Abstract
Background and aims
A study was made to quantify early root development, soil exploitation and nutrient uptake in spring wheat, onion and lettuce, and their variation among cultivars. The goal was to study genetic variation in root traits making cultivars better adapted to organic production systems or other low-input systems.
Methods
Six cultivars of each species were grown in transparent tubes to allow direct observation of early root growth. The tubes were 0.3 m deep, and 0.24 m in diameter. By placing the plants close to the edge rather than at the centre of the tubes, we could quantify the spatial distribution of the root systems as well as the general root growth and nutrient uptake.
Results
Root growth of wheat and lettuce was faster than root growth of onion, and onion showed little capacity for horizontal root system development. Significant variation in early root growth and horizontal spread of the root system was found among cultivars of all three species. In general, cultivars with strong growth and high volume of soil exploitation showed higher average nutrient concentrations.
Conclusion
Early shoot growth, root growth and nutrient uptake are intrinsically linked, making it difficult to determine whether improved root growth was the primary cause of improved performance. However, we did find cultivars where the strong root growth and superior root distribution seemed to be the driver for improved overall growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient availability is a limiting factor in organic plant production systems, and these systems are generally dependent on import of plant nutrients from neighboring organic or conventional farms, as well as urban waste streams such as bio-compost from green waste. With increasing focus on reducing the conventional import to ensure sustainable cropping systems (Oelofse et al. 2013), increased nutrient use efficiency should be an aim in organic agricultural systems. An efficient nutrient uptake is especially important in the plant establishment phase. At this stage plants still have small root systems, and nutrient uptake is thereby restrained by a limited soil root contact. In addition, in early spring nutrient levels in the soil are low, as mineralization of organically bound nutrients such as nitrogen (N), phosphorus (P), and sulphur (S) are limited by low soil temperatures. An extensive root growth and distribution in this critical establishment phase will enable the roots to exploit a larger soil volume, leading to more vigorous plant growth. Such early root traits could therefore be essential, in the search for nutrient efficient cultivars (White et al. 2013).
It is well known that fast and extensive establishment of roots in the top soil is especially important for uptake of immobile nutrients such as P (Gahoonia et al. 1999) as a larger root system will provide greater root-soil contact. During early establishment, root length is also limiting the uptake of more mobile nutrients such as N (Costigan 1987b; Costigan and Heaviside 1988). Though a lower root density is needed for efficient soil exploitation for N than for P, there is still an early period where this critical root density has not been reached, and much of the soil left unexploited by roots. Previous studies showed that early and abundant root growth could improve N uptake in wheat crops (Liao et al. 2004). Wheat cultivars, with extensive root branching and fast horizontal growth in the early growth phase, had an increased uptake of N compared to cultivars with less abundant root growth (Liao et al. 2006). With greater knowledge of the root growth patterns, their variability among cultivars and its effect on early nutrient uptake, plant breeding and agronomic practices such as timing, supply and placement of fertilizer could be improved (Costigan and Heaviside 1988).
Genotypic variation in root characteristics have been shown in many studies (Gahoonia and Nielsen 2004; Kerbiriou et al. 2013b; Manschadi et al. 2008; Ytting et al. 2014). However, the variation in root traits has been sparsely exploited in crop breeding programs, largely because of the difficulties of recording root traits in situ and screening large numbers of plants. Additionally, the structure of the root system is greatly influenced by environmental conditions such as soil type and availability of nutrients (Desnos 2008; Miller et al. 2003). This also makes phenotyping of root traits challenging. High throughput screening of early root growth are often performed in non-soil media such as hydroponics, agar plates and paper rolls, although root characteristics found in such systems are often different and not directly comparable to root growth in a soil environment (Wojciechowski et al. 2009). Another problem with such methods is that they do not provide information about the distribution of the roots in the soil. It is not only the total root length, branching etc. which determines how fast the plants are able to exploit all parts of the upper soil layers; also the 3D spatial distribution is important. The use of tube rhizotrons or other types of soil filled rhizotrons for root studies offers an intermediate approach between the laborious field studies and the use of laboratory techniques.
Current crop cultivars are being bred with focus on aboveground crop traits (Kerbiriou et al. 2014; Narayanan et al. 2014), and in addition, they are bred under conventional conditions of high nutrient availability (Kerbiriou et al. 2013a; Narayanan et al. 2014; Scholten and Kuyper 2012). For example, in lettuce, this has led to cultivars with large heads and small root systems, which perform well in intensive high input systems, but under-perform in conditions with limited resource availability (Kerbiriou et al. 2013a). For breeding genotypes able to perform well in soil with lower nutrient availability, such as many organic farming systems, there is a need for studies of root growth of current as well as older cultivars, to assess genotypic variances in root growth and the effect on nutrient uptake under nutrient limited conditions.
In this study, early root growth of current cultivars of spring wheat, onion and lettuce was studied in rhizotron tubes. The three species have different root growth patterns, and also different planting patterns. Onion and lettuce are both shallow-rooted, with rooting depths of less than 0.5 m. However, the root system of onion grows mainly vertically below the plant, whilst that of lettuce is also capable of horizontal spread (Thorup-Kristensen 2006). In contrast, spring wheat is deeper rooted, (Thorup-Kristensen et al. 2009) and is grown at higher plant density than onion and lettuce. Thus, the three crops studied are exposed to different challenges in the distribution of roots and in the soil exploitation.
