Abstract
Purpose
To evaluated the metabolic profiles and vascular properties in congenital growth hormone (GH) deficiency (GHD) and its replacement in adults.
Patients and methods
Cross-sectional study conducted in a single tertiary center for pituitary diseases. Eighty-one adult subjects were divided into three groups: (1) 29 GHD patients with daily subcutaneous GH replacement therapy (GHRT) during adulthood; (2) 20 GHD patients without GHRT during adulthood and (3) 32 controls. Only patients with adequate adherence to others pituitary hormone deficiencies were included. Anthropometric parameters, body composition by dual-energy X-ray absorptiometry, metabolic profiles and vascular properties (carotid intima media thickness, pulse wave velocity and flow-mediated dilation) were compared among the groups.
Results
Waist-to-height ratio (WHR), body fat percentages and fat mass index (FMI) were lower in patients with GHRT than patients without GHRT during adulthood (0.49 ± 0.06 vs. 0.53 ± 0.06 p = 0.026, 30 ± 10 vs. 40 ± 11 p = 0.003 and 7.3 ± 4 vs. 10 ± 3.5 p = 0.041, respectively). In addition, association between longer GHRT and lower body fat percentage was observed (r = − 0.326, p = 0.04). We found higher triglyceride (113.5 ± 62 vs. 78 ± 36, p = 0.025) and lower HDL cholesterol (51 ± 17 vs. 66 ± 23, p = 0.029) levels in patients without GHRT during adulthood in comparison to controls. No statistical differences were observed for vascular properties among the groups.
Conclusions
No differences in vascular properties were observed in congenital GHD adult patients with or without GHRT despite patients without GHRT had an unfavorable body composition. GHRT currently remains an individualized decision in adults with GHD and these findings bring new insight into the treatment and follow-up of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth hormone (GH) exerts critical biological effects on the metabolic profile and cardiovascular (CV) system [1]. Congenital GH deficiency (GHD) occurs as isolated (IGHD) or combined with other pituitary hormone deficiencies (CPHD), with a prevalence of 1 in 3500–10,000 births [2]. Adult-onset GHD is associated with abnormal body composition, such as increased fat mass and reduced lean mass, impaired physical performance and an impact on the quality of life [3, 4]. Several studies have demonstrated an unfavorable CV risk in adults with untreated GHD, citing impaired glucose and lipid metabolism, arterial hypertension, endothelial dysfunction and arterial stiffness [5,6,7]. However, the impact of GH replacement therapy (GHRT) in adults with GHD has not been well established. Although the effects of GHRT on CV system have been demonstrated [8,9,10], it is not clear if the increased CV risk is due to GHD per se, or to confounding factors of acquired GHD (e.g. other pituitary hormone deficiencies with inadequate replacement, or consequences of pituitary surgery or radiotherapy) [11,12,13,14,15,16,17,18]. In adults with congenital GHD, the impact of GHD and its replacement on CV risk is poorly studied.
Cardiovascular diseases (CVD) are preceded by subclinical endothelial dysfunction, with increased arterial stiffness which results in accelerated atherosclerosis [19, 20]. In this context, carotid ultrasonography, carotid-femoral pulse wave velocity (cfPWV) and flow-mediated dilation (FMD) assessments are used in clinical practice as non-invasive, validated and reproducible techniques for subclinical atherosclerosis assessment [21], to stratify individuals according to CV risk.
However, to the best of our knowledge, no study has yet assessed metabolic profiles, body composition, carotid thickening, arterial stiffness, and endothelial function in adult patients with congenital GHD. Therefore, we evaluated these parameters in adult patients with congenital GHD, with and without GHRT.
Materials and methods
Study design
This cross-sectional single-center study was conducted at Hospital das Clinicas da Universidade de São Paulo, Brazil. Forty-nine adult patients with congenital GHD were included. The study was approved by the medical ethics committee of the Universidade de São Paulo. Written informed consent was obtained from all study subjects.
Subjects
From a congenital GHD cohort comprising 273 subjects, 49 adults with documented congenital GHD were invited to participate in this cross-sectional study, and all agreed to participate (Fig. 1). The inclusion criteria were: (a) proven adult GHD with congenital IGHD or CPHD; (b) outpatient clinic attendance and proven adherence to hormonal replacements for other pituitary deficiencies for at least 1 year; (c) for those with GHRT, IGF1 (insulin-like growth factor 1) levels between − 1 and 1 SD. Exclusion criteria were: (a) active smokers; (b) a diagnosis of diabetes mellitus or arterial hypertension; (c) taking medication known to interfere with glucose and lipid metabolism, blood pressure or the vascular system; and (d) patients with poor compliance.
A GHD diagnosis was based on clinical, laboratory and imaging data. In childhood, short stature (Z-score < − 2 or delta Z-score for target height < − 1.5), low growth velocity, bone age delay, and a GH stimulatory peak after clonidine test of < 3.3 µg/L by immunofluorimetric assay [22]. Magnetic resonance imaging (MRI) of the hypothalamic-pituitary region was performed in all patients at the diagnosis of GHD to identify anatomic alterations. In the transition phase of the condition, which was characterized by the end of linear growth until complete adult maturation, patients were re-evaluated for GHD. In these cases, all patients with MRI abnormalities, low IGF1 values and GH peak < 5 µg/L in the stimulus test were considered as adults with congenital GHD [23].
Additional anterior pituitary hormonal deficiencies were also tested in individuals as recommended [24], and all pituitary hormonal deficiencies were under the appropriate replacement.
