Abstract
Background and aims
Fine-root traits mediate the capacity of plants to acquire soil resources in different environments. This study aimed to examine the changes of fine-root traits when roots proliferate into the litter layer vs. mineral soils, and to determine fine-root trait plasticity of these roots in response to nitrogen (N) addition.
Methods
A one-year N addition experiment was conducted in a 22-year-old broadleaf Mytilaria laosensis (Hamamelidaceae) plantation in subtropical China. Newly produced fine roots were collected monthly from the litter layer and upper mineral soil (0–10 cm) layer to measure root morphological traits and nutrient concentrations. Fine-root production was determined using ingrowth mesh screens in the litter layer.
Results
Fine-root production in the litter layer in the Mytilaria laosensis plantation was 2.6 g m−2 yr.−1 but increased 3- to 5-fold with N addition. Significant differences in fine-root morphological traits and nutrient concentrations were found between the litter layer and 0–10 cm mineral soil layer. Fine roots in the litter layer were thinner, with higher specific root length (SRL), higher specific root area (SRA), a higher proportion of fine-root biomass in lower, more absorptive root orders, and lower root tissue density (RTD) than those in 0–10 cm mineral soil layer. Higher carbon (C), N, phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg) concentrations and lower C:N ratio (C/N) were also observed in fine roots in the litter layer, compared to the 0–10 cm mineral soil layer. Nitrogen addition significantly increased root P, K, and Ca concentrations, but had no effect on Mg concentration. Nitrogen addition did not affect most fine-root morphological traits but did result in decreased root diameter.
Conclusions
Compared with the mineral soil, roots produced in the litter layer generally reflected a more absorptive strategy with smaller root diameter and lower RTD and with higher SRL, SRA, and nutrient concentrations which together are generally associated with more metabolically active, but shorter lived roots. Strong responses of fine-root production and nutrient concentrations to N addition also suggest that N may be a driving factor for fine-root growth into the litter layer. Further studies are required to identify the effect of fine-root growth into the litter layer on microbial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root proliferation into organic horizons plays an important role in nutrient recovery and acquisition for plants and nutrient cycling in forests. Fine roots and associated mycorrhizal fungi often proliferate into organic horizons to obtain nutrients directly before it leaches into mineral soil layers (Laclau et al. 2004; Cotrufo 2006). This “direct acquisition” of nutrients from decomposing organic matter is generally considered as an important adaption to maintain favorable nutrient budgets, especially in infertile and highly leached tropical soils (Sayer et al. 2006). Furthermore, fine roots in organic horizons may mediate decomposition of organic matter, and regulate soil carbon (C) and nutrient cycling through priming effects (Ma et al. 2012), whereby microbial activity and decomposition of organic matter are stimulated by the input of energy-rich carbon (C) from fine roots (Solly et al. 2015). There is also evidence that roots proliferate not only older decomposing organic matter, but also freshly fallen leaves (Jordan and Escalante 1980; Herrera et al. 1978). The subsequent effect of root growth and rhizosphere priming effects on accelerating decomposition rate of litter have been reported to be as much 380% (Cheng 2009). However, most previous studies have focused on root proliferation into mineral soils and root growth into the organic layers, especially the undecomposed and fresh litter layer, has been largely ignored (Sayer et al. 2006).
Fine-root proliferation into the litter layer has been explained partly by high nutrient availability in upper organic layers of forest soils (Fujimaki et al. 2005; Wang et al. 2016a). In the litter layer of the forest floor, substrates are often enriched in nutrients, and sometimes water, relative to the mineral soil layers, and these enrichments provide favorable conditions for root growth (Subke et al. 2004; Sayer et al. 2006; John 2011; Wang et al. 2016b). Fine roots are also expected to adjust their morphological and chemical characteristics in response to high nutrient availability in the litter layer. In previous research conducted in subtropical forests, Wang et al. (2016b) reported higher specific root length (SRL) and lower root diameter in the litter layer relative to the mineral soil layers. Similarly, in a field experiment using root ingrowth cores, Fujimaki et al. (2005) indicated that decomposing litter is more favorable for root growth than mineral soils and root growth into the litter layer coincides with altered fine-root morphology and chemical characteristics. However, it is still unclear to what extent different nutrients can determine fine-root growth into the litter layer and how differences in nutrient availability may drive changes in root morphology and chemistry.
Nitrogen (N), quantitatively the most important nutrient for plants and microbes, is often a limiting nutrient constraining primary productivity in many forest ecosystems (Lebauer and Treseder 2008; Noguchi et al. 2013). As such, N is expected to be an important nutrient driving fine-root growth. In response to N addition, plants usually allocate relatively more photosynthate to fibrous root production (Wang et al. 2013), which may also coincide with higher mean root SRL and root N concentration, as well as lower mean root diameter and mean root tissue density (RTD) (Eissenstat et al. 2015; Zadworny and Eissenstat 2011). Meanwhile, such altered root morphology and chemistry may result in higher root production and turnover (Eissenstat 1991; Wahl and Ryser 2002; Adams et al. 2013). Still, it remains unclear how root production and morphology respond to N addition due to other studies often reported inconsistent results (Nadelhoffer 2000; Mei et al. 2010). For example a recent meta-analysis by Li et al. (2015) reported that N addition significantly decreased fine-root biomass, and increased fine-root respiration and N concentration, but that responses of fine-root production and morphology to N addition were highly uncertain with both positive and negative responses commonly reported.