The objective of this study was to quantify early root growth and soil exploitation in different cultivars of wheat, onion and lettuce. The root growth patterns; total root growth, horizontal and vertical root development, total volume of soil exploited by roots as well as nutrient uptake were determined. The aim was to study the variation in these traits among current commercial cultivars and a few older cultivars. We hypothesized that there were cultivar differences in early root growth and distribution and that cultivars with the largest soil volume exploitation had the highest nutrient uptake.
Materials and methods
Experimental set up
Cultivars of onion (Allium cepa L.), spring wheat (Triticum aestivum L.) and lettuce (Lactuca sativa L.) were included and grown organically. Six different cultivars were studied with four replicates, resulting in a total of 24 experimental units (tube rhizotrons) per crop. The experiment was conducted over three time periods; onions were grown from April 22nd to June 3rd, wheat from June 16th to July 17th and lettuce from August 15th to September 18th 2014, at The University of Copenhagen in Taastrup, Denmark (55°40’N; 12°18’E). The tubes were placed on three racks in a random block design, and placed outside under a rainout shelter. The average temperature in the three growth periods was 12, 17 and 15 °C, respectively.
The rhizotron tubes were 0.39 m high transparent acrylic tubes (PMMA), with an inner diameter of 0.24 m and an outer diameter of 0.25 m. In the bottom of the tubes, a plastic net was attached to hold back the soil while allowing the flow of water and air. Each rhizotron tube was wrapped in non-transparent plastic, black on the inside to prevent light exposure of the soil and the roots and white on the outside to reduce heating of the soil by sunlight. Non-transparent plastic was additionally wrapped around each rack, and the whole set-up was covered with a bird net to prevent damage to the plants.
The transparent material was chosen to allow direct observation of the roots in the tubes, making it possible to quantify the root length development, but also to identify the location of the roots. By planting at one point close to the inner tube surface, rather than placing the plants at the centre of the tube, each root observation could be identified with a vertical depth from the soil surface, as well as a horizontal distance from the planting position, allowing a 2D mapping of the root systems.
Plant material
Current and older cultivars of onion, wheat and lettuce were selected for use in the experiment (Table 1). The onion plants were pre-cultivated before transplanting. They were sown 11 March 2014 in peat plugs, with 6 seeds per plug, in a greenhouse, and moved outside after 3 weeks to improve hardiness before transplanting into the rhizotron tubes. The lettuce plants were pre-cultivated in a greenhouse before transplanting as well. The lettuce cultivars were sown 31 July 2014 except for ‘Intred’ which was sown 3 days earlier 28 July 2014 to ensure transplantation of plants of equal size. The wheat cultivars were sown directly in the rhizotron tubes on 16 June 2014.
Planting, rhizotron tubes and fertilization
Forty-two days old onion plants and 15–18 days old lettuce plants were transplanted into rhizotron tubes, one planting plug per tube. The plants were planted 0.05 m from the inner tube wall. Wheat seeds were sown directly into the rhizotron tubes, four seeds in each tube, 0.03 m from the inner wall of the tube. After 9 days, one representative plant from each tube was chosen, and the other three seedlings removed carefully with a pair of tweezers. At planting of onion and lettuce 3 g organic of fertilizer (Bio grow 10:3:1, Daka Bio-industies, Ringsted, Denmark) was mixed into the soil in the area around the plant. In the tubes with wheat this was done after removing the extra plants.
Growing medium
The growing medium used for the experiment was an arable soil from organically grown fields at the University of Copenhagen, Taastrup, with a texture of 14 % clay (<0.002 mm), 12 % silt (0.002–0.02 mm), 44 % fine sand (0.02–0.2 mm), 29 % coarse sand (0.2–2.0 mm) and 2.3 % organic matter and with an available amount of P, K and Mg content of 16, 67 and 60 mg kg−1, respectively. The soil was air dried, sieved on a 10 mm sieve, homogenized in a pan mixer, and filled by hand into the rhizotron tubes. Approximately 1.5 L soil was packed in the tube at a time, by placing a metal plate on the top of the soil layer, and dropping a 2 kg stone from 0.2 m distance five times for each layer. The total soil weight in each column was approximately 25 kg, giving a bulk density in the columns of 1.4 g cm−3, 1.4 g cm−3 and 1.5 g dry soil cm−3 for onion, wheat and lettuce respectively. The water content in the soil at the time of packing was 10.8 % for the onion batch, 9.4 % for the wheat batch and 3.7 % for the lettuce batch.
Water supply and plant protection
Before transplanting the onion and lettuce plants, each planting hole was irrigated with a total of 200 mL water. At the time of sowing, 20 mL water was added in each hole where the wheat seeds were sown. In addition, bottles with a ceramic tip with a release rate of about 200 mL water every 24 h, was placed in the top soil of all rhizotron tubes. The bottles were filled with 400 mL water every 2–3 days and placed in different positions to provide all areas of the soil with water. A total amount of 5050 mL water per rhizotron was added during the 42 days growth period of onion, a total of 4000 mL water during the 31 days growth period of wheat, and a total of 4600 mL water during the 33 days growth of lettuce. The irrigation applied corresponded to 2.7, 2.9 and 3.1 mm day−1 for onion, wheat and lettuce respectively, which corresponds to the potential daily evaporation in Denmark.
Despite providing approximately the same amount of water to the soil in the lettuce experiment as in the other two experiments, the water was not able to penetrate into the deeper layers of the soil, affecting the root growth in the lowest part of the tube. Due to this the lettuce experiment was terminated before the roots reached the bottom of the tube.