The adult subjects were divided into three groups:
-
(1)
Congenital GHD with GHRT during adulthood 29 patients (14 female), mean age = 36.6 ± 7.4 years, and with GHRT for 8 years (range 2–21 years). GH doses were approximately 1 IU/day (0.33 mcg/day) to maintain IGF1 in the normal range for age and sex. Thirteen patients used GHRT continuously (childhood and transition and adulthood). Sixteen patients used GHRT intermittently throughout life, and at the time of the study, all of them had been using GHRT for more than 2 years. Of these patients, 26 (90%) had CPHD and three (10%) had IGHD. Twenty-five patients (86%) with TSH deficiency had free T4 values (thyroxine) in the normal range (1.31 ± 0.4 ng/dl—reference range 0.9–1.7 ng/dl). Twenty-four patients (76%) had LH/FSH deficiency. Male patients had testosterone levels within the normal range (512 ± 196 ng/dL—reference range 249–740 ng/dL). For female patients, a regular menstrual cycle reflected good adherence to steroid hormone replacement. Of the 17 patients (56%) with ACTH deficiency, all were taking physiological doses of glucocorticoid therapy. The patients did not presented signs of supraphysiological doses. Seven were taking prednisone 5 mg/day, and 10 were taking hydrocortisone 15–20 mg/day.
-
(2)
Congenital GHD without GHRT during adulthood 20 patients (10 female), mean age = 37.4 ± 8.6 years, without GHRT in adulthood for 12 years (range 5–24 years). All patients never used GHRT in adulthood (16 used GHRT only in the childhood and/or transition phase and four never used GHRT during life). All subjects had been without GHRT for more than 5 years. Fourteen patients (70%) had CPHD and 6 (30%) had IGHD. Eleven patients (55%) with TSH (thyroid-stimulating hormone) deficiency had free T4 values in the normal ranges (1.2 ± 0.17 ng/dl—reference range 0.9–1.7 ng/dl). Thirteen patients (65%) had LH/FSH (luteinizing hormone/follicle-stimulating hormone) deficiency. All male patients had testosterone levels within normal ranges (485 ± 208 ng/dL—reference range 249–740 ng/dL). For female patients, menstrual cycles were regular, with good adherence to steroid hormone replacement. Seven patients (35%) with ACTH deficiency (adrenocorticotrophic hormone) were using glucocorticoid therapy at physiological doses. One was using prednisone at 5 mg/day, and six were using hydrocortisone at 15–20 mg/day. None showed clinical signs of supraphysiological glucocorticoid doses.
-
(3)
Control group 32 healthy individuals (17 females), mean age = 37 ± 8.9 years, all of whom had no evidence of cardiovascular disease after clinical and laboratory evaluations. Female controls do not use hormonal contraceptives.
Definition of premature CVD family history
Coronary artery disease in first-degree relative in male under 55 years old and/or female under 65 years old [25].
Definition of GHRT use
GHRT use was considered as follows: (1) For the groups of patients with GHRT during adulthood: the time was the number of years with uninterrupted GHRT; and (2) GHRT use throughout life: for all patients, the sum of years (not continuous) the patient used GHRT throughout their life.
Methods
Anthropometric and blood pressure measurements
Height and weight were measured, and body mass index (BMI) was calculated using the formula; BMI = weight (kg)/height2 (m). Abdominal waist (AW) (cm) was measured at the midpoint between the lower margin of the costal arches and the upper edge of the iliac crest. The waist-to-height ratio (WHR) was calculated using the abdominal waist (cm)/height (cm) formula [26]. Blood pressure (BP) was measured according to recommendations of the 7th edition of the Brazilian Hypertension guidelines [27].
Laboratory assessments
The total cholesterol (TC), high-density lipoprotein cholesterol (HDLc), low-density lipoprotein cholesterol (LDLc), triglycerides (Tg) levels were analyzed with an automatic enzymatic colorimetric method (Cobas Mira; F.Hoffmann-La Roche, Basel, Switzerland). The fasting glucose levels were determined with an automatic enzymatic method using hexokinase (Cobas Integra; Roche, Basel, Switzerland). Glycated hemoglobin (HbA1c) levels were measured by high-performance liquid chromatography (HPLC). The LH, FSH, total testosterone (TT), (17) estradiol and prolactin (PRL) levels were measured with immunofluorometric assays (Autodelfia, Turku, Finland) and more recently by electrochemiluminometric tests (Roche, Mannheim, Germany). The intra- and inter-assay coefficients of variation varied from 5 to 10%. Serum IGF1 levels were measured using a specific immunoradiometric assay (IRMA) or enzyme-labeled chemiluminescent immunometric assay (ICMA) and the values were transformed into SDS adjusted for sex and age (Siemens Healthcare Diagnostics, Dublin, Ireland). TSH and Free T4 were measured by electrochemiluminometric tests (Roche, Mannheim, Germany). For the control group, the following tests were performed: basal glucose, HbA1c, TC, HDLc, LDLc, Tg, IGF1, TSH and TT in men.
Assessment of carotid intima-media thickness
Carotid intima-media thickness (cIMT) measurements were performed by the same professional. A high-resolution ultrasound (GE, Vivid I, USA), with a high-frequency linear transducer, using B mode and a semi-automatic technique was used. A selected image was amplified to optimize visualization of the common carotid posterior wall, and the intima-media complex. The operator manually set measurement area points (start and end), and two lines along the artery were automatically drawn. Automated measurements included an online measure of multiple carotid intima-media data points (cm). The results were represented as the mean number of acquired data points.
Assessment of carotid-femoral pulse wave velocity
Carotid-femoral pulse wave velocity (cfPWV) (m/s) was assessed in participants using a Complior® device (Alam Medical, Vincennes, France). PWV was measured between the carotid and femoral artery using piezoelectric sensors; one was placed on the right side of the neck and the other on the femoral site. Sensor signals were recorded by the device software. The distance between sensors, as measured in a straight line, was used to approximate the arterial distance traveled by pulse waves. The foot of the pulse waves at both locations was used to calculate the mean cfPWV, once every five seconds [28]. This cfPWV measurement technique was previously described [29].
