Abstract
The production of safe foods with little or no artificial preservatives is one of the foremost leading challenges for food manufacturing industries because synthetic antimicrobial agents and chemical food additives can cause severe negative effects on human health. However, there is an ever-increasing interest by consumers towards natural sources that have been aroused recently, and this increased consumer demand for safe food products has forced the food industries to use natural herbal and plant origins preservatives instead of synthetic preservatives for the production of safe foods. Traditionally, essential oils (EOs) obtained from numerous plant sources have been extensively encouraged for their putative health-promoting biological activities. The EOs are composed of complex mixtures encompassing copious individual compounds, which have been extracted by many methods. These diverse compounds display significant biological activities such as antioxidant and antimicrobial through different mechanisms. Nevertheless, their poor solubility in water, oxidation susceptibility, and volatility limit their use. To overcome these constraints, encapsulation is one of the best approaches to preserve the biological activities of EOs and minimize their effects on food sensory qualities. Herein, we have comprehensively enlightened the micro/nanoemulsion loaded with EOs to improve the physical—chemical and microbiological stability of various EOs, and further application of these EOs loaded systems in the food systems. This review confers the importance of EOs in terms of their main components, chemical and biological properties, including mode of action, effectiveness, synergistic effects as antimicrobials, and potential applications in the food system as a preservative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatic plant extracts have a rich history and gained much more acceptance recently, owing to their strong potential in terms of food safety and preservation. Moreover, they have successfully revealed positive responses to clinical treatments against several disorders and diseases such as diabetes, obesity, bowel syndrome, and even different types of cancer (Borborah et al. 2014). These extracts are obtained from different plant parts such as leaves, fruits, wood, nuts, seeds, barks, and roots. One of them is called essential oils (EOs), plant-derived extracts, which have been extended because of their aromatic attributes, used as preservatives in foods, confectionery, and beverages products or flavoring agents (Singh et al. 2018). Besides their aromatic properties, EOs have shown several therapeutic activities such as antioxidant and antimicrobial properties, and innumerable health benefits and they are possessing many applications in the food, pharmaceutical, perfumery, and cosmetic industries (Yousefi et al. 2019).
Generally, EOs compositions are complex mixtures of various secondary metabolites, such as terpenoids, terpenes, phenylpropenes, ketones, phenolics, aldehydes, esters, acids, alcohols, and ethers, which often showed strong potential regarding antioxidant and antimicrobial activity (Prakash et al. 2018b). These are obtained from different plant organs such as leaves (mint, oregano, Thym, Salvia, and eucalyptus), flowers (jasmine, rose, violet, and lavender), buds (clove), herbs, stems, zest (citrus), seeds (cardamom), twigs, roots, and rhizomes (ginger), fruits (orange and lemon), bark (cinnamon), wood (sandal) and branches (camphor) (Fornari et al. 2012; El Asbahani et al. 2015; Tariq et al. 2019), and they are essentially present inside the epidermic cells, canals or glandular trichomes, cavities, and secretory cells in the plants (Tariq et al. 2019), which provides strong defensive systems to the plants (Yousefi et al. 2019). However, their production yields differ because of their different composition of genus and species but remain very low (about 1%), making them exceptional as well as valuable materials (El Asbahani et al. 2015).
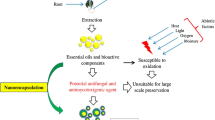
Despite fabulous biological efficiency, most of the EOs fail to employ their full preservative potential in the food system owing to some of the major intrinsic complications such as poor water solubility, lower bioavailability, volatility, and stability. The current developments in micro/nanoencapsulation can efficaciously address these constraints with the controlled release of EOs to deliver their boost effect at the right time and in the right place. Also, these techniques can maintain their biological activity, increase their effective utilization rate, minimize the adverse effects of their odor, and reduce their volatility, in food applications (Ju et al. 2019). Hence, the use of encapsulation approaches in the food industry is one of the intensifying arenas in recent years.
This review encompasses the major bioactive constituents existing in the EOs, chemical, and biological properties, including their mode of action, effectiveness, synergistic effects as an antimicrobial in foods, potential applications in the food system as a preservative. Furthermore, we have discussed the challenges contributing to affecting the production of EOs and declining the use they are as food preservatives, and an overview of methods of packing EOs, focusing on the application of modern procedures to encapsulate antimicrobial EOs in food systems.
Characteristic and chemical composition of EOs
Interestingly, EOs are well characterized based on existing natural compounds about 20 to 60 in different quantities (Buchanan et al. 2015). Because of their hydrophobic nature and often have less density as compared to water, they are generally lipophilic, dissolve in organic solvents, unmatched with water, and can be separated from the water phase by decantation (El Asbahani et al. 2015). Also, EOs can be easily degradable by environmental factors such as light and heat as well as quickly oxidize (Campolo et al. 2020). So, the extraction and dispersion of these substances must be carried out at moderate temperatures, to avert them from thermal decomposition (Fornari et al. 2012).
Based on their chemical composition, EOs components can be broadly classified as terpenoids (e.g., linalool, thymol,), terpenes (e.g., α-pinene, limonene), phenylpropanoids (e.g., cinnamaldehyde, eugenol) and others (e.g., allicin) ( Hyldgaard et al. 2012; Prakash et al. 2018a). Figure 1. on the other side, Tariq et al. (2019) have mentioned that the main components of two or three components are approximately 20 to 70% as compared to other components which in limited quantities due to these major components. These components include two classes that determine the biological properties of the EOs, the primary group consists of terpenoids and terpenes, the other class belongs to the aliphatic and aromatic components which have a lower molecular weight ( Buchanan et al. 2015; Tariq et al. 2019).
Terpenes and terpenoids are referred to as chemical unstable (because of C = C bonds), for that reason, these molecules do not offer the same chemical reorganization (isomerization). The terpenes are subdivided in respect to the numbers of 5 carbon building blocks known as isoprene units Fig. 2. While the skeletons of terpenoids are derived by condensation of multiple units of isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) (Zhang and Hong 2020). On the other hand, special biological properties and the aroma of aromatic oils are attributed to phenylpropanoids and terpenes which are major components of EOs (Raut and Karuppayil 2014). But the terpenes offer less flavor and aroma to the oils as compared to terpenoids. Thus, the removal of terpenes from EOs leads to a more soluble and stable end product (Benvenuti et al. 2001; Espinosa et al. 2005; Gironi and Maschietti 2008).
Biological properties
Antioxidant activity
Recently, it is suspected that synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) have caused potential adverse effects on human health (Oswell et al. 2018). So, EOs were considered a good alternative because the majority of EOs are generally classified as safe (GRAS) (Ribeiro-Santos et al. 2017a). The antioxidant potential of organic matters depends mainly on their chemical composition (Bhavaniramya et al. 2019). Phenols and some other secondary metabolite compounds are bound by double bonds, that are responsible for the intrinsic antioxidant activity of EOs (Koh et al. 2002). EOs extracted from traditional plants are rich sources of oxygenated monoterpenes such as esters, ketones, and aldehydes. Besides, monoterpene hydrocarbons and phenolic terpenoids, such as carvacrol or thymol are the major chemical compounds, which lead to stronger antioxidant activities in EOs obtained from some plants (Bhavaniramya et al. 2019). For example, the oil extracted from medicinal plants such as thyme, oregano, basil, clove, nutmeg, cinnamon, and parsley show significant antioxidant activities due to the presence of major constituents such as carvacrol and thymol (Aruoma 1998). Where, their activities are associated with the presence of phenolic compounds, which have significant properties in oxidation and reduction and play important roles in neutralizing free radicals and in the decomposition of peroxides (Burt 2004). The other components such as ethers, certain alcohols, aldehydes, ketones, and monoterpenes: isomenthone, citronellal, geranial/neral, 1,8- Cineole, linalool, and menthone also play a major role in the antioxidant properties of EOs (Modzelewska et al. 2005).
Mode of action
That EOs have different mechanisms (direct or indirect) to slow down the oxidation reactions including prevention of chain initiation and free-radical scavenging activity (Maqsood et al. 2013; Rodriguez-Garcia et al. 2016). Also, continued hydrogen abstraction and terminators, quenchers of singlet oxygen formation, and binding of transition metal ion catalysts are between their modes of actions (Tongnuanchan and Benjakul 2014). Although, the chemical composition of EOs determines their properties and thus their mode of operation. However, due to the large variety of compounds, its antioxidant activity cannot be attributed only to a single mechanism of action (Pateiro et al. 2018). EOs activity as antioxidants occurs in three phases: initiation, propagation, and termination as shown in Fig. 3.
Antimicrobial activity
The intensive application of antibiotics in livestock production has led to the resistance between commensal flora, opportunistic pathogens, and food-borne pathogens (Kačániová et al. 2017). In addition, continuous consumer demand for safe food has caused a tendency to look for natural additives (Cacho et al. 2016). Therefore, new ways to reduce or eliminate food-borne pathogens are still needed with a smaller impact on the environment to produce safe food (Smid and Gorris 1999). Therefore, the use of EOs being antibacterial additives has successfully been gained more popularity in food industries. Since the EOs are the complex mixture of compounds, which effectively take part to manage food-borne pathogens such as molds, bacteria, and associated toxins without promoting resistance acquisition (Kavanaugh and Ribbeck 2012; Prakash et al. 2013, 2014). As well, EOs are recommended as safe candidates for consumers and the environment with a strong capability to inhibit resistance bacteria (Kačániová et al. 2017). Moreover, mostly EOs have low toxicity of mammals and are ephemeral, making them relatively safe for health and the environment (Calo et al. 2015; Prakash and Kiran 2016). Figures 4 and 5 illustrate the chemical structures of antibacterial terpenoids and polyphenols, respectively.
However, the most active compounds are categorized into four groups based on their diverse nature and chemical composition such as terpenoids (e.g., carvacrol, thymol), terpenes (e.g., limonene, p-cymene), phenylpropenes (e.g., vanillin, eugenol), and other compounds such as isothiocyanates or allicin (Hyldgaard et al. 2012; Zanetti et al. 2018). Conversely, a lot of investigations have exposed the number of compounds like esters, phenols, alcohols, ketones, aldehydes, or hydrocarbons present in EOs shown antimicrobial properties separately when tested, and thus this activity resulting from complex interactions between different classes of compounds (Tariq et al. 2019).
On the other said, Tariq et al. (2019) described that the EOs containing phenols or aldehydes such as eugenol, carvacrol, linanaldehyde, citral, or thymol as a major compound that showed the highest antibacterial activity followed by EOs comprising terpene and alcohol. Other EOs containing esters or ketone such as α-thujone, β- myrcene, or gerenyl acetate have much weaker activity. While EOs encompassing terpene hydrocarbons are typically sedentary (Dorman and Deans 2000; Inouye et al. 2001). Whereas, Kalemba and Kunicka (2003) mentioned that the largest antimicrobial activity in EOs was found due to phenolic compounds, followed by the arrangement of aldehydes, ketones, alcohol, ethers, and hydrocarbons. The phenols’ activity is accredited to the acidic nature of hydroxyl groups (Khorshidian et al. 2018). Therefore, characteristics of antimicrobial shared by EOs have allowed the identification of the effect on pathogenic and commensal microbes as an alternative to antimicrobial agent application (Kačániová et al. 2017).
In line with this, in recent years, alternative sources of antimicrobials have been examined, being extracts and EOs resulting from plants, such as cinnamon (Cinnamomum zeylanicum Blume), oregano (Origanum vulgare L.), sweet fennel (Foeniculum vulgare var. dulce), and rosemary (Rosmarinus officinalis L.), which have successfully been designated and integrated into polymeric matrices (Ribeiro-Santos et al. 2017b). Table 1. Shows the several types of EOs obtained from different families that have been reported and possess a wide range of microorganism inhibitory potential.
Mode of action
The antimicrobial actions of EOs depend mainly on their chemistry, the quantity of major single compounds as well as the methods of assessment, including biogeography, variation, and dilution. In addition, the EOs activity as an antibacterial is also varying due to the difference in the cellular structure of the bacterial cell, such as Gram ( +) and Gram (-) bacteria, which differ in the structure of the cell membrane (Al-Maqtari et al. 2021a). Also, antimicrobial activity is associated with lipophilic or hydrophilic properties of the components of EOs, compositions of the cell wall, and the microorganism type (Khorshidian et al. 2018). On the other hand, the form of bacteria can also be specific to the activity of EOs and it has been reported that cells in the form of rods are more susceptible to the shape of cocci (Nazzaro et al. 2013).
Therefore, the action mode of EOs has not been completely assumed so far, and the foremost consequence of EOs can be linked to naturally occurring chemical compounds in plants carrying EOs (Kačániová et al. 2017). Where, Burt (2004) states that each compound may display different antimicrobial mechanisms thru a series of biochemical reactions inside the bacterial cells, which depends on the type of chemical components existing in the EOs. While Bajpai et al. (2012) testified that the activity of antimicrobial in EOs depended on plant composition and synergy which displayed that the chemistry of EOs was of great importance. Though, Khorshidian et al. (2018) assumed that the hydrophilic nature of functional groups and the lipophilic nature of the structure of hydrocarbons in EOs play significant roles in the antimicrobial effects of these compounds.
