Abstract
Plant-soil feedback is recognized as the mutual interaction between plants and soil microorganisms, but its role on the biological invasion of the Brazilian tropical seasonal dry forest by invasive plants still remains unclear. Here, we analyzed and compared the arbuscular mycorrhizal fungi (AMF) communities and soil characteristics from the root zone of invasive and native plants, and tested how these AMF communities affect the development of four invasive plant species (Cryptostegia madagascariensis, Parkinsonia aculeata, Prosopis juliflora, and Sesbania virgata). Our field sampling revealed that AMF diversity and frequency of the Order Diversisporales were positively correlated with the root zone of the native plants, whereas AMF dominance and frequency of the Order Glomerales were positively correlated with the root zone of invasive plants. We grew the invasive plants in soil inoculated with AMF species from the root zone of invasive (I changed) and native (I unaltered) plant species. We also performed a third treatment with sterilized soil inoculum (control). We examined the effects of these three AMF inoculums on plant dry biomass, root colonization, plant phosphorous concentration, and plant responsiveness to mycorrhizas. We found that I unaltered and I changed promoted the growth of all invasive plants and led to a higher plant dry biomass, mycorrhizal colonization, and P uptake than control, but I changed showed better results on these variables than I unaltered. For plant responsiveness to mycorrhizas and fungal inoculum effect on plant P concentration, we found positive feedback between changed-AMF community (I changed) and three of the studied invasive plants: C. madagascariensis, P. aculeata, and S. virgata.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the feedback between invasive plants and soil communities that influence abiotic and biotic properties of the root zone, which in turn influence biological invasion and the performance of the invasive plant species, is fundamental to predict the course of biological invasion in areas from the Brazilian tropical seasonal dry forest. Plant-soil feedback (hereafter PSF) is recognized as the mutual interaction between plants and soil microorganisms [1], but its role on the biological invasion of the Brazilian xeric shrubland by invasive plant species still remains unclear. According to Carvalho et al. [2], positive PSF may contribute to the success of invasive plants introduced into new habitats by altering soil mutualistic and enemies’ impacts. Invasion success may also depend on the action of abiotic (e.g., water availability, sunlight, soil fertility, soil oxygen, and soil temperature) and biotic conditions (e.g., the community composition of herbivores, decomposers, pathogens, and predators) as described by Johnstone [3] and Agrawal et al. [4].
Biologic invasion by legume and non-legume invasive plant species is recognized as one of the most important events affecting the dynamics of plant communities in the Brazilian semi-arid [5,6,7]. Invasive plant species such as Cryptostegia madagascariensis Bojer ex. Decne, Prosopis juliflora SW. (DC.), Parkinsonia aculeata L., and Sesbania virgata (Cav.) Pers. have been displacing native species (e.g., Copernicia prunifera (Mill.) H. E. Moore. and Mimosa tenuiflora (Willd.)), which might have a negative impact in the soil community (e.g., changing microbial community composition, altering nutrient cycling, and reducing soil community diversity) [2, 7]. Studies from elsewhere have shown that over time, invasive plant species can also affect soil properties, such as soil pH and available phosphorous [8, 9], and alter arbuscular mycorrhizal fungi (hereafter AMF) communities (i.e., changed-AMF community) [10, 11], contributing to a decline of AMF diversity [12,13,14] and influencing the outcome of PFS in the new ranges [2].
PSF may be mediated by symbiotic mutualists, such as AMF [15]. PSF effects among invasive plants and root-associated organisms and saprotrophic organisms have been described in sand dunes, grasslands, and in Mediterranean conditions [16,17,18]. Klironomos [15] and van der Putten et al. [19] reported that invasive plants show positive PSF more frequently than native plants in their new geographic ranges. Invasive plants can also experience stronger positive PSF mediated by AMF and other soil organisms than natives in introduced ranges [16, 20,21,22]. Despite the growing interest in understanding the mechanisms associated with successful plant invasion, little is known about the potential linkages between invasive plant species and AMF in semi-arid conditions.
The main objective of the present study was to investigate the relation established between four invasive plant species (C. madagascariensis, P. aculeata, P. juliflora, and S. virgata) in the Brazilian semi-arid and the AMF communities. Our study addressed the following questions: (1) Do invasive plant species alter the composition of the AMF community in the new range? Based on the enhanced mutualisms hypothesis [20, 22], we expected to find evidence for a changed-AMF community. (2) Is there evidence for differences in the relationship between invasive and native plants and the changed-AMF community in field conditions? We hypothesized that invasive plants would experience a stronger interaction with the changed-AMF community according to the invasion opportunity windows and resource enemy release hypotheses [3, 4, 23]. (3) How do invasive plant species grown from seed respond to the inoculation with AMF from native and invasive root zones? We expected invasive plant species to show a higher mycorrhizal colonization, rhizobial colonization, and plant responsiveness to mycorrhizas after inoculation with changed-AMF communities. This expectation is based on the disturbance-contingent niche creation model [24]. To accomplish this, we combined field sampling of two root zone types, i.e., invasive and native root zones, characterized both the soil chemical properties and AMF communities, and performed bioassays under glasshouse conditions. Fungal inoculum was obtained by establishing a trap culture with Zea mays, a standard host plant used in several mycorrhizal inoculum potential assays according to the International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi, INVAM (http://invam.caf.wvu.edu/). The bioassays allowed us to investigate soil feedback effects on plant dry biomass, root colonization, plant phosphorous concentration, and plant responsiveness to mycorrhizas.
