Abstract
Background and aims
Although plant–soil feedback has been suggested as a mechanism that drives the success of invasive plants, studies that investigate differences in the intensity of plant–soil feedback among native and invasive populations of the same species are still lacking. However, such knowledge is important because it can provide an understanding of the mechanisms responsible for the spread of a species. Rorippa austriaca is a potentially invasive species - a successful range expander in Europe.
Methods
We compared the plant–soil feedback of R. austriaca in populations from its native and invasive range. We explored both intraspecific feedback as well as feedback on a co-occurring grass species.
Results
Our results revealed a strong negative feedback effect as a consequence of soil conditioning by R. austriaca from the native range. On the contrary, a negative feedback effect was not observed for invasive R. austriaca. Interestingly, R. austriaca from the invasive range had a higher biomass than native R. austriaca.
Conclusion
Our results might be explained by pathogen accumulation and soil modification by native R. austriaca, which had strong intra- and interspecific effects that seemed to be lost in the invasive R. austriaca. The loss of negative intraspecific plant–soil feedback and the increased growth of the invasive population may contribute to its successful range expansion. In spite of its increased growth, the co-occurring grass species is expected to successfully coexist with the invasive R. austriaca.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species distributions are changing because of climate change, changes in land use and globalization (Parmesan 2006; Thuiller et al. 2008; Pereira et al. 2010). Because these moving plants may have deep-seated effects on entire ecosystems and also on agricultural production and human health, many studies have attempted to identify the factors that determine which alien species are more likely to succeed in invading and which will likely fail (Seastedt and Pyšek 2011).
A factor that strongly affects species success in a new range is the ability of the plant to modify the soil in which it grows and to suppress the growth of the other plants and/or support its own growth (Callaway et al. 2004; Reinhart and Callaway 2004; Zuppinger-Dingley et al. 2011). This effect can be mediated through the input of chemical compounds and organic matter from the plant to the soil, by impacting hydrological processes and surface soil temperatures and by providing habitats and/or resources for soil biota (Bardgett and Wardle 2010; Lamb et al. 2011). Changes in soil properties caused by plants, which in turn influence the performance of plants, are termed ‘plant–soil feedback’ (Bever et al. 1997; Van der Putten et al. 2013).
Plant–soil feedback characterized by an individual plant affecting itself or other plants of the same species is called direct (intraspecific) plant–soil feedback. It can be negative, neutral or positive depending on the net growth effect of the soil modified by the plant compared to unmodified soil (Van der Putten et al. 2013). Multiple studies (Klironomos 2002; Reinhart et al. 2003; Callaway et al. 2004; Knevel et al. 2004; Bennett et al. 2011; Yang et al. 2013) indicated that intraspecific plant–soil feedback can play an important role in the invasiveness of plant species.
It has been demonstrated that the type of intraspecific feedback can differ depending on where in an invasive species’ distributional range it is studied. Specifically, it was shown that species experience stronger intraspecific negative feedback in their native range than in their invasive range (e.g., Klironomos 2002; Reinhart et al. 2003; Agrawal et al. 2005; Engelkes et al. 2008; Kulmatiski et al. 2008, but see Anacker et al. 2014). In addition to the differences between the intensity of intraspecific feedback in soils from different areas, it has also been shown that individuals of the same species originating from different areas (i.e., the native and invasive range) might experience intraspecific feedback of different intensity (Te Beest et al. 2009; Andonian and Hierro 2011; Yang et al. 2013). Although the majority of studies have shown that species experience stronger intraspecific negative feedback in their native range than in their invasive range, several recent studies have arrived at a different conclusion (Andonian et al. 2011; Birnbaum and Leishman 2013). For example, Andonian et al. (2011) found that Centaurea solstitialis, a globally invasive weed, generated strong negative intraspecific feedback in regions where it is the most invasive, while it generated neutral plant–soil feedback in non-invasive regions.
Differences in the intensity of plant–soil feedback between the native and invasive range could be caused by micro evolutionary changes leading to a change in a range of species traits (Bone and Farres 2001; Maron et al. 2004; Callaway and Ridenour 2004; Bossdorf et al. 2005). Most plant–soil feedback studies have focused on the impact of the soil biota on the invasive and related non-invasive plant species in the non-native range. However, very few studies have explored the differences in plant–soil feedback effects between genotypes of the same species from the native and the invasive range (Te Beest et al. 2009; Seifert et al. 2009; Andonian and Hierro 2011; Birnbaum and Leishman 2013). Theoretically, invasive genotypes could have lost the intraspecific negative feedback that may limit the populations in the native range. This loss in negative feedback compared to the native range may then contribute to the success of the plant’s invasion. Alternatively, plant invasiveness may be promoted by strong interspecific plant–soil feedback. Interspecific plant–soil feedback is feedback from one plant species that affects another species. For example, invasive exotic plants can promote soil pathogens that have a more negative effect on the surrounding native plant species than on the exotics themselves (Mangla et al. 2008). They can also reduce local mycorrhizal fungi with negative consequences for native plant species (Stinson et al. 2006; Lankau and Strauss 2007; Van der Putten et al. 2013). In addition, plants can produce secondary metabolites such as allelopathic root exudates that are relatively ineffective against their natural neighbours because of adaptation but may be highly inhibitory to newly encountered plants in the invaded communities (Callaway and Ridenour 2004).