Assessment of flow-mediated dilation
The ultrasonography transducer was positioned on the brachial artery surface, on the right arm. For FMD (endothelium-dependent) measurements, a sphygmomanometer was inflated to a pressure of at least 50 mmHg above systolic pressure for 5 min, to induce a reactive hyperemia state. Images were captured by the transducer for 3 min after cuff release. For EIV (endothelium-independent vasodilatation) measurements, vasodilatation was evaluated after the administration of sublingual nitrate at dose of 5 mg. The artery diameter was evaluated using ultrasonography (Sequoia Echocardiography System® version 6.0, Siemens, CA, USA). Images were analyzed using an appropriate software package, managed by a professional blinded to group allocation. The basal phase and reactive hyperemia, pre- and post-nitrate diameter were analyzed using FMD Studio (Quipu, Pisa, Italy). This software provides basal diameter, pre-nitrate and post-nitrate diameter, and maximum arterial diameter values in the hyperemia phase. Women underwent the exam during the menstrual phase of their menstrual cycle because endothelial function in women during this period is similar to that observed in men at the same age [30, 31]. Four healthy subjects were also recruited to measure intra-observer reproducibility; they were evaluated at different times of the day by the same evaluator [28].
Assessment of total body densitometry using dual-energy X-ray absorptiometry
Whole-body densitometry was performed using dual-energy X-ray absorptiometry (DXA) on a Hologic DXA (Hologic, Inc. Crosby Drive, Bedford, MA, EUA) scanner to measure fat and lean mass of the whole body, except the head. The following parameters were recorded: (1) an index of total-body adiposity, derived by averaging the body fat percentage for soft tissue regions in spine and hip scans and (2) android/gynoid body aspect. The analysis was based on the following indices: (a) fat mass index (FMI) was calculated as fat mass (kg) divided by height (m) squared, considering normal values of 3–6 kg/m2 in men, and 5–9 kg/m2 in women [32], and (b) the Baumgartner index, which evaluated appendicular skeletal muscle mass, was calculated as the sum of skeletal muscle mass in the arms and legs (kg) divided by height (m) squared. According to Baumgartner’s anthropometric equation, sarcopenia was defined as < 5.5 kg/m2 for women, and < 7.26 kg/m2 for men [33, 34].
Statistical analyses
The Kolmogorov–Smirnov test was applied to test normal distribution. ANOVA or Student’s t-tests were used for parametrical data. ANOVA was followed by Bonferroni multiple comparisons if more than two variables showed significant difference. The Mann Whitney U test was used as a non-parametrical test. Fisher’s exact test and the χ2 test were used to estimate associations between categorical variables. Correlations were analyzed by Pearson's correlation coefficient.
A p value < 0.05 was considered statistically significant. The IBM-SPSS statistical package version 26.0 (Chicago, IL) was used for statistical analysis.
Results
General features
Congenital GHD with GHRT during adulthood
Twenty-nine patients, aged 36.6 ± 7.4 years, with an average height of 161.5 ± 13 cm (males; 168.8 ± 7.7 cm and females; 153.7 ± 13.4 cm) were included in this group. Five out of 29 (17%) had a familial history of CVD (Table 1). The median time of GHRT during childhood, the transition phase, adulthood and GHRT throughout life was 5.8 years (range 0–16 years), 3.1 years (range 0–7 years), 8 years (range 2–21 years) and 16.4 years (range 8.6–32 years), respectively.
TSH deficiency was treated with levothyroxine for a median time of 27 years (range 7–38 years). LH/FSH deficiencies were treated with sex steroids for a median time of 13.8 years (range 2–37 years). ACTH deficiency was treated with glucocorticoids for a median time of 19.3 years (range 1.4–31.5 years).
Congenital GHD without GHRT during adulthood
This group was made up of 20 patients aged 37.4 ± 8.6 years, with an average height of 155.8 ± 15.1 cm (males; 162.2 ± 14.2 cm and females; 149.4 ± 13.7 cm). Seven out of 20 (35%) had a familial history of CVD (Table 1). The median time without GHRT was 12 years (range 5–24 years). GHRT during childhood, the transition phase, and throughout life was 4 years (range 0–17 years), 0 years (range 0–7 years), and 7.6 years (range 0–19 years), respectively.
TSH deficiency was treated with levothyroxine for a median time of 22.5 years (range 13–44 years). LH/FSH deficiencies were treated with sex steroids for a median time of 15.4 years (range 6–38 years). ACTH deficiency was treated with glucocorticoids for a median time of 15.9 years (range 5.6–25.8 years).
Control group
The control group consisted of 32 healthy volunteers. Their mean age was 37 ± 8.9 years, and the mean height was 169 ± 0.1 cm (Table 1).
The patients currently undergoing GHRT had a longer GHRT time during the transition phase and throughout life than those without GHRT (p = 0.018 and p < 0.001, respectively) but not during childhood (p = 0.289). There was no statistical difference in the time of levothyroxine, sex steroids and glucocorticoids replacement between the groups (p = 0.860, p = 0.702 and p = 0.924, respectively).
Anthropometric, blood pressure, metabolic data and vascular properties
There were no significant differences between patients and controls regarding age, gender, CVD family history, AW measurements, systolic and diastolic blood pressure and glycemic profile. Height was statistically different between GHD patients without GHRT and controls (p value after Bonferroni multiple comparison = 0.002) (Table 1).