Material and Methods
Plant Species and Study Sites
Field sampling was carried out in four different areas in Brazil (Ibaretama, Ceará, 06° 51′ 11.3″ S; 35° 55′ 51.5″ W; Juazeirinho, Paraíba, 07° 02′ 3.4″ S; 36° 30′ 16.6″ W; Monteiro, Paraíba, 06° 51′ 12.6″ S, 35° 55′ 50.6″ W; and Natuba, Paraíba, 07° 26′ 86″ S, 35° 32′ 73″ W). These study areas are classified as hot semi-arid climate (type Bsh) following Köppen-Geiger climate classification, i.e., hot semi-arid with hot summers and mild to warm winters, annual precipitation, and temperature of 600 mm and 30 °C, respectively. In these sites, rainfall is highly low, unpredictable, and irregular [25]. Data on the monthly rainfall and main temperature between January and December 2012 were obtained from the Brazilian National Institute of Meteorology (Fig. 1; http://www.inmet.gov.br). With the exception of Ibaretama site where the soil was classified as eutric vertisol, soil types from the remaining areas were classified as sandy loam dystric fluvisols [26].
Mean temperature (black line) and rainfall amount (dotted line) in each studied site from January to December 2012; the arrows indicate the sampling period. Data were obtained from the website: http://www.inmet.gov.br
We selected the invasive plant species C. madagascariensis, P. juliflora, P. aculeata, and S. virgata, because C. madagascariensis co-occur with C. prunifera, which is an endangered native species from Ceará, while the remaining invaders co-occur with M. tenuiflora that is a common native tree from the Brazilian xeric shrubland [27]. Seeds from the invasive plants were directly collected from the studied areas, kept in paper bags, and dried at room temperature.
Field Sampling, Soil, and AMF Communities’ Characterization
In each study area, we established 40 plots of 100 m2 according to Fortin and Dale [28]. Within each plot, we selected 80 plant species (i.e., 40 invasive plant species plus 40 native plant species) according to the following criteria: (1) the plant had a diameter near soil surface >3 cm and (2) no individual from a different plant species were growing in a 3-m radius to the sampling point in all directions [28,29,30]. Root zone samples (including soil and root fragments) were collected near the drip line and beyond (0–20 cm deep), during the dry period, i.e., at the beginning of September 2012. By sampling during the dry season, we guaranteed that we sampled the largest number of AMF species, because fungal sporulation is expected to be higher at this time of the year in semi-arid environments [31]. Samples from each plant species in each plot were bulked, mixed, and stored at 4 °C until host-plant bioassays. During sampling and handling of each soil sample, precautions (e.g., sterilization with ethanol and gloves) were undertaken to avoid cross contamination. Later, each sample collected from the field was divided into portions intended for chemical soil characterization, AMF community assessment, and mounting trap cultures.
To chemically characterize the soil from each plot, we analyzed soil pH, total organic carbon, total nitrogen, and available phosphorus (N = 40 by plant species). Soil pH was measured in a suspension of soil and distilled water (1:2.5 v:v, soil:water suspension) [32]. Total organic carbon was determined by rapid dichromate oxidation method according to the methodology described by Okalebo et al. [33]. To quantify total nitrogen, soil samples were first digested with sulfuric acid plus potassium sulfate, and we then followed the protocol described in Kjeldahl [32]. Available phosphorus (Olsen’s P) was determined colorimetrically using a spectrophotometer at 882 nm by extraction with sodium bicarbonate for 30 min [34].
AMF communities extracted from native and invasive plant species were classified as “changed-AMF community” if they occurred in the root zone of invasive plant species only, and as “unaltered-AMF community” if they occurred in the root zone of native plant species only. These were used to establish trap cultures of each AMF community for each studied root zone grown in field soil in sterilized 2-L plastic pots to later be propagated on a common host-plant (Z. mays L.). To establish the initial fungal inoculum (i.e., soil, roots, and spores), we used a portion of soil (500 g) from the field. We sterilized the seeds of Z. mays in 10% sodium hypochlorite for 10 min, and then these were thoroughly rinsed with deionized water. This species is a standard host-plant used for mycorrhiza trap cultures and inoculum potential assays according to the INVAM (http://invam.caf.wvu.edu/). Z. mays plants were grown in a mixture 3:1 (m:m) of sterilized sand to field soil during 4 months in a glasshouse at the University of Coimbra. This was the period required until sporulation occurred. The average temperature in the glasshouse was 28 °C, ranging from 20 to 35 °C, an irradiance of up to 70% of full sun, relative humidity ranging between 65 and 75%, and photoperiod of 16 h/8 h light/dark. The host-plants received weekly amounts of a modified nutrient solution [35] containing 554.0 mg L−1 KCl, 200.0 mg L−1 NaH2PO4·H2O, 2.24 mg L−1 MgSO4, 520.0 mg L−1 CaCl2·H2O, 1.7 mg L−1 MnSO4, 0.25 mg L−1 CuSO4·5H2O, 0.30 mg L−1 ZnSO4·7H20, 5.0 mg L−1 NaCl, 3.0 mg L−1 H3O3, 0.09 mg L−1 (NH4)6Mo7O24·4H2O, and 32.9 mg L−1 Na-Fe EDTA. The nutrient solution was previously sterilized by UV radiation to avoid external contamination.
