1) were able to form clear halo zone on the Pikovskaya’s (PVK) media and showed growth on Jensen’s medium. Out of ten strains only three (KIS2, KUS3 and CHS2) were rhizospheric in origin while the rest were endophytic in origin, all of which were further screened for other quantitative PGP traits and ACC deaminase activity. The variation in microbial population in the rhizosphere and roots may be attributed to the age of plant, cultivar type, sampling time, physico-chemical properties of soil, positive influence of root exudates that act as regulators of microbial growth and environmental conditions of study area (Sood et al. 2018a, b, 2019).
Molecular (16s rDNA) Identification
Identification of the best performing strain (SIR1) under net house conditions was confirmed by 16s rDNA gene sequencing. Phylogenetic tree generated by the neighbour-joining method for the SIR1 is shown in Fig. 2. Based on the nucleotide homology and phylogenetic tree generated, SIR1 strain was found to be Bacillus subtilis (GenBank Accession Number: KX379531) by securing 99% homology with Bacillus subtilis strain SBMP4 (GenBank Accession Number: NR118383). Similar molecular characteristics for the member of genus Bacillus were reported (Kumar et al. 2015; Wahyudi et al. 2011; Majeed et al. 2015). Further, it has also been demonstrated that bacteria belonging to genus Bacillus, not only proliferate rhizosphere, but also reside inside the root tissue of crop plants (Hallmann et al. 1997).
Plant growth promoting traits
Microorganisms possessing multifarious PGP traits are beneficial for enhancing plant growth under abiotic stress. Significant variations were observed in the plant growth promoting activities of the isolated PGPR (Table 1). The phosphate solubilization ranged from 93.00 to 190.00 µg/ml in liquid PVK medium. The maximum (190.00 µg/ml) P-solubilisation was noted for SIR1 strain which was at par with KUR2 (181.00 µg/ml) whereas the minimum (93.00 µg/ml) was recorded for SHS1. Possible mechanisms adopted by the strains for phosphate solubilisation includes different array of solubilization reactions, which include acidification, chelation, exchange reactions, and production of gluconic acid, to release monobasic and dibasic forms of phosphorus from insoluble phosphorus (Ngumbi and Kloepper 2016; Sood et al. 2018a, b, 2019), which might have resulted in the increased available P content, thus enhancing the growth of plants under drought stress. The indole acetic acid (IAA) and siderophore production ranged from 17.33 to 29.67 µg/ml and 25.00 to 57.00%, respectively. The maximum IAA (29.67 µg/ml) and siderophore production (57.00%) was shown by SIR1 strain, followed by KUR2. Auxin, also referred to as indole-3-acetic acid (IAA), is an important regulator of plant growth and development (Glick et al. 2007; Glick 1995). Recently, Chandra et al. (2018) have reported the effect of bacterial IAA on root growth for enhanced uptake of nutrients, thereby, anchoring tall sugarcane plants with soil under drought stress. Similar studies on the effect of bacterial IAA on plant growth under abiotic stress have also been conducted in wheat, maize, pepper and Arabidopsis thaliana etc. (Sorty et al. 2016; Vardharajula et al. 2011; Lim and Kim 2013; Cohen et al. 2015). The plants facing stressed conditions are more susceptible to phytopathogens. The possible solution to combat this susceptibility is to provide defense system against these phytopathogens by using the strains that were efficient producers of bacterial siderophores. This can be achieved either directly by supplying iron to plants or indirectly by depriving the fungal pathogens of iron, thereby limiting their growth (Glick 2012; Bagyaraj 2011). All the strains showed ammonia production and antagonism against Fusarium graminearum, Alternaria triticina and Claviceps purpurea. Ammonia produced by bacterial strains, suppresses the growth of certain fungi due to it potent antagonistic effect, which complemented the biocontrol activity of the tested strains. Only three strains namely KIR2, LSR1 and SHR1 were not found to be ACC deaminase producers. The active ACC deaminase enzyme of bacterial origin might have played a significant role in the reduction of ACC levels in roots, as reported in previous research (Glick 2014). Onset of stress signals triggers the enhanced production of ethylene (ET) and 1-Aminocyclopropane-1-carboxylate (ACC) during the initial phase of stress induction. Bacteria exert beneficial effects on plants under abiotic stress by regulating the ET level (Saleem et al. 2018). PGPR possessing ACC deaminase activity results in hydrolysis of ACC into ammonia and alpha-ketobutyrate. As a result, the levels of ethylene get reduced and plants are able to maintain normal growth (Glick and Bashan 1998). The results are in line with the previous findings (Khan et al. 2016). Similarly, Saikia et al. (2018) reported consortium effect of three ACC-deaminase producingrhizobacteria (Ochrobactrum pseudogrignonense RJ12, Pseudomonas sp. RJ15 and Bacillus subtilis RJ46) in enhancing the crop physiological parameters of Vigna mungo L. and Pisum sativum L. under drought stress. On the basis of PGPR traits and antifungal activity, SIR1 and KUR2 were found to possess the maximum PGP traits and were thus selected for net house studies.