Most studies compare intraspecific and interspecific plant–soil feedback effects of invasive exotic plants that were introduced from other continents (Klironomos 2002; Knevel et al. 2004; Van Grunsven et al. 2009, but see Van Grunsven et al. 2007). However, not all exotic plants arise from other continents. In past decades, many species have moved to higher latitudes within the same continent, partly driven by climate change (Parmesan 2006; Morriën and van der Putten 2013). Recently, it has been shown that plant–soil feedback can play an important role in the spread of invasive species within continents as well (Engelkes et al. 2008; van Grunsven et al. 2010).
The aim of this study was to understand the importance of plant–soil feedback in the spread of range expanding Rorippa austriaca (Crantz) Besser (Austrian yellowcress). R. austriaca is a successful intra-continental range expander, which has spread northwards and westwards from central and south-eastern Europe within the Eurasian continent (Jonsell 1993; Tutin et al. 1993; Bleeker 2003). In the Netherlands, R. austriaca was first discovered around the 1920s and has strongly increased in abundance since the 1980s and particularly since 2000 (Tamis et al. 2005; Engelkes et al. 2008). Meisner et al. (2012) tested how the legacy of litter from invasive R. austriaca affects its own performance in comparison to that of its congeneric native R. sylvestris, which co-occurred in an invaded habitat in the Netherlands. Their results suggested that both invasive and native Rorippa species may benefit from the litter of invasive R. austriaca. In the closely related species R. sylvestris and R. indica, negative effects of root exudates on lettuce growth were recorded (Yamane et al. 1992a; Yamane et al. 1992b). Negative effects of root exudates of R. austriaca on plant growth of other co-occurring species could thus be expected. In this study, we explored differences in plant–soil feedback between native and invasive populations of R. austriaca and asked the following questions:
-
A)
What are the differences in intraspecific plant–soil feedback effects between plants of R. austriaca from the native and invasive range?
-
B)
Is there any interspecific plant–soil feedback effect of R. austriaca on co-occurring grass species and does this effect differ between R. austriaca from the native and invasive range?
To answer these questions, we performed two pot experiments on plant–soil feedback in the common garden in the native range of R. austriaca. In the first step, we conditioned the soil with native and invasive R. austriaca and co-occurring grass species. In the second step, this soil was used for the cultivation of native and invasive R. austriaca and a co-occurring grass species to test the effects of soil conditioning on different populations within the same species and on co-occurring species.
Methods
Study species
R. austriaca is a polycarpic herbaceous perennial with a semi rosette growth form and relatively deep storage roots (Oberdorfer 1990). It combines clonal growth by lateral roots with the ability to regenerate from root fragments (Dietz et al. 2002). It can also reproduce via seeds and is an obligate outcrossing species (Bleeker and Matthies 2005). Stands of R. austriaca can be found growing in sandy soils as well as in nutrient rich loamy soils in habitats with greatly different vegetation structures ranging from open, intensively or patchily disturbed sites to sites with dense, more successional herbaceous vegetation. In its invasive range, it predominantly occurs along riversides.
Collection of plant material
Root fragments of R. austriaca were collected in 5 populations in both the native range in the Czech Republic (central Europe) and the invasive range in Western Europe in the Netherlands in 2011 (Table 1). In each range and population, we took root fragments from 5 distinct rosettes of adult R. austriaca plants to reflect possible genotypic variability within populations. We refer to these rosettes as different individuals. The root fragments were put in 3 L pots with common garden soil mixed with sand (at a ratio of 1:1). Plants resprouting from these root fragments were subsequently cultivated for 12 months in the experimental garden.
Soil conditioning (first experimental phase)
Three root fragments of similar size (length 4 cm, diameter 0.3–0.5 cm) from each plant of R. austriaca cultivated in the experimental garden in 2011 were placed in the 2 L pots filled with unsterilized common garden soil that had never been exposed to R. austriaca mixed with steam-sterilized river sand at a ratio of 1:1. The experiment contained 150 pots in total; i.e., pots with plants from 2 ranges × 5 populations × 5 individuals × 3 clonal replicates. Because plants of R. austriaca were planted from root fragments and not from the seeds, it was not possible to sterilize them in the experiment. However, we grew them for 12 months in the same soil and in the same experimental garden prior to the experiment. We assumed that any possible microbial differences originating from the original soil in which the plants were naturally growing were likely to be greatly diminished or absent. However, we cannot completely exclude the possibility that beneficial or detrimental microbes were carried over from the original growth location. Additionally, we grew Agrostis capillaris in 2 L pots in the same soil mixture (150 pots); 0.1 g of A. capillaris seeds (obtained from the Planta Naturalis company, Czech Republic) were sown in each pot. A. capillaris is a common grass species that is easy to cultivate, often forming nearly monodominant stands that occur at the localities occupied by R. austriaca in both ranges.
All 150 pots of R. austriaca and 150 pots of A. capillaris were grown for 8 weeks in the experimental garden located in the Institute of Botany, the Czech Academy of Sciences in Průhonice (322 m asl, 49°99′N, 14°57′E) from late May to late July 2012, when all of the plants were harvested. The environmental conditions of experimental garden were similar to those at natural localities in the Czech Republic, in which R. austriaca and A. capillaris grow. During cultivation, both species substantially increased their aboveground and belowground biomass, but they were not limited by the 2 L pots.