Anthropogenic N deposition has doubled the atmospheric input of N in terrestrial systems since 1950 (Galloway et al. 2008). Excessive N deposition may cause a suite of negative effects, such as soil acidification (Tian and Niu 2015), loss of soil cations (Matson et al. 2002), and shift from N to P limitation (Galloway et al. 2008; Huang et al. 2016), all of which may induce root stress (Smithwick et al. 2013), influence fine-root distribution and production, and alter root functional traits related to nutrient foraging. Fine roots in the litter layer may play an importance role in forest health to absorb excess inorganic N before it leaches into the mineral soils where may strip base cations from mineral surfaces and alter soil pH (Ma et al. 2012). Although numerous studies have sought to determine the effects of N addition on fine-root traits in the mineral soil layer (Nadelhoffer 2000; Majdi and Öhrvik 2004; Kou et al. 2015), it is not known whether similar responses of fine-root production and other functional traits can be expected in the litter layer. Given the escalating rates of N deposition in tropical and subtropical areas, understanding how fine-root production and other functional traits related to nutrient foraging may be altered in the litter layer will provide significant insights into mechanisms underlying adaptation of plants in these systems.
Anthropogenic N deposition in subtropical China is among the highest rates observed globally (Chen and Mulder 2007). Within the Chinese subtropical biome, the Mytilaria laosensis (Hamamelidaceae) forest is an important native broad-leaf species common among the regional flora and often used in forest plantations (Huang et al. 2014). It is often characterized by having a relatively high abundance of fine roots in organic horizons, particularly in the weakly decomposed, surficial litter layer (Liu et al. 2015b). Previous research on leaf litter decomposition conducted in our research sites revealed that fine roots would proliferate into litter bags and that bags with such proliferation showed strong reduction in the leaf litter mass and nutrients (Liu et al. 2015b). This suggested that the roots were actively scavenging throughout this litter layer and their presence resulted in a priming of litter decomposition. However, it is unclear if the potential availability of nutrients was specifically responsible for root proliferation. A better understanding of how fine roots in the litter layer respond to N deposition is critical to determine their role in ecosystem nutrient cycling in both natural and plantation forests and this information can then be used to improve forest management strategies (e.g. tree species selection) in response to chronic atmospheric N deposition and to global climate change.
In this study, we investigated characteristics of growth, morphological traits and nutrient concentrations (including C, N, phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg)) of fine roots in litter layer exposed to different levels of N addition and in the upper mineral soil layer (0–10 cm). We hypothesized that fine roots in the litter layer express a more nutrient acquisitive trait strategy as opposed to a relatively more nutrient conservative trait strategy of roots in the mineral soil layer such that (1) fine roots in leaf litter layer would be thinner with lower RTD and have higher SRL, SRA, and would have higher percentages of fine roots in the lower orders than those in 0–10 cm mineral soil layer; and that (2) fine roots in leaf litter layer would have significantly higher nutrient concentrations than those in 0–10 cm mineral soil layer. Because N is a key limiting nutrient regulating root growth, we hypothesized that (3) root production and growth into the litter layer will increase with N addition. Finally, we hypothesized that (4) N addition will increase root nutrient concentrations and SRL, as well as decrease RTD and average root diameter in the litter layer as roots displaying traits more associated with an acquisitive strategy would be produced with increased N availability.
Materials and methods
Site description and experimental design
In March 2014, a chronic N addition experiment was initiated in a 22-year-old Mytilaria laosensis (Hamamelidaceae) plantation at the Xiayang forest farm (26°48′N, 117°58′E), northwest Fujian Province, in Southeastern China. The site has a deep red soil classified as a sandy clay loam Ferric Acrisol according to the FAO/UNESCO classification. The experimental site has a humid subtropical climate, with short and mild winters in January and February, and long, hot and humid summers between June and October (Huang et al. 2014). Spring and autumn are warm transitional periods. Annual precipitation is concentrated in spring and summer (Wan et al. 2015). Mean annual rainfall and average temperature are 1940 mm and 20.5 °C, respectively (Fig. 1). Stand density at the study site is 2500 stems ha−1 (Huang et al. 2014). We collected the data of monthly mean air temperature and monthly mean precipitation from Nanping’s Bureau of Meteorology 10 km from the experimental site.
A randomized complete block design was employed with three replicate blocks with approximately 10 m buffers left between blocks. Each block contained three 10 × 10 m plots (a total of 9 plots) with >3 m distances between the plots within each block. One of the three following treatments was randomly assigned to one plot in each block: 1) control, no N addition; 2) 10 kg N ha−1 applied monthly as NH4Cl for one year; and 3) 30 kg N ha−1 applied monthly as NH4Cl for one year. Three 0.5 × 0.5 m sub-plots within the plots (a total of 27 sub-plots) were randomly set-up to quantify root proliferation into the litter layer. The leaf litter was cleared from the sub-plots, 0.5 × 0.5 m nylon nets of 2 mm mesh window screen were then placed horizontally on the soil surface in the sub-plots and the mesh anchored firmly to the soil surface using corrosion-resistant PVC tubes of 3 cm diameter in each corner (method see Sayer et al. 2006). N addition started on March 15th, 2014, fertilizers were applied once per month as solid powder on non-rainy days. The locations of the mesh screens were marked with red nylon rope to ensure that they would not be disturbed, and the equivalent mass of leaf litter was replaced on the mesh screens.