Efficacy of bacterial strains on physiological growth parameters, antioxidant enzyme activities and nutrient uptake
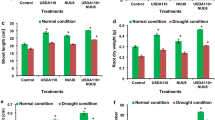
The adverse effect of drought stress was noticed in uninoculated water stress (drought) imposed wheat plants with reduced growth, relative water content and less chlorophyll content (Table 2). However, significant (p = 0.05) plant growth and development was noticed in bacteria-inoculated plants under drought stress (Table 2). The action of SIR1 strain was found to be more promising than the action of KUR2. At 80% FC soil moisture level, plants receiving SIR1 inoculation increased the shoot and root length of wheat significantly (p = 0.05) by 14.22% and 23.51% over uninoculated control maintained at same stress level (80% FC). However, these values in KUR2 inoculated plants were 8.10% and 4.60% for shoot and root length, respectively. Similar trend was observed in SIR1 and KUR2 inoculated treatments maintained at 60 and 40% FC soil moisture levels in contrast to uninoculated controls at respective stress levels (60 and 40% FC). This is also confirmed by significant increase in shoot and root biomass (dry weight) compared to non-inoculated plants. Compared to uninoculated control plants at 80% FC soil moisture, 39.09% and 66.00% increase in shoot and root biomass was observed in SIR1-treated stressed plants at 80% FC. However, this rate for KUR2 inoculated stressed plants was 3.64% and 11.00%, respectively (Table 2). 33.33%, 15.56% increase in shoot biomass and 66.67%, 50.00% in root biomass was observed in the treatments receiving SIR1 and KUR2 inoculation at 60% FC compared to their uninoculated control stressed plants. This increase for plants inoculated with SIR1 and KUR2 strains at 40% FC was noted as 26.67%, 16.67% (shoot biomass) and 40.00%, 20.00% (root biomass), respectively. The possible reason for enhanced shoot/root length and their biomass in the inoculated treatments is the release of variety of plant growth regulators i.e. phytohormones in presence of different plant flavonoids in the rhizospheric region, which results in alteration of root architecture that may lead to an increase in the total root surface area, and consequently improved water and nutrient uptake, particularly N (asymbiotic N-fixation) and P (P-solubilization), with positive effects on plant growth as a whole (Montano et al. 2014). For example, Timmusk et al. (2014) reported that wheat plants treated with PGPR had 78% higher biomass than non-treated plants under drought stress, exhibiting the potential of PGPR to enhance plant performance under stress conditions. Significant (p = 0.05) increase of 2.22%, 6.17%, 4.23% and 1.11%, 2.06%, 2.82% in RWC (relative water content) was observed in SIR1 and KUR2 inoculated stress (80, 60 and 40% FC) imposed wheat plants, as compared to the uninoculated stressed plants at respective stress levels. Bacterial inoculation had a direct effect on plant osmolytes (Table 2). Treatments receiving SIR1 inoculation at respective stress levels (80, 60 and 40% FC) showed significant increase of 15.63%, 5.00% and 8.51% in total amino acid content over their uninoculated stressed plants maintained at 80, 60 and 40% FC, followed by KUR2 with 9.30%, 10% and 4.26% increase. The increased concentration of solutes and osmolytes are key elements of plant response facing abiotic stress. Osmolyte accumulation and cellular osmotic potential are correlated i.e. as the osmolyte concentration increases, there is a decrease in the cellular osmotic potential. This decrease helps in absorption of water from the drying soil and as a result, turgor pressure of the cell gets increased thereby improving the physiological efficacy of plants under drought stress (Harb et al. 2010). Total chlorophyll content was determined to examine the impact of the rhizobacterial strains on the photosynthetic potential of host plants. Plants receiving SIR1 and KUR2 inoculation at 80%, 60% and 40% FC showed significant (p = 0.05) increase of 33.33%, 12.50%, 40.00% and 16.67%, 6.25%, 10% in total chlorophyll content in comparison to uninoculated stressed plants (negative controls at respective stress levels). This indicates the competence of select strains at upholding the chlorophyll content under drought conditions as reported by Glick (2014).