A wick made of a double layer of Vattex (Garta A/S, Denmark) sheet (0.1 × 0.3 m) was placed under each tube, and hanging 0.05 m down from the bottom of the tube, to drain away excess water and prevent water logging of the soil in the bottom of the tubes.
The lettuce plants were sprayed with an insect soap, approved for organic production in Denmark (Tanaco insektsæbe, Tanaco A/S, Esbjerg, Denmark), two times during a 7 day period, to prevent a build-up of aphid colonies on the leaves.
Root measurements
A grid of 0.02 × 0.02 m squares, printed on transparent plastic sheets (0.30 × 0.78 m), was placed on the rhizotron surface, covering the whole outer area of the tube from the soil surface to the bottom. Roots were observed by eye and each root observation was combined with its vertical and horizontal distribution. The root intensity (RI = root crossings m−1) was assessed by counting the number of roots crossing the grid lines. Root assessments were made every 3–4 days after the appearance of the first roots on the rhizotron surface, and ended when the roots reached the bottom of the tubes. In the case of lettuce, the experiment was terminated when the roots reached the level of the waterfront in the tube, which in all tubes were between 0.20 and 0.24 m from the soil surface.
Plant harvest and analysis
The plants were harvested 31, 42 and 34 days after transplanting or sowing for wheat, onion and lettuce respectively, by cutting the plants at soil level. The plant material was oven dried at 70 °C for 48-72 h to determine dry weight. Total nitrogen content was determined by flash combustion (FLASH 2000 HT Elemental Analyzer (Thermo Scientific)) and plant material was analyzed for content of other important macro and micro nutrients (P, K, S, Mg, Ca, Al, B, Cu, Fe, Mn, Zn) (Agilent 5100 ICP-OES).
Calculations
Thermal time (°C day) was calculated as the accumulated average daily temperature over the whole growing period, with a base temperature of 0 °C.
The vertical and horizontal root growth was assessed as the vertical or horizontal distance from the plant where a minimum of five roots were recorded. The vertical and horizontal growth rate (mm °C day−1) was calculated as the average growth rate during a period including four measuring days. Selection criteria for these days were that the growth speed was at its highest for four succeeding measuring dates without being limited by the space in the tube. For wheat this was after 220–345 °C days, for onion after 224–356, and for lettuce after 303–456 °C days. For lettuce, vertical growth rate was not assessed, due to the problem with dry soil in the bottom of the tube.
Total root intensity was calculated as the total number of observed root crossings per meter grid line (crossings m−1) at a given time point in the experiment.
As the tube rhizotrons had an inner diameter of 0.24 m and the plants were positioned either 0.05 m (onion and lettuce) or 0.03 m (wheat) from the edge, we were able to record data for roots of onion and lettuce situated between 0.05 and 0.19 m away from the plants, and wheat roots between 0.03 and 0.21 m away from the plants. The tubes allowed us to record roots down to 0.3 m below the soil surface. Thereby the observations represented a soil volume of 0.0102 m2 for wheat and 0.0080 m3 for onion. Due to the problems in the lower parts of the soil in the lettuce experiment we could use only data from the top 0.2 m, and the total soil volume represented by the root observations was then 0.0053 m3. To study the root growth in different vertical and horizontal distances from the plant, the position of each observation on the tube was recorded, allowing the calculation of vertical as well as horizontal distance from the plant basis. The root intensity of different areas on the rhizotrons was calculated as number or roots observed in the area per m grid line within the relevant area. Volume of soil exploited by the root system was calculated based on the horizontal spread within each vertical soil layer. Each 2D position on the tubes was considered out of reach by the root system until a root appeared. When a root appeared, the volume of soil represented by this position horizontally and vertically was considered as within the soil volume exploited by the root system. As roots observed at increasing distance from the plant basis will represent larger volumes of soil exploited, the volume was calculated based on the distance of the observed roots from the plant multiplied by a weighting factor calculated from the area of the annulus (ring) these roots could exploit. This value was multiplied by the height of each square in the grid, giving the volume of soil exploited.
The area of each annulus was calculated as:
where A is the area of the different annuli and ri is radius of the inner circle and rj the radius of the outer circle of the annulus.
Statistical analysis
Significant differences in root growth, in aboveground biomass and in nutrient uptake were tested by analysis of variance. Multiple comparisons were based on values of least significant difference (LSD) derived from analyses of variance (Proc GLM, SAS Institute Inc., Cary, NC). In assessing differences between results, tests with p < 0.05 were considered statistically significant (* = p < 0.05; ** = p < 0.01; *** = p < 0.001).
Results
Root development over time
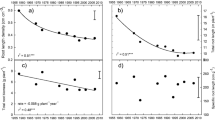
During the experiment, the total root growth was assessed every two to 3 days after the appearance of the first roots at around 120 °C days for all three species. Cultivar differences in root development were registered already at an early stage. The cultivar difference in total root intensity (Fig. 1), root distribution (Fig. 2) and volume soil exploited (Fig. 3) was consistent during the period of measurement, until the cessation of the experiment.