GHD patients with GHRT and controls had statistically lower WHR than patients without GHRT (p value after Bonferroni multiple comparison = 0.026 and < 0.001, respectively). Patients without GHRT had statistically higher overweight and obesity rates than patients with GHRT (40% vs.13.8%, respectively) (p = 0.048) (Table 1).
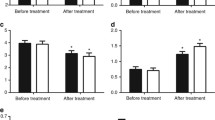
We observed statistically higher triglycerides and lower HDLc levels in patients without GHRT when compared with controls (p value after Bonferroni multiple comparison = 0.025 and = 0.029, respectively) but no statistical differences were found with GHRT group (Fig. 2).
No statistical differences were observed in large artery structural and functional vascular parameters between patients and controls (Table 2). However, a strongly and linear correlation existed between chronological age and cIMT (r = 0.645, p < 0.001) values in patients with congenital GHD, regardless GHRT (Fig. 3).
Body composition by DXA
Fat percentages were lower in patients with GHRT, when compared to patients without GHRT (p = 0.003). Similarly, patients with GHRT had a significantly lower fat index, when compared to patients without GHRT (p = 0.041) (Table 3).
We observed negative association between body fat percentage and FMI with GHRT throughout life (r = − 0.378 p = 0.012 and r = − 0.334 p = 0.029, respectively), which was independent of age. The Baumgartner index correlated inversely with age in patient with congenital GHD in adulthood (r = − 0.388, p = 0.01) (Fig. 4).
a Negative association between body fat mass percentage in all adult patients with congenital GHD and GHRT throughout life (r = − 0.378 p = 0.012). b Negative association between fat mass index (FMI) in all adult patients with congenital GHD and GHRT throughout life (r = − 0.334 p = 0.029). c Negative associations between Baumgartner index in all adult patients with congenital GHD and age (r = − 0.388 p = 0.01)
Discussion
Several studies have identified associations between GHD and vasculometabolic impairments in adults [10]. Nevertheless, adult patients with congenital GHD have been under-represented in these studies. In the present study, we used strict exclusion criteria in congenital GHD adult patients to avoid confounding factors that could interfere with data interpretation. Thus, these patients are an interesting model to explore the impact of both GHD and GHRT on metabolic profiles and the CV system. There were neither structural nor functional large arterial differences in adult patients with congenital GHD, with or without GHRT, and healthy controls. However, the group without GHRT showed disturbances in lipid profiles and body composition reflected by WRH, BMI, fat mass percentages and fat mass index.
The impact of untreated GHD and GHRT on CV health remains controversial. A retrospective study of 1411 patients with untreated GHD observed an increase in general mortality, myocardial infarction and cerebrovascular events compared with the normal population [35]. This higher occurrence of CV events could be related to greater development of premature subclinical atherosclerosis evidenced by endothelial dysfunction [36], increased arterial stiffness and more atheromatous plaques in the carotid and femoral arteries in patients with GHD [37,38,39]. Low IGF-I levels were associated with increased cIMT, a recognized sign of premature subclinical atherosclerosis directly related to mortality from coronary artery disease [40]. Moreover, GHD is associated with increased levels of inflammatory cytokines, homocysteine and free radicals and reduced nitric oxide production [41]. Thus, these mechanisms appear to be important in endothelial homeostasis and vasodilation regulation.
Endothelial dysfunction occurs at the beginning of the atherosclerotic process, even before structural changes in vessel walls become evident. GHRT improves endothelial function by increasing flow-mediated dilation in patients with acquired GHD [10, 42,43,44]. A putative mechanism whereby GHRT improves vascular function occurs via IGF1-mediated stimulation of nitric oxide synthesis in endothelial cells [45]. A randomized, double-blind, placebo-controlled study observed the effect of GHRT over 6 months on arterial stiffness (assessed by PWV of the radial artery) and endothelial function (assessed by FMD of the brachial artery) in 32 adults with GHD, paired with controls of the same age and sex. The authors concluded that GHD in adults is associated with increased arterial stiffness and endothelial dysfunction and that GHRT improves these parameters. These findings suggest an important role for this treatment in reducing CV risk in these patients [10].
However, these beneficial results of the effect of GHRT on vascular properties have not been confirmed in all studies, and we did not observe them in our congenital patients. Van der Klaauw et al. [43] reported no change in cfPWV after 1.5 years of GHRT in 14 patients with acquired GHD. However, another study showed a significant reduction in cfPWV (8.1 to 6.7 m/s) during 6 months of GHRT in 16 patients with acquired GHD [46]. The discrepancies between these studies can be justified by the characteristics of the patients in the first study: older people, greater inclusion of men and higher BMI, factors related to a more disadvantageous CV profile.
A study in adults with congenital IGHD due to GHRHR gene mutation showed no evidence of premature atherosclerosis [47]. Furthermore, adults with congenital IGHD and never treated with GHRT have normal longevity [48]. In the same cohort, another study revealed increased CV risk, carotid thickening and atherosclerotic plaque development after GHRT with bimonthly depot of GHRT for 6 months [49]. Five years after GHRT withdrawal, carotid thickening had decreased to baseline values, but atherosclerotic plaque quantities did not change [50]. This study presents a notable contrast with most studies that had been published. Although we also found no changes in vascular properties, the effects of GHRT in IGHD were different to our patients, perhaps due to the application form of GHRT (daily versus depot) and the use of long-acting GHRT [51], which may have exposed body tissues to constant high GH levels [52].
Although GHD and its replacement had no impact on cIMT in our patients, aging was associated with increased carotid thickness, as observed in individuals without GHD. The influence of aging on vascular properties is well established [53].