The activation of a plant’s inherent enzymatic and non-enzymatic systems is always crucial for the detoxification of the ROS under stress conditions. The results of this study clearly indicate that treatments receiving SIR1 and KUR2 inoculation (p = 0.05) stimulates the SOD activity in wheat plants much significantly over uninoculted stressed controls (Fig. 3). An increase of 84.40%, 42.60% at 80% FC, 16.94%, 0.10% at 60% FC and 33.16%, 13.79% at 40% FC was recorded in SIR1 and KUR2 inoculated plants in comparison to the uninoculated stressed controls maintained at 80%, 60% and 40% FC soil moisture, respectively. POD and CAT were located in every ROS-producing compartment and functioned as a fine regulator of intracellular ROS level. As with SOD, a similar increasing trend was observed in POD and CAT activities, with 134.73%, 31.3% increase at 80% FC, 74.95%, 0.00% at 60% FC and 35.72%, 13.73% at 40% FC in case of CAT and 25.85%, 10.20% at 80% FC, 24.68%, 15.58% at 60% FC and 7.77%, 2.91% at 40% FC in case of POD. Moreover, an increasing trend was also noticed in stress-induced plants without inoculation, but this trend was not as prominent as it was in the SIR1 and KUR2 inoculated plants (Fig. 3). This increase in enzymatic activity may serve to minimize oxidative injury and contribute to the drought stress tolerance by degradation of reactive oxygen species (ROS), such as hydrogen peroxide, singlet oxygen, superoxide radical, and hydroxyl radical (Hasanuzzaman et al. 2014). Our studies are in accordance with Kasim et al. (2013) who reported that priming of wheat seeds with plant-growth promoting bacteria (Bacillus amyloliquefaciens 5113 and Azospirillum brasilense NO40) significantly alleviated the deleterious effect of drought stress on wheat.