Spores from field and trap cultures were extracted by the wet sieving technique [36] followed by sucrose centrifugation [37]. For this, we used 100 g of field soil and 100 g from the trap cultures. Initially, the extracted spores were examined in water under a dissecting microscope and they were separated based on morphology. Subsequently, they were mounted in polyvinyl alcohol lacto-glycerol (PVLG) with or without the addition of Melzer’s reagent [38]. Species identification was based on the descriptions provided by Schenck & Perez [39], publications with descriptions of new families and genera [40], and by consulting the INVAM database (http://invam.caf.wvu.edu). In this work, we followed the classification proposed by Oehl et al. [40], including recently new described taxa [41, 42]. In addition to species identification, we also assessed spore abundance by counting the total number of spores, spore abundance of each AMF species by recording the number of spores of each AMF species recorded in the samples, and the species occurrence frequency (FO i) of each AMF species. FO i was calculated using the following equation:
where n i is the number of times an AMF species was observed and N is the total of AMF spores observed from each studied area. We classify the FO i of each AMF species based on Zhang’s [43] frequency of occurrence classification: dominant (FO i > 50%), most common (31 ≤ FO i ≤ 50%), common (10 ≤ FO i ≤ 30%), and rare (FO i < 10%).
Bioassay Experiments
The bioassay experiments were performed in a completely randomized design with three treatments: sterilized inoculum (control) and two non-sterilized inoculum treatments, one with inoculum of AMF communities from native plant root zone (unaltered-AMF), and the other with inoculum from invasive plant root zone (changed-AMF). The control received 500 mL of filtrate from 500 g of AMF-inoculum with no mycorrhizal spores obtained by sieving through a 25-μm mesh and 500 g of sterilized mixed AMF inoculum (a mix of all AMF inoculums by plant species), thereby controlling for potential mineral and non-mycorrhizal microbial components of the AMF inoculum. Each treatment had 30 replicates, each from 30 independent replicates at trap culture stage.
This experiment was conducted in a glasshouse at the University of Coimbra with average temperatures of 25 °C/16 °C (day/night). Seeds of all invasive plant species were sterilized in 10% sodium hypochlorite for 10 min, and then thoroughly rinsed with deionized water. Seeds were then germinated in sterilized trays containing sterilized sand (sterilized twice at 121 °C for 20 min each in two consecutive days). Seven days after emergence, seedlings (plant height varied between 2 and 4 cm) were selected and individually transferred to sterilized plastic pots containing 2000 g of substrate obtained by mixing 1500 g of sterilized sand with 500 g of soil containing fungal inoculum. The number of infective propagules was determined following the protocol described by Habte and Osorio [44] and ranged between 5.35 and 6.18 propagules per gram of soil. All pots were covered with aluminum wrap around the seedling to prevent dehydration and external contamination, and plants were watered with sterilized water as necessary and fertilized using the same nutrient solution as in the trap culture [35].
Five months after planting, the invasive plant species were harvested. Roots were separated from shoots, and the fresh roots were weighed immediately. A total of 0.5 g of each fresh root sample was used for determination of root colonization following the grid line intersect method [45, 46]. Under a compound microscope at 200× magnification, 100 intersects were examined and hyphae were scored as “mycorrhizal” based on the presence of vesicles, arbuscules, spores, and the morphology of the mycelium. Thus, if any of the structures referred to were found in one of the 100 microscope intersections per replicate, they were scored as mycorrhizal. To avoid error associated with the observer, all microscopic examinations were carried out by the same individual.
To estimate shoot and root dry biomass, the remaining root material and shoots were oven-dried at 72 °C for 48 h. The plant responsiveness to mycorrhizas (PRM) was calculated using the values of dry biomass from the non-sterilized treatment and the control, by using the formula:
where B AMF is the dry biomass of model plants in the non-sterilized treatment and B control is the dry biomass of the control treatment [47]. To estimate the total P, a known mass of the dry material was first digested in a 10:1:4 mixture of HNO3:H2SO4:HCl (60%), and then analyzed using the vanadate molybdate colorimetric method [48]. We also calculated the AMF inoculum effect on plant P concentration (IEP) by using the formula:
where P AMF is plant P concentration in non-sterilized treatment and P Control refers to plant P concentration in the control [49]. The fungal inoculum effect can only range between −1 and +1, with positive values indicating positive effects of AMF on plant P concentration and negative values indicating negative effects [49].
Statistical Analysis
The Kolmogorov-Smirnov test was applied to assess the normality of the data distribution. To assess for correlations between the chemical soil properties and the ecological indexes of the AMF community, we used Pearson’s correlation. To explore the variability and similarities among soils and AMF community composition among plant species, principal component analysis (PCA) was used. Student t test for independent samples was carried out to investigate differences between native and invasive root zones in each parameter from field collection. One-way ANOVA was used to test for the effect of fungal inoculum on plant dry biomass and plant P concentration for all studied plant species in the bioassay. Data sets were arcsin square root transformed for percentage variables and log10(x) for the remaining [50]. Notwithstanding, the results are presented in their original scale of measurement (mean ± standard deviation). In the control treatment, mycorrhizal colonization, PRM, and IEP were zero and consequently, they were excluded from the statistical analyses. When necessary, Tukey’s HSD post hoc comparison tests were conducted. All statistical analyses were conducted using SAS 9.1.3 Portable, while the ecological indexes and PCA analysis were conducted using MVSP 3.1 [51].
Results
Effects of Invasive Plants on Soil Characteristics
Significant differences between native and invasive root zones were found for soil chemical properties in all studied areas (Table 1). Across the investigated areas, the root zone of the invasive plant species had a soil pH ranging from acid (e.g., S. virgata) to neutral (e.g., P. aculeata and P. juliflora), whereas all root zone of the native plant species had acid pH. Total organic carbon (P < 0.01), total nitrogen (P < 0.01), and available phosphorous (P < 0.01) were significantly larger in the root zone of the invasive plant species. However, no differences between C. madagascariensis and C. prunifera were obtained for total organic carbon (P = 0.1150). Not only total organic carbon was particularly high in the root zone of P. aculeata (9.99 ± 0.57 g kg−1) and S. virgata (9.73 ± 1.78 g kg−1) but also total nitrogen (0.98 ± 0.09 and 1.51 ± 0.17 g kg−1, respectively). The highest value of available phosphorous was found in the root zone of P. juliflora (10.70 ± 0.52 mg dm−3) (Table 1).