However, diverse mechanisms of antibacterial activity of EOs have been suggested, For example, the EOs are supposed to be less effective against Gram (-) bacteria compared to those Gram ( +) bacteria due to the presence of a hydrophilic outer membrane that contains a hydrophilic polysaccharide chain that acts as a barrier to the hydrophobic compounds (Al-Maqtari et al. 2020). Moreover, the outer boundary of this cell is charged, so it has a hydrophilic nature. But, some non-hydrophilic compounds can easily penetrate through this barrier (Nikaido 1994; Nazzaro et al. 2013). On the other side, EOs can penetrate cells easily and then work on the cell wall and interact with the membrane of the cell (Trombetta et al. 2005; Khorshidian et al. 2018), then easily can be passed to the cytoplasm (Khorshidian et al. 2018). After entering the cell, these compounds alter the permeability of the cell and interfere with the enzymes contributory in energy production (Basim et al. 2000). Moreover, these compounds cannot just affect many of the enzymes contributory to energy production, but also damage the internal proteins at higher concentrations (Omonijo et al. 2018), and stop the proton motive force that eventually leads to cell death (Basim et al. 2000). Figure 6 elaborates the targets of EOs in bacterial cells and showing different mechanisms of antimicrobial activity.
Targets of EOs in bacterial cells, showing different mechanisms of antimicrobial activity: degradation of the cell wall; damage to the cell membrane; leakage of cell contents; cytoplasmic protein coagulation or inhibition; and depletion of the protonmotive force. (Gutiérrez-del-Río et al. 2018)
To sum up, the degree of antimicrobial activity shown by EOs may affect their ability to penetrate bacterial membranes and exhibit activity that inhibits functional properties of the cell (Guinoiseau et al. 2010; Bajpai et al. 2012). As a result, disruption of the cell membrane by EOs may affect many biochemical processes, secretion of growth regulators, synthesis of structural molecules, and nutrient manipulation. (Tariq et al. 2019).
Applications of EOs as preservatives
At present, the excessive use of synthetic antimicrobial additives in food processing has become questionable in their safety, and many of them may have residual toxicity (Bonomo et al. 2017). In addition, mostly synthetic preservatives are being restricted to their use or under evaluation due to resistance and adverse influence on health and the environment (Prakash and Kiran 2016; Prakash et al. 2018b). Furthermore, chemical additives have negative side effects on human health. Consumers have begun to recognize these facts and rejected products that contain unnatural additives (Cacho et al. 2016). Therefore, the food industries are currently looking for some new and safer ways to save food to meet the challenges of existing chemical preservatives (Prakash et al. 2018b).
A wide range of extracts contains effective antioxidant and antimicrobial agents, which are alternatives to the traditional chemicals used as preservatives (Zanetti et al. 2018). It is estimated that among 500,000 species of plants in the world, only about 1 to 10% (approximately 50–500 species) are being used as preservatives for food (Sauceda 2011). One of these extracts is recognized to be EOs. The use of EOs as natural food supplements has been the focus of vast reports, due to that, these active mixtures have successfully been replaced the chemical food additives (Ribeiro-Santos et al. 2017a). Many EOs offer strong potential food conservation applications, and the use of EOs in the food industry can help to reduce the addition of chemical preservatives as well as the intensity of thermal treatments, leading to foods that are naturally preserved and richer in organic characteristics (Gutierrez et al. 2008).
In the same line, traditionally used EOs and their biologically active compounds have antimicrobial activity against food-borne pathogens, which can provide a new source of food preservation (Prakash et al. 2018b). Thus, they can be combined into food packaging to release their compounds into food over time (Ribeiro-Santos et al. 2017a). In fact, a large variety of EOs of different plants such as chamomile flowers (Matricaria chamomilla L.), basil (Ocimum basilicum L.), cardamom seeds (Elettaria cardamomum L.), and rosemary (Rosmarinus officinalis L.), are being used in the packaging materials of foodstuffs as antimicrobials, antioxidants and is considered a GRAS (Food and Administration 2016). Table 2. Summarize details of some recent studies about the application of EOs as a preservative in the food system.
Synergism of EOs
During the manufacturing process, the Food manufacturers are being used traditional methods for food preservation such as sterilization, drying, freezing, and pasteurization, as well as preservation agents that are used in great amounts potential to ensure food safety (Zanetti et al. 2018). Currently, a combination of two or more factors interacting in an additive or synergistic manner to control microorganisms is sought while avoiding the application of a single preservation factor, which improves the sensory and nutritional quality of the food and produces fresh and healthy products with the lowest of prepare cost and processed additives (Alzamora 1997). Many investigations available that show the added effects of some EOs and organic acids (Zhou et al. 2007; Souza et al. 2009; Hulánková and Bořilová 2012). As an example, Zhou et al. (2007) reported that EOs with acetic acid or citric acid but not with lactic acid had a better effect against Gram (-) bacteria (Salmonella typhimurium) when compared to individual EOs or organic acids alone. Also, in current studies, the findings have demonstrated in vivo effectiveness of these synergistic in food strategies on pigs meats, and poultry (Diao et al. 2015; Balasubramanian et al. 2016; Walia et al. 2017; Liu et al. 2017). Besides, the inclusion of EOs mixed with organic acids in pig feed before slaughter can inhibit Salmonella and seroprevalence proliferation (Noirrit and Philippe 2016; Walia et al. 2017).
On the whole, the mechanisms behind this potential synergy between some EOs and organic acids are still unclear (Omonijo et al. 2018). But many factors may be linked between EOs and organic acids to support this co-synergy to influence microbes. Where, various factors subsidize the inhibition of microorganisms by organic acids, including the proportion of non-organic forms of organic acids, pH reduction, physiology/metabolism, string length, and branching level (Booth 1985). For example, it is known that the phenols present in the EOs can change the functions and structure of the membrane of the bacterial cells, which leads to bulge and increase the permeability of the membrane (Omonijo et al. 2018). Thus, the lipophilic nature of the weak organic acids permits them to enter the plasma membrane easily and thereby reduce the pH of the cell, leading eventually to the bacteria's death (Abdelhamid and El-Dougdoug 2020). Furthermore, the hydrophobic nature of EOs is increased when the pH is reduced, allowing it to pass more easily through the membrane of the bacterial cell (Karatzas et al. 2001).
On the other said, EOs also contain a mixture of active compounds that synergize with each other to affect the food-borne pathogens according to their mechanism effect, as the key constituents in EOs can form up to 85% of oils and usually determine their biological properties (Ribeiro-Santos et al. 2017a), and the other 15% are composed of simple components, although they are present at low levels, they show significant character to confer biological activities, working in synergy with key components (Pavela 2015). Where, the influence of synergies occurs when the effect of the combined material is greater than the sum of the individual effects (Burt 2004), for example, numerous studies are supporting that phenolic compounds of EOs can stimulate the response of antimicrobial against food-borne pathogens by change permeability of microbial cell, destructive of membranes of cytoplasm, interfering in the power generation of the cellular system (ATP), and disrupt the force of the proton drive that lead towards inhibition of several cellular actions and functional properties including the production of energy and leakage of internal cellular contents and other metabolic functions ( Friedly et al. 2009; Bajpai et al. 2012; Kačániová et al. 2017; Tariq et al. 2019). As an example, Helander et al. (1998) testified that carvacrol is a non- hydrophilic compound affecting the cell membrane by changing the composition of fatty acids that affect the membrane's permeability and fluidity. Another mode of action is through trans-cinnamaldehyde, which enters the periplasm of cells and damage cellular functions. Similarly, cinnamaldehyde (EOs) has been reported to constrain ATPase and damage the outer membrane of the cell (Tariq et al. 2019). Other studies have shown that vanillin has shown antimicrobial activity by blocking bacterial breathing pathways, disrupting flow ions (K+), and pH gradients. In the same line, following EOs (carvon, careveol, and citronilal) were introduced to modify hydrophobicity and damage membrane solidity, leading to loss of ions (K+) (Lopez-Romero et al. 2015). Moreover, the p-cymene has a greater link to bacterial cell membranes and thus may destabilize the integrity of the membrane (Cristani et al. 2007). So, these mechanisms facilitate the entry of substances used as preservatives alongside EOs, whether they are organic acids, chemically active compounds, or other EOs into the bacterial cell and work to destroy them.
On the other hand, some studies also have shown that a combination of inorganic compounds such as gold, silver, chitosan, zinc, iron, platinum, carbon nanotubes, copper, and numerous inorganic more have been used with EOs to evaluate their antimicrobial activity (Van Long et al. 2016; Gaspar et al. 2017; Jogee et al. 2017).
The factors affecting the production of EOs
Usually, it is believed that the EOs produced by plants are the consequence of various stresses (Theis and Lerdau 2003). Although all parts of the plant may contain EOs, the composition, quality, and quantity vary according to many factors as representing the EOs less than 5% of the plant's dry matter (Fornari et al. 2012). Therefore, stressors and growth conditions may affect the yield and content of EOs in plants and play a vital part in their quality (Theis and Lerdau 2003). Also, the differences among the plant species, ecotypes, and cultivar affect concentrations of EOs (Kholif and Olafadehan 2021). Furthermore, the chemical composition of EOs also depends on the parts of the plant, the geographical origin, age, preparation, climate conditions, stocking time, agrochemicals used, the composition of the soil (Borges et al. 2018). For example, Badi et al. (2004) studied the effects of spacing between plants and harvest time on the yield of EOs in thyme. The plants were harvested either at the beginning of blooming, full bloom, or fruit group. The area of planting did not significantly affect EO content, but harvest time had a significant impact. The maximum yield was obtained from EO and thymol content when the plants were placed 15 cm from each other and harvested at the beginning of the blooming phase. On the other side, Celiktas et al. (2007) examined different sources of variability in the uncritical extraction of rosemary leaves, including location (different cities of Turkey) and harvest time (December, March, June, and September). They have revealed that even when applying the same pre-treatment of raw materials and the same process conditions, the extracts obtained from the collected papers at different locations and harvest times have a somewhat different composition. For example, the concentration of carnosic acid, one of the most abundant antioxidants found in rosemary, ranges from 0.5 to 11.6% (W/ W) in the extracts obtained from different samples of the plant matrix. Besides, they observed that the plants harvested in September had antioxidant capabilities greater than those collected at other harvest times. Similarly, Hidalgo et al. (1998) reported that for the rosemary plants harvested in Córdoba (Spain), the carnosic acid content increased progressively during the spring and peaked in the summer months. Ioannou et al. (2014) studied EOs of fresh needles for 46 pine species that were extracted by hydrodistillation. the EOs obtained were showed quantitative, and qualitative differences between the samples.
Also, the compositions of the EOs are determined primarily by the homogenization of primary materials, whose properties can be affected by a large number of factors (Zhai et al. 2018). For example, Senatore (1996) reported that the total content of mono-terpenes hydrocarbons (especially p-cymene and γ-terpinene) and phenol terpenes (specially carvacrol and thymol) ranges from 57.3 to 62.5% of the EOs from a Thymus pulegioides L., and it is relatively constant in times of harvesting, but phenols’ content starts to increase at the beginning of blooming and reach a higher value during the plant’s full flowering period. Besides, González et al. (2019) noted that the quantitative and qualitative of EOs from Artemisia magellanica obtained under the same conditions except the time plants collocation were different. The EOs of the plant that collected in February and March 2015 were contained γ-costol (21.0%), 2-methylbutyl 2-methylbutyrate (17.8%), (Z)-β-ocimene (7.6%), and selina-4,11-diene (5.9%), while EOs of the plant that collected in February and March 2014 were contained γ-costol (43.5%), selina-4,11-diene (8.8%), 2-methylbutyl-2-methylbutyrate (8.2%), and α-selinene (4.3%). Moreover, Aprotosoaie et al. (2017) reported that the high yield of lavandin plants is determined according to a storage capacity and different morphological features like localization and higher density of secretory structures such as peltate glandular trichomes.
Other parameters such as water can also determine the quality and composition of EOs. Rebey et al. (2012) found that the number of seeds produced by cumin plants increased due to the moderate water deficit (MWD) and the severe water shortage (SWS) resulted in lower yield. By comparison, the EOs production arose 1.4 times under MWD but fell by 37.2% under SWS. Also, water deficits modified the profile components in the EOs that were γ-terpinene/cuminaldehyde in stressed ones in the control seeds to predominantly γ-terpinene/phenyl-1,2 ethanediol.
Furthermore, the drying methods, extraction techniques (Calo et al. 2015), and conditions of analysis affect EOs yield and composition of aromatic plants' chemical (Negi 2012; Riahi et al. 2013). Fathi and Sefidkon (2012) obtained fresh leaves of Eucalyptus sargentii and five different drying methods were used: drying the shade, drying the oven (at 30, 40, and 50 °C), and drying the sun. The methods of extraction included HD and steam-distillation. The oils analyses showed that the dried samples in the shade produced the highest oil yield and the content of 1.8-cineole between all drying methods used, while HD produced the highest oil yield. Also, Rojek et al. (2021) mentioned that drying by oven yields the highest EOs content, while drying at shade with well-ventilated generates less amount of oil but with higher citral (A) and citral (B) content, which enhances the quality of the EOs of lemongrass.
Additionally, the solvent of extraction also affects the quantity and quality of EOs (Kholif and Olafadehan 2021). Al-Maqtari et al. (2021a) found that the major compounds of volatile compounds in carbon dioxide (CO2) companion ethanol extract of P. jaubertii were carvotanacetone, α- linolenic acid, cerotin, and palmitic acid, respectively, while, Fawzy et al. (2013) noted that the major compound in the methanolic extract was oxygenated monoterpenes. Also, Tariku et al. (2010) reported that the major volatile compounds of EOs obtained from A. abyssinica were Yomogi alcohol (38.47%), artemisyl acetate (24.88%), artemisia alcohol (6.70%), santolina triene (1.78%), and 1,8-cineole (1.56%), while, Asfaw and Demissew (2015) found that the EOs of A. abyssinica obtained using hydrodistillation were contained Yomogi alcohol (37.6%), artemisyl acetate (22.4%), and artemisia alcohol (8.8%) as the major components.