At the end of this first experimental phase, we had three soil types: soil from pots with 1) R. austriaca from the native range (150 L) 2) R. austriaca from invasive range (150 L) and 3) A. capillaris (300 L). Soil from all pots within the three variants was mixed to create specific soil types (see Hawkes et al. 2013 and Sun et al. 2014 for similar approach). We mixed the soils because we wanted to study the overall effects of native and invasive populations of R. austriaca, rather than looking at the effect of individual genotypes or populations, and we wanted to keep the number of replicates reasonable. In addition, the growth of the plants in the pots was uneven (i.e., very large plants or many A. capillaris seedlings were present in some pots and small plants and few seedlings were present in others). By mixing the soil we thus ensured that all of the soil used in the second experimental phase experienced the same intensity of conditioning.
Plant–soil feedback (second experimental phase)
A) Plant–soil feedback on Rorippa
In the second experimental phase, we explored the differences in the intraspecific plant–soil feedback effects between populations of R. austriaca from the native and invasive range. We grew R. austriaca from the native and invasive range in 2 L pots with the soil type conditioned by R. austriaca from native and invasive range and the soil type conditioned by A. capillaris. R. austriaca plants were established from root fragments taken from the 5 individuals of each population grown in the first experimental phase. There were 3 soil types × 2 ranges × 5 populations × 5 individuals, i.e., 150 pots in total.
B) Plant–soil feedback on co-occurring species
In a complementary experiment, we compared the interspecific plant–soil feedback effects of populations of R. austriaca from native and invasive range on growth of the co-occurring species A. capillaris. We grew A. capillaris plants in 2 L pots in the three soil types from the first experimental phase (conditioned by R. austriaca native, R. austriaca invasive, A. capillaris). We used A. capillaris plants from the first experimental phase and chose plants as similar as possible for planting with leaves 4–6 cm long. After a week, dead plants of A. capillaris were replaced by new plants.
To check for the possible effects of allelopathic secondary metabolites of R. austriaca on competing plants, we included an activated carbon treatment of all three soil types by adding 20 mL of activated carbon per litre of soil (particle size <0.075 mm; Resorbent Ostrava). Activated carbon has high affinity for potentially toxic organic compounds and is commonly used to test for allelopathic effects (Callaway and Aschehoug 2000; Dostál 2011). There were 3 soil types (R. austriaca native, R. austriaca invasive, A. capillaris) × 2 soil treatments (with and without activated carbon addition) × 25 plant replicates, i.e., 150 pots with A. capillaris in total.
The plants from both experiments in the second experimental phase were cultivated for 8 weeks from late July to late September 2012 outdoors in the experimental garden. At the end of September, the plants were harvested and the above- and belowground biomass was separated. After drying to a constant weight (at 70 °C for 48 h), the biomass was weighed.
Data analyses
In the second experimental phase, the total biomass of R. austriaca was closely correlated with its aboveground and belowground biomass (r = 0.80, P < 0.001 and r = 0.98, P < 0.001, respectively) and total biomass of A. capillaris was closely correlated with its aboveground and belowground biomass (r = 0.95, P < 0.001 and r = 0.99, P < 0.001, respectively). Furthermore, the aboveground and belowground biomass was closely related for R. austriaca (r = 0.66, P < 0.001) and A. capillaris (r = 0.92, P < 0.001) as well. Therefore, we used only total biomass of both species in further analyses.
To explore the differences in the intraspecific plant–soil feedback effects between R. austriaca from the native and invasive range, the effects of range, population, individual, and soil type were tested using Generalized Linear Models (GLM). Further, we also tested the effect of the interaction between soil type and range and the interaction between soil type and population on the total biomass of R. austriaca. Range and soil type were fixed factors and population nested within range and individual identity nested within population were random factors. Differences between soil types within each range were tested with Tukey’s HSD test.
To compare interspecific plant–soil feedback effects of populations of R. austriaca from the native and invasive range on the growth of co-occurring A. capillaris grass, the effects of soil type, activated carbon addition and their interaction on total biomass of A. capillaris were tested in a full factorial ANOVA. A. capillaris total biomass data were square root transformed to meet the assumptions of the analyses. Differences between soil types within each activated carbon treatment were tested with Tukey’s HSD test.
To see how plant–soil feedback might potentially affect species interactions within the community, we further explored the strength of pairwise plant–soil feedback interactions between native and invasive R. austriaca and the co-occurring grass A. capillaris as suggested by Bever et al. (1997). The interaction coefficient, IS, which represents the net pairwise feedback, is defined as IS = αA – βA – αB + βB. The variables α and β represent the growth of the two plant species, respectively, and the subscripts A and B indicate which plant species was used for the soil conditioning (soil A being conditioned by species α and soil B being conditioned by species β) (Bever et al. 1997; Bever et al. 2010; Mangan et al. 2010; Shannon et al. 2011). Feedback interaction between the two species is indicated when the interaction coefficient is significantly different from 0 (by a t test). Because we had 25 individuals of R. austriaca (5 populations × 5 individuals) from each range in each soil type, we were able to calculate an IS for each individual (resulting in 25 IS values for each pairwise comparison). We then used a t test to determine whether the IS values were significantly different from zero (i.e., no feedback interaction). When IS is positive, the feedback increases the relative performance of the locally abundant plant species generating a positive feedback dynamic that would lead to loss of diversity at a local scale. Conversely, when IS is negative, feedback decreases the relative performance of the locally abundant plant species, leading to coexistance through net negative feedback. A negative IS indicates net negative feedback (coexistence), and a positive Is indicates positive feedback (exclusion). The IS was calculated for the three possible pairs of plants in our data, i.e., native R austriaca and A. capillaris, invasive R. austriaca and A. capillaris, native and invasive R. austriaca.
