Abstract
Essential oils (EOs) are natural products obtained from aromatic plants. Steam distillation and hydrodistillation are the most commonly used methods for the extraction of EOs at laboratory scale. They have been widely studied due to their potential in the food industry. EO can be used in food in order to prolong the shelf-life, and additionally, they can reduce or replace synthetics additives. Their effectiveness can be confirmed in antimicrobial and antioxidant tests performed, in general, by diffusion test in agar and DPPH• assay, respectively. Volatile compounds are present in EOs, a role in their biological activities. In this line of thought, chromatography techniques can be applied to identify the main volatile compounds present in EOs. In general, EOs extend food stability during storage, inhibiting the growth of spoilage or pathogenic microorganisms and protecting against oxidation. It is important to evaluate the responsible compounds for the biological activities of EOs and determine their utilization limits, including their safety. Highly variable composition with source species, plant parts, and/or extraction methods appears to play important roles in the variability of EO biological activities. This review provides a concise and critical insight in the use of EOs with emphasis in food applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, consumers’ concern over the quality and safety of food has significantly increased. The increasing demand for healthier, nutritious, and safer foodstuffs is reflected in the research on newer technologies for their preservation during production, transport, and storage. In addition, efforts have been made in order to prevent foodborne diseases that affect thousands of people around the world and are a major public health concern (Centers for Disease Control and Prevention 2015; Organização Mundial de Saúde 2015).
In this sense, new alternatives from natural sources have been studied such as the use of essential oils (EOs). EOs are secondary metabolites from aromatic plants and have been the focus of extensive research, not only due to their natural nature but also because they have demonstrated benefits in food and in human health. EOs have been used for centuries as perfume fragrances, in culinary as flavoring, and in folk medicine. Currently, they are mainly studied for their different biological properties such as antioxidant, antimicrobial, antitumor, analgesic, insecticidal, antidiabetic, and antiinflammatory (Ocaña-Fuentes et al. 2010; Yen et al. 2015; Brahmi et al. 2016; Periasamy et al. 2016).
In general, the EOs’ major components are the main responsible for their biological properties, but it is known that the minor compounds can also contribute for these properties and, together, they can exhibit a synergetic activity (Burt 2004). In this line of thought, the identification and quantification of the EOs’ active compounds are very important. The most common analytical technique used for the determination of volatile active compounds of EOs is gas chromatography (GC) coupled with mass spectrometry (MS).
In food systems, EOs have demonstrated antimicrobial and antioxidant capacity, which allows increasing shelf-life of the product and ensure its quality (Oussalah et al. 2004, 2007; Zinoviadou et al. 2009). Additionaly, EOs have been well regarded as an alternative to reduce or replace the use of synthetic additives which are associated with various human health adverse effects (Proestos et al. 2006).
Factors such as lipid oxidation and microbial growth are responsible for the loss of quality and reduced expiration date of the food product. The oxidation causes the degradation of the basic structure of lipids, which are responsible for the texture and the taste of foods rich in fat, among others (Sanches-Silva et al. 2004). Food contaminated by fungi and bacteria has social and economic implications, not only because they reduce the quality of food but also because they cause foodborne diseases (Centers for Disease Control and Prevention 2015).
For this reason, a large variety of EOs from different aromatic plants has been applied to food or incorporated in food packaging (Oussalah et al. 2007; Zinoviadou et al. 2009; Salgado et al. 2013). In this study, an extensive review of scientific publications, in which EOs perform antioxidant and antimicrobial function, was conducted. Different EOs were reviewed and discussed in what regards to their biological functions, main active compounds, and techniques used for the study of their composition. The main objective of this review was to access the current status and the recent advances regarding the use of EOs in food systems.
Essential Oils
EOs are aromatic substances produced by plants generally belonging to angiospermic families that can be used by several industries for different purposes (Pavela 2015). Thus, EOs are natural products obtained from aromatic plant materials such as flowers, buds, seeds, leaves, stem, bark, and herbs (Dvaranauskaité et al. 2009; Aidi Wannes et al. 2010; Lv et al. 2012; Hill et al. 2013).
For EO preparation, there are different methods that can be used which can enhance or reduce the above-mentioned properties. EOs can be extracted by hydrodistillation, steam distillation, solvent extraction, microwave extraction, and supercritical fluid extraction. Steam distillation and hydrodistillation are the most commonly used methods for the extraction of EOs at laboratory scale (Khajeh et al. 2005; Edris 2007; Bendahou et al. 2008; Dvaranauskaité et al. 2009; Romano et al. 2009).