In general, many chemical and physical parameters can affect the composition and amount of EOs obtained from plants (Calo et al. 2015). This difference is usually more quantitative than qualitative. Because of it, articles that discuss EOs should always provide a biological description of the plant material and the phytochemical profile of the oil, allowing the illustration and accuracy of the data (Freires et al. 2015; Borges et al. 2018).
Challenges contributing to declining the use of plant EOs as food preservatives
Although various kinds of EOs and their naturally active compounds have successfully been permitted by regulators such as the EC and FDA for intentional consumption being therapeutic agents in food products (Hyldgaard et al. 2012), and, despite the enormous effectiveness of antimicrobials in the laboratory, most EOs fail to exercise their full potential in the food system because of some major intrinsic obstacles such as bioavailability, the low solubility of water, stability, and volatility (Kujur et al. 2017; Prakash et al. 2018b). In contrast, high cost and t threat of biodiversity loss, shortage of raw materials, inconsistent efficiency, chemical variation, the negative impact on the food matrix, and the absence of a molecular mechanism of action are some of the major challenges facing to EOs that limit their application in the broad spectrum (Prakash and Kiran 2016). Bucar et al. (2013) stated that the collection, assessment of the quality of raw materials, and plants' identification required specialists to become increasingly. Moreover, the amount of materials obtained from raw materials is often insufficient for their commercial application (Prakash et al. 2018b).
On the other side, the use of food preservation by EOs is often limited by application costs and other defects, such as severe odor and potential toxicity (Yuan et al. 2016). Noori et al. (2018) assumed that the severe odor of most EOs, even at a low dose, may adversely affect the sensory properties such as color, flavor, texture, and taste of the applicable food, leading to a decrease in consumer acceptance. So, there are perceived sensory attributes that might be correlated with EOs composition. For example, some EOs compounds like linalool and nerolidol are associated with unfavorable attributes such as metallic, iodophor-like, soapy, and floral attributes. Simultaneously, the γ-terpinene and 1,8-cineole are associated with a cooling aftertaste, but caryophyllene and 1,8-cineole are connected with a sweet taste (Septiana et al. 2020). Wang et al. (2020) noted that the sensory attributes (taste, flavor, and overall acceptability) of oxidized sunflower oil were added by EOs of Angelica dahurica cv. Yubaizhi were memorably elevated because the myrcene was demonstrated to be its active compound in the EOs used. Also, Solomakos et al. (2008) observed that the greater acceptability of sensory properties in minced beef was when lower concentrations (0.3 and 0.6%) of EOs of thyme were used. However, there was rejection in samples with a higher concentration (0.9%).
Moreover, in the food system, EOs may bind to carbohydrates, lipids, and proteins, thus require high doses to achieve the antimicrobial effect that may change the sensory threshold levels (Prakash et al. 2018b). For example, higher concentrations of peppermint EOs decreased the overall acceptability of minced meat due to the strong odor of EOs (Smaoui et al. 2016). The intense EOs of garlic, applied in combination with allyl isothiocyanate and nisin, resulted in an acceptability index below 70% for the odor attribute in fresh sausage (Araújo et al. 2018). Also, Yuan et al. (2016) mentioned that the addition of EOs to food directly might negatively affect the perception of the foods applied.
Furthermore, EOs may lose effectiveness over time (Yuan et al. 2016). As the lipophilic and volatile compounds of EOs are prone to polymerization and oxidative degradation throughout food processing and storage, that may alter the functional composition of active ingredients and antimicrobial properties (Turek and Stintzing 2013). Fernández-López and Viuda-Martos (2018) reported that EOs are unstable, so volatile compounds that can be degraded easily by oxidation, volatilization, heating, light, etc. when they are added to the food matrix. Thus, it must be taken into account that most of the food elaboration processes include heat treatment or air and light exposition, all of those factors that increase their degradation.
Additionally, the food matrix that contains protein, fat, water, carbohydrates, pH, and salt content with external factors such as types of microorganisms, gaseous composition, and temperature can decrease the antimicrobial force in EOs (Calo et al. 2015; Prakash et al. 2018b). For example, meat nutrients are a relevant factor since they can favor the recovery of microorganisms that have suffered some injury and stress due to the antimicrobial action of EOs (da Silva et al. 2021). Smith-Palmer et al. (1998) found that foods with higher lipid content required more EOs to inhibit bacterial growth. According to Burt (2004), high pH can decrease the solubility and stability of EOs.
Although the vast majority of the most common EOs have been well tried and tested and safety levels have been determined, some EOs are more likely to cause adverse reactions than others, and that have limited their use in food systems. Sharmeen et al. (2021) mentioned that the oxidation of EOs constituents can increase the risk of causing allergic reactions because the oxides and peroxides formed are more reactive. This can be seen with α-pinene, ( +)-limonene, and δ-3-carene, and arise due to the formation of oxidation products, some of which are more sensitizing than the parent compound (Tisserand and Young 2013). On the other hand, many EOs that are considered non-toxic can have a toxic effect on some people; this can be influenced by preceding sensitization to a particular EOs, a group of EOs having similar components, or some adulterants in EOs (Sharmeen et al. 2021).
Given the above challenges, the interest in EOs based preservatives has gradually decreased in the last decade, and have been recent concerns about the standardization and safety of these natural products.
The safety performance of EOs
Food additives, according to the World Health Organization (WHO), are either natural or artificial substances added to food to maintain or improve the texture, taste, freshness, safety, or appearance of food. Nevertheless, there are approximately 320 approved food additives in Europe classified into 26 functional categories. Various chemical preservatives such as thiocyanates, pyrrolidines, imidazoles, formaldehyde, sorbates, benzoates, sodium benzoate, sodium nitrite, sulfites, and sulfur dioxide have made a significant contribution to controlling bacterial contamination of foodstuffs (Gutiérrez-del-Río et al. 2018). However, these chemical preservatives have aroused consumer concerns because of potential hazards of carcinogenesis, environmental toxins, re-emergence of pests, their long-term degradation cycles, and teratogenesis in humans and animals (Falleh et al. 2020). Excessive preservatives can also affect the human body's regular metabolism and harm the liver. Pickled vegetables, for example, are frequently found to have benzoic acid (BA) preservatives (Ling et al. 2015). The recommended daily intake (ADI) of BA and benzoates, according to the Joint Food and Agriculture Organization (FAO)/WHO Expert Committee on Food Additives (JECFA), is 0–5 mg/kg. However, even with an ADI of less than 5 mg/kg (body weight), pseudoallergy or increased hyperactivity in children has been documented in extremely sensitive groups. (Lin et al. 2020). Therefore, one of the alternatives that have been studied in depth is the natural preservation by means of natural antimicrobial preservatives produced from plants, animals, or microorganisms, in particular those extracted from different types of plants and their parts that are used as flavoring agents in some foods (Laranjo et al. 2017), as EOs from medicinal and aromatic plants (MAPs) have been proven to contribute to food safety (Laranjo et al. 2019). Plant-based preservatives, particularly EOs and their active ingredients extracted from important aromatic and medicinal plants, are gaining cumulative interest in the food industry because of broad-spectrum antioxidant properties, anti-mycotoxigenic, antifungal, and antibacterial (Maurya et al. 2021). Also, active packaging containing natural antioxidants such as polyphenols, EOs, etc., is a cost-saving alternative and also has the ability to eliminate food safety hazards (Sharma et al. 2020). Therefore, the increasing consumer interest in natural products and synthetic additives is being replaced by natural substances such as polyphenols, EOs, and other natural extracts (Vinceković et al. 2017). Moreover, the application of EOs expands a new environmentally friendly approach towards food protection due to its inclusion in the generally recognized safe category and the exemption from mammalian toxicity by the FDA (Chaudhari et al. 2020).
Nevertheless, the application of natural preservatives in food legislation and their subsequent approval for commercial use in the food industry requires notification, pack labeling, ingredient warning, compliance with the Good Manufacturing Practice (GMP), the safety report of the product, manufacturing, and storage methods, a full description of the product, and comprehensive science-based evidence (Dreger and Wielgus 2013). The European Food Safety Authority (EFSA) of the European Union and the FDA are two of the most powerful regulating agencies in the world that legislate, enforce the law, and monitor the licensing and regulation of food additives. The Joint FAO/WHO Expert Committee on Food Additives and the Codex Alimentarius are two additional significant organizations that are active in risk assessment and studies on food preservatives and routinely release comments about food preservatives (Carocho et al. 2018). Safety Data Sheets (SDS) under EU Directives 91/155/EEC and 93/112/EC are intended to provide professional users of hazardous materials and preparations with the information necessary for the safe handling of products in the workplace and the environment (Geyer et al. 1999). In general, four index systems were created with the goal of comparing additives based on risks. The indices create ratings that represent the relative combined additive hazard intensity based on information about the additive's environmental, health, and physical risks. A number of data sources are utilized to obtain information on danger categories/intensity in existing index systems. Both the Jordan and Sanjel systems, for example, employ the Globally Harmonized System (GHS) and SDSs as a basis for hazard description (Hurley et al. 2016).
In the past few years, several studies have been published on the biological properties and therapeutic applications of EOs such as one covering the scientific literature from 2000 onwards, up to the first half of 2007 (Adorjan and Buchbauer 2010). Based on toxicological potential, the toxicity of EOs components has been classified into three structural groups (Regnault-Roger et al. 2012). The Oral toxicity is minimal in Class I compounds with little functionality, such as the aliphatic compound limonene; intermediate toxicity is in Class II compounds with moderate functionality; high potential toxicity is in Class III compounds with reactive functionality (Baptista-Silva et al. 2020). Elemicin, for example, is classified as a Class III compound because it is an allyl substituted benzene derivative with a reactive benzylic/ allylic position (Regnault-Roger et al. 2012). On the other said, rosemary, carotenes (E 160a), bixin, annatto, norbixin (E 160b), δ-tocopherol (E 309), γ-tocopherol (E 308), and tocopherol-rich extracts (E 306) are among the natural preservatives presently allowed as food additives in the EU, according to this regulation. In the case of meat, however, particular natural preservatives have been recognized as food additives (Beya et al., 2021). This includes rosemary to be used only in dried sausages at a maximum level of 100 mg/kg or 150 mg/kg in dried meats only and carotene to be used in sausages and pies with a maximum level of 20 mg/kg (Manessis et al. 2020). Moreover, The FDA classifies natural preservatives such as estragole as GRAS but is banned as flavorings in the European Union. On the other hand, the Codex Alimentarius suggested a maximum level of carotene of 20 mg/kg in fresh (ground) meat, poultry, and game, but the USA did not adopt this suggestion in its legislation. Despite all this, in the United States and other countries, manufacturers of consumer products and fragrance formulations are not required to disclose all ingredients to the public, because the Consumer Product Safety Act (CPSA), which is administered by the Consumer Product Safety Commission, does not require product labels mention any or all ingredients (USEPA 2005). So, consumable product components are exempt from disclosure of product labels and Material Safety Data Sheets (MSDS) in several ways (Steinemann et al. 2011). For example, Kwon et al. (2008) mentioned that out of 133 unique volatile compounds identified across products, only 1 volatile compound (ethanol in 2 products) was listed on any of the product labels, and only 2 volatile compounds (namely ethanol in five products, and 2-butoxyethanol in one product) in any of the MSDS.
Although, exposure to volatile compounds has been associated with health effects such as contact allergies, mucosal symptoms, headaches, and asthmatic exacerbations. For example, according to Steinemann et al. (2011), 24 of the 133 distinct volatile chemicals discovered in their investigation were categorized as toxic or hazardous by at least one federal legislation. Consequently, Thus, information on product labels and Material Safety Data Sheets (MSDS) are essential to understanding product ingredients and their potential relationships with effects, exposures, and policies. As a result, in the European Union (EU), Regulation EC1333/2008 on Food Additives has established a list of approved food additives which is fully published in EU Regulation 1129/2011. However, additional research is needed to assess the safety performance of these natural compounds in order to ensure their safe application in the food, agriculture, and pharmaceutical fields, as well as to understand their mechanism of action and any side effects.
Essential requirements for quality assurance of EOs
Purified EOs have become more popular as additions in foods, cosmetics, and fragrances in recent decades. (Rasekh et al. 2021). The food flavoring and perfume industries utilize around 90% of the entire global production of EOs (Sharmeen et al. 2021). Moreover, MAPs make up a large percentage of natural plants and 80% of the world's population use it as traditional medicines to treat various human health problems, where more than 9000 local plants have been identified and registered for their therapeutic properties, and about 1500 species are known for their flavor and aroma (Laranjo et al. 2017). However, when using EOs, there are some issues that should be considered. For instance, EOs are obtained from suppliers or corporations all over the world, who generally collect them from farmers or wholesalers they have grown to trust over time for their methods and honesty (Baptista-Silva et al. 2020). Therefore, as a result of the commercial value and importance of EOs, products made with them are subject to regulatory standards that require the detection of counterfeit or adulterated products in commercial markets to prevent the illegal sale of plant products at prices higher than their commercial value. Indeed, there have been numerous recorded examples of EOs adulteration, including the addition of a non-volatile component, low-cost synthetic chemicals, volatile or EOs from other natural sources, and vegetable oils to increase their weight. Different than that, it has been reported that other plants have been used to replace all or part of the original plant from which the EOs is extracted (Salgueiro et al. 2010). All of these adulteration techniques can impair the quality of EOs, and adulteration can lead to safety problems or non-compliance with natural labeling by introducing one or more synthetic chemicals. Furthermore, Adulteration of EOs can also have an impact on the regulatory side, since an EOs may no longer meet standardization criteria, as adulterants are usually introduced at a low level (5–8%) to evade detection it by standard analytical techniques (Pellati et al. 2013). As a result, authenticity is a crucial subject for consumer safety and EOs manufacturing quality in the future (Mosandl 2004). Consequently, rapid detection of counterfeit raw materials is critical for quality assurance and quality control (QA/QC) procedures, as well as for health concerns related to the avoidance of consumption of artificial or unknown harmful products in industrial production processes, but current analytical methods for QA/QC are often costly and time-consuming.