All statistical analyses were carried in R version 3.0.2 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
A) Plant–soil feedback on Rorippa
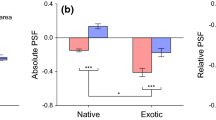
In the second experimental phase, R. austriaca plants from the invasive range produced 32 % more biomass than R. austriaca plants from the native range (P = 0.013; Table 2a). Furthermore, the biomass of R. austriaca was significantly different between the three soil types conditioned by R. austriaca from the native and invasive range and by A. capillaris (P < 0.001; Table 2a). We found a significant negative effect from soil conditioned by native R. austriaca, however, this effect significantly influenced the biomass of native R. austriaca (i.e., an average decrease of 30 % compared to soil type conditioned by A. capillaris) but did not affect the biomass of invasive R. austriaca (Fig. 1). Soil types conditioned by invasive R. austriaca and by A. capillaris were not significantly different in their effects on the biomass of both native and invasive R austriaca (GLM, Tukey’s HSD test, P > 0.256 in all cases; Fig. 1). The biomass was also different between individual R. austriaca plants within populations and marginally different between populations within ranges (P = 0.017 and P = 0.080, respectively; Table 2a, Fig. 2). The interactions between range and population and soil type were not significant, which indicates that the effects of soil type were similar in both ranges and all populations (P = 0.916 and P = 0.221, respectively; Table 2a, Fig. 2).
Mean total biomass of R. austriaca from the native and the invasive range in different soil types from the plant–soil feedback experiment. Bars are means ± SEM. Significant differences in R. austriaca biomass between the three soil types within the range are indicated by different lowercase letters (GLM, Tukey’s HSD, P < 0.05). Columns sharing the same letter are not significantly different (P > 0.05)
B) Plant–soil feedback on co-occurring species
The biomass of A. capillaris differed between the soil types (P < 0.001). Activated carbon addition had a marginally significant effect leading to a weak (12 %) overall increase in the biomass of A. capillaris (P = 0.080). There was no interaction between the soil type and the addition of activated carbon (P = 0.120; Table 2b, Fig. 3). While we recorded no differences between A. capillaris biomass in soil types conditioned by A. capillaris and invasive R. austriaca, we found a significant decrease of biomass in soil type conditioned by native R. austriaca (i.e., an average biomass decrease of 28 and 46 % compared to soil type conditioned by A. capillaris and invasive R. austriaca, respectively). The effects were similar in treatments with and without activated carbon (ANOVA, Tukey’s HSD test, P < 0.05, Fig. 3, Table 2b).
Effects of different soil types conditioned by R. austriaca plants and by the co-occurring grass species A. capillaris and activated carbon addition on growth of the co-occurring grass A. capillaris. Significant differences (ANOVA, Tukey’s HSD, P < 0.05) between soil types within treatments with (activated carbon +) and without (activated carbon –) activated carbon are indicated by different lowercase letters. Bars are means ± SEM. Columns sharing the same letter are not significantly different from each other (P > 0.05)
Effect of plant–soil feedback on possible interactions within the community
In the analyses of plant–soil feedback interactions between native R. austriaca and A. capillaris, we found a consistent negative effect of soil type conditioned by native R. austriaca with very similar negative consequences for the growth of both native R. austriaca and A. capillaris; i.e., no significant feedback interaction was evident (IS = −0.18, P = 0.221; Fig. 4a). In the comparison of plant–soil feedback of invasive R. austriaca and A. capillaris, A. capillaris grew better in soil type conditioned by invasive R. austriaca compared to its own soil. Invasive R. austriaca performed slightly worse in its own soil type as well, resulting in significant negative feedback interaction (IS = −0.41, P = 0.005; Fig. 4b). Both species in the community were suppressed by the negative effect of its own soil type and neither had a competitive advantage. On the contrary, in comparison of native and invasive R. austriaca, we found marginally significant positive plant–soil feedback interaction (IS = 0.28, P = 0.057; Fig. 4c), when native R. austriaca grew more poorly in its own soil while no difference was noted for invasive R. austriaca. This ability might provide a competitive advantage to invasive R. austriaca, which is not limited by intraspecific negative plant–soil feedback such as native R. austriaca.