Root intensity (root crossings m−1) per degree day in six different cultivars of a wheat, b onion and c lettuce. Results are means of 4 replicates per cultivar and error bars show standard error. Asterisks indicate statistical significant differences (* = P < 0.05, ** = P < 0.01 and *** = P < 0.001)
Volume of soil (cm3) exploited by roots in six different cultivars of wheat, onion and lettuce. Results are means of four replicates of each cultivar. Asterisks indicate statistical significant differences (* = P < 0.05, ** = P < 0.01 and *** = P < 0.001). The variables and the correlation coefficients of the linear regressions are given in the table above the figure
Total root growth and root distribution
The total root intensity and the root distribution varied among the cultivars of the three species. At the cessation of the experiment, the wheat cultivar ‘Bittern’ had significantly (p < 0.01) higher total root intensity than the five other cultivars (Table 2). The total root intensity of 14.9 crossings m−1 was approximately twofold higherthan ‘Økilde’ and ‘Quintus’ after 502 °C days. The wheat cultivars also distributed the roots differently in the soil. At cessation of the experiment ‘Koga’ and ‘Økilde’ had more than 50 % of the roots within a distance of 0.03 to 0.08 m (Fig. 2a) from the plant, and only 8–9 % of the roots in a distance of more than 0.15 m (Fig. 2c) from the plant, while ‘Bittern’ had 14 % of the roots at this distance (Fig. 2c, Table 2). ‘Quintus’ was the cultivar with the lowest proportion of roots between 0.03 and 0.08 m from the plant (32 %), while having a significant (p < 0.01) higher proportion of roots (28 %) than the other five cultivars at a distance of more than 0.15 m from the plant (Fig. 2c, Table 2). Although ‘Quintus’ was found to have low total root intensity, this cultivar had the most horizontally distributed root system, which was also reflected in the fastest horizontal growth rate.
In onion, ‘Hylander’ had significantly (p < 0.001) higher root intensity than the other five cultivars after 531 °C days, with a total root intensity of 30.6 root crossings m−1. This was more than twice as much as ‘Bajosta’ which had the lowest total root intensity (Table 2). All onion cultivars had a high proportion of roots close to the plant, between 61 and 71 % of the roots at a distance of 0.05 to 0.08 m from the plant (data not shown). Differences were also found in root intensity of the lettuce cultivars (Table 2). The lettuce cultivar ‘Cegoline’ had the highest root intensity of 12.7 crossings m−1 after 488 °C days, followed by ‘Xaroma’ and ‘Olite’. The root intensity of ‘Cegoline’ was three times higher than the red lettuce cultivar ‘Intred’, and twice as high as ‘Ralph’ (Table 2). ‘Khan’, had the highest amount of roots (17 %) at a distance of more than 0.14 m from the plant, despite having the third lowest total root intensity (Table 2).
The results revealed that the wheat cultivar ‘Bittern’, the onion cultivar ‘Hylander’ and the lettuce cultivars ‘Cegoline’, ‘Xaroma’ and ‘Olite’ combine a fast growth with an extensive root distribution (Table 2). To some extent, a linear relationship was found between dry weight and total root intensity with r2 of 0.61, 0.82 and 0.69 respectively for wheat, onion and lettuce (data not shown). The results additionally showed that cultivars with high horizontal growth rates such as ‘Quintus’ were able to develop high root intensity in the area furthest away from the plant.
In general, variability between the species (Fig. 4) and between cultivars of each of the species (Fig. 5) was large. On average, wheat had more horizontal root growth initially, while onion had the majority of the roots right below the plant (Fig. 4). Lettuce had fast horizontal growth as well, while the dry conditions in the lower part of the rhizotron tube hampers firm conclusions about the vertical root growth. Substantial variability was found in amount and distribution of roots between the cultivars (Fig. 5).
Root intensity and root distribution in soil; means of six cultivars with 4 replicates of each cultivar of a wheat, b onion and c lettuce after 502, 531 and 488 °C days, respectively. The increasing darkness of the contours indicates increasing root intensity, the lightest color shows no roots (0 root crossings m−1 grid line) while the darkest color shows a root intensity of >60 root crossings m−1. Wheat seeds are sown 0.03 m from the inner tube wall, and the measured horizontal distance from the plant varies between 0.03 to 0.21 m. Onion and lettuce are transplanted 0.05 m from the inner tube wall, resulting in a measured horizontal distance from the plant from 0.05 to 0.19 m
Variability in root intensity and root distribution in three selected wheat cultivars; a Koga, b Bittern and c Quintus after 502 °C days, three onion cultivars; d Hybelle, e Hylander, f Bajosta after 531 °C days, and lettuce cultivars; g Intred, h Cegoline and i Khan after 488 °C days. Results are presented as means of 4 replicates of each cultivar. The increasing darkness of the contours indicates increasing root intensity, the lightest color shows no roots (0 root crossings m−1 grid line) while the darkest color shows a root intensity of >60 root crossings m−1. Wheat seeds are sown 0.03 m from the inner tube wall, and the measured horizontal distance from the plant varies between 0.03 to 0.21 m. Onion and lettuce are transplanted 0.05 m from the inner tube wall, resulting in a measured horizontal distance from the plant from 0.05 to 0.19 m
Among the crops, wheat had faster average growth rates than both onion and lettuce (Table 3). But although wheat grew faster, onion produced more roots. With an average total root intensity of 20.4 root crossings m−1 grid line, onion had twice the root intensity of wheat and lettuce, which had average total root intensities of 10.8 and 8.9 crossings m−1, respectively (Table 2). Onion generally had a large proportion of its roots in the soil volume close to the plant. On average only 7.8 % of the roots was found at a distance of more than 0.14 m from the plant. Wheat and lettuce on the other hand, had 14.7 and 15.3 % of the roots extending to a distance of more than 0.14 m at cessation of the study.