Currently, traditional methods of categorizing aromatic compounds entail sensory analysis by human experimental panels consisting of several experts with a list of key features and samples for documentation. Although this approach appears to be simple at first look, plant-product sampling is generally limited or compromised owing to expensive costs, a lack of accuracy or measurement standards, human tester score variability, and low repeatability (Karami et al. 2020a). Furthermore, there are no simple generalizations about the key odorants in EOs; the odor of some EOs is due to a large amount of a single compound, whereas trace amounts can determine the odor of others, and true odor is the manifestation of a complex mixture of compounds in general (Sharmeen et al. 2021). So, instrumental procedures such as gas chromatography (GC) provide an alternate QA approach and provide high objective and accuracy, but it is expensive, time-consuming, requires destructive sampling, and must be conducted by skilled experts (Rusinek et al. 2021). Also, high-performance liquid chromatography-ultraviolet (HPLC–UV) may be used as a complement or even an alternative technique for analyzing volatile oils because of its versatility, sensitivity, flexibility, and selectivity. In most cases, HPLC is the method of choice for analyzing essential oil's less volatile components (Sharmeen et al. 2021). HPLC–MS offers significant information on the amount and type of elements in natural complex matrices, such as EOs, but it is also must be performed by trained professionals, involves destructive sampling, time-consuming, and expensive. As a result, developing low-cost, accurate, and fast techniques for assessing and categorizing EOs is critical for successful commercial QA/QC testing. Electronic odor detection (EAD) techniques and tools and associated methods provide a good alternative testing method due to avoidance of operator fatigue by human testers, reproducibility, accuracy, and low costs (Gorji-Chakespari et al. 2017). Advances in electronic olfactory technologies have resulted in the fast growth and deployment of artificial intelligence (AI) devices, such as electronic-nose (e-nose) devices, as non-invasive and speedy detection techniques (Karami et al. 2020b). E-nose devices are especially well adapted for the detection and analysis of volatile chemicals, and they have a long history of successful usage in a variety of industries, including food safety, food quality, agriculture, pharmaceutical, and medicine (Rasekh et al. 2021). Inaddionally, the industrial sector is growing fastaly and food producers are under constant pressure to enhance both goods and processes, thus predictive modeling in the food business can provide a significant competitive advantage (Kourkoutas and Proestos 2020).
Gas chromatography (GC-O), on the other hand, is a well-known standard technique for determining odor active components in complex mixtures, based on the correlation between the chromatographic peaks of removed substances that are simultaneously perceived by two detectors, one of which is the electronic olfactory apparatus. So, over the years, GC-O has been frequently used in the analysis of EOs (Breme et al. 2009). The results of a GC-O analysis can provide information about the absence or presence of an odor in a composite, describe the quality of a perceived odor, measure the duration of odor activity, and determine the strength of a specific odor that may have the final purpose in the application of these compounds in the fragranceand flavor industries. GC-O is also used to identify odor-active areas on a chromatogram and to create an odor profile for the entire EOs sample (Eyres et al. 2007).
Modern methods of packing EOs
The phenomenon of globalization has allowed access to innumerable foods from all over the world, thus the importance of maintaining its original sensory characteristics is increasing day by day. So, food factories were enforced to develop innovative methods and techniques to meet consumer’s needs. In line with this, packaging has emerged in food systems (Ribeiro-Santos et al. 2017a). Food packaging is designed to protect the food matrix from external factors, such as light, temperature, and humidity, which can lead to spoilage (Carocho et al. 2015). Additionally, the packaging as well protects its contents from other environmental impacts such as microorganisms, odors, dust, shocks, pressure forces, and vibration (Robertson 2005). Therefore, a lot of studies have engrossed in new packaging techniques because of the increasing population and limited natural resources (Krochta 2002).
However, as noted earlier, the primary use of EOs in foods is largely limited by their low stability and strong flavor that affect the sensory properties of food (Prakash et al. 2018a). Currently, the packaging of EOs in the food systems has encouraged new ways to protect it from internal and external factors and extended the shelf life for it, thus led this to food industries to develop new packaging concepts (Ribeiro-Santos et al. 2017b). These difficulties can be overcome by packaging EOs inappropriate delivery systems that can help to maintain biological activity, stability, and help at the same time to reduce the sensory impact on foods (Donsi and Ferrari 2016).
In this section, two EOs delivery systems that may have practical applications in the food industry are briefly discussed.
Micro and nanoemulsions
Instead of applying most EOs directly to foods, one of the most encouraging approaches is to integrate EOs into colloidal delivery systems (Donsì et al. 2011; Rao et al. 2019). These delivery systems provide the best solution to improve water solubility and the mass transfer of EOs in foods, which may enhance the effectiveness of antimicrobials (Ghosh et al. 2014; El-Sayed et al. 2017; Rao et al. 2019). Where delivery of EOs in the colloidal delivery systems such as emulsion-based systems with food ingredients can be a promising tool for the direct application of EOs in food systems (Donsì et al. 2011).
In general, the emulsion-based systems can be divided into three types according to the size of the droplets and their thermodynamic stability: (1) microemulsions, (2) nanoemulsions, (3) traditional emulsions. Each of them has its features and disadvantages in terms of physical stability and antimicrobial activity (Rao et al. 2019).
Microemulsions are dynamically stable systems consisting of very small molecules that include surface molecules and EOs molecules (McClements 2012). These systems offer excellent stability and prolonged storage that tend to be visually visible due to the relatively small particle size as compared to the wavelength of light. The main disadvantages of microemulsions as a delivery system for EOs are that they can usually be manufactured only from synthetic surface materials that must be used at comparatively high levels (Rao et al. 2019). Furthermore, it must be carefully formulated so that it is stable in its initial form and after dilution in the system food. For this reason, much researches on the formulation of EOs emulsifiers has focused on improving their performance, stability, and manufacture (Ma and Zhong 2015; Ma et al. 2016; Rao et al. 2019). However, there are several types of encapsulation techniques, such as spray drying, freeze-drying, co-crystallization, vibrational nozzle, and centrifugal extrusion (Mahdi et al. 2020). On the other side, the formation of the emulsion is non-spontaneous and energy input is needed to produce the droplets. There are many mechanical devices used in this process such as ultrasound generators, high shear stirring, and high-pressure homogenizers, designed as dispersion or high-energy emulsification methods (Al-Maqtari et al. 2021b).
Modern improvements in the arena of nanotechnology can successfully face these challenges by controlling the release of EOs in the food system to provide them with timely and appropriate impact. Also, nanoparticles (size < 100 nm) can be used as a stand to mask the effect of unwanted odors in EOs and improve their interaction with nutrients (Donsì et al. 2011). Therefore, recently, there has been an increasing interest in the use of nanoemulsions as a system for the delivery of EOs (Rao et al. 2019).
As of now, research on the application of EOs nanoemulsions as antimicrobial agents to the food system is in its rapid growth phase and requires further exploration for commercial exploitation (Prakash et al. 2018a). If the average droplet size of these emulsions in a nanometer size (10–100 nm), referred to as nanoemulsions (Ghosh et al. 2013; Rehman et al. 2019a), and this non-metric volume led to be the better diffusion of emulsions and have enhanced biological activity and reduced the impact on the sensory properties of food products. Therefore, the use of nanoemulsions as an agent for EOs is a promising way to improve the quality, safety, and functionality of food products (Prakash et al. 2018b). Because of their small size, nanoemulsions exhibit many distinctive properties. First, it is very stable to separate gravity as the smallest particle size confirms that the effects of Brownian motion dominate the forces of gravity (McClements 2015). Secondly, the bioavailability of the entrapped components is enhanced in nanoemulsions because of its high surface-to-volume ratio and small particle size (Silva et al. 2012). For similar reasons, the biological activity of the nanoemulsions of the encapsulated lipophilic component was improved through amended interaction and superior transfer of molecules across membranes (Salvia-Trujillo et al. 2015a). Thirdly, the nanoparticles are either transparent or considerably turbid because the light scattering is weak in the nanodroplets and therefore can be easily used in the food systems (Salvia-Trujillo et al. 2015b). Fourth, surface factors that are food-grade, such as polysorbates, lecithin, biopolymers such as (natural gums, modified starch, vegetable proteins, or animal) and sugar esters, are commonly used as emulsifying agents for the fabrication of nanoemulsions for food applications (Rehman et al. 2019b). Table 3. presents some recent studies about the application of EOs emulsions as delivery systems in the food industry.
In addition, these factors also give some of the expected advantages to the nanoparticles in terms of their interpersonal behavior (steric repulsion, rheology, and electrostatic forces), loading capacity, and response to environmental stresses (Donsi and Ferrari 2016). Therefore, the application of nanotechnology in the food industry is one of the fastest-growing scopes in the last few years (Prakash et al. 2018b).
As displayed in Fig. 7, oil in water nanoemulsions contain tiny oil droplets affixed to the surface agent molecules that are dispersed within the continuous water phase (Prakash et al. 2018a). Apart from EOs, nanoparticles can also act as delivery systems for other important compounds containing fat such as flavors, medications, antioxidants, and food nutrients (Ezhilarasi et al. 2013).
Despite these advantages, the main drawbacks are the strict restriction on the use of surfactant material types, oils, and the requirement of the higher surface to oil ratio for the production of stable nanoemulsions and applying it in the food systems (Sessa et al. 2015). As the long-term stability of nanoemulsions represents a major obstacle to its widespread application. Karthik et al. (2017) mention that the nanoemulsions are dynamically unfavorable because of the positive free energy associated with the construction of the oil and water interface, and thus tend to collapse over time. The mechanisms of flocculation, separation of gravity, coalescence, and/or maturation of Ostwald are diverse physicochemical mechanisms associated with the breakdown of nanoemulsions. The mechanisms of destabilization of nanoemulsions are associated with various forces in the system such as the flow forces, the forces of attraction and dissonance between particles, molecular forces, and gravity forces.
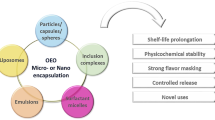
Micro and nanoencapsulation
The food industry is evaluating best practices to include EOs, such as natural antimicrobials, in polymeric materials to obtain their advantages and reduce their imperfections. Encapsulation is one such technique, which is formed in a process in which small molecules are coated with a layer to form a capsule (Ribeiro-Santos et al. 2017b; Riaz et al. 2019).
Micro/nanoencapsulation of various bioactive components of food plays an important role in protecting these nutrients from unfavorable processes and conditions of storage such as high humidity, high temperatures, high oxygen levels, exposure to light, and some pH values (Assadpour and Jafari 2019). Also, this technique can be used to produce custom ingredients, supplements, and additives, with a long lifespan that can be applied to pharmaceuticals, food products, and cosmetics (Katouzian et al. 2017; Akhavan et al. 2018; Assadpour and Jafari 2019).
The encapsulation of the active components has appeared quite recently, but its rapid and important development has allowed it to be used in the most diverse branches of industry, especially the food industries, cosmetics, and pharmaceutical (Zanetti et al. 2018). It is a process that involves an active agent in another material, the wall material, and produces particles in the nanometer, micrometer, or millimeter by many packaging techniques (Burgain et al. 2011).
Generally, the classified encapsulation can depend on the size where the fine particle size is ranging between 1 and 1000 mm and can be formed by natural or synthetic materials. However, nanomaterials range in size between 1 and 100 nm (Ribeiro-Santos et al. 2017b). But, choosing the right wall material is a critical factor in the encapsulation of active components. Where, These substances should protect the underlying material from degeneration, must-have thermal properties compatible with the product's characteristics, be compatible with the food type, and it is required mechanical strength, allow controlled release (Gómez et al. 2018). Some of the common wall materials used to encapsulate food compounds are maltodextrin, gum arabic, gelatin, sodium caseinate, whey protein, chitosan, and modified starch. This technology requires core competencies in materials science, colloidal chemistry, and a deep understanding of the stability of active agents (Vinceković et al. 2017).
There are many proposed encapsulation proceedings, but none of them can be considered universally applicable. The proper encapsulation favors the delivery of active components of interest Properly and effectively, where they may be allocated in a suitable carrier to deliver it to the target, with still contain a version-controlled by different time intervals independent of the molecular nature of biomass, which improves safety and effectiveness because it benefits the protection of the bio compound (Zanetti et al. 2018). In addition to protecting some of the target ingredients through the work of one or more wall materials, also encapsulation is suitable for the development of end products containing plant antioxidants that have seamy smells or bad flavors such as rosemary or sage extracts (Tavares et al. 2014).
Applying these microcapsules may be a viable option for maintaining the functional features of bioactive components (Franco et al. 2017). Furthermore, active ingredients in microencapsulated can be living cells such as probiotics (Bifidobacterium, Lactobacillus) (Gómez et al. 2018). There are many techniques used to fine-tune food ingredients to overcome some limitations and increase solubility, applicability, and bioavailability. These techniques are freeze-drying, spray drying, coacervation, liposomes, polymer micelles, molecular inclusion, supercritical fluids, extrusion processes, evaporation of solvents, or nanostructured lipid (Aguiar et al. 2016; Franco et al. 2017). Besides, powders with low water activity can be used as additives, ensuring microbiological stability, and transport, facilitating packaging, marketing, and storage (Gómez et al. 2018).