The uses of EOs as natural food additives have been the focus of countless investigation reports due to the possibility of these active mixtures to replace the synthetic food additives. As mentioned previously, synthetic additives have negative side effects to human health and the consumers are beginning to be aware of those facts and are starting to reject products with non-natural additives (Cacho et al. 2016). These oils have preservative properties related to the presence of compounds with antimicrobial and antioxidant capacity in their composition, which minimize or eliminate the presence of microorganisms and/or reduce the lipid oxidation phenomenon. Additionally, EOs are considered as generally recognized as safe (GRAS) by the Food And Drug Administration (FDA) (2016).
A large variety of EOs from different plants such as basil (Ocimum basilicum L.), oregano (Origanum vulgare L.), cinnamon (Cinnamomum zeylanicum Blume), and rosemary (Rosmarinus officinalis L.) has been applied to food in order to extend food shelf-life (Zinoviadou et al. 2009; Viteri Jumbo et al. 2014; Gaio et al. 2015a; Ribeiro-Santos et al. 2015).
Several foods showed positive protection when using EO as additives such as meat, fish, and vegetables (Table 1).
Active Compounds
The composition and quality of EOs are naturally affected by the plant characteristics, such as development stage, variety, geographical origin, part of the plant used, age, season, and condition of the plant when harvested. But they are also affected by the extraction method (namely the solvent used) and analysis conditions (Khajeh et al. 2005; Hussain et al. 2008; Negi 2012; Riahi et al. 2013). For instance, Elzaawely et al. (2007) revealed that the main components of the EO of Alpinia zerumbet obtained from flowers are different from the EO obtained from seeds.
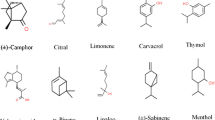
The technique used to identify and quantify the compounds of the numerous EOs is, in general, GC coupled with MS detector (Khajeh et al. 2005; Riahi et al. 2013). These oils contain a mixture of compounds which includes terpenes and aroma compounds (phenols, aldehydes, alcohols, methoxy derivatives, and methylene dioxy compounds), being the oxygenated compounds responsible for the odor. This mixture of compounds provides the biological properties of EOs, but they are extremely dependent on external factors affecting the preparation procedure such as light, heat, and oxygen (Nakatsu et al. 2000; Handa et al. 2008; Diaz et al. 2012; Hossain et al. 2012; Tisserand and Young 2014; Pavela 2015). Major components can constitute up to 85% of an EO and, usually, define the biological properties of EOs. However, the other 15% are composed by minor components that, although are present in small levels, have a significant role in the biological activities acting in synergism with the major constituents (Burt 2004; Pavela 2015). Table 2 compiles some EOs and their major compounds.
Terpenes are organic compounds present in large quantities in all kinds of plants, formed by isoprene (C5H8) units (Calogirou et al. 1999). Monoterpenes, such as linalool, p-cymene, and limonene, are C-10 abundant natural compounds that represent approximately 90% of EOs and are associated with their biological activity (Olagnier et al. 2007; Pavela 2015). Sesquiterpenes (C-15 terpenes), such as α-cadinene, β-caryophyllene, and β-elemene, are also present in EOs, although in lower quantities (Pavela 2015).
Biological Properties of Essential Oils
Since ancient times, EOs are used as fragrances and flavoring agents in the perfume and food industries (Nakatsu et al. 2000; Tung et al. 2008; Unlu et al. 2010; Viteri Jumbo et al. 2014; Ali et al. 2015). In the cosmetic industry, EOs are used for skin and hair care and perfumes due to their biological properties that can protect our bodies against exogenous or endogenous harmful agents (Aburjai and Natsheh 2003). As to their pharmaceutical and medicinal uses, they are present in traditional Indian (Ayurveda) and Chinese (Zhong Yo) medicines (Nakatsu et al. 2000). The biological properties of EOs have drawn attention for their anticarcinogenic potential and the possible suppression of tumors including glioma, colon and gastric cancer, liver tumor, pulmonary tumor, breast cancer, and leukemia. EOs are also known for their potential activity against cardiovascular diseases (like atherosclerosis and thrombosis), bacteria and virus, oxidation, and diabetes, and they are also used for aromatherapy and massage (Edris 2007). They are also well-known for their preservative and antioxidant properties, offering protection against various chronic diseases and they can be a natural alternative to conventional therapy (Table 3).