Pairwise comparison of the average biomass of native and invasive R. austriaca and the co-occurring grass A. capillaris grown in the three soil types (soil conditioned by native or invasive R. austriaca or the co-occurring grass A. capillaris). The interaction coefficient IS indicates the strength of pairwise plant–soil feedback interaction between a) A. capillaris and native R. austriaca, b) A. capillaris and invasive R. austriaca and c) native and invasive R. austriaca. Significance was tested using a t test to determine whether the IS values were different from zero (i.e., no feedback interaction). A negative IS indicates net negative feedback (coexistence), and a positive IS indicates positive feedback (exclusion)
Discussion
Our results revealed a strong effect from soil conditioning on the biomass of native R. austriaca and the grass A. capillaris. Specifically, the plants performed more poorly in soil conditioned by native R. austriaca compared to soil conditioned by invasive R. austriaca or by A. capillaris. A negative effect from soil conditioned by native R. austriaca on the biomass of the co-occurring grass A. capillaris was detected both in the presence and absence of activated carbon.
Similarly to this study, it has been shown that performance of Ailanthus altissima was significantly different in soil conditioned by populations of single species of different origin (Felker-Quinn et al. 2011). This difference might be explained by changes in soil nutrients and/or in the composition of the soil microbial community (Seifert et al. 2009; Felker-Quinn et al. 2011). It has been repeatedly shown that plants affect soil nutrients and the soil microbial community, which affects the colonization success of conspecifics and/or heterospecifics (e.g., Klironomos 2002; Callaway et al. 2004; Jordan et al. 2008; Perkins and Nowak 2013). Perkins and Nowak (2013) found that native species produced plant–soil feedback that benefited other species more than themselves and non-native invasive species tended to produce plant–soil feedback that benefited themselves more than other species. This mechanism increases the potential of non-native species to become invasive. This is somewhat similar to our results, which indicated that invasive R. austriaca did not experience intraspecific negative soil feedback and may not be as limited as native R. austriaca when colonizing new localities. The reason for this difference between the native and invasive populations remains unknown. One explanation could be that rapid evolutionary change of the plant occurred in the new range; i.e., the plants in the new range might have evolved a different type of plant–soil feedback. For example, Seifert et al. (2009) showed that introduced North American and native European populations of Hypericum perforatum differed in their mycorrhizal responsiveness. North American populations benefited less from inoculation with a cosmopolitan arbuscular mycorrhizal fungal species than did European populations. North American populations also had finer root systems, invested more in reproductive biomass and less in below-ground biomass than European populations.
The less negative intraspecific plant–soil feedback might be because invasive populations of R. austriaca exhibit more extensive clonal growth compared to native R. austriaca, i.e., invasive populations more often reproduce vegetatively rather than generatively, thus remaining closer together and more likely grow in their own soil (N. Bihler & M. Macel, unpublished data). The selection against negative plant–soil feedback could thus be stronger in the invasive range. Alternatively, there may be high variation in the intensity of plant–soil feedback in the native range (Peña et al. 2009; Felker-Quinn et al. 2011; Lankau 2013) and genotypes that have less negative intraspecific plant–soil feedback in the native range could also be the ones that are spreading or are spreading more successfully into the new range. This explanation is consistent with our results, as we also found differences among soil feedbacks of plants grown from different individuals within populations of R. austriaca (Table 2a). This topic, however, requires further study. There is also a possibility that R. austriaca from the invasive range could experience negative plant–soil feedback in soil from invasive range because local soil pathogens are adapted to the local genotypes of R. austriaca (Thrall et al. 2002). However, soil used in our experiment did not have any previous exposure to R. austriaca, and the closest populations were several tens of kilometres from the experimental garden. Unless we repeat the experiments using soil from the invasive range, we cannot exclude this option.
Negative soil feedback caused by native R. austriaca affected not only R. austriaca but also the co-occurring grass A. capillaris, which might be caused by production of allelopathic chemicals by native R. austriaca (Bais et al. 2003). Previous studies on related Rorippa species (Yamane et al. 1992a; Yamane et al. 1992b) revealed a negative effect on the growth of other plant species as a consequence of production of root exudates and suggested possible allelopathic effects. However, allelopathic effects are usually reported for exotic species when such effects facilitate the invasion of exotics in the new range; the so called Novel Weapons Hypothesis (Callaway and Ridenour 2004; Thorpe et al. 2009; Barto et al. 2010). Here, we show that native R. austriaca also negatively affects the growth of a co-occurring species. We have only recorded slight non-significant increases in A. capillaris biomass in soil conditioned by native R. austriaca after activated carbon addition (see Fig. 3). Because the effect of the activated carbon on plant growth was relatively small and non-significant, it does not seem very likely that native R. austriaca produced high concentrations of toxic root exudates that negatively affected A. capillaris. Rather, other, non-chemical, mechanisms might play a role, such as pathogen accumulation of the native R. austriaca. An alternative explanation may be that native R. austriaca takes up more nutrients from the soil than invasive R. austriaca, which negatively affected the growth of A. capillaris (Kardol et al. 2006). However, if R. austriaca plants from the native range took up more nutrients than A. capillaris and R. austriaca from the invasive range, it should probably grow larger than invasive R. austriaca, which was not the case. We did not include a fertilization treatment in our experiments so that we could control for this well-known confounding factor in plant–soil feedback experiments (see, e.g., Te Beest et al. 2009 for such an approach). Future experiments should control for the potential effect of nutrient uptake on the plant–soil feedback observed here.