Root growth rates
We found significant cultivar differences in root growth rates, both vertically and horizontally. In wheat, ‘Bittern’ and ‘Trappe’ had the highest vertical growth rate, while ‘Quintus’ had the highest horizontal growth rate (Table 3). The horizontal growth rate was generally lower than the vertical growth rate. Yet the difference in growth rates of ‘Quintus’ was low with an average vertical growth rate of 0.68 mm day °C−1 and an average horizontal growth rate of 0.60 mm day °C−1. In contrast, the average vertical growth rate of ‘Bittern’ was high, 0.81 mm day °C−1, whereas the average horizontal growth rate was 0.55 mm day °C−1 (Table 3). In onion, the cultivar ‘Hylander’ had higher growth rates than the five other cultivars both vertically and horizontally. The vertical growth rate was not determined in lettuce, due to the lack of water in the lower layers, but ‘Xaroma’ had the highest horizontal growth rate, followed by ‘Khan’, ‘Cegoline’ and ‘Olite’ (Table 3).
Soil exploitation and nutrient uptake
Differences in the volume of soil exploitation were found between cultivars as well. The volume soil exploited developed in a linear way during the observation period of the three crops, and a rate of daily increase could be estimated (Fig. 3). In wheat, there was a tendency towards ‘Bittern’ and ‘Quintus’ having the highest soil exploitation rates with 5.47 × 10−6 and 4.78 × 10−6 m3 °C day−1. At cessation of the experiment, ‘Bittern’ and ‘Quintus’ roots exploited 2.74 × 10−3 m3 and 2.4 × 10−3 m3 soil. The volume of soil exploited by ‘Bittern’ was 81 % larger than by ‘Økilde’, and 45 % larger than ‘Koga’ (p < 0.05). Some of the difference was caused by the distribution of roots, i.e. ‘Koga’ showed a high total root growth, but as its root growth was more vertical, many roots exploited the same volume right beneath the plant, while soil at larger horizontal distance from the plant was not effectively exploited. In onion, ‘Hylander’ was superior in soil exploitation. ‘Hylander’ had the fastest exploitation rate and the total volume of soil exploited, 3.05 × 10−3 m3, was 47–95 % larger than for the other five cultivars (p < 0.01). Also in lettuce there were large differences, ‘Cegoline’ and ‘Xaroma’ exploited 1.71–1.85 × 10−3 m3 soil, which were two-threefold the volume of ‘Ralph’ and ‘Intred’ (p < 0.05).
As with the volume of soil exploited, a large cultivar variation in DW was seen. The average DW ranged from 0.6 to 0.9, 1.7 to 3.5 and 0.3 to 1.5 g plant−1 for wheat, onion and lettuce, respectively, and there was a positive correlation between the DW and the volume of soil exploited in all three crops (Fig. 6). The volume of soil exploited was also positively correlated to the root intensity, although in wheat, ‘Quintus’ had a low total root intensity and a high volume of soil exploited while ‘Koga’ had the opposite (Fig. 6).
Linear regression of the volume of soil (cm3) exploited by roots and shoot dry weight (g) in a wheat, c onion and e lettuce, and a linear regression of the volume of soil and root intensity (crossings m−1) in b wheat, d onion and f lettuce. Results are means of four replicates of each cultivar, and error bars show the standard error
The results on root growth were compared to the nutrient acquisition by the plants, both in terms of nutrient concentrations and total nutrient uptake. We found a significant relationship between the volume of soil exploited and the nutrient uptake. The N content (g plant−1) was positively correlated to the volume of soil exploited (Fig. 7) in all three crops and the same result was found for P, K, S, and Mg content (results not shown).
Linear regression of the volume of soil (cm3) exploited by roots and shoot N-content (g plant−1) in a wheat, c onion and e lettuce, and a linear regression of the volume of soil (cm3) and shoot N-concentration (mg g−1) in b wheat, d onion and f lettuce. Results are means of four replicates of each cultivar, and error bars show the standard error
The nutrient concentrations did not correlate to the volume of soil exploited, yet we found cultivars which combined a fast and extensive root development with high nutrient concentrations. The wheat cultivar with the most extensive root growth, ‘Bittern’, also had high concentrations of N, P, K, S and Mg compared to the average nutrient concentrations of the cultivars, and the same tendency was found for the lettuce cultivar ‘Olite’ (Fig. 8). ‘Xaroma’ also had high concentrations of P, K, S and Mg although the N concentration was low (Fig. 8). In contrast, the onion cultivar with the most widespread root growth, ‘Hylander’, had low concentrations of nutrients in the plant biomass (Fig. 8).
Nutrients (N, P, K, S, Mg) shown as % relative to the average nutrient concentration in shoots set as 100 % among the six cultivars of each species; a spring wheat, b onion, and c lettuce. Results are means of four replicates of each cultivar. Coefficient of variation (cv in %) for each nutrient is shown in brackets
Discussion
It is well documented that crops differ in root growth (Thorup-Kristensen 2006), and the findings of this study showed differences in early root growth patterns among spring wheat, onion and lettuce. Onion was found to have the majority of its roots in the soil volume close to the base of the shoot, while lettuce was much better at spreading its root system horizontally during initial growth. This was in accordance to previous findings of root growth in field grown crops of onion, lettuce, carrot and cabbage (Thorup-Kristensen 2006). Wheat was better than onion and as good as lettuce in spreading the root system horizontally, at least within the c. 0.2 m horizontal distance studied here. This is surprising as wheat is normally grown at lower plant to plant distance, supposedly making horizontal root development less important. Still, Manschadi et al. (2008) found that at later growth stages two wheat genotypes were both able to build high root densities to 0.3 m distance from the plant, and one of the genotypes even had a considerable root density at 0.3 to 0.45 m distance.