Previously, microencapsulation methods were divided into the following basic groups: chemical, physicochemical and physicomechanical. No single encapsulation process is adaptable to all core materials or product applications (Wilson and Shah 2007). Each method can offer its advantages and also can offer its disadvantages, but most commonly used can present many disadvantages as unfavorable conditions for the complexity of the procedure, basic material, reproductive problems, or low encapsulation efficiency (Das et al. 2011). For example, coacervation offers high-efficiency of encapsulation and control by particle size but is an expensive technique, such as emulsification also associated with high production of residual solvents and low-efficiency of encapsulation (Gómez et al. 2018). On the other hand, coating the fluid layer has low operating costs, but it is a time-consuming method (Carvalho et al. 2016).
In short, microencapsulation consists of a technological process of integrating a compound/s into another material, commonly known as active/core components and encapsulating /coating/wall, with so small "packages"(Gómez et al. 2018). The forms of the morphology of molecules depend on the encapsulated and active materials and the technique used to prepare them. Currently, many components that include different antioxidant compounds are subject to encapsulation. Some studies have already focused on encapsulating additives that benefit specific products and improve product shelf life (Domínguez et al. 2018).
In general, two main forms of coated systems can be distinguished, namely the type of core–shell (capsules) and the type of matrix (balls). In the first type, the core material is a continuous phase surrounded by a shell (liquid or solid), while the matrix type contains active compounds distributed uniformly within the solid phase of a homogeneous matrix (Gómez et al. 2018). (Fig. 8).
On the other hand, nanoencapsulation of EOs can also improve their biological properties by increasing their biological availability by increasing the ratio of surface to size by reducing the particle size to the nanoscale (Esfanjani and Jafari 2016).
The nanocapsule or polymer acts as a protective film, isolates the inner nucleus containing the active agent, and avoids the effect of unsuitable exposure (Suave et al. 2006). An important point in the production of a nanoencapsulation product is the selection of wall material, generally, a polymer is selected based on the physical and chemical properties of the active agent and the intended application (Ribeiro-Santos et al. 2017b). A wide variety of natural and synthetic polymeric matrices were used such as carbohydrates (chitosan, starch, and cellulose), polyethylene, proteins (gelatin, casein, and albumin), gum (carrageenan, Arabic gum, and alginate), and lipids (paraffin, fatty acids, and wax) (Silva et al. 2014). Table 4. presents some recent studies on the application of EOs encapsulation in the food industry and the wall materials used with them.
Spray drying is one of the most common operations of the unit during the process of nanoencapsulation (Botrel et al. 2015). This technique includes the formation of an emulsion containing EOs and a basic wall. Then, the emulsion turns into a large number of small droplets that fall into the spray chamber synchronized with the hot circulating air (Ribeiro-Santos et al. 2017b). Then, the water immediately evaporates when it touches the hot air, and these droplets become solid particles (Laohasongkram et al. 2011).
Conclusion and future perspective
In this review, we have reviewed the studies of EOs and their importance in the food industry and some biological activities such as antimicrobial activities and antioxidants, and the basic mechanisms for the work of its main active compounds. It is interesting to note that EOs are exploring many areas. Plenty of researches showed that many EOs can be used as preservatives in food applications, which are often more abundant and have many benefits such as antimicrobial effects and antioxidants and have many mechanisms through which they can affect foodborne pathogens. In general, many challenges are facing the use of EOs in food applications, for example, antimicrobial activity in EOs may decrease, due to several factors that can affect the composition of EOs such as geographical area, diversity Plant, harvest, extraction method, and others that can leads to decrease their application. Furthermore, the oil composition plays an important role in its biological activities. Therefore, the phytochemical characterization of the EOs that has been evaluated is still necessary because of the difference in biological activity according to the composition of the oil. where it is important to remember that the EOs also contains a large mixture of secondary metabolites that can mask the activity of major compounds; this can explain higher doses of EOs needed for a particular biological activity compared to its isolated molecules. Moreover, the lipophilic nature of EOs contributes to this low activity, and the synergies and hostility between EOs and food ingredients require further study before these substances can be used reliably in commercial applications.
Recent studies, however, suggest that this can be overcome by using modern methods of packing EOs in the form of micro/nanoemulsions or micro/nanoencapsulation as an alternative to improving their biological activity and effectiveness and reducing their interaction with different nutrients in food systems. Also, this technology can improve the commercial potential of EOs due to the low doses of oil needed to operate in food technology and reduce their effects on the sensory characteristics of food. Generally, although the EOs can increase the stability of food during storage, prevent the growth of foodborne pathogens and spoilage microorganisms, alleviate oxidation, thereby working as a good natural preservative. However, their safety and potential side effects should be conducted in future studies.
References
Abdelhamid AG, El-Dougdoug NK (2020) Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies. Heliyon 6(9):e05020
Adorjan B, Buchbauer G (2010) Biological properties of essential oils: an updated review. Flavour Fragr J 25:407–426
Aguiar J, Estevinho BN, Santos L (2016) Microencapsulation of natural antioxidants for food application–The specific case of coffee antioxidants–A review. Trends Food Sci Technol 58:21–39
Akarca G (2019) Composition and antibacterial effect on food borne pathogens of Hibiscus surrattensis L. calyces essential oil. Ind Crops Prod 137:285–289
Akhavan S, Assadpour E, Katouzian I, Jafari SM (2018) Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci Technol 74:132–146
Al-Hajj NQM, Algabr M, Raza H, Thabi R, Ammar A-F, Aboshora W, Wang H (2017) Antibacterial activities of the essential oils of some aromatic medicinal plants to control pathogenic bacteria and extend the shelf-life of seafood. Turkish J Fish Aquat Sci 17(1):181–191
Al-Maqtari QA, Al-Ansi W, Mahdi AA, Al-Gheethi AAS, Mushtaq BS, Al-Adeeb A, Wei M, Yao W (2021) Supercritical fluid extraction of four aromatic herbs and assessment of the volatile compositions, bioactive compounds, antibacterial, and anti-biofilm activity. Environ Sci Pollut Res 28:25479
Al-Maqtari QA, Ghaleb AD, Mahdi AA, Al-Ansi W, Noman AE, Wei M, Aladeeb A, Yao W (2021) Stabilization of water-in-oil emulsion of pulicaria jaubertii extract by ultrasonication: fabrication, characterization, and storage stability. Food Chem 350:129249
Al-Maqtari QA, Mahdi AA, Al-Ansi W, Mohammed JK, Wei M, Yao W (2020) Evaluation of bioactive compounds and antibacterial activity of Pulicaria jaubertii extract obtained by supercritical and conventional methods. J Food Meas Charact 15:449
Alimpić A, Pljevljakušić D, Šavikin K, Knežević A, Ćurčić M, Veličković D, Stević T, Petrović G, Matevski V, Vukojević J (2015) Composition and biological effects of Salvia ringens (Lamiaceae) essential oil and extracts. Ind Crops Prod 76:702–709
Almasi H, Azizi S, Amjadi SJFH (2020) Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L) essential oil. Food Hydrocoll 99:105338
Alzamora S (1997) Preservación I. Alimentos conservados por factores combinados. Temas En Tecnología De Alimentos 1:45–89
Aprotosoaie AC, Gille E, Trifan A, Luca VS, Miron A (2017) Essential oils of Lavandula genus: a systematic review of their chemistry. Phytochem Rev 16(4):761–799
Arasu MV, Viayaraghavan P, Ilavenil S, Al-Dhabi NA, Choi KC (2019) Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind Crops Prod 133:54–62
Araújo MK, Gumiela AM, Bordin K, Luciano FB, de Macedo REF (2018) Combination of garlic essential oil, allyl isothiocyanate, and nisin Z as bio-preservatives in fresh sausage. Meat Sci 143:177–183
Aruoma OI (1998) Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 75(2):199–212
Asfaw N, Demissew S (2015) Essential oil composition of four Artemisia Species from Ethiopia. Bull Chem Soc Ethiop 29(1):123–128
Assadpour E, Jafari SM (2019) Advances in spray-drying encapsulation of food bioactive ingredients: from microcapsules to nanocapsules. Annu Rev Food Sci Technol 10:103–131
Badi HN, Yazdani D, Ali SM, Nazari F (2004) Effects of spacing and harvesting time on herbage yield and quality/quantity of oil in thyme. Thymus Vulgaris l Ind Crops Prod 19(3):231–236
Bajpai VK, Baek K-H, Kang SC (2012) Control of Salmonella in foods by using essential oils: a review. Food Res Int 45(2):722–734
Bajpai VK, Yoon JI, Bhardwaj M, Kang SC (2014) Anti-listerial synergism of leaf essential oil of Metasequoia glyptostroboides with nisin in whole, low and skim milks. Asian Pac J Trop Med 7(8):602–608
Balasubramanian B, Park JW, Kim IH (2016) Evaluation of the effectiveness of supplementing micro-encapsulated organic acids and essential oils in diets for sows and suckling piglets. Ital J Anim Sci 15(4):626–633
Baptista-Silva S, Borges S, Ramos OL et al (2020) The progress of essential oils as potential therapeutic agents: a review. J Essent Oil Res 32:279–295
Basak S (2018) The use of fuzzy logic to determine the concentration of betel leaf essential oil and its potency as a juice preservative. Food Chem 240:1113–1120
Basim H, Yegen O, Zeller W (2000) Antibacterial effect of essential oil of Thymbra spicata L. var. spicata on some plant pathogenic bacteria. J Plant Dis Prot 107(3):279–284
Benvenuti F, Gironi F, Lamberti L (2001) Supercritical deterpenation of lemon essential oil, experimental data and simulation of the semicontinuous extraction process. J Supercrit Fluids 20(1):29–44
Beya MM, Netzel ME, Sultanbawa Y et al (2021) Plant-based phenolic molecules as natural preservatives in comminuted meats: a review. Antioxidants 10:263
Bhavaniramya S, Vishnupriya S, Al-Aboody MS, Vijayakumar R, Baskaran D (2019) Role of essential oils in food safety: antimicrobial and antioxidant applications. Grain Oil Sci Techno 2(2):49–55
Bonomo MG, Russo D, Cristiano C, Calabrone L, Di Tomaso K, Milella L, Salzano G (2017) Antimicrobial activity, antioxidant properties and phytochemical screening of echinacea angustifolia, fraxinus excelsior and crataegus oxyacantha mother tinctures against food-borne bacteria. EC Microbiol 7(5):173–181
Booth IR (1985) Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49(4):359
Borborah K, Baruah S, Borthakur S (2014) Plant masticatories and their medicinal importance from Assam & Meghalaya. Int J Herb Med 2(3):21–25
Borges RS, Ortiz BLS, Pereira ACM, Keita H, Carvalho JCT (2018) Rosmarinus officinalis essential oil: a review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J Ethnopharmacol 229:29–45
Botrel DA, de Barros Fernandes RV, Borges SV (2015) Microencapsulation of essential oils using spray drying technology In Microencapsulation and microspheres for food applications. Academic Press, San Diego
Boudjedjou L, Ramdani M, Zeraib A, Benmeddour T, Fercha A (2019) Chemical composition and biological activities of Algerian Santolina africana essential oil. Sci Afr 4:e00090
Breme K, Tournayre P, Fernandez X et al (2009) Identification of odor impact compounds of Tagetes minuta L. essential oil: comparison of two GC-olfactometry methods. J Agric Food Chem 57:8572–8580
Bucar F, Wube A, Schmid M (2013) Natural product isolation–how to get from biological material to pure compounds. Nat Prod Rep 30(4):525–545
Buchanan BB, Gruissem W, Jones RL (2015) Biochemistry and molecular biology of plants. Wiley, New Jersey
Bukvicki D, Giweli A, Stojkovic D, Vujisic L, Tesevic V, Nikolic M, Sokovic M, Marin PD (2018) Cheese supplemented with Thymus algeriensis oil, a potential natural food preservative. J Dairy Sci 101(5):3859–3865
Burgain J, Gaiani C, Linder M, Scher J (2011) Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J Food Eng 104(4):467–483
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94(3):223–253
Cacho JI, Campillo N, Viñas P, Hernández-Córdoba M (2016) Determination of synthetic phenolic antioxidants in edible oils using microvial insert large volume injection gas-chromatography. Food Chem 200:249–254