Because R. austriaca in its invasive range does not experience negative plant–soil feedback, it may gain competitive advantage over other plants in the community in its new range and thus may become invasive. However, the co-occurring grass species A. capillaris also benefits from soil conditioned by invasive R. austriaca, compared to soil conditioned by A. capillaris. This is seen from the results of plant–soil feedback interactions analyses indicating that invasive R. austriaca gains no advantage in competition with A. capillaris. These two species should thus be able to coexist. However, this conclusion should be verified by an experiment in which the effect of soil conditioned by A. capillaris is compared to the effect of soil conditioned by both A. capillaris and invasive R. austriaca. This experiment would determine how conditioning by invasive R. austriaca can remove the negative home soil effect of A. capillaris. Moreover, further studies should also include other species occurring in the communities with R. austriaca so that we could generalize our results.
Our results thus indicate that other factors contribute to R. austriaca expansion. In this study, we found that R. austriaca plants from the invasive range grew significantly larger than the plants from the native range, which could confer an important competitive advantage. In agreement with this finding, Buschmann et al. (2005) compared R. austriaca from the introduced range in North America and from the native range (central Europe) and found that the invasive North American plants grew larger than the native plants. This also agrees with other studies that indicate that plants from the invasive range grow larger than plants from the native range (e.g., Blumenthal and Hufbauer 2007; Abela-Hofbauerová and Münzbergová 2011). For R. austriaca, the larger size of plants from the invasive range does not seem to be related to a shift in resource allocation from shoot defence to growth as predicted by the evolution of increased competitive ability (EICA) hypothesis (Blossey and Nötzold 1995 but see Felker-Quinn et al. 2013). Native and invasive populations were equally damaged by shoot herbivores and had similar concentrations of shoot defences (Buschmann et al. 2005; Huberty et al. 2014). Currently, nothing is known about the root defences of this species.
Conclusions
We found negative intra- and interspecific plant–soil feedback in R. austriaca from the native range but not from the invasive range. This could be explained by pathogen accumulation by the native R. austriaca. In contrast, R. austriaca from the invasive range induced no intra- or interspecific plant–soil feedback and was less affected by soil conditioned by native R. austriaca than native R. austriaca was. Furthermore, the invasive population showed increased vigour compared to the native population. This thus suggests that, contrary to our expectation, plants from the invasive range did not increase but diminished their negative effects on other species via soil modification. The co-occurring grass species is thus likely to co-exist with R. austriaca in the invasive range.
References
Abela-Hofbauerová I, Münzbergová Z (2011) Increased performance of Cirsium arvense from the invasive range. Flora - Morphol Distrib Funct Ecol Plants 206:1012–1019. doi:10.1016/j.flora.2011.07.007
Agrawal AA, Kotanen PM, Mitchell CE et al (2005) Enemy release? an experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 86:2979–2989. doi:10.1890/05-0219
Anacker BL, Klironomos JN, Maherali H et al (2014) Phylogenetic conservatism in plant-soil feedback and its implications for plant abundance. Ecol Lett 17:1613–1621. doi:10.1111/ele.12378
Andonian K, Hierro JL (2011) Species interactions contribute to the success of a global plant invader. Biol Invasions 13:2957–2965. doi:10.1007/s10530-011-9978-x
Andonian K, Hierro JL, Khetsuriani L et al (2011) Range-expanding populations of a globally introduced weed experience negative plant-soil feedbacks. PLoS One 6, e20117. doi:10.1371/journal.pone.0020117
Bais HP, Vepachedu R, Gilroy S et al (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:1377–1380. doi:10.1126/science.1083245
Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press, Oxford
Barto EK, Powell JR, Cipollini D (2010) How novel are the chemical weapons of garlic mustard in North American forest understories? Biol Invasions 12:3465–3471. doi:10.1007/s10530-010-9744-5
Bennett AE, Thomsen M, Strauss SY (2011) Multiple mechanisms enable invasive species to suppress native species. Am J Bot 98:1086–1094. doi:10.3732/ajb.1000177
Bever JD, Westover KM, Antonovics J (1997) Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J Ecol 85:561–573. doi:10.2307/2960528
Bever JD, Dickie IA, Facelli E et al (2010) Rooting theories of plant community ecology in microbial interactions. Trends Ecol Evol 25:468–478. doi:10.1016/j.tree.2010.05.004
Birnbaum C, Leishman MR (2013) Plant-soil feedbacks do not explain invasion success of Acacia species in introduced range populations in Australia. Biol Invasions 15:2609–2625. doi:10.1007/s10530-013-0478-z
Bleeker W (2003) Hybridization and Rorippa austriaca (Brassicaceae) invasion in Germany. Mol Ecol 12:1831–1841. doi:10.1046/j.1365-294X.2003.01854.x