In wheat we found that the plants produced few roots right below the plant, whereas higher root densities were developed at some distance from the plant basis. This should not be surprising, as seminal roots appear at an angle from vertical (Manschadi et al. 2006), and the initial root system is developed from them. However, this effect is often obscured by the 2D representations made of the 3D reality of root systems. In the 2D representations roots growing into or away from the plane are shown as if they grow vertically, as can be seen in the detailed images of young maize and sorghum root systems (Singh et al. 2010). Although in rice, root traits measured in 2D and 3D systems were found to be significantly correlated (Clark et al. 2011). Towards the end of our experiment, new more vertically developing main root axes were appearing. These were apparently shoot borne roots from newly developing tillers, growing into the otherwise low root density volume right below the plants as normally seen in graminaceous plants. In a field grown wheat crop, this soil volume may already be exploited by roots of neighbor plants, thus differing from the conditions in our observations on single plants.
The observed root growth rates differ somewhat from estimated growth rates based on previous field studies. Most markedly, onion had a faster vertical development rate compared to previous studies while wheat had a slower development than previous studies on wheat root growth (Kirkegaard and Lilley 2007; Thorup-Kristensen 2006; Thorup-Kristensen et al. 2009). This may be related to the early growth stages studied within this experiment, stages not covered by field data of whole season root growth. In addition, the fact that the onions were “group planted”, which is now a common practice in organic onion production in Denmark, may have affected their root growth. In a previous field study where onions were group planted, the early root growth of onion appeared to be fast, followed by much slower root growth during most of the growth period (Thorup-Kristensen et al. 2012).
Differences in total root growth among cultivars was strong, almost two fold among wheat cultivars, and more than two fold among onion and lettuce cultivars, even when not considering the slow growing ‘Intred’ lettuce. In the horizontal root growth and ability to exploit soil at larger distance from the plant, the difference was again approximately two fold among onion and lettuce cultivars, but it was stronger among the wheat cultivars, where 28 % of the roots of ‘Quintus’ were seen at a distance of more than 0.14 m from the plant while only 8 % of the roots of ‘Koga’ and ‘Økilde’ was observed so far from the plant basis. This larger variability in horizontal root growth among wheat cultivars may be related to the fact that wheat is grown at much lower plant to plant distance than onion and lettuce, creating less selection pressure for traits related to horizontal root growth during wheat breeding.
At cessation of the experiment, the calculated volume of exploited soil was between 10 and 38 % of the soil volume represented by the measurements, highest for onion and lettuce, which were transplanted into the tubes, while wheat was sown directly. With a wheat plant density of 250 plants m2 under field conditions, there is on average 1.2 × 10−3 m3 for each plant to exploit in the top 0.3 m of the soil. This level was reached by wheat after 21 days, whereas at the end of the experiment 8 days later, each wheat plant exploited twice the volume available in a normal crop stand. Considering the actual distribution of the roots in a crop stand, there would be zones of overlap between root systems of the plants, and still be zones with no or few roots. With onion and lettuce the field plant density is less than 10 plants m−2, or for group-planted onions, 10 plant groups m−2, and with an exploitation of approximately 3.5 × 10−3 m3 soil by onion, each plant group still only has roots in c. 11 % of the available soil volume in the field.
Thus, the results show the initial growth period when the plant roots are still in the process of colonizing even the topsoil layer, and reveal that only a limited volume of the soil is exploited initially making breeding for early root distribution relevant. The duration of the initial growth period was shown to be longer for onion than for wheat, but also show significant variation among cultivars within the species. Lettuce is intermediate, with a root growth which may be faster than that of wheat, but it is grown at a low plant density as with the onions. In this study, the root intensity of lettuce reached up to about 50 root intersections per m−1 during a period of 480 °C days, while Thorup-Kristensen (2006) found that after 680 °C days lettuce had reached root intensities around 60 root intersections m−1 both in the plant row and in the inter-row soil 0.25 m away.
There are only few studies to compare our results to. During the last years quite a few studies have focused on root angles, both in lab setups during early growth (Richard et al. 2015), and under field conditions (Trachsel et al. 2011). However, while such results have shown genotypic differences in root angles, they do not show the effects on actual root distribution in the soil, or show how much of the soil volume the plants are able to exploit. More extensive studies have only been conducted for a few species such as wheat and cauliflower (Manschadi et al. 2006; Thorup-Kristensen and Van den Boogaard 1998).
While we found significant differences in root growth parameters among the six cultivars of each species studied, the relationship between these differences in root growth and variable soil resource use is less clear. We found strong correlations between shoot and root growth, and between shoot growth and nutrient uptake at the end of the experiment. It is not immediately clear whether the driving factor for better performance was better root growth leading to more resources for shoot growth, or stronger shoot growth leading to stronger nutrient demand and more photosynthesis products available for root growth. In short, it is a challenge to determine what the causal relationship is, as also noted by Pang et al. (2015). Further, a number of other traits could also be important for determining the genetic variability in the efficiency of nutrient uptake of the root system. Differences between cultivars could also be due to differences in the excretion of protons, organic acids and other exudates as well as efficiency of the nitrate transporter systems (Lynch 2011; Pang et al. 2015; White et al. 2013).