Effect of liquid bacterial inoculum on antioxidant enzymes activities a superoxide dismutase, b peroxidase and c catalase. T1: 80% of field capacity; T2: 80% of field capacity + SIR1; T3: 80% of field capacity + KUR2; T4: 60% of field capacity; T5: 60% of field capacity + SIR1; T6: 60% of field capacity + KUR2; T7: 40% of field capacity; T8: 40% of field capacity + SIR1; T9: 40% of field capacity + KUR2
Induction of drought stress hindered the nutrient uptake from soil and a decrease in nutrient (NPK) content of wheat plants was observed with increase in imposed drought stress (Table 3). A decrease of 32.50%, 23.40% in N, 11.36%, 9.30% in P and 18.31%, 15.33% in K content was observed in the stressed plants grown at 80% FC over SIR1 and KUR2 inoculated plants at same water stress regime. Similar decrease was observed in uninoculated treatments over SIR1 and KUR2 inoculated ones, maintained at 60 and 40% FC soil moisture levels [18.75%, 17.20% (N), 29.41%, 27.27% (P) and 15.04%, 5.83% (K) at 60% FC; 5.63%, 4.29% (N), 20.51%, 18.42% (P) and 12.21%, 8.00% (K) at 40% FC]. Drought stress also significantly reduced nutrient uptake in the uninoculated plants over the inoculated ones. This decrease in nutrient uptake was 53.76%, 29.53% for N uptake, 39.54%, 16.33% (P uptake) and 42.27%, 21.97% (K uptake) in the uninoculated stressed plants grown at 80% FC as compared to the ones that received SIR1 and KUR2 inoculation. Similar trend for nutrient uptake was observed in uninoculated treatments maintained at 60% and 40% FC soil moisture levels over plants receiving SIR1 and KUR2 inoculation (40.37%, 30.17% (N uptake), 28.87%, 16.44% (P uptake) and 37.58%, 21.15% (K uptake) at 60% FC ; 26.13%, 18.48% (N uptake), 38.07%, 29.68% (P uptake) and 31.62%, 22.33% (K uptake ) at 40% FC). The significant increase in nutrient (NPK) content in the PGPR inoculated treatment as compared to uninoculated controls might be attributed to the increase in different fractions of available N, P and K in soil, stimulation of root formation or increase in plant growth stimulating mechanisms (direct mechanisms), which can positively influence plant growth and crop yields. Similarly, the plant growth promoting effect of strains favored the wheat plants accumulating more macronutrients in the biomass. Our results are in accordance with the findings of Kumar et al. (2014) who reported maximum nutrients (macro and micronutrients) acquisition and content in grain of wheat receiving different combinations of microbial strains (Bacillus megaterium, Arthrobacter chlorophenolicus and Enterobacter sp.) under pot and field experiments. The results of in-vivo experiments revealed tremendous improvements in plant growth promotion of wheat plants under water deficit conditions when the two strains (SIR1 and KUR2) were applied at different levels of water deficit stress as compared to uninoculated treatments (Fig. 4).
Conclusions
It can be concluded from current study that inoculation of wheat plants with endophyte (Bacillus subtilis) significantly alleviated drought stress and consequently improved growth. The application of Bacillus subtilis may thus be recommended for field trials in high hills and dry temperate zone of Himachal Pradesh for sustainable crop production. It can further help in improving soil health and conservation of natural resources.
References
Aneja KR (2010) Experiments in microbiology, plant pathology and biotechnology. New Age International, New Delhi
Bagyaraj DJ (2011) Microbial biotechnology for sustainable agriculture, horticulture & forestry. Nipa, New Delhi
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Chandlee JM, Scandalios JG (1984) Analysis of variants affecting the catalase development program in maize scutellum. Theor Appl Genet 69:71–77
Chandra P, Tripathi P, Chandra A (2018) Isolation and molecular characterization of plant growth-promoting Bacillus spp. and their impact on sugarcane (Saccharum spp. hybrids) growth and tolerance towards drought stress. Acta Physiol Plant 40:199
Cohen A, Bottini R, Pontin M, Berli F, Moreno D, Boccanlandro H, Travaglia C, Picocoli P (2015) Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol Plant 153:79–90
Dimkpa C, Weinand T, Asch F (2009) Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32:1682–1694
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 963401:1–15
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Glick BR, Bashan Y (1998) Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15:353–378
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339
Hallmann J, Hallmann QA, Mahafee WE, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Harb A, Krishnan A, Ambavaram MMR, Pereira A (2010) Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol 154:1254–1271
Hasanuzzaman M, Nahar K, Gill SS, Gill R, Fujita M (2014) Drought stress responses in plants, oxidative stress, and antioxidant defense. In: Tuteja N, Gill SS (eds) Climate change and plant abiotic Stress tolerance. Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim, pp 209–249