Different degrees of GHD may result in different effects on the vascular wall [54]. We hypothesize that the pathophysiology of GHD in patients with congenital GHD is different from those with acquired GHD. The residual GH secretion may exert some metabolic actions, resulting in differences between these disease models. While a slight reduction in IGF1 in the general population (observed in patients with acquired GHD) has been associated with an increased risk of ischemic heart disease [55, 56], a more intense reduction in IGF1 (observed in patients with congenital GHD) can be protective against atherosclerosis [54]. These previous results are consistent with our findings.
The duration of the GHRT in GHD could be an important contributing factor to the results observed in this study. Patients with GHRT in adulthood were those who most replenished GH through their life, including during the transition phase, when GHRT improves changes in metabolic profile and body composition, reinforcing the importance of replacement during this period of life [57].
CfPWV and FMD are the gold standard methods to assess arterial stiffness and endothelial dysfunction in humans, respectively [21]. To the best of our knowledge, this is the first study to evaluate arterial stiffness and endothelial function in adult patients with congenital GHD. In our assistance, untreated GHD and the effects of GHRT in adults with congenital GHD did not accelerate subclinical atherosclerosis.
Modifications in lipid and glycemic profiles can also aggravate CV risk markers and contribute to CVD. Holdaway et al. [58], in their 3-year GHRT follow-up study, reported no significant changes in TC, LDLc, Tg, basal glucose and HbA1C levels. Fifteen years of GH replacement in GHD adults induced sustained improvements in serum lipid levels: TC and LDLc decreased and HDLc increased with no change in serum Tg level [9]. In our study, the group without GHRT showed higher Tg and lower HDLc levels compared with controls; the outcome displays a metabolic benefit of GHRT.
The literature data are inconsistent with regard to the glycemic profile, both in untreated and treated GHD patients. Adult patients with untreated GHD have increased visceral fat mass and often have an impaired glucose metabolism together with insulin resistance [59]. This association is based on studies with heterogeneous cohorts, different etiologies and severity of GHD. It is possible that the insulin resistance observed in these patients is due to the metabolic, inflammatory and body composition changes associated with GHD, but not directly caused by this condition. Castillo et al. [60], in their cross-sectional study, evaluated 15 patients with acquired GHD without GHRT, with adequate replacement of other pituitary hormone deficiency, and compared this cohort to a healthy group. They demonstrated that insulin sensitivity was similar to individuals with normal pituitary function, despite higher fat mass percentages in patients with GHD without GHRT. GHRT induces beneficial effects on body composition, findings that provide a rationale for improvement in insulin resistance with treatment. However, the glycemic effects of GHRT in GHD are conflicting. Whereas some studies have documented an improvement in glucose metabolism and insulin sensitivity [61], other investigations have observed no effect [62, 63].
In untreated congenital IGHD due to GHRHR gene mutation, insulin sensitivity is increased despite abdominal obesity [64]. Increased insulin sensitivity plays a fundamental role in longevity, possibly explaining the normal life span of these subjects [35]. As vascular properties, residual GH secretion can exert some metabolic actions, resulting in differences between these disease models (acquired vs. congenital). Visceral adiposity requires a minimal level of GH secretion to promote increased insulin resistance, which is not seen in patients with congenital GHD [64].
The absence of residual GH secretion and the adequate hormonal replacement of the other hormone deficient sectors of our patients, could explain the absence of glycemic alterations. These observations and controversies raise an important issue regarding insulin sensitivity and the role of GHD.
Lower GH levels are associated with an unfavorable impact on body composition as reported in acquired GHD patients [65]. In our study, there was a favorable effect of GHRT on WHR. The WHR metric is of great value because it is strongly associated with several chronic diseases, cardiovascular events and mortality rate [26].
The most consistent effect of GHRT in patients with GHD is a decrease in fat mass [9]. The impact of GHRT on body composition in adults with GHD was previously investigated in a randomized placebo-controlled trial (study duration range 2–18 months), which demonstrated a positive effect of GHRT on total lean and fat body mass, while BMI remained unaffected [6]. In our study, we identified lower BMI, WHR, fat percentage and FMI in the group with GHRT. Furthermore, prolonged use of GHRT was related to reduced fat body percentage. Our DXA approach evaluated fat and lean mass as a whole rather than compartmentalizing them; therefore, we were unable to differentiate total fat from visceral fat. With this limitation, we indirectly assessed visceral obesity using WHR. From the literature, WHR is the best predictor of whole-body fat percentages and visceral fat in men and women [66]. However, the ideal anthropometric parameter to predict CV risk in adults with congenital GHD is still unknown; thus, more studies must address whether WHR is a reliable marker of CV risk in these patients.
It is notable that GHRT did not impact lean mass in our study. Patients with untreated IGHD by GHRHR gene inactivation had better muscle strength parameters adjusted for weight and fat-free mass than controls and exhibited satisfactory muscle function [67]. In one open-label prospective study, patients with acquired GHD (61 men, mean age 50.0 years; range 22–74 years) were under GHRT for 10 years; they presented increased muscle strength during the first half of the study and were partially protected against normal age-related decline in strength during the last 5 years [68]. In a randomized, placebo-controlled crossover trial, 60 patients after more than 3 years of GHRT, a 4-month period of placebo treatment decreased the measured thigh muscle mass but without changing muscle strength [69]. Observed increases in lean mass are susceptible to measurement error and thus may occur without an improvement in patient strength [70].