Calo JR, Crandall PG, O’Bryan CA, Ricke SC (2015) Essential oils as antimicrobials in food systems–A review. Food Control 54:111–119
Campolo O, Giunti G, Laigle M, Michel T, Palmeri V (2020) Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind Crops Prod 157:112935
Carocho M, Morales P, Ferreira IC (2015) Natural food additives: quo vadis? Trends Food Sci Technol 45(2):284–295
Carocho M, Morales P, Ferreira IC (2018) Antioxidants: reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci Technol 71:107–120
Carvalho IT, Estevinho BN, Santos L (2016) Application of microencapsulated essential oils in cosmetic and personal healthcare products–a review. Int J Cosmet Sci 38(2):109–119
Casiglia S, Riccobono L, Bruno M, Senatore F, Senatore F (2016) Chemical composition of the essential oil from Pulicaria vulgaris var graeca (Sch-Bip) Fiori (Asteraceae) growing wild in Sicily and its antimicrobial activity. Nat Prod Res 30(3):259–267
Celiktas OY, Bedir E, Sukan FV (2007) In vitro antioxidant activities of Rosmarinus officinalis extracts treated with supercritical carbon dioxide. Food Chem 101(4):1457–1464
Chaib F, Allali H, Bennaceur M, Flamini G (2017) Chemical composition and antimicrobial activity of essential oils from the aerial parts of Asteriscus graveolens (FORSSK) LESS and Pulicaria incisa (LAM) DC Two asteraceae herbs growing wild in the hoggar. Chem Biodivers 14(8):e1700092
Charfi S, Boujida N, Abrini J, Senhaji N (2019) Study of chemical composition and antibacterial activity of Moroccan Thymbra capitata essential oil and its possible use in orange juice conservation. Mater Today Proc 13:706–712
Chaudhari AK, Singh VK, Das S et al (2020) Improvement of in vitro and in situ antifungal AFB1 inhibitory and antioxidant activity of Origanum majorana L essential oil through nanoemulsion and recommending as novel food preservative. Food Chem Toxicol 143:111536
Chemsa AE, Zellagui A, Öztürk M, Erol E, Ceylan O, Duru ME, Lahouel M (2018) Chemical composition, antioxidant, anticholinesterase, antimicrobial and antibiofilm activities of essential oil and methanolic extract of Anthemis stiparum subsp. sabulicola (Pomel) Oberpr. Microb Pathogen 119:233–240
Cristani M, D’Arrigo M, Mandalari G, Castelli F, Sarpietro MG, Micieli D, Venuti V, Bisignano G, Saija A, Trombetta D (2007) Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J Agric Food Chem 55(15):6300–6308
Cruz EM, Mendonça MC, Blank AF, Sampaio TS, Pinto JA, Gagliardi PR, Junior LFO, de Lima RS, Nunes RS, Warwick DR (2018) Lippia gracilis Schauer essential oil nanoformulation prototype for the control of Thielaviopis paradoxa. Ind Crops Prod 117:245–251
Cui H, Bai M, Lin L (2018) Plasma-treated poly (ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohydr Polym 179:360–369
da Silva BD, Bernardes PC, Pinheiro PF, Fantuzzi E, Roberto CD (2021) Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. https://doi.org/10.1016/j.meatsci.2021.108463
Das S, Singh VK, Dwivedy AK, Chaudhari AK, Upadhyay N, Singh P, Sharma S, Dubey NK (2019) Encapsulation in chitosan-based nanomatrix as an efficient green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int J Biol Macromo 133:294–305. https://doi.org/10.1016/j.ijbiomac.2019.04.070
Das SK, Nakka S, Rajabalaya R, Mukhopadhyay H, Halder T, Palanisamy M, Khanam J, Nanda A (2011) Microencapsulation techniques and its practice. Int J Pharm Sci Tech 6(2):1
Devrnja N, Anđelković B, Aranđelović S, Radulović S, Soković M, Krstić-Milošević D, Ristić M, Ćalić D (2017) Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S Afr J Bot 111:212–221
Diao H, Zheng P, Yu B, He J, Mao X, Yu J, Chen D (2015) Effects of benzoic acid and thymol on growth performance and gut characteristics of weaned piglets. Asian-Australas J Anim Sci 28(6):827
Domínguez R, Barba FJ, Gómez B, Putnik P, Kovačević DB, Pateiro M, Santos EM, Lorenzo JM (2018) Active packaging films with natural antioxidants to be used in meat industry: a review. Food Res Int 113:93–101
Donsì F, Annunziata M, Sessa M, Ferrari G (2011) Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT 44(9):1908–1914
Donsi F, Ferrari G (2016) Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol 233:106–120
Dorman H, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88(2):308–316
Dreger M, Wielgus K (2013) Application of essential oils as natural cosmetic preservatives. Herba polonica 59:142–156
El-Sayed HS, Chizzola R, Ramadan AA, Edris AE (2017) Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water based delivery systems. Food Chem 221:196–204
El Asbahani A, Miladi K, Badri W, Sala M, Addi EA, Casabianca H, El Mousadik A, Hartmann D, Jilale A, Renaud F (2015) Essential oils: from extraction to encapsulation. Int J Pharm 483(1–2):220–243
Ertas A, Boga M, Yilmaz MA, Yesil Y, Tel G, Temel H, Hasimi N, Gazioglu I, Ozturk M, Ugurlu P (2015) A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymusnummularius (Anzer tea): Rosmarinic acid. Ind Crops Prod 67:336–345
Esfanjani AF, Jafari SM (2016) Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf, B 146:532–543
Espinosa S, Diaz M, Brignole E (2005) Process optimization for supercritical concentration of orange peel oil. Lat Am Appl Res 35(4):321–326
Eyres G, Marriott PJ, Dufour J-P (2007) The combination of gas chromatography–olfactometry and multidimensional gas chromatography for the characterisation of essential oils. J Chromatogr A 1150:70–77
Ezhilarasi P, Karthik P, Chhanwal N, Anandharamakrishnan C (2013) Nanoencapsulation techniques for food bioactive components: a review. Food Bioproc Tech 6(3):628–647
Falleh H, Jemaa MB, Saada M et al (2020) Essential oils: a promising eco-friendly food preservative. Food Chem 330:127268
Fathi E, Sefidkon F (2012) Influence of drying and extraction methods on yield and chemical composition of the essential oil of Eucalyptus sargentii. J Agric Sci Technol 14(5):1035–1042
Fawzy GA, Al Ati HY, El Gamal AA (2013) Chemical composition and biological evaluation of essential oils of Pulicaria jaubertii. Pharmacogn Mag 9(33):28
Fernández-López J, Viuda-Martos M (2018) Introduction to the special issue: application of essential oils in food systems. Foods 7(4):56. https://doi.org/10.3390/foods7040056
Feyzioglu GC, Tornuk F (2016) Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 70:104–110
Foddai M, Marchetti M, Ruggero A, Juliano C, Usai M (2018) Evaluation of chemical composition and anti-inflammatory, antioxidant, antibacterial activity of essential oil of Sardinian Santolina corsica Jord & Fourr. Saudi J Biol Sci 26(5):930–937
Food, Administration D (2016) Code of Federal Regulations (CFR) Title 21-food and drugs chapter I -food and drug administration department of health and human services subchapter B - food for human consumption part 135 frozen desserts. CFR - Code of Federal Regulations Title 21 (fda.gov). Accessed 1 Jan 2020
Fornari T, Vicente G, Vázquez E, García-Risco MR, Reglero G (2012) Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J Chromatogr A 1250:34–48
Franco D, Antequera T, Pinho SCd, Jiménez E, Pérez-Palacios T, Fávaro-Trindade CS, Lorenzo JM (2017) The use of microencapsulation by spray-drying and its aplication in meat products Strategies for obtaining healthier foods. Nova Science Publishers, New York, pp 334–362
Freires I, Denny C, Benso B, de Alencar S, Rosalen P (2015) Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: a systematic review. Molecules 20(4):7329–7358
Friedly E, Crandall P, Ricke S, Roman M, O’bryan C, Chalova V (2009) In vitro antilisterial effects of citrus oil fractions in combination with organic acids. J Food Sci 74(2):M67–M72
Garcia-Sotelo D, Silva-Espinoza B, Perez-Tello M, Olivas I, Alvarez-Parrilla E, González-Aguilar GA, Ayala-Zavala JF (2019) Antimicrobial activity and thermal stability of rosemary essential oil: β− cyclodextrin capsules applied in tomato juice. LWT 111:837–845
Gaspar AS, Wagner FE, Amaral VS, Lima SAC, Khomchenko VA, Santos JG, Costa BF, Durães L (2017) Development of a biocompatible magnetic nanofluid by incorporating SPIONs in Amazonian oils. Spectrochim Acta A Mol Biomol Spectrosc 172:135–146
Geyer A, Kittel G, Vollebregt L et al (1999) Assessment of the usefulness of material safety data sheets (MSDS) for SMEs SAFE project file no SOC 97201817
Ghaderi-Ghahfarokhi M, Barzegar M, Sahari MA, Azizi MH (2016) Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food Bioprocess Technol 9(7):1187–1201
Gharenaghadeh S, Karimi N, Forghani S, Nourazarian M, Gharehnaghadeh S, Jabbari V, Khiabani MS, Kafil HS (2017) Application of salvia multicaulis essential oil-containing nanoemulsion against food-borne pathogens. Food Biosci 19:128–133
Gherib M, Chahrazed B, El-Haci IA, Chaouche TM, Bekkara FA (2016) Antioxidant and antibacterial activities of aerial part essential oil and some organic extracts from the Algerian medicinal plant Pulicaria mauritanica coss. Int J Pharm Sci Res 7(1):76
Ghosh V, Mukherjee A, Chandrasekaran N (2013) Formulation and characterization of plant essential oil based nanoemulsion: evaluation of its larvicidal activity against Aedes aegypti. Asian J Chem. 25 (Supplementary Issue):S321
Ghosh V, Mukherjee A, Chandrasekaran N (2014) Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf, B 114:392–397
Gironi F, Maschietti M (2008) Continuous countercurrent deterpenation of lemon essential oil by means of supercritical carbon dioxide: experimental data and process modelling. Chem Eng Sci 63(3):651–661
Giunti G, Palermo D, Laudani F, Algeri GM, Campolo O, Palmeri V (2019) Repellence and acute toxicity of a nano-emulsion of sweet orange essential oil toward two major stored grain insect pests. Ind Crops Prod. 142:111869
Gómez B, Barba FJ, Domínguez R, Putnik P, Kovačević DB, Pateiro M, Toldrá F, Lorenzo JM (2018) Microencapsulation of antioxidant compounds through innovative technologies and its specific application in meat processing. Trends Food Sci Technol 82:135–147
Gonçalves ND, de Lima PF, Sartoratto A, Derlamelina C, Duarte MCT, Antunes AEC, Prata AS (2017) Encapsulated thyme (Thymus vulgaris) essential oil used as a natural preservative in bakery product. Food Res Int 96:154–160
González SB, Gastaldi B, Catalán C, Di Leo Lira P, Retta D, van Baren CM, Bandoni AL (2019) Artemisia magellanica chemical composition of the essential oil from an unexplored endemic species of Patagonia. Chem Biodivers 16(7):e1900125
Gorji-Chakespari A, Nikbakht AM, Sefidkon F et al (2017) Classification of essential oil composition in Rosa damascena Mill. genotypes using an electronic nose. J Appl Res Med Arom Plants 4:27–34
Guinoiseau E, Luciani A, Rossi P, Quilichini Y, Ternengo S, Bradesi P, Berti L (2010) Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 29(7):873–879
Gutiérrez-del-Río I, Fernández J, Lombó F (2018) Plant nutraceuticals as antimicrobial agents in food preservation: terpenoids, polyphenols and thiols. Int J Antimicrob Agents 52(3):309–315
Gutierrez J, Barry-Ryan C, Bourke P (2008) The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int J Food Microbiol 124(1):91–97
Hasheminejad N, Khodaiyan F (2020) The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem 309:125520
Helander IM, Alakomi H-L, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LG, von Wright A (1998) Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem 46(9):3590–3595
Herculano ED, de Paula HCB, de Figueiredo EAT, Dias FGB, Pereira VdA (2015) Physicochemical and antimicrobial properties of nanoencapsulated Eucalyptus staigeriana essential oil. LWT 61(2):484–491
Hidalgo P, Ubera J, Tena M, Valcarcel M (1998) Determination of the carnosic acid content in wild and cultivated Rosmarinus officinalis. J Agric Food Chem 46(7):2624–2627
Hulánková R, Bořilová G (2012) In vitro combined effect of oregano essential oil and caprylic acid against Salmonella serovars, Escherichia coli O157: H7, Staphylococcus aureus and Listeria monocytogenes. Acta Vet Brno 80(4):343–348
Hurley T, Chhipi-Shrestha G, Gheisi A et al (2016) Characterizing hydraulic fracturing fluid greenness: application of a hazard-based index approach. Clean Technol Environ Policy 18:647–668
Hyldgaard M, Mygind T, Meyer RL (2012) Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol 3:12
Inouye S, Yamaguchi H, Takizawa T (2001) Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infect Chemother 7(4):251–254
Ioannou E, Koutsaviti A, Tzakou O, Roussis V (2014) The genus Pinus: a comparative study on the needle essential oil composition of 46 pine species. Phytochem Rev 13(4):741–768
Jesser E, Lorenzetti AS, Yeguerman C, Murray AP, Domini C, Werdin-González JO (2020) Ultrasound assisted formation of essential oil nanoemulsions: Emerging alternative for Culex pipiens pipiens Say (Diptera: Culicidae) and Plodia interpunctella Hübner (Lepidoptera: Pyralidae) management. Ultrason Sonochem. 61:104832
Jogee PS, Ingle AP, Rai M (2017) Isolation and identification of toxigenic fungi from infected peanuts and efficacy of silver nanoparticles against them. Food Control 71:143–151