Helrich K (1990) Official methods of analysis of the Association of official Analytical Chemists. William Star Wetglad, Washington
Jackson ML (1967) Soil chemical analysis. Oxford and IBH Publishing House, Bombay, p 38
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd., New Delhi
Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J (2013) Control of drought stress in wheat using plant growth promoting bacteria. J Plant Growth Regul 32:122–130
Kaushal M, Wani SP (2016) Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol 66:35–42
Khan AL, Halo BA, Elyassi A, Ali S, Al-Hosni K, Hussain J, Al-Harrasi A, Lee I (2016) Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron J Biotechnol 21:58–64
Kumar A, Maurya BR, Raghuwanshi R (2014) Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal Agric Biotechnol 3:121–128
Kumar A, Maurya BR, Raghuwanshi R (2015) Charachterization of bacterial strains and their impact on plant growth promotion and yield of wheat and microbial population of soil. Afr J Agric Res 10:1367–1375
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J Exp Biol 20:412–416
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529:84–87
Lim JH, Kim SD (2013) Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol J 29:201–208
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N (2015) Isolation and characterization of plant growth- promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol 6:1–10
Mancosu N, Snyder RL, Kyriakakis G, Spano D (2015) Water scarcity and future challenges for food production. Water 7:975–992
Mishra IG, Sapre S, Kachare S, Tiwari S (2017) Molecular diversity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing PGPR from wheat (Triticum aestivum L.) rhizosphere. Plant Soil 414:213–227
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Montano PF, Villeges AC, Bellogin RA, Cerro DP, Espuny MR, Guerrero JI, Baena LFJ, Ollero FJ, Cubo T (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169:325–326
Naseem H, Bano A (2014) Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance in maize. J Plant Interact 9:689–701
Ngumbi E, Kloepper J (2016) Bacterial-mediated drought tolerance: current and future prospects. Appl Soil Ecol 105:109–125
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiology 17:362–370
Saikia J, Sarma RK, Dhandia R, Yadav A, Bharali R, Gupta VK, Saikia R (2018) Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci Rep 8:1–16
Saleem AR, Brunetti C, Khalid A, Rocca GD, Raio A, Emiliani G, Carlo AD, Mahmood T, Centritto M (2018) Drought response of Mucuna pruriens (L.) DC. Inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS One 13:e0191218
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sood G, Kaushal R, Chauhan A, Gupta S (2018a) Effect of conjoint application of indigenous PGPR’s and chemical fertilizers on productivity of maize (Zea mays L.) under mid hills of Himachal Pradesh. J Plant Nutr 41:297–303
Sood G, Kaushal R, Chauhan A, Gupta S (2018b) Indigenous plant growth–promoting Rhizobacteria and chemical fertilizers: impact on wheat (Triticumaestivum L.) productivity and soil properties in North Western Himalayan Region. Crop Pasture Science 69:460–468
Sood G, Kaushal R, Panwar G, Dhiman M (2019) Effect of indigenous plant growth- promoting rhizobacteria on wheat (Triticum aestivum L.) productivity and soil nutrients. Commun Soil Sci Plant Anal 2:141–152
Sorty AM, Meena KK, Choudhary K, Bitla UM, Minhas PS, Krishnani KK (2016) Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L) on germination and seedling growth of wheat under saline conditions. Appl Biochem Biotechnol 180:872–882
Timmusk S, Abd El-Daim IA, Copolovici L, Tanilas T, Kannaste A, Behers L, Nevo E, Seisenbaeva G, Stenstrom E, Niinemets U (2014) Drought-tolerance of wheat improved by Rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9:e96086
Vardharajula S, Ali SZ, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6:1–14
Vincent JM (1947) Distortion of fungal hyphae in the presence of certain inhibitors. Nature 4051:159–850
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16:123–132
Wahyudi AT, Astuti RP, Widyawati A, Meryandini AN, Abdjad A (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrob 3:34–40
Witham FH, Bladydes DF, Delvins RM (1971) Experiments in plant physiology. Van Nostrand Reinhold, New York, p 245
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Acknowledgements
This research did not receive any specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sood, G., Kaushal, R. & Sharma, M. Significance of inoculation with Bacillus subtilis to alleviate drought stress in wheat (Triticum aestivum L.). Vegetos 33, 782–792 (2020). https://doi.org/10.1007/s42535-020-00149-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-020-00149-y