Root production
Fine-root production in the leaf litter layer was measured by harvesting fine roots which had grown through the mesh screen each month from April 15th, 2014 to March 15th, 2015 (except February 2015). This was done by first carefully removing the leaf litter on the nets before harvesting fine roots. The roots were then cut from the screen using a knife. Afterwards, we placed an equivalent mass of leaf litter back onto the screens, after which the N addition treatments were applied. All fine roots were oven-dried (60 °C, for 48 h) and weighed. The repeated harvesting used in this approach may have biased production estimates higher due to the repeated wounding during harvests. However, the repeated harvests represented a tractable way to examine treatment effects on fine-root traits in the litter layer without confounding effects of microsite, and it also provided the clearest sense to total production together with monthly patterns. Additionally, we acknowledge that there may be some artifact of repeated sampling from the in-growth methods due to the repeated wounding and young root age (always <1 month) which could impact production rates and root morphology. To minimize impacts on root morphology (see methods below), we were careful to not include roots that appeared to still be actively growing (based on appearance; short, bright white in color, stunted architecture) in our morphological analyses. Previous observations of root morphology generally indicate minimal changes once active growth/elongation is complete until later senescence and decomposition.
Root sampling, morphology and chemistry
Fine-root biomass (diameter < 2 mm) in the 0–10 cm mineral soil layer was determined with the coring method (Vogt et al. 1998) from April 15th, 2014 to March 15th, 2015 (except February 2015). Each time, ten soil cores were randomly extracted from each block using a 3.5-cm-diameter hole saw. Fine roots were carefully removed from the soil by tweezers and placed in water to clean off the adhering soil and organic matter particles.
Fine roots collected on August 15, 2014 from the litter layer and upper mineral soils were kept intact, and scanned at a resolution of 400 dpi on a desktop scanner (Epson Expression 10000XL scanner). Background impurities in each image were removed using Adobe Photoshop version 8.0 LE (Adobe Systems) prior to further analysis. Total root length and root morphological traits (mean specific root length (SRL), mean specific root area (SRA) and mean root diameter) were quantified by analyzing scanned images with WinRHIZO Arabidopsis version 2012b (Regents Instruments Inc., Quebec Canada). Roots were then oven-dried (60 °C, for 48 h) and weighed and mean root tissue density (RTD) was determined by dividing root weight by root volume estimated by WinRHIZO.
Dried root samples were ground in a Wiley mill to pass a 40-mesh screen, digested in HNO3-H2O2. The concentration of fine-root phosphorus (P) was measured using pushing continuous-flow autoanalyzer (Autoanalyzer 3, Bran and Luebbe, Germany). The concentrations of fine-root potassium (K), calcium (Ca), and magnesium (Mg) were measured using atomic absorption spectroscopy (TAS-990, China). The concentrations of fine-root C and N were determined by using a Vario MAX elemental analyzer (Elementar, Germany). Fine-root nutrient concentrations were measured from each monthly sample, except January 15, 2015 as a result of low root production and not enough samples.
All live and intact root branches sampled at August 15, 2014 were hierarchically dissected into branch orders after scanning the bulk roots to determine patterns of biomass partitioning within the fine-root system, following the protocol described by Pregitzer et al. (2002). The most distal roots were defined as the first order roots, where two first order roots derived was considered the second order roots, etc. Fine roots were sorted into four orders (first order, second order, third order and then all higher orders were grouped together) before drying. Once dissected, roots were oven-dried (60 °C, for 48 h) and weighed.
Soil chemistry
Mineral soil N was extracted from field moist samples from each monthly sample by shaking with 2 M KCl at a soil to solution ratio of 1:10 (w/w), followed by centrifuging at 2000 rpm for 20 min. The supernatant was filtered through Whatman 42 filter paper. Soil dissolved organic N (DON) and dissolved organic C (DOC) was extracted monthly in cold water. Water extracts were prepared by mixing 10 g field moist soil and 40 ml distilled water on an end-to-end shaker for 1 h. The mixture was then centrifuged at 3500 rpm for 20 min and filtered through Whatman 42 paper and then a 0.45 μm filter membrane. The organic C concentrations in the water extracts were determined using a SHIMADZU TOC-VCPH/CPN analyzer (Germany), and mineral N and DON was determined in the supernatant using an automated ion analyzer (Quik Chem method 10–107-064-D for NH4 + and 10,107–04-1-H for NO3 −, Germany).
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine the impact of treatments on fine-root morphology and order structure. Effects of treatments on fine-root production and nutrient concentrations were determined by using repeated-measures ANOVA. Pearson correlation analysis was performed to determine the relationships between fine roots of litter layer and soil nutrient concentrations (soil DOC, DON and mineral-N concentrations), and environmental variables (mean monthly temperature and precipitation). Statistical analyses were performed using SPSS 11.5. Throughout the text, differences were considered significant if P ≤ 0.05.