There is a growing interest in the study of natural products for the discovery of active compounds not only with antimicrobial activity but also with antioxidant capacity to extend the storage stability of food, by inhibiting the growth of microorganisms and protecting food from oxidation. Several authors have tested EOs as an alternative to the synthetic additives used in foods (Khajeh et al. 2005; Hossain et al. 2008, 2012; Djabou et al. 2013).
The variability of EOs (related with their chemical composition) influence, mainly, their distinctive physico-chemical properties. The biological effect of EOs is dependent on the concentration used (in general, high concentration increases the biological effect), the microorganisms tested (fungal are more sensitive than bacteria) (Singh et al. 2007), tests performed, and on the main constituents of the oil. However, minor components may be critical to the activity (Burt 2004).
The possibility of synergism between a combination of EOs and among their constituents increases their spectrum of action enhancing the EO effectiveness (Goñi et al. 2009; Azeredo et al. 2011; Souza et al. 2013). These synergism effects, between major and minor compounds present in the EOs, should be taken into consideration when assessing their biological activity (Bouaziz et al. 2009). An antagonistic effect may also be observed when the combined effect is lower than individual effect (Goñi et al. 2009). An additional effect can be generated when the combined effect of the EO compounds is the same as the sum of the individual effects. Synergism occurs when the combined effect of the substances is higher than the sum of their individual effects. These effects may depend on the concentration of individual components of the EOs and on the concentration of each EO in the mixture (Goñi et al. 2009). A synergism advantage is that the individual concentration required to achieve the same effect can be significantly lower than the combination of two or more EOs. It may result in the reduction of undesirable sensory impact due to the strong aroma of some oils (Stojkovic et al. 2013). Therefore, synergism and antagonistic relationships among the EOs components might affect positively and negatively, respectively, their biological activity (Adrar et al. 2016; Ud-Daula et al. 2016).
Stojković et al. (2013) studied and proved the antimicrobial synergism of a combination of thyme and oregano EOs. Azeredo et al. (2011) showed also the synergism of O. vulgare and R. officinalis EOs against bacteria: Listeria monocytogenes, Yersinia enterocolitica, and Aeromonas hydrophilla and additive effect of combination of these EOs against Pseudomonas fluorescens. Goñi et al. (2009), reported that combination of cinnamon and clove EOs showed an antagonism effect on the inhibition of Escherichia coli. However, at higher concentrations, these individual EOs and their combinations presented the same activity against the growth of E. coli. Thus, a synergistic, antagonistic or additive effect could be concentration-dependent (Goñi et al. 2009).
Adrar et al. (2016) showed a synergistic antioxidant interaction between α-tocopherol and Thymus numidicus EO (Adrar et al. 2016). Pei et al. (2009) reported synergistic effects of the combination of components of several EOs and their antimicrobial interactions against E. coli. Synergistic effects were found between cinnamaldehyde/eugenol, thymol/eugenol, carvacrol/eugenol, and thymol/carvacrol (Pei et al. 2009).
Thus, identification and quantification of the EO compounds followed by antioxidant and antimicrobial capacity assays of the EO and their individual compounds are important to evaluate the constituents that contribute the most for their antioxidant and antimicrobial properties and to evaluate possible synergism. In addition, a chemical profile can be reproduced in laboratory based on this knowledge.
Essential Oils as Antimicrobial Agents
EOs have shown promising antimicrobial action against a wide range of microorganisms. Table 4 compiles the EOs tested against several microorganisms and their minimal inhibitory concentrations (MIC). The MIC can be defined as the lowest concentration of a sample at which the tested microorganisms do not demonstrate any visible growth (Bozin et al. 2006; Naik et al. 2010; Ye et al. 2013).
EOs components have been applied due to their capacity in generating pleasant odors and for providing an effective action against several food pathogens thereby reducing foodborne diseases (Bouaziz et al. 2009).