There is a range of interactions between plant size, root system size and distribution and nutrient uptake which makes it difficult to conclude whether truly improved root systems have been identified. Nutrient uptake is governed by crop demand in combination with the supply from the soil through the root system and any trait leading to increased shoot growth will in itself lead to increased nutrient uptake. After some time, such plants will also have larger root systems. Based on the results of nutrient interruption experiments (Burns 1992, 1994), it is contended that if stronger growth of a genotype is driven by shoot traits leading to increased nutrient demand, nutrient concentrations in the plant are likely to be lower than if growth were being driven by improved root traits that increase nutrient supply to the plant. In the comparison between the wheat cultivars, such a difference was seen between ‘Bittern’ with its large and well distributed root system and high nutrient concentrations, and ‘Koga’ with low soil exploitation by the roots and also generally low nutrient concentrations. However, ‘Quintus’ with its well distributed root system does not clearly follow this pattern and showed only moderate nutrient concentrations. Further complicating the interpretations is the fact that small plants have higher optimal nutrient concentrations than larger plants, as exemplified in the critical nutrient concentration curves used in field diagnostics of crop nutrient status (Zhao 2014). In the present data, the relatively high nutrient concentrations of the wheat cultivar ‘Økilde’ may simply be the result of its small size.
In spite of the uncertainties caused by differences in size and the question of cause and effect, the measurements of nutrient concentration show promising results. In lettuce as well as in wheat, cultivars with strong growth and high volume of soil exploitation (‘Xaroma’ and ‘Bittern’) showed higher average nutrient concentrations, compared to cultivars with less biomass production and low volume of soil exploitation (‘Ralph’ and ‘Koga’). In onion this was less clear, but still ‘Hylander’ with fast soil exploitation was able to produce much more biomass than the other cultivars, but maintaining an average nutrient content. In contrast, ‘Hybelle’ with a low volume of soil exploitation, combined a low biomass production with a low nutrient content. Thus, we were able to find cultivars within all three species, where high nutrient supply capacity from the root system seemed to be a main cause of increased biomass production, with high nutrient concentrations as also indicated in the results of Kerbiriou et al. (2013b).
The study was made under low-fertility conditions, and as a result of this, the cultivars were tested under conditions where root traits are critical for the ability of the plants to take up sufficient nutrients for vigorous growth. Studies by Costigan (1987a, b) showed that early growth may still be limited by soil nutrient levels, even in highly fertilized soils (Costigan 1987b) as the root systems need time to exploit the soil volume and get into contact with the available nutrients (Costigan 1987a). A next step towards testing whether we have in fact identified root traits and variability leading to better crop establishment under low nutrient availability as in organic farming, would be to test the response of these cultivars in soil with different levels of fertility. Modern crop cultivars are being bred under conventional conditions with high nutrient availability, limiting the selection for the ability to cope with low nutrient soil conditions as in organic farming (Kerbiriou et al. 2013b; Tiemens-Hulscher et al. 2014). Cultivars selected under high nutrient availability are also grown in organic production systems today, even though the conditions are different from conditions in conventional farming. However, previous studies have shown that cultivars bred under conditions experienced in organic agriculture do not necessarily perform better than conventionally-bred cultivars when grown under organic conditions (Hildermann et al. 2009). Our results show that a relatively simple experiment protocol can be used to study 2D aspects of root growth. Ultimately it may be feasible to screen root growth traits by using the protocol in genotype breeding. As no breeding for these root traits has occurred to date, we would expect to find significant genotype variability, as found in our study. Screening for root growth traits will still be laborious, but the results also indicate that an initial, simpler screening may be made by identifying genotypes that combine vigorous early growth with relatively high nutrient concentrations when grown under low nutrient availability in the field.
Rhizotron tubes provide a non-destructive method for directly viewing and studying root growth. It is useful for root phenotyping (Ytting et al. 2014), as well as for studies of other aspects of root function and development (e.g. Andersen et al. 2014), as it allows repeated observation of root development in time and space. Compared to laboratory methods using hydroponics and other growth media, the tube rhizotrons have the advantage of allowing studies to be made in real field soils. A disadvantage is that this method is laborious, but when used for phenotyping, time consumption can be reduced substantially compared to the detailed observations of this study.
Conclusion
Significant differences in root growth patterns were found among the three crops studied; spring wheat, onion and lettuce. In combination with the differences in crop patterns and plant density in the field, this leads to large differences in the time needed for a young crop to develop roots exploiting all of the upper soil layers. Within each of the three crops there were significant cultivar differences in early root growth, in total root growth as well as in the spatial distribution of their root systems in the soil. In addition, within each of the three crops, cultivars were identified which combined strong root growth with strong shoot growth and high nutrient concentrations, indicating that their improved performance was caused by their superior root traits. The rhizotron method for studying spatial root development by non-centric planting was very useful for measuring differences in early soil exploitation by root systems, both among species, and for studying differences among cultivars within species.