Results
Environmental variables and soil nutrient concentrations
Monthly mean air temperature ranged from 10.1 °C in December 2014 to 29.7 °C in August 2014 (Fig. 1). Monthly mean precipitation ranged from 18.6 mm in December 2014 to 443.3 mm in May 2014 (Fig. 1). Monthly patterns of available soil nitrogen were irregular but tended to peak in winter (Fig. 2). Soil mineral-N ranged from 3.8 mg kg−1 in September 2014 to 10.1 mg kg−1 in January 2015. Soil DON varied between 3.2 mg kg−1 in March 2015 and 11 mg kg−1 in December 2014. Soil DOC ranged from 26.4 mg kg−1 in April 2014 to 70.9 mg kg−1 in January 2015 (Fig. 2).
Fine-root production and biomass
Repeated-measures analysis of variance (ANOVA) identified significant effects of N addition on fine-root production in the litter layer (Table 3, P = 0.007, F = 12.5, df = 2). Fine-root production in the litter layer in the Mytilaria laosensis plantation was 2.6 g m−2 yr.−1 in the control treatment, and increased to 9.0 and 13.1 g m−2 yr.−1 in the 10 kg N ha−1 month−1 and 30 kg N ha−1 month−1 treatments, respectively (Fig. 3). Overall, fine-root production in the 10 kg N ha−1 month−1 and 30 kg N ha−1 month−1 treatments was 3.4- and 5.0-fold higher than the control treatment, respectively, but there was no significant difference between 10 kg N ha−1 month−1 and 30 kg N ha−1 month−1 treatments. Maximum production was 0.46 g m−2 in September 2014 in the control treatment and in July 2014 for the fertilized treatments while the minimum was 0 g m2 yr.−1 in January for the control and the 10 kg N ha−1 month−1 treatment (Fig. 3a).
Monthly patterns of fine-root production in the litter layer (a) and standing biomass of fine roots (b) in 0–10 cm mineral soils in a Mytilaria laosensis plantation. The plots were subject to the following treatments: Control treatment in the leaf litter layer without any N addition; N addition at rate of 10 kg N ha−1 month−1 (or N1); N addition at rate of 30 kg N ha−1 month−1 (or N2) in the leaf litter layer. Different lowercase letters above bars show significant difference under different levels of N addition (P < 0.05). Vertical bars represent the standard error (the same below)
Mean fine-root standing biomass in the 0–10 cm mineral soil layer was 157.2 g m−2. Standing biomass reached its maximum in June (220.5 g m−2) and the minimum in April (118.6 g m−2) (Fig. 3b). There was no significant correlation between fine-root production in the litter layer and standing fine-root biomass in the 0–10 cm mineral soil layer (P > 0.05, R = 0.109). Pearson correlation analysis also revealed no relationship between climate (monthly mean air temperature and monthly mean precipitation) and edaphic factors (soil DOC, DON and mineral-N), and fine-root production of the litter layer (P > 0.05).
Fine-root morphology and root order structure
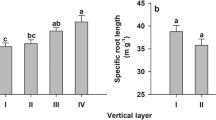
Fine roots in the litter layer were significantly thinner, had significantly higher specific root length (SRL), specific root area (SRA) and lower root tissue density (RTD) than those in the 0–10 cm mineral soil layer (Table 1, P < 0.05). N addition had no significant effect on fine-root morphological traits except for root diameter. The 30 kg N ha−1 month−1 treatment significantly decreased fine-root diameter relative to the control treatment, but no significant difference was observed between the 10 kg N ha−1 month−1 and the 30 kg N ha−1 month−1 treatments. There were significantly higher percentages of fine-root biomass in the absorptive first and second orders compared to higher order roots in the litter layer than in the 0–10 cm mineral soil layer. The 30 kg N ha−1 month−1 treatment significantly increased percentage of fine roots in the first and second orders, but had no significant effect on other root orders (Table 2, P > 0.05).
Fine-root nutrient concentrations
Fine roots in the litter layer had significantly higher carbon (C) and N concentrations, and lower C:N ratio (C/N) than those in the 0–10 cm mineral soil layer. Repeated-measures ANOVA showed that N addition significantly decreased C/N and increased N concentration of fine roots in the litter layer compared to the control treatment (Table 3, P < 0.01, F = 21.0, df = 2). Greater fine-root N concentration and slightly lower fine-root C/N were found in the 30 kg N ha−1 month−1 treatment than the 10 kg N ha−1 month−1 treatment. No significant effect of N addition was observed on fine-root C concentration (Fig. 4, P > 0.05, F = 0.9, df = 3). Similarly, higher phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) concentrations were observed in fine roots of the litter layer compared to those in the 0–10 cm mineral soil layer (Fig. 5, P < 0.05). N addition also significantly increased P, K and Ca concentrations of fine roots in the litter layer (Table 3, P < 0.05). There was a significant difference in fine-root Ca concentration between the 10 kg N ha−1 month−1 and the 30 kg N ha−1 month−1 treatments, but no difference in fine-root P and K concentrations between the two treatments (Fig. 5, P > 0.05). There was no significant effect of N addition on fine-root Mg concentration (Table 3, P > 0.05, F = 0.3, df = 2).