In the current study, we only included patients with adequate adherence to hormonal treatment from other pituitary sectors. Inadequate pituitary hormone replacement therapies could also contribute to the CV risk. It has been shown that daily glucocorticoid doses higher than 20 mg of hydrocortisone are associated with an adverse metabolic profile in CPHD [71]. Furthermore, acquired CPHD patients with secondary adrenal insufficiency may frequently have residual cortisol secretion [72], which does not occur in congenital CPHD. In our cohort, all patients with ACTH deficiency were replaced with glucocorticoid at physiological doses. In addition, untreated hypogonadism might also exert an unfavorable impact on metabolism [73]. In our cohort, all patients with LH/FSH deficiency were receiving sex steroids. Only women with a regular menstrual cycle and men with normal testosterone levels were selected for the study. Finally, all patients were in replacement therapy with levothyroxine, with free T4 levels within the normal range. Adequate treatment of all pituitary hormonal sector deficits may have benefited from the absence of vasculometabolic changes in our patients. Our findings support further efforts to optimize replacement therapy for all deficiencies without compromising patient safety and well-being in the short and long term.
One strength of our study was the availability of data from a specifically well-selected sub-group of GHD patients. All patients had congenital GHD and were taking pituitary hormone replacements with adequate doses of glucocorticoids, levothyroxine and sex steroids. However, some limitations need to be considered. Due to the rarity of the congenital disease and the strict exclusion criteria adopted, our cohort was small. Another limitation is the cross-sectional nature of this study. Nevertheless, the design differs from many studies in the literature that have evaluated GHRT in vascular properties for small periods of therapy, without assessing the long-term effect on vasculature in GHD with and without GHRT. Given these limitations, prospective studies with larger patient samples and longer GHRT during adulthood are necessary to validate our results.
In conclusion, our study revealed no differences in structural and functional large arterial properties in congenital GHD patients with or without GHRT in adulthood. However, patients without GHRT had an unfavorable body composition. Adequate hormonal replacement of other pituitary sectors is also essential for vasculometabolic health. Furthermore, the data found in the current literature on acquired hypopituitarism are not directly applicable to the management of congenital GHD. These findings bring new insights into the treatment and follow-up of these patients and may guide GHD patients in their individual decision for GHRT in adulthood.
References
Aguiar-Oliveira MH (2011) Lifetime growth hormone (GH) deficiency: impact on growth, metabolism, body composition and survival capacity. In: Salvatori R (ed) Handbook of growth and growth monitoring in health and disease. Springer, New York
Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT (2009) Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829
de Boer H, Blok GJ, Van der Veen EA (1995) Clinical aspects of growth hormone deficiency in adults. Endocr Rev 16:63–86
Cuneo RC, Salomon F, McGauley GA, Sönksen PH (1992) The growth hormone deficiency syndrome in adults. Clin Endocrinol (Oxf) 37:387–397
van der Klaauw AA, Biermasz NR, Feskens EJ, Bos MB, Smit JW, Roelfsema F et al (2007) The prevalence of the metabolic syndrome is increased in patients with GH deficiency, irrespective of long-term substitution with recombinant human GH. Eur J Endocrinol 156:455–462
Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P et al (2004) Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a Metaanalysis of Blinded, Randomized, Placebo-Controlled Trails. J Clin Endocrinol Metab 89:2192–2199
Yuen KCJ, Llahana S, Miller BS (2019) Adult growth hormone deficiency: clinical advances and approaches to improve adherence. Expert Rev Endocrinol Metab 14:419–436
Salomon F, Cuneo RC, Hesp R, Sönksen PH (1989) The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 321:1797–1803
Elbornsson M, Götherström G, Bosæus I, Bengtsson B, Johannsson G, Svensson J (2013) Fifteen years of GH replacement improves body composition and cardiovascular risk factors. Eur J Endocrinol 168:745–753
Smith JC, Evans LM, Wilkinson I, Goodfellow J, Cockcroft JR, Scanlon MF et al (2002) Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: a randomized, double-blind, placebo-controlled study. Clin Endocrinol (Oxf) 56:493–501
Zueger T, Kirchner P, Herren C, Fischli S, Zwahlen M, Christ E et al (2012) Glucocorticoid replacement and mortality in patients with nonfunctioning pituitary adenoma. J Clin Endocrinol Metab 97:E1938–E1942
Yuen KCJ, Mattsson AF, Burman P, Erfurth EM, Camacho-Hubner C, Fox JL et al (2018) Relative risks of contributing factors to morbidity and mortality in adults with craniopharyngioma on growth hormone replacement. J Clin Endocrinol Metab 103:768–777
Bülow B, Attewell R, Hagmar L, Malmström P, Nordström CH, Erfurth EM (1998) Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J Clin Endocrinol Metab 83:3897–3904
Burman P, Mattsson AF, Johannsson G, Höybye C, Holmer H, Dahlqvist P et al (2013) Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J Clin Endocrinol Metab 98:1466–1475
Mahmood SS, Nohria A (2016) Cardiovascular complications of cranial and neck radiation. Curr Treat Options Cardiovasc Med 18:45
Amado A, Araújo F, Carvalho D (2018) Cardiovascular risk factors in acromegaly: what’s the impact of disease control? Exp Clin Endocrinol Diabetes 126:505
Aranda G, Fernandez-Ruiz R, Palomo M, Romo M, Mora M, Halperin I et al (2018) Translational evidence of prothrombotic and inflammatory endothelial damage in Cushing syndrome after remission. Clin Endocrinol (Oxf) 88:415–424
Feldt-Rasmussen U, Klose M (2016) Central hypothyroidism and its role for cardiovascular risk factors in hypopituitary patients. Endocrine 54:15–23