For all study areas, the number of spores, root colonization, diversity, and dominance of AMF species were significantly different between invasive and native plant species (Table 2). The number of AMF spores (P < 0.01) and diversity of AMF species (P < 0.01) were significantly higher in the root zone of native plants than in the invaders. The highest number of spores was found in the root zone of M. tenuiflora (11.70 ± 1.42 spores g soil−1; P < 0.05) from Natuba, PB, whereas the highest diversity in AMF species (2.98 ± 0.22; P < 0.05) was found in the root zone of the same native plant species from Monteiro, PB. In contrast, root colonization (P < 0.01) and dominance of AMF species (P < 0.01) were significantly higher in the root zone of invasive than native plants (Table 2). The highest values were detected in the root zone of S. virgata (40.2 ± 3.1% and 0.91 ± 0.01 for root colonization and dominance of AMF species, respectively) (Table 2).
In total, we identified 29 different AMF species corresponding to 12 genera—Acaulospora (3), Ambispora (1), Claroideoglomus (2), Dentiscutata (2), Entrophospora (1), Funneliformis (3), Gigaspora (4), Glomus (3), Quatunica (1), Racocetra (3), Rhizoglomus (4), and Scutellospora (2). The most abundant taxa were species from the Order Diversisporales in the root zone of all studied native plant species, whereas AMF species from the Order Glomerales, such as AMF species from the genus Claroideoglomus, Funneliformis, and Rhizoglomus, were mostly found in the root zone of the invasive plant species (Table S1). For this reason, we decided to only include the values of frequency of occurrence from these three genera and from the Order Diversisporales in the PCA analysis (Table 3).
The PCA analysis showed a clear plant species separation on the basis of sampling root zone, with all studied invasive plant species on one side and all studied native plant species on the other side (Fig. 2). Number of AMF spores, AMF diversity, and frequency of Order Diversisporales were positively correlated with the root zone of the native plants, whereas chemical soil properties, AMF dominance, and the frequency of occurrence of AMF species from genera Funneliformis and Rhizoglomus were positively correlated with the root zone of the invasive plants (Fig. 2).
Score plot for the PCA of AMF ecological indices (diversity and dominance), chemical soil properties, and frequency of occurrence of AMF species from the Order Diversisporales and genera Claroideoglomus, Funneliformis, and Rhizoglomus from the root zones of the studied plant species. The two axes explained 88.79% of the variation in the samples. Points represent samples in each studied root zone of the plant species. Invasive plants: C. madagascariensis, P. aculeata, P. juliflora, and S. virgata; native plants: C. prunifera and M. tenuiflora. Circles represent samples in root zones from legume plant species
Fungal Inoculum Effect on Plant Growth
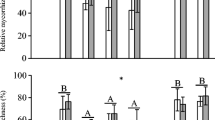
The fungal inoculum (i.e., control, I unaltered, and I changed) had a significant effect on biomass for all studied invasive plant species (C. madagascariensis, F 2,27 = 12.78, P < 0.0001; P. aculeate, F 2,27 = 22.02, P < 0.0001; P. juliflora, F 2,27 = 15.91, P < 0.0001; S. virgata, F 2,27 = 32.81, P < 0.0001). In the invasive C. madagascariensis, no differences between control and I unaltered were found in plant biomass, whereas I changed had a significant positive effect. For the remaining invasive species, both I changed and I unaltered had a significant positive effect on plant growth, but it was significantly larger for I changed than I unaltered (Fig. 3A).
a AMF inoculation effect on plant dry biomass (mg/plant). b Plant phosphorous concentration (g/kg) for invasive plant species growing in soil inoculated with control (non-inoculated); I unaltered (fungal inoculum from the root zone of native plant species) and I changed (fungal inoculum from the root zone of invasive plant species). Values are mean and standard deviation of the mean. Different letters indicate significant differences after Tukey’s HSD post hoc test (P < 0.05) among AMF inoculum treatments for each invasive plant species
While we detected significant effects of AMF inoculum on the AMF root colonization of C. madagascariensis (P < 0.01), P. juliflora (P < 0.05), and S. virgata (P < 0.01), no significant effect was found for P. aculeata (P = 0.0833). There was a drastic reduction in AM colonization caused by AMF inoculum from I unaltered compared to I changed. For the control treatments, we did not find any root colonization for all studied plant species.
Plant P concentration significantly differed among AMF inoculation treatments (C. madagascariensis, F 2,27 = 10.99, P < 0.0001; P. aculeata, F 2,27 = 12.78, P < 0.0001; P. juliflora, F 2,27 = 11.28, P < 0.0001; S. virgata, F 2,27 = 14.01, P < 0.0001). For C. madagascariensis plants, the I unaltered and control treatments showed lower plant P concentration than I changed (P < 0.05). For P. aculeata and P. juliflora, plant P concentration was larger after I unaltered and I changed treatments than in the control (P < 0.05). S. virgata experienced larger positive effects growing with I changed than I unaltered inoculation (P < 0.05; Fig. 3B).