Fine-root carbon (C), nitrogen (N) concentrations and C:N ratio among different treatments in a Mytilaria laosensis plantation. The plots were subject to the following treatments: Control treatment in the leaf litter layer without any N addition; N addition at rate of 10 kg N ha−1 month−1; and N addition at rate of 30 kg N ha−1 month−1 in the leaf litter layer. The soil layer symbol represents nutrient concentrations of roots in the 0–10 cm mineral soil layer
Fine-root phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) concentrations among different treatments in a Mytilaria laosensis plantation. The plots were subject to the following treatments: Control treatment in the leaf litter layer without any N addition; N addition at rate of 10 kg N ha−1 month−1; and N addition at rate of 30 kg N ha−1 month−1 in the leaf litter layer. The soil layer symbol represents nutrient concentrations of roots in the 0–10 cm mineral soil layer
Discussion
Root proliferation into the litter layer
Root production in the litter layer has been largely ignored in previous studies (Sayer et al. 2006). This is partly due to the fact that most other research methods such as minirhizotron methods generally exclude fine roots in the litter layer from their monitoring scope. In this study we utilized mesh screens to estimate of root production in the litter layer and observed an annual production rate of 2.6 g dry weight m−2. This estimate is lower than the results of Sayer et al. (2006) (5.7 g dry weight m−2), which may result from higher overall fine-root biomass in their tropical experimental site (e.g. an average of 219 vs. 157 g dry weight m−2 in 0–10 cm mineral soil layer in their study and our study, respectively). Assuming steady state (i.e. annual production ~ annual mortality), production amounts observed in our study would equate to 10.5 kg C ha−1 yr.−1 contributed by turnover of fine roots within the litter layer (calculated by root production × root carbon (C) concentration, data not shown). This indicates that a large mass of fine-root C is neglected by methods that do not directly account for this fine-root pool.
Fine-root proliferation into relatively intact, undecomposed and fresh leaf litter in our study is consistent with observations of several previous studies (Herrera et al. 1978; Jordan and Escalante 1980; Cuevas and Medina 1988; Sayer et al. 2006). In addition to fine-root proliferation into old established organic layers, which has been well recognized (Chuyong et al. 2002; Coomes and Grubb 1996; LaClau et al. 2004; Stark and Jordan 1978; Stark and Spratt 1977), Sayer et al. (2006) suggested that roots may also respond rapidly to relatively recent additions of fresh leaf litter. Fine-root proliferation into litter layers has generally been seen as an adaption to acquire nutrients in infertile and highly leached soils when growth is limited by nutrient availability (Laclau et al. 2004; Vogt et al. 1995). However, Sayer et al. (2006) and Cotrufo (2006) argued that root proliferation into the litter layer occurs in response to the more easily obtainable nutrients in the organic matter relative to the mineral soils, and is not necessarily an adaptation to low soil fertility. Thus, root proliferation into the litter layer could occur at any site regardless of soil fertility provided there is a persistent organic layer and sufficient moisture. Total mass of standing litter in our experimental site averages 7.9 Mg ha−1, and receives annual litter inputs of 9.5 Mg ha−1 (Huang et al. 2014) which represent a significant and pool of potentially available nutrients.
In addition to observing significant amounts of fine-root production biomass in the litter layer, our study observed that these roots had higher nutrient concentrations, SRL, specific root area (SRA), thinner diameter, and lower root tissue density (RTD) than fine roots in the upper mineral soil layer. These changes in fine-root traits may reflect adaptation to higher nutrient availability in the litter layer and are consistent with the increased proportion of lower order fine roots which are more active in nutrient acquisition. Moreover, the significant effect of N addition on fine-root production in the litter layer and the absence of a significant correlation between seasonal fine-root production and mineral soil nutrient concentrations also support the conclusions of Sayer et al. (2006) and Cotrufo (2006) that root exploitation of the litter layer is controlled more by the quality and relatively consistent availability of the surface organic matter than by the nutrient availability of the mineral soil layers.
Although no significant correlation was observed between climate factors and root production, the lowest rate of fine-root growth in the litter layer was found in January. This is consistent with a previous study from this site which reported that fine-root length production in the mineral soil layer was lowest during the same period and corresponds to the annual minimum for soil temperature (Huang et al. 2014). Other studies have reported that soil temperature can influence root production, including the initiation and cessation of root growth, cell elongation, root length and diameter extension, and root branching patterns (McMichael and Burke 1998). If soil moisture and nutrient availability are adequate, rates of root length extension and root mortality increase with increasing soil temperature, at least up to an optimal temperature for root growth (Burke and Raynal 1994; Pregitzer et al. 2000). The lack of correlation observed in this study may be due to the lack of a distinct cold season with persistent temperature approaching freezing.