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA et al (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39:257–265
Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM et al (1995) Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 26:485–490
Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F et al (2015) The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 241:507–532
Silva EG, Slhessarenko N, Arnhold IJ, Batista MC, Estefan V, Osorio MG et al (2003) GH values after clonidine stimulation measured by immunofluorometric assay in normal prepubertal children and GH-deficient patients. Horm Res 59:229–233
Clayton PE, Cuneo RC, Juul A, Monson JP, Shalet SM, Tauber M (2005) Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol 152:165–170
Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R et al (2016) Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 101:3888–3921
Malachias MVB, Póvoa RMS, Nogueira AR, Souza D, Costa LS, Magalhães ME (2016) 7th Brazilian guideline of arterial hypertension: chapter 3—clinical and complementary assessment. Arq Bras Cardiol 107(Suppl 3): 14–7
Ashwell M, Gunn P, Gibson S (2012) Waist-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev 13:275–286
Malachias MVB, Bortolotto LA, Drager LF, Borelli FAO, Lotaif LAD, Martins LC (2016) 7th Brazilian guideline of arterial hypertension: chapter 12—secondary arterial hypertension. Arq Bras Cardiol 107(Suppl 3), 67–74
Misse RG, Borges IBP, Costa-Hong VA, Bortolotto LA, Shinjo SK (2019) Functional and structural arterial vessel features of female patient with stable dermatomyositis and antisynthetase syndrome. Open J Rheumatol Autoimune Dis 9:101–110
Muela HCS, Costa-Hong VA, Yassuda MS, Moraes NC, Memória CM, Machado MF et al (2018) Higher arterial stiffness is associated with lower cognitive performance in patients with hypertension. J Clin Hypertens (Greenwich) 20:22–30
Sader MA, Celermajer DS (2002) Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res 53:597–604
Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K et al (1995) Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92:3431–3435
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 4:e7038
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Walsh MC, Hunter GR, Livingstone MB (2006) Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17:61–67
Svensson J, Bengtsson BA, Rosén T, Odén A, Johannsson G (2004) Malignant disease and cardiovascular morbidity in hypopituitary adults with or without growth hormone replacement therapy. J Clin Endocrinol Metab 89:3306–3312
Gazzaruso C, Gola M, Karamouzis I, Giubbini R, Giustina A (2014) Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH–an update. J Clin Endocrinol Metab 99:18–29
Leonsson M, Hulthe J, Oscarsson J, Johannsson G, Wendelhag I, Wikstrand J et al (2002) Intima-media thickness in cardiovascularly asymptomatic hypopituitary adults with growth hormone deficiency: relation to body mass index, gender, and other cardiovascular risk factors. Clin Endocrinol (Oxf) 57:751–759
Markussis V, Beshyah SA, Fisher C, Parker KH, Nicolaides AN, Johnston DG (1997) Abnormal carotid arterial wall dynamics in symptom-free hypopituitary adults. Eur J Endocrinol 136:157–164
Capaldo B, Guardasole V, Pardo F, Matarazzo M, Di Rella F, Numis F et al (2001) Abnormal vascular reactivity in growth hormone deficiency. Circulation 103:520–524
Bonithon-Kopp C, Touboul PJ, Berr C, Leroux C, Mainard F, Courbon D et al (1996) Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. The Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol 16:310–316
Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P et al (2008) Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295:1882–1894
Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N et al (2000) Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med 133:111–122
van der Klaauw AA, Pereira AM, Rabelink TJ, Corssmit EP, Zonneveld AJ, Pijl H et al (2008) Recombinant human GH replacement increases CD34+ cells and improves endothelial function in adults with GH deficiency. Eur J Endocrinol 159:105–111
Evans LM, Davies JS, Anderson RA, Ellis GR, Jackson SK, Lewis MJ et al (2000) The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol 142:254–262
Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS (1994) Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int 45:598–604
McCallum RW, Sainsbury CA, Spiers A, Dominiczak AF, Petrie JR, Sattar N et al (2005) Growth hormone replacement reduces C-reactive protein and large-artery stiffness but does not alter endothelial function in patients with adult growth hormone deficiency. Clin Endocrinol (Oxf) 62:473–479
Costa UM, Oliveira CR, Salvatori R, Barreto-Filho JA, Campos VC, Oliveira FT et al (2016) Brazilian adult individuals with untreated isolated GH deficiency do not have accelerated subclinical atherosclerosis. Endocr Connect 5:41–46
Aguiar-Oliveira MH, Oliveira FT, Pereira RM, Oliveira CR, Blackford A, Valenca EH et al (2010) Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J Clin Endocrinol Metab 95:714–721
Oliveira JL, Aguiar-Oliveira MH, D’Oliveira A, Pereira RM, Oliveira CR, Farias CT et al (2007) Congenital growth hormone (GH) deficiency and atherosclerosis: effects of GH replacement in GH-naive adults. J Clin Endocrinol Metab 92:4664–4670
Araujo VP, Aguiar-Oliveira MH, Oliveira JL, Rocha HM, Oliveira CR, Rodrigues TM et al (2012) Arrest of atherosclerosis progression after interruption of GH replacement in adults with congenital isolated GH deficiency. Eur J Endocrinol 166:977–982
Cook DM, Biller BM, Vance ML, Hoffman AR, Phillips LS, Ford KM et al (2002) The pharmacokinetic and pharmacodynamic characteristics of a long-acting growth hormone (GH) preparation (nutropin depot) in GH-deficient adults. J Clin Endocrinol Metab 87:4508–4514
Lombardi G, Di Somma C, Grasso LF, Savanelli MC, Colao A, Pivonello R (2012) The cardiovascular system in growth hormone excess and growth hormone deficiency. J Endocrinol Investig 35:1021–1029