Significant differences between I unaltered and I changed treatments on PRM and IEP for C. madagascariensis (t = 11.13, P < 0.001) and S. virgata (t = 10.79, P < 0.001). For P. aculeata and P. juliflora, there were no significant differences (t = 1.99, P = 0.098, and t = 2.01, P = 0.1055, respectively). The analysis of the effects of AMF inoculation by plant species on PRM and IEP revealed that C. madagascariensis, P. juliflora, and S. virgata experienced significant positive effects when growing with I changed inoculation, and negative effects when growing with I unaltered (t = 23.73, P < 0.001; t = 12.65, P < 0.001; and t = 11.98, P < 0.001, respectively). For P. juliflora, we found no significant differences of both inoculation treatments (t = 2.09, P = 0.1123; Fig. 4A and B).
a AMF inoculation effect on plant responsiveness to mycorrhizas. b IEP for invasive plant species growing in soil inoculated with I unaltered (fungal inoculum from the root zone of native plant species) and I changed (fungal inoculum from the root zone of invasive plant species). Values are mean and standard deviation of the mean. *** indicated significant differences and ns indicated no significant differences by unpaired t test (P < 0.001) among AMF inoculum treatment
Discussion
Our results provided evidence for changes in soil properties caused by invasive plant species and to differences in the AMF community composition between sites with native and invasive plant species in the Brazilian semi-arid region. In fact, invasive plant species may influence AMF community composition in different ways [52]. In our study sites, the introduction of invasive plant species significantly affected AMF abundance and diversity. These results are in agreement with previous studies [8, 9, 52, 54, 55, 60, 61] and support our hypothesis that invasive plants are associated with a changed-AMF community. The whole of these studies showed negative effects of exotic plant species on AMF abundance and diversity. Zubek et al. [52] reported that the number of AMF spores and the number of AMF species (species richness) decreased 35.5 and 20.74%, respectively because of invasion by three exotic plant species in southern Poland. Examining other studies around the world, we found negative effects of Eucalyptus litter on AMF symbiotic status (mycorrhization) [8] and a significant reduction (on average 24%) on AMF diversity as a result of C. maculosa invasion [60] in Africa and California grasslands, respectively. Vogelgang and Bever [61] also reported reduction of AMF density (on average 28%) in the non-native-conditioned soil relative to the native-conditioned soil. In total, when we compare our results against these studies, we found in our case reduction on number of AMF spores (on average 44.1%), AMF diversity (on average 22.4%), and AMF species richness (on average 54.57%) as a result of plant invasion, and we can suggest that the biological invasion process is more impactful in the Brazilian semi-arid compared to other areas where exotic species have taken place. As a consequence, invaders seemed to be in advantage comparing with natives (e.g., emergence rate, survival rate, plant growth, or nutrient uptake) by profiting from beneficial AMF species (e.g., AMF from the Order Glomerales) [53]. The differences in AMF community structure between natives and invasive plant species were revealed by the decreased AMF species richness in all invasive plant species root zone and a lesser root colonization of the natives. According to studies from elsewhere [52, 54, 55], we hypothesize that three different mechanisms may be involved in the detected AMF-changed community. First, invasive species form large monospecific plant populations, as was the case in our study, thus reducing the diversity of host-plants available to the AMF community. Consequently, (1) this changes soil organic carbon inputs [5, 7, 56], and (2) monospecific stands of exotic plant species result in a reduction of mycorrhizal plant communities that impact negatively the AMF growth because the reduction of possible host-plants [57].
Second, metabolites produced by invaders negatively affect native plant growth by disrupting their mutualistic associations with the unaltered-AMF community [52]. Studies by Stinson et al. [58], Callaway et al. [54], and Yuan et al. [59] have provided evidence that invasive plant species produce metabolites that are novel for unaltered-AMF community in their introduced areas, and these secondary compounds directly limit AMF growth, spore germination, and root colonization [54]. Consequently, the most beneficial AMF (e.g., AMF species from Order Glomerales) from the unaltered-AMF community composition are favored, while the growth of the less favorable ones (e.g., AMF species from Order Diversisporales) is inhibited.
Finally, the introduction of invasive plant species might cause changes in soil chemical properties that may indirectly affect AMF community composition and thus, contribute to a successful establishment and spread of invasive plant species [53]. As revealed by our study, soil pH and available P were higher in the root zone of all invaders compared with native plants, whereas total organic carbon and total nitrogen were higher in the root zone of P. aculeata, P. juliflora, and S. virgata than in the native’s root zone. These results are in agreement with previous work [8, 62] that reported higher values of soil pH and available P in the root zone of invasive plants, such as Acacia senegal, Acacia seyal, Acacia albida, Eragostis albensis, and Olpidium spp. By altering the chemical properties of the soil below their canopy, invasive plant species may alter the nutrient cycle [63] and thus, are responsible for the modification from an unaltered-AMF community to a changed-AMF community in the invaded sites [52, 57]. Our results are in agreement with the mechanisms described; once the invasive plant species significantly changed plant community structure, soil properties that turn directly and indirectly altered AMF community structure (number of AMF spores, root colonization, AMF diversity, AMF dominance, and the frequency of occurrence of AMF species).
The PCA analysis revealed that AMF species from the Order Glomerales (e.g., genus Claroideoglomus, Funneliformis, and Rhizoglomus), the extent of root colonization, and AMF dominance are positively correlated with the root zone of invasive species, supporting our hypothesis that invasive plants would experience a stronger interaction with the changed-AMF community. According to Ramos et al. [64] and Carneiro et al. [65], AMF species from the Orders Diversisporales and Gigasporales are commonly found in acid soil with low available P, and changes in these two variables might be more favorable to AMF species from the Order Glomerales, such as Claroideoglomus claroideum, Funneliformis caledonium, Funneliformis mosseae, and Rhizophagus intraradices.