Responses of root traits to N addition
Exogenous N addition elicited variable responses among the fine-root traits studied in our experiment. The significant effect of N addition on fine-root production suggests that N may be a driving factor regulating fine-root growth into the litter layer (Lõhmus et al. 2006). Increased fine-root production in the litter layer under N addition is consistent with most studies, including a recent meta-analysis (Li et al. 2015), which reported the positive response of fine-root production to N addition. According to previous studies, plants often modify root growth and enhance root turnover to maintain efficient soil nutrient foraging strategy under exogenous N addition (Adams et al. 2013; Kou et al. 2015; Mei et al. 2010). Due to the difficulty in monitoring root dynamics in the litter layer, we could not directly measure root longevity and turnover rates in this horizon. However, the decreased root diameter as well as enhanced root N may indicate decreased root longevity and increased turnover rate of fine roots (Valverde-Barrantes et al. 2007; McCormack et al. 2012) in the litter layer and under N addition, though the specific effects of N addition on root lifespan are difficult to predict and may be determined by background levels of N availability as well as total tree N demand (McCormack and Guo 2014).
Contrary to our expectation, we observed few changes in root morphology with N addition. While we did observe changes in root diameter, there were no significant changes in SRL, SRA and STD in the litter layer in response to N addition. The lack of change in SRL under N addition is consistent with the study of Tobner et al. (2013) who reported that SRL did not vary with changing soil conditions. Moreover, SRL is inherently codetermined by root diameter and RTD (Ostonen et al. 2007). Thus, slightly higher root RTD under N addition relative to the control treatment may counterbalance the decrease in root diameter and provide an alternative explanation for the absent response of root SRL to N addition. Conservatism in root SRA under N addition may also result from the decrease in root diameter being counterbalanced by a trend toward enhanced root length.
Changes in root branching architecture are often accompanied by changes in root morphology and root chemistry. Plants may allocate relatively more C to low-order (absorptive/fibrous) roots, and reduce C allocation to high-order (transport/framework) roots when N becomes more available (Wang et al. 2013), due to higher absorptive capacity of fibrous roots (Zadworny and Eissenstat 2011). This phenomenon provides an additional explanation for the decrease in average root diameter with N addition as well as the increase in root tissue N as distal, absorptive roots tend to be smaller in diameter and higher in N concentration than higher order roots. Supporting our experimental results, other studies reported enhanced root N concentration and decreased root C:N ratio (C/N) with N addition (Hendricks et al. 2000; Li et al. 2015).
Although responses of fine-root N concentration to N addition have been extensively measured (Persson et al. 1998; Hendricks et al. 2000), the effects of N addition on other nutrients of fine roots are less commonly reported. Stimulated absorption of P, K and Ca of fine roots in the litter layer may occur with increased fine-root N concentration as tissues aim to keep proper stoichiometric balance between N and other elements. This may also indicate the existence of multiple nutrient limitation at the root or whole-plant level (Wright and Corre 2011; Wurzburger and Wright 2015). In our study, both levels of N addition (10 and 30 kg N ha−1 month−1) increased root N, P, K, and Ca concentrations relative to the control treatment. However, while root N concentration was further increased in the highest level of fertilization (30 kg N ha−1 month−1 treatment) relative to the lower level (10 kg N ha−1 month−1 treatment), the concentrations of P and K did not further increase with the 30 kg N ha−1 month−1 treatment relative to the 10 kg N ha−1 month−1 treatment. This indicates the possibility that these nutrients become increasingly limiting with chronic N additions (Smithwick et al. 2013).
Future directions
Ma et al. (2012) highlighted the multiple functions of fine roots in litter layers including their roles in stimulating microbial activity and subsequent litter decomposition in forests. In this study, nutrient concentrations were generally higher and C/N ratio lower among roots in the litter layer compared to the mineral soil layer. This may have been partly due to age differences between root samples collected from the litter layer, which were all less than one month old, and roots in the mineral soil which may have been substantially older. Based on previous studies, roots with high root N concentration and low C/N ratio are generally associated with greater root production, shorter root longevity and greater root decomposition rate (McCormack et al. 2012; Silver and Miya 2001; Tjoelker et al. 2005). Therefore, the persistent differences in root N concentration as well as their thinner diameters may indicate that fine roots in the litter layer are associated with faster turnover rate compared to fine roots of mineral soil layer. Increased fine-root production in the litter layer with N addition may also suggest an increasingly important role for these roots in forest nutrient cycles under increased anthropogenic N deposition in the future. However, feedbacks between fine-root dynamics and microbial function in organic horizons remain unclear, but may also have significant impacts on forest nutrient cycles.
Colonization of fine roots by mycorrhizal fungi also plays an important role in plant nutrient acquisition and nutrient cycling of forest. Mycorrhizal fungi have some advantages over roots in exploring and exploiting soil nutrient heterogeneity because their hyphae can provide a greater surface area per unit mass than absorptive roots (Chen et al. 2016). Conversely, plant fine roots often live much longer and require less frequent replacement than do fungal hyphae. This ultimately leads to a tradeoff, or complementarity among roots and mycorrhizal fungi enabling different strategies for nutrient acquisition and different degrees of plant reliance on mycorrhizal fungi that may shift as soil nutrient availability changes (Liu et al. 2015a; Cheng et al. 2016). Previous work has demonstrated a general reduction of mycorrhizal colonization with fertilization (Treseder 2004; Liu et al. 2015a), but similar studies about mycorrhizal colonization of fine roots in the litter layer are rare. Therefore, microbial or/and fungal responses together with changes in fine-root growth into litter layers under anthropogenic N deposition require further research.