References
Andersen SN, Dresboll DB, Thorup-Kristensen K (2014) Root interactions between intercropped legumes and non-legumes-a competition study of red clover and red beet at different nitrogen levels. Plant Soil 378:59–72
Burns IG (1992) Influence of plant nutrient concentration on growth rate: use of a nutrient interruption technique to determine critical concentrations of N, P and K in young plants. Plant Soil 142:221–233
Burns IG (1994) A mechanistic theory for the relationship between growth rate and the concentration of nitrate-N or organic-N in young plants derived from nutrient interruption experiments. Ann Bot 74:159–172
Clark RT, MacCurdy RB, Jung JK, Shaff JE, McCouch SR, Aneshansley DJ, Koachian LV (2011) Three-dimensional root phenotyping with novel imaging and software platform. Plant Physiol 156:455–465
Costigan PA (1987a) A model to describe the pattern of availability of broadcast phosphorus fertilizer during the growth of a crop. Plant Soil 101:281–285
Costigan PA (1987b) The importance of seedling nutrient stress in producing site to site variations in yield. J Plant Nutr 10:1523–1530
Costigan PA, Heaviside M (1988) The effects of starter fertilizer on the early growth and yield of transplanted crisp lettuce on fertile soils. J Hort Sci 63:247–253
Desnos T (2008) Root branching responses to phosphate and nitrate. Curr Opin Plant Biol 11:82–87
Gahoonia TS, Nielsen NE (2004) Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 262:55–62
Gahoonia TS, Nielsen NE, Lyshede OB (1999) Phosphorus (P) acquisition of cereal cultivars in the field at three levels of P fertilization. Plant Soil 211:269–281
Hildermann I, Thommen A, Dubois D, Boller T, Wiemken A, Mäder P (2009) Yield and bakin quality of winter wheat cultivars in different farming systems of the DOK long-term trials. J Sci Food Agric 89:2477–2491
Kerbiriou P, Stomph T, Van Bueren E, Struik P (2013a) Influence of transplant size on the above- and below-ground performance of four contrasting field-grown lettuce cultivars. Front Plant Sci 4
Kerbiriou P, Stomph T, Van Der Putten P, Van Bueren E, Struik P (2013b) Shoot growth, root growth and resource capture under limiting water and N supply for two cultivars of lettuce (Lactuca sativa L.). Plant Soil 371:281–297
Kerbiriou P, Stomph T, Van Bueren E, Struik P (2014) Modelling concept of lettuce breeding for nutrient efficiency. Euphytica 199:167–181
Kirkegaard JA, Lilley JM (2007) Root penetration rate - a benchmark to identify soil and plant limitations to rooting depth in wheat. Aust J Exp Agric 47:590–602
Liao MT, Fillery IRP, Palta JA (2004) Early vigorous growth is a major factor influencing nitrogen uptake in wheat. Funct Plant Biol 31:121–129
Liao M, Palta JA, Fillery IR (2006) Root characteristics of vigorous wheat improve early nitrogen uptake. Aust J Agric Res 57:1097–1107
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049
Manschadi AM, Christopher JT, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33:823–837
Manschadi AM, Hammer GL, Christopher JT, deVoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129
Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP (2003) Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Funct Plant Biol 30:973–985
Narayanan S, Mohan A, Gill KS and Prasad PV 2014 Variability of root traits in spring wheat Germplasm. PLoS One 9
Oelofse M, Jensen L, Magid J (2013) The implications of phasing out conventional nutrient supply in organic agriculture: Denmark as a case. Org Agric 3:41–55
Pang J, Milroy SP, Rebetzke GJ, Palta JA (2015) The influence of shoot and root size on nitrogen uptake in wheat is affected by nitrate affinity in the roots during early growth. Funct Plant Biol 42:1179–1189
Richard CAI, Hickey LT, Fletcher S, Jennings R, Chenu K, Christopher JT (2015) High-throughput phenotyping of seminal root traits in wheat. Plant Methods 11:13
Scholten OE and Kuyper TW 2012 Onions: breeding onions for low-input and organic agriculture. in organic crop breeding. pp. 263–272. Wiley-Blackwell
Singh V, van Oosterom EJ, Jordan DR, Messina CD, Cooper M, Hammer GL (2010) Morphological and architectural development of root systems in sorghum and maize. Plant Soil 333:287–299
Thorup-Kristensen K (2006) Root growth and nitrogen uptake of carrot, early cabbage, onion and lettuce following a range of green manures. Soil Use Manag 22:29–38
Thorup-Kristensen K, Van den Boogaard R (1998) Temporal and spatial root development of cauliflower (Brassica oleracea L. var. botrytis L.). Plant Soil 201:37–47
Thorup-Kristensen K, Salmeron Cortasa M, Loges R (2009) Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 322:101–114
Thorup-Kristensen K, Dresboll DB, Kristensen HL (2012) Crop yield, root growth, and nutrient dynamics in a conventional and three organic cropping systems with different levels of external inputs and N re-cycling through fertility building crops. Eur J Agron 37:66–82
Tiemens-Hulscher M, van Bueren ETL, Struik PC (2014) Identifying nitrogen-efficient potato cultivars for organic farming. Euphytica 199:137–154
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341:75–87
White PJ, George TS, Dupuy LX, Karley AJ, Valentine TA, Wiesel L, Wishart J (2013) Root traits for infertile soils. Front Plant Sci 4:193
Wojciechowski T, Gooding MJ, Ramsay L, Gregory PJ (2009) The effects of dwarfing genes on seedling root growth of wheat. J Exp Bot 60:2565–2573
Ytting NK, Andersen SB, Thorup-Kristensen K (2014) Using tube rhizotrons to measure variation in depth penetration rate among modern North-European winter wheat (Triticum aestivum L.) cultivars. Euphytica 199:233–245
Zhao B (2014) Determining of a critical dilution curve for plant nitrogen concentration in winter barley. Field Crop Res 160:64–72
Acknowledgments
The authors gratefully acknowledge funding through the Organic RDD program from the Ministry of Environment and Food of Denmark, The Danish Agrifish Agency and ICROFS (International Centre for Research in Organic Food Systems).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Richard J. Simpson.
Rights and permissions
About this article
Cite this article
Andresen, M., Dresbøll, D.B., Jensen, L.S. et al. Cultivar differences in spatial root distribution during early growth in soil, and its relation to nutrient uptake - a study of wheat, onion and lettuce. Plant Soil 408, 255–270 (2016). https://doi.org/10.1007/s11104-016-2932-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2932-z