van den Munckhof ICL, Jones H, Hopman MTE, de Graaf J, Nyakayiru J, van Dijk B et al (2018) Relation between age and carotid artery intima-medial thickness: a systematic review. Clin Cardiol 41:698–704
Menezes Oliveira JL, Marques-Santos C, Barreto-Filho JA, Ximenes Filho R, de Oliveira Britto AV, Oliveira Souza AH et al (2006) Lack of evidence of premature atherosclerosis in untreated severe isolated growth hormone (GH) deficiency due to a GH-releasing hormone receptor mutation. J Clin Endocrinol Metab 91:2093–2099
Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D (2004) The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab 89:114–120
Vasan RS, Sullivan LM, D’Agostino RB, Roubenoff R, Harris T, Sawyer DB et al (2003) Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med 139:642–648
Radovick S, DiVall S (2007) Approach to the growth hormone-deficient child during transition to adulthood. J Clin Endocrinol Metab 92:1195–1200
Holdaway IM, Hunt P, Manning P, Cutfield W, Gamble G, Ninow N et al (2015) Three-year experience with access to nationally funded growth hormone (GH) replacement for GH-deficient adults. Clin Endocrinol (Oxf) 83:85–90
Johansson JO, Fowelin J, Landin K, Lager I, Bengtsson BA (1995) Growth hormone-deficient adults are insulin-resistant. Metabolism 44:1126–1129
Castillo AR, de Souza AL, Alegre SM, Atala YB, Zantut-Wittmann DE, Garmes HM (2019) Insulin sensitivity is not decreased in adult patients with hypopituitarism without growth hormone replacement. Front Endocrinol (Lausanne). 10:534
Rochira V, Mossetto G, Jia N, Cannavo S, Beck-Peccoz P, Aimaretti G et al (2018) Analysis of characteristics and outcomes by growth hormone treatment duration in adult patients in the Italian cohort of the Hypopituitary Control and Complications Study (HypoCCS). J Endocrinol Investig 41:1259–1266
Weber MM, Biller BM, Pedersen BT, Pournara E, Christiansen JS, Höybye C (2017) The effect of growth hormone (GH) replacement on blood glucose homeostasis in adult nondiabetic patients with GH deficiency: real-life data from the NordiNet. Clin Endocrinol (Oxf) 86:192–198
Yuen KC, Roberts CT, Frystyk J, Rooney WD, Pollaro JR, Klopfenstein BJ et al (2014) Short-term, low-dose GH therapy improves insulin sensitivity without modifying cortisol metabolism and ectopic fat accumulation in adults with GH deficiency. J Clin Endocrinol Metab 99:E1862–E1869
Gomes-Santos E, Salvatori R, Ferrão TO, Oliveira CR, Diniz RD, Santana JA et al (2014) Increased visceral adiposity and cortisol to cortisone ratio in adults with congenital lifetime isolated GH deficiency. J Clin Endocrinol Metab 99:3285–3289
Ciresi A, Radellini S, Guarnotta V, Giordano C (2017) The visceral adiposity index is associated with insulin sensitivity and IGF-I levels in adults with growth hormone deficiency. Endocrine 56:579–588
Swainson MG, Batterham AM, Tsakirides C, Rutherford ZH, Hind K (2017) Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 12:e0177175
Andrade-Guimarães AL, Aguiar-Oliveira MH, Salvatori R, Carvalho VO, Alvim-Pereira F, Daniel CRA et al (2019) Adult individuals with congenital, untreated, severe isolated growth hormone deficiency have satisfactory muscular function. Endocrine 63:112–119
Götherström G, Elbornsson M, Stibrant-Sunnerhagen K, Bengtsson BA, Johannsson G, Svensson J (2009) Ten years of growth hormone (GH) replacement normalizes muscle strength in GH-deficient adults. J Clin Endocrinol Metab 94:809–816
Filipsson Nyström H, Barbosa EJ, Nilsson AG, Norrman LL, Ragnarsson O, Johannsson G (2012) Discontinuing long-term GH replacement therapy–a randomized, placebo-controlled crossover trial in adult GH deficiency. J Clin Endocrinol Metab 97:3185–3195
He X, Barkan AL (2020) Growth hormone therapy in adults with growth hormone deficiency: a critical assessment of the literature. Pituitary 23:294–306
Filipsson H, Monson JP, Koltowska-Häggström M, Mattsson A, Johannsson G (2006) The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab 91:3954–3961
Graziadio C, Hasenmajer V, Venneri MA, Gianfrilli D, Isidori AM, Sbardella E (2018) Glycometabolic alterations in secondary adrenal insufficiency: does replacement therapy play a role? Front Endocrinol (Lausanne) 9:434
Miljić D, Popovic V (2018) Metabolic syndrome in hypopituitarism. Front Horm Res 49:1–19
Acknowledgements
We thank the unit of Endocrinology, Hospital das Clinicas da Faculdade de Medicina da Universidade São Paulo, (São Paulo) Brazil, the Instituto do Coração, Hospital das Clinicas da Faculdade de Medicina da Universidade São Paulo, (São Paulo), Brazil, as well as all participants of this study for their assistance in successfully completing this study.
Funding
This study was partially financed by the Coordenacão de Aperfeicoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001. The project was also sponsored by a Grant (W1230416) from Pfizer. Funding organizations had no role in the study design, data analysis, or manuscript writing.
Author information
Authors and Affiliations
Contributions
IPB, VACH, LAB, BBM and LRSC contributed to study design; IPB and LRSC collected the data; VACH performed all vascular exams (cIMT, cfPWV and FMD) in all subjects. IPB and RLB performed the statistical analyses; IPB drafted the manuscript, and all authors provided intellectual input and critically reviewed the manuscript; LAB and LRSC supervised the work. All authors approved the final version of the manuscript and agree with its submission.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no potential conflict of interest in relation to this study.
Ethical approval
The study was approved by the medical ethics committee of the Universidade de São Paulo. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all study subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biscotto, I.P., Costa Hong, V., Batista, R.L. et al. Vasculometabolic effects in patients with congenital growth hormone deficiency with and without GH replacement therapy during adulthood. Pituitary 24, 216–228 (2021). https://doi.org/10.1007/s11102-020-01099-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01099-z