Conclusion
The vital role of fine roots in the litter layer has been previously determined, but appreciation of their specific root traits and their responses to N addition in subtropical area is quite limited. In our study, we found significant differences in morphological traits and nutrient concentrations among litter and mineral soil layers. Meanwhile, strong responses of fine-root production, root diameter and nutrient concentrations to N addition were observed in the litter layer. These observations suggest that N is a key factor determining fine-root growth into the litter layer and that these roots may ultimately play an important role in resource allocation and acquisition strategies in forest ecosystems.
Reference
Adams TS, Mccormack ML, Eissenstat DM (2013) Foraging strategies in trees of different root morphology: the role of root lifespan. Tree Physiol 33(9):940–948
Burke MK, Raynal DJ (1994) Fine root growth phenology, production, and turnover in a northern hardwood forest ecosystem. Plant Soil 162(1):135–146
Chen XY, Mulder J (2007) Indicators for nitrogen status and leaching in subtropical forest ecosystems, South China. Biogeochem 82(2):165–180
Chen W, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016) Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. PNAS 113(31):8741–8746
Cheng L, Chen W, Adams TS, Wei X, Li L, McCormack ML, DeForest J, Koide R, Eissenstat DM (2016) Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology. doi:10.1002/ecy.1514
Cheng W (2009) Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C–N budgets. Soil Biol Biochem 41(9):1795–1801
Chuyong GB, Newbery DM, Songwe NC (2002) Litter breakdown and mineralization in a central African rain forest dominated by ectomycorrhizal trees. Biogeochem 61(1):73–94
Coomes DA, Grubb PJ (1996) Amazonian caatinga and related communities at La Esmeralda, Venezuela: forest structure, physiognomy and floristics, and control by soil factors. Plant Ecol 122(2):167–191
Cotrufo MF (2006) Quantity of standing litter: a driving factor of root dynamics. Plant Soil 281(1–2):1–3
Cuevas E, Medina E (1988) Nutrient dynamics within Amazonian forests. II: fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76(2):222–235
Eissenstat DM (1991) On the relationship between specific root length and the rate of root proliferation: a field study using citrus rootstocks. New Phytol 118(1):63–68
Eissenstat DM, Kucharski JM, Zadworny M, Adams TS, Koide RT (2015) Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol 208(1):114–124
Fujimaki R, McGonigle TP, Takeda H (2005) Soil micro-habitat effects on fine roots of Chamaecyparis obtusa Endl.: a field experiment using root ingrowth cores. Plant Soil 266(1–2):325–332
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320(5878):889–892
Hendricks JJ, Aber JD, Nadelhoffer KJ, Hallett RD (2000) Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 3(1):57–69
Herrera R, Jordan CF, Klinge H, Medina E (1978) Amazon ecosystems: their structure and functioning with particular emphasis on nutrients. Interciencia 3(4):223–232
Huang ZQ, Liu B, Davis MR, Sardans J, Peñuelas J, Billings S (2016) Long-term nitrogen deposition linked to reduced water use efficiency in forests with low phosphorus availability. New Phytol 210:431–442
Huang ZQ, Yu ZP, Wang MH (2014) Environmental controls and the influence of tree species on temporal variation in soil respiration in subtropical China. Plant Soil 382(1–2):75–87
John TVS (2011) Response of tree roots to decomposing organic matter in two lowland Amazonian rain forests. Can J For Res 13(2):346–349
Jordan CF, Escalante G (1980) Root productivity in an Amazonian rain forest. Ecology 61(1):14–18
Kou L, Guo D, Yang H, Gao W, Li S (2015) Growth, morphological traits and mycorrhizal colonization of fine roots respond differently to nitrogen addition in a slash pine plantation in subtropical China. Plant Soil 391(1–2):1–12
Lõhmus K, Truu M, Truu J, Ostonen I, Kaar E, Vares A, Uri V, Alama S, Kanal A (2006) Functional diversity of culturable bacterial communities in the rhizosphere in relation to fine-root and soil parameters in alder stands on forest, abandoned agricultural, and oil-shale mining areas. Plant Soil 283(1):1–10
Laclau JP, Toutain F, M’Bou AT, Arnaud M, Joffre R, Ranger J (2004) The function of the superficial root mat in the biogeochemical cycles of nutrients in Congolese Eucalyptus plantations. Ann Bot 93(3):249–261
Lebauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89(2):371–379
Li W, Jin C, Guan D, Wang Q, Wang A, Yuan F, Wu J (2015) The effects of simulated nitrogen deposition on plant root traits: a meta-analysis. Soil Biol Biochem 82:112–118
Liu B, Li H, Zhu B, Koide RT, Eissenstat DM, Guo D (2015a) Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol 208(1):125–136
Liu RQ, Huang ZQ, He ZM, Wan XH, Yu ZP, Zheng LJ, Xiao HY (2015b) Effect of root removal on litter decomposition in plantations of Mytilaria laosensis and Cunninghamia lanceolata. Sci Silv Sin 51(9):1–8 In Chinese, Abstract in English
Ma CE, Kong DL, Chen ZX (2012) Root growth into litter layer and its impact on litter decomposition: a review. Chin J Plant Ecol 36(11):1197–1204 In Chinese, Abstract in English