Bleeker W, Matthies A (2005) Hybrid zones between invasive Rorippa austriaca and native R. sylvestris (Brassicaceae) in Germany: ploidy levels and patterns of fitness in the field. Heredity 94:664–670. doi:10.1038/sj.hdy.6800687
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. Ecology 83:887–889
Blumenthal DM, Hufbauer RA (2007) Increased plant size in exotic populations: a common-garden test with 14 invasive species. Ecology 88:2758–2765. doi:10.1890/06-2115.1
Bone E, Farres A (2001) Trends and rates of microevolution in plants. In: Hendry AP, Kinnison MT (eds) Microevolution Rate, Pattern, Process. Springer, Netherlands, pp 165–182
Bossdorf O, Auge H, Lafuma L et al (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11. doi:10.1007/s00442-005-0070-z
Buschmann H, Edwards PJ, Dietz H (2005) Variation in growth pattern and response to slug damage among native and invasive provenances of four perennial Brassicaceae species. J Ecol 93:322–334. doi:10.1111/j.1365-2745.2005.00991.x
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523. doi:10.1126/science.290.5491.521
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443. doi:10.2307/3868432
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427:731–733
de la Peña E, Bonte D, Moens M (2009) Evidence of population differentiation in the dune grass Ammophila arenaria and its associated root-feeding nematodes. Plant Soil 324:307–316. doi:10.1007/s11104-009-9958-4
Dietz H, Köhler A, Ullmann I (2002) Regeneration growth of the invasive clonal forb Rorippa austriaca (Brassicaceae) in relation to fertilization and interspecific competition. Plant Ecol 158:171–182. doi:10.1023/A:1015567316004
Dostál P (2011) Plant competitive interactions and invasiveness: searching for the effects of phylogenetic relatedness and origin on competition intensity. Am Nat 177:655–667. doi:10.1086/659060
Engelkes T, Morriën E, Verhoeven KJF et al (2008) Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456:946–948
Felker-Quinn E, Bailey JK, Schweitzer JA (2011) Soil biota drive expression of genetic variation and development of population-specific feedbacks in an invasive plant. Ecology 92:1208–1214. doi:10.1890/10-1370.1
Felker-Quinn E, Schweitzer JA, Bailey JK (2013) Meta-analysis reveals evolution in invasive plant species but little support for Evolution of Increased Competitive Ability (EICA). Ecol Evol 3:739–751. doi:10.1002/ece3.488
Hawkes CV, Kivlin SN, Du J, Eviner VT (2013) The temporal development and additivity of plant-soil feedback in perennial grasses. Plant Soil 369:141–150. doi:10.1007/s11104-012-1557-0
Huberty M, Tielbörger K, Harvey JA et al (2014) Chemical defenses (Glucosinolates) of native and invasive populations of the range expanding invasive plant Rorippa austriaca. J Chem Ecol 40:363–370. doi:10.1007/s10886-014-0425-1
Jonsell B (1993) Taxonomy and distribution of Rorippa (Cruciferae) in the southern USSR. Sven Bot Tidskr 67:281–302
Jordan NR, Larson DL, Huerd SC (2008) Soil modification by invasive plants: effects on native and invasive species of mixed-grass prairies. Biol Invasions 10:177–190. doi:10.1007/s10530-007-9121-1
Kardol P, Martijn Bezemer T, van der Putten WH (2006) Temporal variation in plant–soil feedback controls succession. Ecol Lett 9:1080–1088. doi:10.1111/j.1461-0248.2006.00953.x
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70. doi:10.1038/417067a
Knevel IC, Lans T, Menting FBJ et al (2004) Release from native root herbivores and biotic resistance by soil pathogens in a new habitat both affect the alien Ammophila arenaria in South Africa. Oecologia 141:502–510. doi:10.1007/s00442-004-1662-8
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant–soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992. doi:10.1111/j.1461-0248.2008.01209.x
Lamb EG, Kennedy N, Siciliano SD (2011) Effects of plant species richness and evenness on soil microbial community diversity and function. Plant Soil 338:483–495. doi:10.1007/s11104-010-0560-6
Lankau RA (2013) Species invasion alters local adaptation to soil communities in a native plant. Ecology 94:32–40. doi:10.1890/12-0675.1
Lankau RA, Strauss SY (2007) Mutual feedbacks maintain both genetic and species diversity in a plant community. Science 317:1561–1563. doi:10.1126/science.1147455
Mangan SA, Schnitzer SA, Herre EA, Mack KML, Valencia MC, Sanchez EI, Bever JD (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466:752–755. doi:10.1038/nature09273
Mangla S, Inderjit, Callaway RM (2008) Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J Ecol 96:58–67. doi:10.1111/j.1365-2745.2007.01312.x
Maron JL, Vilà M, Bommarco R et al (2004) Rapid evolution of an invasive plant. Ecol Monogr 74:261–280. doi:10.1890/03-4027
Meisner A, de Boer W, Cornelissen JHC, van der Putten WH (2012) Reciprocal effects of litter from exotic and congeneric native plant species via soil nutrients. PLoS One 7, e31596. doi:10.1371/journal.pone.0031596
Morriën E, van der Putten WH (2013) Soil microbial community structure of range-expanding plant species differs from co-occurring natives. J Ecol 101:1093–1102. doi:10.1111/1365-2745.12117