Ju J, Chen X, Xie Y, Yu H, Guo Y, Cheng Y, Qian H, Yao W (2019) Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci Technol 92:22–32
Juliano C, Marchetti M, Campagna P, Usai M (2019) Antimicrobial activity and chemical composition of essential oil from Helichrysum microphyllum Cambess subsp tyrrhenicum Bacch, Brullo & Giusso collected in South-West Sardinia. Saudi J Biol Sci 26(5):897–905
Kačániová M, Terentjeva M, Vukovic N, Puchalski C, Roychoudhury S, Kunová S, Klūga A, Tokár M, Kluz M, Ivanišová E (2017) The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp isolated from fish. Saudi Pharm J 25(8):1108–1116
Kalemba D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem 10(10):813–829
Karami H, Rasekh M, Mirzaee-Ghaleh E (2020) Application of the E-nose machine system to detect adulterations in mixed edible oils using chemometrics methods. J Food Process Preserv 44:e14696
Karami H, Rasekh M, Mirzaee-Ghaleh E (2020) Comparison of chemometrics and AOCS official methods for predicting the shelf life of edible oil. Chemom Intel Lab Syst 206:104165
Karatzas A, Kets E, Smid E, Bennik M (2001) The combined action of carvacrol and high hydrostatic pressure on Listeria monocytogenes Scott A. J Appl Microbiol 90(3):463–469
Karthik P, Ezhilarasi P, Anandharamakrishnan C (2017) Challenges associated in stability of food grade nanoemulsions. Crit Rev Food Sci Nutr 57(7):1435–1450
Katouzian I, Esfanjani AF, Jafari SM, Akhavan S (2017) Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci Technol 68:14–25
Kavanaugh NL, Ribbeck K (2012) Selected antimicrobial essential oils eradicate Pseudomonas spp and Staphylococcus aureus biofilms. Appl Environ Microbiol 78(11):4057–4061
Kavaz D, Idris M, Onyebuchi C (2019) Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int J Biol Macromo 123:837–845
Kholif AE, Olafadehan OA (2021) Essential oils and phytogenic feed additives in ruminant diet: chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem Rev. https://doi.org/10.1007/s11101-021-09739-3
Khorshidian N, Yousefi M, Khanniri E, Mortazavian AM (2018) Potential application of essential oils as antimicrobial preservatives in cheese. Innov Food Sci Emerg Technol 45:62–72
Koh K, Pearce A, Marshman G, Finlay-Jones J, Hart P (2002) Tea tree oil reduces histamine-induced skin inflammation. Br J Dermatol 147(6):1212–1217
Kourkoutas Y, Proestos C (2020) Food preservation: challenges and efforts for the future. Foods. https://doi.org/10.3390/foods9040391
Krochta JM (2002) In: Gennadios A (ed) Proteins as raw materials for films and coatings: definitions, current status, and opportunities. Protein-based films and coatings, 1st edn. Taylor & Francis Group, Boca Raton
Kuhn D, Ziem R, Scheibel T, Buhl B, Vettorello G, Pacheco LA, Heidrich D, Kauffmann C, de Freitas EM, Ethur EM (2019) Antibiofilm activity of the essential oil of Campomanesia aurea O. Berg against microorganisms causing food borne diseases. LWT 108:247–252
Kujur A, Kiran S, Dubey N, Prakash B (2017) Microencapsulation of Gaultheria procumbens essential oil using chitosan-cinnamic acid microgel: improvement of antimicrobial activity, stability and mode of action. LWT 86:132–138
Kwon K-D, Jo W-K, Lim H-J et al (2008) Volatile pollutants emitted from selected liquid household products. Environ Sci Pollut Res 15:521–526
Laohasongkram K, Mahamaktudsanee T, Chaiwanichsiri S (2011) Microencapsulation of Macadamia oil by spray drying. Procedia Food Sci 1:1660–1665
Laranjo M, Fernández-Léon AM, Potes ME, Agulheiro-Santos AC, Elias M (2017) Use of essential oils in food preservation, pp 177–188 In Antimicrobial research: novel bioknowledge and educational programs (Microbiology Book Series #6). Edited by A. Méndez-Vilas, Formatex Research Center, Badajoz, Espanha. ISBN-13 978–84–947512–0–2
Laranjo M, Fernández-León AM, Agulheiro-Santos AC, Potes ME, Elias M (2019) Essential oils of aromatic and medicinal plants play a role in food safety J Food Process Preserv. https://doi.org/10.1111/jfpp.14278
Lee JY, Garcia CV, Shin GH, Kim JT (2019) Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT 106:164–171
Li Z-h, Cai M, Liu Y-s, Sun P-l (2018) Development of finger citron (Citrus medica L. var. sarcodactylis) essential oil loaded nanoemulsion and its antimicrobial activity. Food Control 94:317–323
Lin DY, Tsai CH, Huang Y et al (2020) Novel strategy for food safety risk management and communication: risk identification for benzoic acid residues in pickled vegetables. Food Sci Nutr 8:5419–5425
Lin L, Wang X, Cui H (2019) Synergistic efficacy of pulsed magnetic fields and Litseacubeba essential oil treatment against Escherichia coli O157: H7 in vegetable juices. Food Control 106:106686
Ling M-P, Lien K-W, Wu C-H et al (2015) Dietary exposure estimates for the food preservatives benzoic acid and sorbic acid in the total diet in Taiwan. J Agric Food Chem 63:2074–2082
Liu Q, Zhang M, Bhandari B, Xu J, Yang C (2020) Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 107:106771
Liu Y, Yang X, Xin H, Chen S, Yang C, Duan Y, Yang X (2017) Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim Sci J 88(9):1414–1424
Lopez-Romero JC, González-Ríos H, Borges A, Simões M (2015) Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid Based Complement Alternat Med 2015:795435
Ma Q, Davidson PM, Zhong Q (2016) Antimicrobial properties of microemulsions formulated with essential oils, soybean oil, and Tween 80. Int J Food Microbiol 226:20–25
Ma Q, Zhong Q (2015) Incorporation of soybean oil improves the dilutability of essential oil microemulsions. Food Res Int 71:118–125
Mahdi AA, Mohammed JK, Al-Ansi W, Ghaleb AD, Al-Maqtari QA, Ma M, Ahmed MI, Wang H (2020) Microencapsulation of fingered citron extract with gum arabic, modified starch, whey protein, and maltodextrin using spray drying. Int J Biol Macromo 152:1125–1134
Malik S, de Mesquita LSS, Silva CR, de Mesquita JWC, de Sá RE, Bose J, Abiri R, de Maria Silva FigueiredoCosta-Júnior PLM (2019) Chemical profile and biological activities of essential oil from Artemisia vulgaris L Cultivated in Brazil. Pharm 12(2):49
Manessis G, Kalogianni AI, Lazou T et al (2020) Plant-derived natural antioxidants in meat and meat products. Antioxidants 9:1215
Maqsood S, BenjakulShahidi SF (2013) Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit Rev Food Sci Nutr 53(2):162–179
Matshetshe KI, Parani S, Manki SM, Oluwafemi OS (2018) Preparation, characterization and in vitro release study of β-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int J Biol Macromo 118:676–682
Maurya A, Prasad J, Das S et al (2021) Essential oils and their application in food safety. Front Sustain Food Syst 5:133
McClements DJ (2012) Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 8(6):1719–1729
McClements DJ (2015) Food emulsions: principles, practices, and techniques. CRC Press, New York
Mendes J, Norcino L, Martins H, Manrich A, Otoni C, Carvalho E, Piccoli R, Oliveira J, Pinheiro A, Mattoso LJFH (2020) Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocoll 100:105428
Modzelewska A, Sur S, Kumar SK, Khan SR (2005) Sesquiterpenes: natural products that decrease cancer growth. Curr Med Chem-Anti-Cancer Agents 5(5):477–499
Mosandl A (2004) Authenticity assessment: a permanent challenge in food flavor and essential oil analysis. J Chromatogr Sci 42:440–449
Nasrollahi S, Ghoreishi SM, Ebrahimabadi AH, Khoobi A (2019) Gas chromatography-mass spectrometry analysis and antimicrobial, antioxidant and anti-cancer activities of essential oils and extracts of Stachys schtschegleevii plant as biological macromolecules. Int J Biol Macromo 128:718–723
Nazari M, Ghanbarzadeh B, Kafil HS, Zeinali M, Hamishehkar H (2019) Garlic essential oil nanophytosomes as a natural food preservative: its application in yogurt as food model. Colloid Interface Sci Commun 30:100176
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V (2013) Effect of essential oils on pathogenic bacteria. Pharm 6(12):1451–1474
Negi PS (2012) Plant extracts for the control of bacterial growth: efficacy, stability and safety issues for food application. Int J Food Microbiol 156(1):7–17
Nikaido H (1994) Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264(5157):382–388
Noirrit M, Philippe F (2016) Reduction of Salmonella prevalence on sows and finisher pigs by use of a protected mix of organic acids and essential oils in the feed of lactating sows and weaned piglets. Journées De La Recherche Porcine En France 48:351–352
Noori S, Zeynali F, Almasi H (2018) Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 84:312–320
Omonijo FA, Ni L, Gong J, Wang Q, Lahaye L, Yang C (2018) Essential oils as alternatives to antibiotics in swine production. Anim Nutr 4(2):126–136
Osanloo M, Amani A, Sereshti H, Abai MR, Esmaeili F, Sedaghat MM (2017) Preparation and optimization nanoemulsion of Tarragon (Artemisia dracunculus) essential oil as effective herbal larvicide against Anopheles stephensi. Ind Crops Prod 109:214–219
Oswell NJ, Thippareddi H, Pegg RB (2018) Practical use of natural antioxidants in meat products in the US: a review. Meat Sci 145:469–479
Pandey BP, Thapa R, Upreti A (2017) Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of Artemisia vulgaris and Gaultheria fragrantissima collected from Nepal. Asian Pac J Trop Med 10(10):952–959
Pateiro M, Barba FJ, Domínguez R, Sant’Ana AS, Khaneghah AM, Gavahian M, Gómez B, Lorenzo JMJFri (2018) Essential oils as natural additives to prevent oxidation reactions in meat and meat products: a review. Food Res Int 113:156–166
Pavela R (2015) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crops Prod 76:174–187
Pellati F, Orlandini G, van Leeuwen KA et al (2013) Gas chromatography combined with mass spectrometry, flame ionization detection and elemental analyzer/isotope ratio mass spectrometry for characterizing and detecting the authenticity of commercial essential oils of Rosa damascena Mill. Rapid Commun Mass Spectrom 27:591–602
Pongsumpun P, Iwamoto S, Siripatrawan U (2020) Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason Sonochem 60:104604
Prakash A, Baskaran R, Paramasivam N, Vadivel V (2018a) Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: a review. Food Res Int 111:509–523
Prakash B, Kiran S (2016) Essential oils: a traditionally realized natural resource for food preservation. Curr Sci 110(10):1890–1892
Prakash B, Kujur A, Yadav A, Kumar A, Singh PP, Dubey N (2018b) Nanoencapsulation: an efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 89:1–11
Prakash B, Mishra PK, Kedia A, Dubey N (2014) Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L fruits. LWT 56(2):240–247
Prakash B, Singh P, Yadav S, Singh S, Dubey N (2013) Safety profile assessment and efficacy of chemically characterized Cinnamomum glaucescens essential oil against storage fungi, insect, aflatoxin secretion and as antioxidant. Food Chem Toxicol 53:160–167
Rahman A, Shanta ZS, Rashid M, Parvin T, Afrin S, Khatun MK, Sattar M (2016) In vitro antibacterial properties of essential oil and organic extracts of Premna integrifolia Linn. Arabian J Chem 9:S475–S479
Rahman MM, Sultana T, Ali MY, Rahman MM, Al-Reza SM, Rahman A (2017) Chemical composition and antibacterial activity of the essential oil and various extracts from Cassia sophera L. against Bacillus sp. from soil. Arabian J Chem 10:S2132–S2137
Raimundo KF, de Campos BW, Glamočlija J, Soković M, Gonçalves JE, Linde GA, Colauto NB, Gazim ZC (2018) Antifungal activity of Gallesia integrifolia fruit essential oil. Braz J Microbiol 49:229–235
Rao J, Chen B, McClements DJ (2019) Improving the efficacy of essential oils as antimicrobials in foods: mechanisms of action. Annu Rev Food Sci Technol 10:365–387
Rasekh M, Karami H, Wilson AD et al (2021) Classification and identification of essential oils from herbs and fruits based on a MOS electronic-nose technology. Chemosensors 9:142
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crops Prod 62:250–264
Rebey IB, Jabri-Karoui I, Hamrouni-Sellami I, Bourgou S, Limam F, Marzouk B (2012) Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L) seeds. Ind Crops Prod 36(1):238–245
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424
Rehman A, Ahmad T, Aadil RM, Spotti MJ, Bakry AM, Khan IM, Zhao L, Riaz T, Tong Q (2019a) Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci Technol 90:35–46
Rehman A, Tong Q, Jafari SM, Assadpour E, Shehzad Q, Aadil RM, Iqbal MW, Rashed MM, Mushtaq BS, Ashraf W (2019) Carotenoid-loaded nanocarriers: a comprehensive review. Adv Colloid Interface Sci 275:102048
Reis PML, Mezzomo N, Aguiar GPS, Senna EMTL, Hense H, Ferreira SR (2019) Ultrasound-assisted emulsion of laurel leaves essential oil (Laurus nobilis L.) encapsulated by SFEE. J Supercrit Fluids 147:284–292
Riahi L, Chograni H, Elferchichi M, Zaouali Y, Zoghlami N, Mliki A (2013) Variations in Tunisian wormwood essential oil profiles and phenolic contents between leaves and flowers and their effects on antioxidant activities. Ind Crops Prod 46:290–296