Majdi H, Öhrvik J (2004) Interactive effects of soil warming and fertilization on root production, mortality, and longevity in a Norway spruce stand in northern Sweden. Glob Chang Biol 10(2):182–188
Matson P, Lohse KA, Hall SJ (2002) The globalization of nitrogen deposition: consequences for terrestrial ecosystems. Ambio 31(2):113–119
McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM (2012) Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195(4):823–831
McCormack ML, Guo D (2014) Impacts of environmental factors on fine root lifespan. Front in Plant Sci 5:205
McMichael BL, Burke JJ (1998) Soil temperature and root growth. Hort Sci 33(6):947–951
Mei L, Gu JC, Zhang ZW, Wang ZQ (2010) Responses of fine root mass, length, production and turnover to soil nitrogen fertilization in Larix gmelinii and Fraxinus mandshurica forests in northeastern China. J For Res 15(3):194–201
Nadelhoffer KJ (2000) The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol 147(1):131–139
Noguchi K, Nagakura J, Kaneko S (2013) Biomass and morphology of fine roots of sugi (Cryptomeria japonica) after 3 years of nitrogen fertilization. Front in Plant Sci 4(1):347
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM (2007) Pronk a (2007) specific root length as an indicator of environmental change. Plant Biosyst 141:426–442
Persson H, Ahlström K, Clemensson-Lindell A (1998) Nitrogen addition and removal at Gårdsjön — effects on fine-root growth and fine-root chemistry. For Ecol Manag 101(1):199–205
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine north American trees. Ecol Monogr 72(2):293–309
Pregitzer KS, King JS, Burton AJ, Brown SE (2000) Responses of tree fine roots to temperature. New Phytol 147(1):105–115
Sayer EJ, Tanner EVJ, Cheesman AW (2006) Increased litterfall changes fine root distribution in a moist tropical forest. Plant Soil 281(1–2):5–13
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129(3):407–419
Smithwick EAH, Eissenstat DM, Lovett GM, Bowden RD, Rustad LE, Driscoll CT (2013) Root stress and nitrogen deposition: consequences and research priorities. New Phytol 197(3):712–719
Solly EF, Schöning I, Herold N, Trumbore SE, Schrumpf M (2015) No depth-dependence of fine root litter decomposition in temperate beech forest soils. Plant Soil 393(1–2):1–10
Stark N, Spratt M (1977) Root biomass and nutrient storage in rain forest oxisols near San Carlos de Rio Negro. Trop Ecol 39:1004–1015
Stark NM, Jordan CF (1978) Nutrient retention by the root mat of an Amazonian rain forest. Ecology 59(3):434–437
Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559
Tian D, Niu S (2015) A global analysis of soil acidification caused by nitrogen addition. Environ Res Lett 10(2):24019–24028
Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167(2):493–508
Tobner CM, Paquette A, Messier C (2013) Interspecific coordination and intraspecific plasticity of fine root traits in north American temperate tree species. Funct Ecol 4(1):242
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164(2):347–355
Valverde-Barrantes OJ, Raich JW, Russell AE (2007) Fine-root mass, growth and nitrogen concentration for six tropical tree species. Plant Soil 290(1–2):357–370
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 200(1):71–89
Vogt KA, Vogt DJ, Palmiotto PA, Boon P, O’Hara J, Asbjornsen H (1995) Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 187(2):159–219
Wahl S, Ryser P (2002) Root tissue structure is linked to ecological strategies of grasses. New Phytol 148(3):459–471
Wan X, Huang Z, He Z, Yu Z, Wang M, Davis MR, Yang Y (2015) Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387(1–2):103–116
Wang FC, Fang XM, Ding ZQ, Wan SZ, Chen FS (2016a) Effects of understory plant root growth into the litter layer on the leaf litter decomposition of two woody species in a subtropical forest. Forest Ecol Manag 364:39–45
Wang G, Fahey TJ, Xue S, Fang L (2013) Root morphology and architecture respond to N addition in Pinus tabuliformis, West China. Oecologia 171(2):583–590
Wang W, Wu X, Hu K, Liu J, Tao J (2016b) Understorey fine root mass and morphology in the litter and upper soil layers of three Chinese subtropical forests. Plant Soil 406(1):1–12
Wright SJ, Corre MD (2011) Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 92(8):1616–1625
Wurzburger N, Wright SJ (2015) Fine root responses to fertilization reveal multiple nutrient limitation in a lowland tropical forest. Ecology 96(8):2137–2146
Zadworny M, Eissenstat DM (2011) Contrasting the morphology, anatomy and fungal colonization of new pioneer and fibrous roots. New Phytol 190(1):213–221
Acknowledgement
The research was supported by a National Natural Science Foundation of China (41371269, 31570604 and 31625007) and the National “973” Program of China (2014CB954002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alexia Stokes.
Rights and permissions
About this article
Cite this article
Liu, R., Huang, Z., Luke McCormack, M. et al. Plasticity of fine-root functional traits in the litter layer in response to nitrogen addition in a subtropical forest plantation. Plant Soil 415, 317–330 (2017). https://doi.org/10.1007/s11104-016-3168-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3168-7