Oberdorfer E (1990) Pflanzensoziologische Exkursionsflora. Stuttgart: Ulmer 1050p.-illus.. ISBN 3800134764 Ge Icones, Chromosome numbers, Keys. Plant records. Geog
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Pereira HM, Leadley PW, Proença V et al (2010) Scenarios for global biodiversity in the 21st Century. Science 330:1496–1501. doi:10.1126/science.1196624
Perkins LB, Nowak RS (2013) Native and non-native grasses generate common types of plant–soil feedbacks by altering soil nutrients and microbial communities. Oikos 122:199–208. doi:10.1111/j.1600-0706.2012.20592.x
Reinhart KO, Callaway RM (2004) Soil biota facilitate exotic acer invasions in Europe and north america. Ecol Appl 14:1737–1745. doi:10.1890/03-5204
Reinhart KO, Packer A, Van der Putten WH, Clay K (2003) Plant–soil biota interactions and spatial distribution of black cherry in its native and invasive ranges. Ecol Lett 6:1046–1050. doi:10.1046/j.1461-0248.2003.00539.x
Seastedt TR, Pyšek P (2011) Mechanisms of plant invasions of North American and European Grasslands. Annu Rev Ecol Evol Syst 42:133–153. doi:10.1146/annurev-ecolsys-102710-145057
Seifert EK, Bever JD, Maron JL (2009) Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90:1055–1062. doi:10.1890/08-0419.1
Shannon S, Flory SL, Reynolds H (2011) Competitive context alters plant–soil feedback in an experimental woodland community. Oecologia 169:235–243. doi:10.1007/s00442-011-2195-6
Stinson KA, Campbell SA, Powell JR et al (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol 4, e140. doi:10.1371/journal.pbio.0040140
Sun B, Wang P, Kong C-H (2014) Plant-soil feedback in the interference of allelopathic rice with barnyardgrass. Plant Soil 377:309–321. doi:10.1007/s11104-013-2004-6
Tamis WLM, Zelfde MV, Meijden RVD, Haes HAUD (2005) Changes in vascular plant biodiversity in the Netherlands in the 20th Century explained by their climatic and other environmental characteristics. Clim Chang 72:37–56. doi:10.1007/s10584-005-5287-7
Te Beest M, Stevens N, Olff H, Van Der Putten WH (2009) Plant–soil feedback induces shifts in biomass allocation in the invasive plant Chromolaena odorata. J Ecol 97:1281–1290. doi:10.1111/j.1365-2745.2009.01574.x
Thorpe AS, Thelen GC, Diaconu A, Callaway RM (2009) Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97:641–645. doi:10.1111/j.1365-2745.2009.01520.x
Thrall PH, Burdon JJ, Bever JD (2002) Local adaptation in the Linum marginale—Melampsora lini host-pathogen interaction. Evolution 56:1340–1351. doi:10.1111/j.0014-3820.2002.tb01448.x
Thuiller W, Albert C, Araújo MB et al (2008) Predicting global change impacts on plant species’ distributions: Future challenges. Perspect Plant Ecol Evol Syst 9:137–152. doi:10.1016/j.ppees.2007.09.004
Tutin TG, Burges NA, Chater AO et al (1993) Flora europaea, 2nd edn. Cambridge University Press, Cambridge
Van der Putten WH, Bardgett RD, Bever JD et al (2013) Plant–soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276. doi:10.1111/1365-2745.12054
Van Grunsven RHA, Van Der Putten WH, Bezemer TM et al (2007) Reduced plant–soil feedback of plant species expanding their range as compared to natives. J Ecol 95:1050–1057. doi:10.1111/j.1365-2745.2007.01282.x
Van Grunsven RHA, Bos F, Ripley BS et al (2009) Release from soil pathogens plays an important role in the success of invasive Carpobrotus in the Mediterranean. South Afr J Bot 75:172–175. doi:10.1016/j.sajb.2008.09.003
Van Grunsven RHA, van der Putten WH, Martijn Bezemer T et al (2010) Plant–soil interactions in the expansion and native range of a poleward shifting plant species. Glob Chang Biol 16:380–385. doi:10.1111/j.1365-2486.2009.01996.x
Yamane A, Fujikura J, Ogawa H, Mizutani J (1992a) Isothiocyanates as alleopathic compounds fromRorippa indica Hiern. (Cruciferae) roots. J Chem Ecol 18:1941–1954. doi:10.1007/BF00981918
Yamane A, Nishimura H, Mizutani J (1992b) Allelopathy of yellow fieldcress (Rorippa sylvestris): Identification and characterization of phytotoxic constituents. J Chem Ecol 18:683–691. doi:10.1007/BF00994606
Yang Q, Carrillo J, Jin H et al (2013) Plant–soil biota interactions of an invasive species in its native and introduced ranges: Implications for invasion success. Soil Biol Biochem 65:78–85. doi:10.1016/j.soilbio.2013.05.004
Zuppinger-Dingley D, Schmid B, Chen Y et al (2011) In their native range, invasive plants are held in check by negative soil-feedbacks. Ecosphere 2:art54. doi:10.1890/ES11-00061.1
Acknowledgments
We are grateful to Bohdana Frantíková for her technical assistance with the experiment. Participants in the POPEKOL seminars, Michael Van Nuland and two anonymous reviewers provided us with many useful comments. The study was supported by the Charles University in Prague, project GA UK No. 400611, and partly by institutional projects RVO 67985939 and MŠMT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juha Mikola.
Rights and permissions
About this article
Cite this article
Dostálek, T., Münzbergová, Z., Kladivová, A. et al. Plant–soil feedback in native vs. invasive populations of a range expanding plant. Plant Soil 399, 209–220 (2016). https://doi.org/10.1007/s11104-015-2688-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2688-x