Riaz T, Iqbal MW, Saeed M, Yasmin I, Hassanin HA, Mahmood S, Rehman A (2019) In vitro survival of Bifidobacterium bifidum microencapsulated in zein-coated alginate hydrogel microbeads. J Microencapsul 36(2):192–203
Ribeiro-Santos R, Andrade M, de Melo NR, Sanches-Silva A (2017a) Use of essential oils in active food packaging: recent advances and future trends. Trends Food Sci Technol 61:132–140
Ribeiro-Santos R, Andrade M, Sanches-Silva A (2017b) Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr Opin Food Sci 14:78–84
Ribes S, Fuentes A, Barat JM (2019) Effect of oregano (Origanum vulgare L. ssp. hirtum) and clove (Eugenia spp.) nanoemulsions on Zygosaccharomyces bailii survival in salad dressings. Food Chem 295:630–636
Robertson GL (2005) Food packaging: principles and practice. CRC Press, New York
Rodriguez-Garcia I, Silva-Espinoza B, Ortega-Ramirez L, Leyva J, Siddiqui M, Cruz-Valenzuela M, Gonzalez-Aguilar G, Ayala-Zavala JF (2016) Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit Rev Food Sci Nutr 56(10):1717–1727
Rojek K, Serefko A, Poleszak E, Szopa A, Wróbel A, Guz M, Xiao J, Skalicka-Woźniak K (2021) Neurobehavioral properties of Cymbopogon essential oils and its components Phytochem Rev. https://doi.org/10.1007/s11101-020-09734-0
Rostami H, Nikoo AM, Rajabzadeh G, Niknia N, Salehi S (2018) Development of cumin essential oil nanoemulsions and its emulsion filled hydrogels. Food Biosci 26:126–132
Rusinek R, Gawrysiak-Witulska M, Siger A et al (2021) Effect of supplementation of flour with fruit fiber on the volatile compound profile in bread. Sensors 21:2812
Ryu V, Corradini MG, McClements DJ, McLandsborough L (2019) Impact of ripening inhibitors on molecular transport of antimicrobial components from essential oil nanoemulsions. J Colloid Interface Sci 556:568–576
Salem MZ, Ali HM, El-Shanhorey NA, Abdel-Megeed A (2013) Evaluation of extracts and essential oil from Callistemon viminalis leaves: antibacterial and antioxidant activities, total phenolic and flavonoid contents. Asian Pac J Trop Med 6(10):785–791
Salgueiro L, Martins A, Correia H (2010) Raw materials: the importance of quality and safety: a review. Flavour Fragr J 25:253–271
Salvia-Trujillo L, Rojas-Graü A, Soliva-Fortuny R, Martín-Belloso O (2015a) Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll 43:547–556
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2015b) Use of antimicrobial nanoemulsions as edible coatings: impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol Technol 105:8–16
Sauceda ENR (2011) Uso de agentes antimicrobianos naturales en la conservación de frutas y hortalizas. Ra Ximhai: Revista Científica De Sociedad, Cultura y Desarrollo Sostenible 7(1):153–170
Senatore F (1996) Influence of harvesting time on yield and composition of the essential oil of a thyme (Thymus pulegioides L) growing wild in Campania (Southern Italy). J Agric Food Chem 44(5):1327–1332
Septiana S, Yuliana ND, Bachtiar BM, Putri SP, Fukusaki E, Laviña WA, Wijaya CH (2020) Metabolomics approach for determining potential metabolites correlated with sensory attributes of Melaleuca cajuputi essential oil, a promising flavor ingredient. J Biosci Bioeng 129(5):581–587
Sessa M, Ferrari G, Donsì F (2015) Novel edible coating containing essential oil nanoemulsions to prolong the shelf life of vegetable products. Chem Eng Trans 43:55–60
Shahat EA, Bakr RO, Eldahshan OA, Ayoub NA (2017) Chemical composition and biological activities of the essential oil from leaves and flowers of pulicaria incisa sub candolleana (Family Asteraceae). Chem Biodivers. 14(4):e1600156
Shange N, Makasi T, Gouws P, Hoffman LC (2019) Preservation of previously frozen black wildebeest meat (Connochaetes gnou) using oregano (Oreganum vulgare) essential oil. Meat Sci 148:88–95
Sharifi-Rad J, Miri A, Hoseini-Alfatemi SM, Sharifi-Rad M, Setzer WN, Hadjiakhoondi A (2014) Chemical composition and biological activity of Pulicaria vulgaris essential oil from Iran. Nat Prod Commun. 9(11):1934578X1400901126
Sharma S, Barkauskaite S, Jaiswal AK et al (2020) Essential oils as additives in active food packaging. Food Chem 343:128403
Sharmeen JB, Mahomoodally FM, Zengin G, Maggi F (2021) Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 26(3):666
Shetta A, Kegere J, Mamdouh W (2019) Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int J Biol Macromo 126:731–742
Siddiqui SA, Islam R, Islam R, Jamal A, Parvin T, Rahman A (2017) Chemical composition and antifungal properties of the essential oil and various extracts of Mikania scandens (L.) Willd. Arabian J Chem 10:S2170–S2174
Silva HD, Cerqueira MÂ, Vicente AA (2012) Nanoemulsions for food applications: development and characterization. Food Bioproc Tech 5(3):854–867
Silva PTd, Fries LLM, Menezes CRd, Holkem AT, Schwan CL, Wigmann ÉF, Bastos JdO, Silva CdBd (2014) Microencapsulation: concepts, mechanisms, methods and some applications in food technology. Ciência Rural 44(7):1304–1311
Singh A, Dwivedy AK, Singh VK, Upadhyay N, Chaudhari AK, Das S, Dubey NK (2018) Essential oils based formulations as safe preservatives for stored plant masticatories against fungal and mycotoxin contamination: a review. Biocatal Agric Biotechnol 17:313–317
Smaoui S, Hsouna AB, Lahmar A, Ennouri K, Mtibaa-Chakchouk A, Sellem I, Najah S, Bouaziz M, Mellouli L (2016) Bio-preservative effect of the essential oil of the endemic Mentha piperita used alone and in combination with BacTN635 in stored minced beef meat. Meat Sci 117:196–204
Smid EJ, Gorris LG (1999) In: Rhaman MS (ed) Natural antimicrobials for food preservation, Handbook of Food Preservation, 1st edn. Marcel Dekker, New York
Smith-Palmer A, Stewart J, Fyfe L (1998) Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Appl Microbiol 26(2):118–122
Snene A, El Mokni R, Jmii H, Jlassi I, Jaïdane H, Falconieri D, Piras A, Dhaouadi H, Porcedda S, Hammami S (2017) In vitro antimicrobial, antioxidant and antiviral activities of the essential oil and various extracts of wild (Daucus virgatus (Poir.) Maire) from Tunisia. Ind Crops Prod 109:109–115
Šojić B, Pavlić B, Tomović V, Ikonić P, Zeković Z, Kocić-Tanackov S, Đurović S, Škaljac S, Jokanović M, Ivić M (2019) Essential oil versus supercritical fluid extracts of winter savory (Satureja montana L.)–assessment of the oxidative, microbiological and sensory quality of fresh pork sausages. Food Chem 287:280–286
Solomakos N, Govaris A, Koidis P, Botsoglou N (2008) The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157: H7 in minced beef during refrigerated storage. Meat Sci 80(2):159–166
Sotelo-Boyás M, Correa-Pacheco Z, Bautista-Baños S, y Gómez GY (2017) Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int J Biol Macromo 103:409–414
Souza ELd, Barros JCd, Conceição MLd, Gomes Neto NJ, Costa ACVd (2009) Combined application of Origanum vulgare L essential oil and acetic acid for controlling the growth of Staphylococcus aureus in foods. Braz J Microbiol 40(2):387–393
Steinemann AC, MacGregor IC, Gordon SM et al (2011) Fragranced consumer products: chemicals emitted, ingredients unlisted. Environ Impact Assess Rev 31:328–333
Suave J, Dall’Agnol E, Pezzin A, Silva D, Meier M, Soldi V (2006) Microencapsulação: inovação em diferentes áreas. Revista Saúde e Ambiente/Health Enviro J 7(2):12–20
Tariku Y, Hymete A, Hailu A, Rohloff J (2010) Essential-oil composition, antileishmanial, and toxicity study of Artemisia abyssinica and Satureja punctata ssp punctata from Ethiopia. Chem Biodivers 7(4):1009–1018
Tariq S, Wani S, Rasool W, Bhat MA, Prabhakar A, Shalla AH, Rather MA (2019) A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb Pathogen 134:103580
Tavares GM, Croguennec T, Carvalho AF, Bouhallab S (2014) Milk proteins as encapsulation devices and delivery vehicles: applications and trends. Trends Food Sci Technol 37(1):5–20
Teixeira B, Marques A, Ramos C, Batista I, Serrano C, Matos O, Neng NR, Nogueira JM, Saraiva JA, Nunes ML (2012) European pennyroyal (Mentha pulegium) from Portugal: chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind Crops Prod 36(1):81–87
Theis N, Lerdau M (2003) The evolution of function in plant secondary metabolites. Int J Plant Sci 164(S3):S93–S102
Tisserand R, Young R (2013) Essential oil safety-e-book: a guide for health care professionals. Elsevier, London
Tongnuanchan P, Benjakul S (2014) Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci 79(7):R1231–R1249
Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, Saija A, Mazzanti G, Bisignano G (2005) Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother 49(6):2474–2478
Turek C, Stintzing FC (2013) Stability of essential oils: a review. Compr Rev Food Sci Food Saf 12(1):40–53
USEPA M 1 (2005) 'Guidelines for carcinogen risk assessment' Risk Assessment Forum US Environmental Protection Agency, Washington, DC EPA/630/P-03 F
Vafania B, Fathi M, Soleimanian-Zad S (2019) Nanoencapsulation of thyme essential oil in chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to reduce nitrite in sausages. Food Bioprod Process 116:240–248
Van Long NN, Joly C, Dantigny P (2016) Active packaging with antifungal activities. Int J Food Microbiol 220:73–90
Vinceković M, Viskić M, Jurić S, Giacometti J, Kovačević DB, Putnik P, Donsì F, Barba FJ, Jambrak AR (2017) Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci Technol 69:1–12
Walia K, Argueello H, Lynch H, Leonard FC, Grant J, Yearsley D, Kelly S, Duffy G, Gardiner GE, Lawlor PG (2017) Effect of strategic administration of an encapsulated blend of formic acid, citric acid, and essential oils on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev Vet Med 137:28–35
Wan J, Zhong S, Schwarz P, Chen B, Rao J (2019) Enhancement of antifungal and mycotoxin inhibitory activities of food-grade thyme oil nanoemulsions with natural emulsifiers. Food Control 106:106709
Wang D, Meng Y, Wang C, Wang X, Blasi F (2020) Antioxidant activity and sensory improvement of Angelica dahurica cv Yubaizhi essential oil on sunflower oil during high-temperature storage. Processes 8(4):403
Wilson N, Shah N (2007) Microencapsulation of Vitamins. ASEAN Food J 14(1):1
Xiang F, Bai J, Tan X, Chen T, Yang W, He F (2018) Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl et Van var argyi cv. Qiai Ind Crops Prod 125:582–587
Yadav A, Kujur A, Kumar A, Singh PP, Gupta V, Prakash B (2019a) Encapsulation of bunium persicum essential oil using chitosan nanopolymer: preparation, characterization, antifungal assessment, and thermal stability. Int J Biol Macromo 142:172–180
Yadav A, Kujur A, Kumar A, Singh PP, Prakash B, Dubey NK (2019b) Assessing the preservative efficacy of nanoencapsulated mace essential oil against food borne molds, aflatoxin B1 contamination, and free radical generation. LWT 108:429–436
Yousefi M, Rahimi-Nasrabadi M, Pourmortazavi SM, Wysokowski M, Jesionowski T, Ehrlich H, Mirsadeghi S (2019) Supercritical fluid extraction of essential oils. TrAC Trends Anal Chem 118:182–193
Yuan G, Chen X, Li D (2016) Chitosan films and coatings containing essential oils: the antioxidant and antimicrobial activity, and application in food systems. Food Res Int 89:117–128
Zanetti M, Carniel TK, DalcaCnton F, dos Anjos RS, Riella HG, de Araujo PH, de Oliveira D, Fiori MA (2018) Use of encapsulated natural compounds as antimicrobial additives in food packaging: a brief review. Trends Food Sci Technol 81:51–60
Zhai H, Liu H, Wang S, Wu J, Kluenter A-M (2018) Potential of essential oils for poultry and pigs. Anim Nutr 4(2):179–186
Zhang C, Hong K (2020) Production of terpenoids by synthetic biology approaches. Front Bioeng Biotechnol 8:347
Zhang H, Li X, Kang H (2019) Chitosan coatings incorporated with free or nano-encapsulated Paulownia Tomentosa essential oil to improve shelf-life of ready-to-cook pork chops LWT 116:108580
Zhou F, Ji B, Zhang H, Jiang H, Yang Z, Li J, Li J, Ren Y, Yan W (2007) Synergistic effect of thymol and carvacrol combined with chelators and organic acids against Salmonella Typhimurium. J Food Prot 70(7):1704–1709
Acknowledgements
This review was supported by the China Scholarship Council (CSC) (2018GXZ018756), Key R&D Program of Jiangsu Province (BE2019362), Science and Technology Plan of Suzhou City (SS2019016), China Postdoctoral Science Foundation Funded Project (2018M642165), Science and Technology Project of Jiangsu Bureau of Quality and Technical Supervision (KJ175923 and KJ185646), National Key R&D Program of China (2018YFC1602300), National First-class Discipline Program of Food Science and Technology (JUFSTR20180509), The Natural Science Foundation of Jiangsu Province (BK20171139), and Forestry Science and Technology Innovation and Extension Project of Jiangsu Province [No. LYKJ (2017) 26].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Maqtari, Q.A., Rehman, A., Mahdi, A.A. et al. Application of essential oils as preservatives in food systems: challenges and future prospectives – a review. Phytochem Rev 21, 1209–1246 (2022). https://doi.org/10.1007/s11101-021-09776-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-021-09776-y