In our bioassay, all fungal inoculum (I unaltered and I changed) were capable of root colonization of the invasive plant species. Plant dry biomass and plant phosphorous concentration indicated that the invasive plants responded positively to the I changed treatment. The results from these two variables also indicated a low responsiveness of the studied invasive plants to I unaltered and control treatments. In fact, we found a significantly positive effect of I changed on the root colonization, which might promote the growth of the invasive plants studied here. These results support our hypotheses that (1) invasive plants are strongly associated with I changed, and that (2) the fungal inoculum would result in a high mycorrhizal colonization and rhizobial colonization. However, we cannot exclude the hypothesis that a rhizobial population colonizing legume invasive species after I changed inoculation may be also involved in the increased plant dry biomass, plant P concentration, and root colonization. The ability to nodulate in the presence of a changed-AMF community may play a very important role in the first stages of biological invasion [66] and deserves further consideration.
Fungal inoculum from the root zone of the invasive plant species (I changed) played a role in the early growth of invasive species in our study, since there was a large increasing in AMF root colonization, PRM, and IEP. Conversely, the fungal inoculum from native plant species rhizosphere (I unaltered) promoted the reduction on plant biomass accompanied by the reduction in plant P concentration and AMF root colonization. These results suggest that the studied invasive plant species (legume or non-legume) may benefit from I changed, but C. madagascariensis and S. virgata experienced higher benefits from the I changed treatment than P. aculeata and P. juliflora.
Our results from field samples suggest an increase in promiscuous AMF propagules more associated with invasive than native plants, such as AMF species from the Order Glomerales (e.g., C. claroideum, F. caledonium, F. mosseae, and R. intraradices). The results showing higher benefits for the invasive plant growth after inoculation with I changed fungal inoculum than when inoculated with I unaltered fungal inoculum support previous studies by Grümberg et al. [67] and Ortiz et al. [68], which concluded that AMF benefit plants by mediating the availability of important plant nutrients, promoting plant growth, and offering protection against drought and soil pathogens, thus highly benefiting fast-growing invaders [24]. This largely supports our hypothesis that the spread, invasion, and dominance of invasive species would result in positive feedbacks for their own growth [16, 24]. Evidences for direct modification of soil communities induced by plant invasion thus generating self-facilitative conditions for invaders have been found in other study systems [4, 16, 20]. Our results also suggest that the sites invaded by the exotic plant species present a changed-AMF community that are in general characterized by low microbiological activity as described by Zhang et al. [16] and Zubek et al. [52]. The decreased emergence, survival rate, plant growth, or nutrient uptake of several native plant species were found on soils with changed-AMF community by biological invasion process in comparison to soils with unaltered-AMF in North America and Asia [16, 54, 58, 61].
As is highly recognized, AMF increase nutrient acquisition, growth, and vitality of their hosts, independently of the plant species, thus playing an important role in the survival of their plant host. In semi-arid conditions as is the case in our study, AMF are also crucial for the protection of their hosts against abiotic stresses, such as drought and salinity, and have been found to determine plant community composition and function in other study systems [69]. The main point of our study was to assess the changes in soil properties caused by invasive plants that in turn affect AMF community composition given as abundance and species richness. Despite the clear pattern of a changed-AMF community associated with the invasive species, at this stage, we cannot prove that changes in AMF community composition in the Brazilian semi-arid region are detrimental to native plant species and accelerate plant invasions as described by Reinhart and Callaway [22] and Shah et al. [53].
The main findings of this study may be summarized as follows: (1) invasive plant species alter soil chemical properties and AMF community composition in field conditions from the Brazilian semi-arid region, and (2) the inoculation with a changed-AMF community (I changed) in a bioassay experiment increased the growth, P uptake, AMF colonization, and rhizobial colonization of all invasive plant species. Our findings suggest that a changed-AMF community can facilitate the establishment and subsequent spread of invasive plants in the Brazilian semi-arid region. Despite our results are an important contribution to our understanding on the importance of considering ecological mechanisms underlying how PSF affects biological invasions, we do not know for sure that the patterns obtained under controlled glasshouse conditions would be the same in a dynamic plant community. Thus, future studies should include fungal inoculation under field conditions to fully understand its effects on the dominance of invasive plants over natives in natural communities.
References
Kardol P, Veen GF, Teste FP, Perring MP (2015) Peeking into the black box: a trait-based approach to predicting plant-soil feedback. New Phytol. 206:1–4
Carvalho LM, Antunes PM, Martins-Loução MA, Klironomos JN (2010) Disturbance influences the outcome on plant-soil biota interactions in the invasive Acacia longifolia and in native species. Oikos 119:1172–1180
Johnstone IM (1986) Plant invasion windows: a time-based classification of invasion potential. Brol Rev 61:369–394
Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J (2005) Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 86:2979–2989
Andrade LA, Fabricante JR, Oliveira FX (2009) Invasão biológica por Prosopis juliflora (Sw.) DC.: impactos sobre a diversidade e a estrutura do componente arbustivo-arbóreo da caatinga no estado do Rio Grande do Norte, Brasil. Acta Botanica Brasílica 23:935–943
Silva JL, Barreto RW, Pereita OL (2008) Pseudocercospora cryptostegiae-madagascariensis sp. nov. on Cryptostegia madagascariensis, an exotic vine involved in major biologial invasion in Northeast Brazil. Mycopathologia 166:87–91
Sousa VC, Andrade LA, Bezerra FTC, Fabricante JR, Feitosa RC (2011) Avaliação populacional de Sesbania virgata (Cav.) Pers. (Fabaceae Lindl.) nas margens do rio Paraíba. Agrária (Recife Online) 6:314–320
Soumare A, Manga A, Fall S, Hafidi M, Ndoye I (2015) Effects of Eucaplyptus camaldulensis amendment on soil chemical properties, enzymatic activity, Acacia species growth and roots symbioses. Agrofor. Syst. 89:97–106
Ayanu Y, Jintsch A, Müller-Mahn D, Rettberg S, RomanKiewicz C, Koellner T (2015) Ecosystem engineer unleashed: Prosopis juliflora threatening ecosystem services? Reg. Environ. Chang. 15:155–167
Day NJ, Antunes PM, Dunfield KE (2015) Changes in arbuscular mycorrhizal fungal communities during invasion by an exotic invasive plant. Acta Oecologia 67:66–74
Bunn RA, Ramsey PW, Lekberg Y (2015) Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. J. Ecol. 103:1547–1556
Taylor DL, Bruns TD, Hodges SA (2004) Evidence for mycorrhizal races in a cheating orchid. Proc Royal Soc London B 271:35–43
Shah MA, Callaway RM, Shah T, Houseman GR, Pal RW, Xiao S, Luo W, Rosche C, Reshi ZA, Khasa DP, Chen S (2014) Conyza canadensis suppresses plant diversity in its nonnative ranges but not at home: a transcontinental comparison. New Phytol. 202:1286–1296
Shah MA, Beaulieu M-E, Reshi ZA, Qureshi S, Khasa DP (2015) A cross-city molecular biogeographic investigation of arbuscular mycorrhizas in Conyza canadensis rhizosphere across native and non-native regions. Ecol. Process. doi:10.1186/s13717-015-0034-0
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Zhang Q, Yang R, Tang J, Yang H, Hu S, Chen X (2010) Positive feedback between mycorrhizal fungi and plants influences plant invasion success and resistance to invasion. PLoS One 5:e12380
Abbott KC, Karst J, Biederman LA, Borrett SR, Hastings A, Walsh V, Bever JD (2015) Spatial heterogeneity in soil microbes alters outcomes of plant competition. PLoS One 10:e0125788
Mehrabi Z, Bell T, Lewis OT (2015) Plant-soil feedbacks from 30-year family-specific soil cultures: phylogeny, soil chemistry and plant life stage. Ecology and Evolution 5:2333–2339
van der Putten WH, Kowalchuk GA, Brinkman EP, Doodeman GTA, van der Kaaij RM, Kamp AFD, Menting FBJ, Veenendaal EM (2007) Soil feedbacks of exotic savanna grass relates to pathogen absence and mycorrhizal selectivity. Ecology 88:978–988
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmánek M (2000) Plant invasions-the role of mutualisms. Biol. Rev. 75:65–93
Mummey DL, Rillig MC, Holben WE (2005) Neighboring plant influences on arbuscular mycorrhizal fungal community composition as assessed by T-RFLP analysis. Plant Soil 271:83–90
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol. 170:445–457
Blumenthal D (2005) Interrelated causes of plant invasion. Science 310:243–244
Kulmatiski A, Kardol P (2008) Getting plant-soil feedbacks out of the greenhouse: experimental and conceptual approaches. In: Lüttige U et al. (eds) Progress in botany 69. Springer, Berlin, pp. 449–472
Alves JJA, Araújo MA, Nascimento SS (2009) Degradação da Caatinga: uma investigação ecofisiográfica. Revista Caatinga, Mossoró 22:126–135
WRB (IUSS Working Group) (2006) World reference base for soil. World Soil Resources Reports, 103. FAO, Rome
Mello CMA, Silva IR, Pontes JS, Goto BT, Silva GA, Maia LC (2012) Diversidade de fungos micorrízicos arbusculares em área de Caatinga, PE, Brasil. Acta Botanica Brasilica 26:938–943
Fortin M, Dale MR (2005) Spatial analysis: a guide for ecologists. Cambridge University Press, Cambridge
Caifa AN, Martins FR (2007) Taxonomic identification, sampling methods, and minimum size of the tree sampled: implications and perspectives for studies in the Brazilian Atlantic Rainforest. Functional Ecosystems and Communities 1:95–104
Durigan G (2009) Estrutura e diversidade de comunidades florestais. In: Martins SV (ed) Ecologia de florestas tropicais do Brasil. Editora UFV, Vicoça, pp. 185–215
Silva IRS, Mello CMA, Ferreira Neto RA, Silva DKA, Melo AL, Oehl F, Maia LO (2014) Diversity of arbuscular mycorrhizal fungi along an environmental gradient in the Brazilian semi-arid. Appl. Soil Ecol. 84:166–175
Black CA (1965) Methods of soil analysis, part 2. In: Black CA (ed) Agronomy monograph no. 9. American Society of Agronomy, Madison, pp. 771–1572
Okalebo JR, Gathua KW, Woomer PL (1993) Laboratory methods of plant and soil analysis: A working manual. Soil Science Society East Africa technical publication 1:22–29
Olsen SR, Cole CV, Watanable FS, Dean LA (1954) Estimation of available phosphorous in soils by extraction with sodium bicarbonate. US Department of Agriculture, Washigton DC, USA
Hoagland DR, Arnon DI (1939) The water culture method for growing plant without soil. Calif Agric Exp Stn Circ 347:1–32
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1:43–66
Jenkins WR (1964) A rapid centrifugal flotation technique for separating nematodes from soil. Plant Dis. Rep. 48:692
Walker C, Vestberg M, Demircik F, Stockinger H, Saito M, Sawari H, Nishmura I, Schüßler A (2007) Molecular phylogeny and new taxa in the Archaeosporales (Glomeromycota): Ambispora fennica gen. sp. nov., Ambisporaceae fam. nov., and emendation of Archaeospora and Archaeosporaceae. Mycol. Res. 111:137–153
Schenck NC, Perez Y (1987) Manual for the identification of VA mycorrhizal fungi, Second edn. International Culture Collection of VA Mycorrhizal Fungi (INVAM), University of Florida, Gainesville
Oehl F, Souza FA, Sieverding E (2008) Revision of Scutellospora and description of five new genera and three new families in the arbuscular mycorrhiza-forming Glomeromycetes. Mycotaxon 106:311–360
Goto BT, Silva GA, Assis DMA, Silva DKA, Souza RG, Ferreira ACA, Jobim K, Mello CMA, Vieira HEE, Maia LC, Oehl F (2012) Intraornatosporaceae (Gigasporales), a new family with two new genera and two new species. Mycotaxon 119:117–132
Sieverding E, Silva GA, Berndt R, Oehl F (2014) Rhizoglomus, a new genus of the Glomeraceae. Mycotaxon 129:373–386
Zhang Y, Gui LD, Liu RJ (2004) Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujiangyan, southwest China. Plant Soil 261:257–263
Habte M, Osorio NW (2001) Arbuscular mycorrhizas: producing and applying arbuscular mycorrhizal inoculum. University of Hawaii, Honolulu
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55:158–160
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 84:489–500
Janos DP (2007) Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17:75–91
Jackson ML (1973) Estimation of phosphorous content. Soil chemical analysis. Printer Hall Inc., New Delhi
Armas C, Ordiales R, Pugnaire F (2005) Measuring plant interactions: a new comparative index. Ecology 85:2682–2886
Zar JH (1984) Biostatistical analysis. Prentice Hall, New Jersey
Kovach WL (2007) MVSP—a multivariate statistical package for Windows, ver. 3.1. Kovach Computing Services, Pentraeth
Zubek S, Majewska ML, Błaszkowski J, Stefanowicz AM, Nobis M, Kapusta P (2016) Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol. Fertil. Soils 52:879–893
Shah MA, Reshi ZA, Khasa D (2009) Arbuscular mycorrhizas: drivers or passengers of alien plant invasion. Bot. Rev. 75:397–417
Callaway RM, Cipolini D, Barto K, Thelen GC, Hallett SG, Prati D, Stinson K, Klironomos J (2008) Novel weapons: invasive plant suppresses fungal mutualists in American but not in its native Europe. Ecology 89:1043–1055
Tanner RA, Gange AC (2013) The impact of two non-native plant species on native flora performance: potential implications for habitat restoration. Plant Ecol. 214:423–432
Souza TAF, Rodriguez-Echeverría S, Andrade LA, Freitas H (2016) Could biological invasion by Cryptostegia madagascariensis alter the composition of the arbuscular mycorrhizal fungal community in semi-arid Brazil? Acta Botanica Brasilica 30. doi:10.1590/0102-33062015abb0190
Zubek S, Błaszkowski J, Seidler-Łożykowska K, Bąba W, Mleczko P (2013) Arbuscular mycorrhizal fungi abundance, species richness and composition under the monocultures of five medicinal plants. Acta Sci Pol-Hortoru 12:127–114
Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol. 4:e140
Yuan Y, Tang J, Leng D, Hu S, Yong JWH, Chen X (2014) An invasive plant promotes its arbuscular mycorrhizal symbioses and competitiveness through its secondary metabolites: indirect evidence from activated carbon. PLoS One 9:e97163
Mummey DL, Rillig MC (2006) The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant Soil 288:81–90
Vogelsang KM, Bever JD (2009) Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology 90:399–407
Majewska ML, Błaszkowski J, Nobis M, Role K, Nobis A, Lakomiec D, Czachura P, Zubek S (2015) Root-inhabiting fungi in alien plant species in relation to invasion status and soil chemical properties. Symbiosis 65:101–115
Follstad Shah JJ, Harner MJ, Tibbets TM (2010) Elaeagnus angustifolia elevates soil inorganic nitrogen pools in riparian ecosystems. Ecosystems 13:46–61
Ramos AC, Façanha AR, Feijó JA (2008) Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol. 178:177–188
Carneiro MAC, Ferreira DA, Souza ED, Paulino HB, Saggin Junior OJ, Siqueira JO (2015) Arbuscular mycorrhizal fungi in soil aggregates from fields of “murundus” converted to agriculture. Pesquisa Agropecuária Brasileira, Brasília 50:313–321
Rodríguez-Echeverría S, Crisóstomo JA, Nabais C, Freitas H (2009) Belowground mutualists and the invasive ability of Acacia longifólia in coastal dunes of Portugal. Biol. Invasions 11:651–661
Grümberg BC, Urcelay C, Shroider MA, Vargas-Gil S, Luna CM (2015) The role of inoculum identity in drought stress mitigation by arbuscular mycorrhizal fungi in soybean. Biol. Fertil. Soils 51:1–10
Ortiz N, Armada E, Duque E, Roldán A, Azcón A (2015) Contribution of arbuscular mycorrhizal fungi and/or bactéria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthnous strains. J. Plant Physiol. 174:87–96
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press and Elsevier, London
Acknowledgements
Special thanks to Joana Costa and Susana Rodriguez-Echeverría for the valuable discussions and checking of English grammar. The authors also thank the two anonymous reviewers for the helpful comments, which greatly improved a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Table S1
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
de Souza, T.A.F., de Andrade, L.A., Freitas, H. et al. Biological Invasion Influences the Outcome of Plant-Soil Feedback in the Invasive Plant Species from the Brazilian Semi-arid. Microb Ecol 76, 102–112 (2018). https://doi.org/10.1007/s00248-017-0999-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-0999-6