The disk diffusion test, an antimicrobial in vitro evaluation test, is standardized by CLSI (Clinical and Laboratory Standards Institute) and it has been used in several research studies (Hussain et al. 2008; Jordán et al. 2013; Jrah Harzallah et al. 2011; Lv et al. 2011; Melo et al. 2012; Ojeda-Sana et al. 2013). Besides this method, the hole-plate agar diffusion method is another diffusion method that is commonly applied (Bozin et al. 2006; Naik et al. 2010; Al Abbasy et al. 2015). However, researchers adapt experimental methods to have a better representation of the possible future applications of a product or substance in their particular field (Burt 2004). The antimicrobial activity assay can be also carried in broth by the broth microdilution susceptibility assay (Longaray Delamare et al. 2007; Kelen and Tepe 2008).
Antimicrobial assays can be performed in direct contact with the microorganism or by vapor phase. When both methods are compared, the best effectiveness is dependent on the microorganism, the concentration, and type of EO (Fisher and Phillips 2006; Dimić et al. 2014). Lemon EO demonstrated more efficiency in the vapor phase against Cladosporium cladosporioides and equal inhibitory effect against Penicillium chrysogenum (Dimić et al. 2014).
Viuda-Martos et al. (2011) observed that thyme EO, followed by black cumin EO, presented higher inhibition against Listeria innocua and P. fluorescens than fennel, parsley, and lavender EO.
Basil (O. basilicum) EO did not show inhibition against Pseudomonas aeruginosa (Bozin et al. 2006; Al Abbasy et al. 2015; Gaio et al. 2015a), Staphylococcus epidermidis, and Klebsiella pneumonia (Al Abbasy et al. 2015). On the other hand, Bozin et al. (2006) found a positive antimicrobial effect against S. epidermidis, and Melo et al. (2012) showed that the species of basil, Ocimum micranthum and Ocimum selloi, presented antimicrobial action against P. aeruginosa.
Singh et al. (2007) reported an increase in the inhibition zone (measure of the effectiveness of an antimicrobial compound) when the highest volume (6 μL) of cinnamon EO was used. Cinnamon leaf EO presented a better antifungal (Aspergillus niger, Aspergillus flavus, Aspergillus ochraceus, Aspergillus terreus, Fusarium moniliforme, Fusarium graminearum, Penicillium citrinum, and Penicillium viridicatum) activity than cinnamon bark EO and the opposite was observed for bacteria (Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, E. coli, Salmonella typhi, P. aeruginosa). Cinnamon EO compounds such as E-cinnamaldehyde and eugenol showed a higher antimicrobial activity (Singh et al. 2007) than cinnamic acid (Ooi et al. 2006a).
The antimicrobial activity of sage EO (Salvia officinalis L. and Salvia triloba L.) against some foodborne microorganisms was proved by Longaray Delamare et al. (2007). These authors found different MIC against B. cereus, B. subtilis, E. coli, P. aeruginosa, P. fluorescens, Salmonella typhimurium, and S. aureus from that found by Bouaziz et al. (2009) for S. officinalis EO. This discrepancy can be attributed to the bacterial strains and the composition of the EO of different species used in each particular study (Longaray Delamare et al. 2007; Bouaziz et al. 2009).
Gomes Neto et al. (2012) found a high MIC for rosemary EO against S. aureus, while Fernández-Pan et al. (2014) found no efficacy for the same oil against this microorganism and observed low inhibition against L. innocua, Salmonella enteritidis, and Pseudomonas fragi (Gomes Neto et al. 2012; Fernández-Pan et al. 2014).
The antifungal and antibacterial activity of the EO from different parts of Etlingera fimbriobracteata was studied by Ud-Daula et al. (2016). Rhizome oils from E. fimbriobracteata showed the highest antibacterial activity, followed by basal stem oils, aerial stem oils, and leaf oils. E. fimbriobracteata EO presented a higher antifungal activity against Saccharomyces cerevisiae than the antifungal, miconazole (Ud-Daula et al. 2016).
The biological activities of EOs are affected by many factors, such as chemical composition, species, tested microorganisms, method of analysis, method of EO extraction, and other factors, that limit the reproduction of the results. In addition, normalized methods are necessary in order to facilitate the comparison of the results from different authors.
Essential Oils as Antioxidant Agents
In today’s world, with the globalization phenomenon and the technology advances in the food industry, people have access to all foods in every part of the world. In order to make this food availability possible, it is necessary to use an extremely organized and timed transport chain in order to reduce food waste at the lowest levels. Food packaging is designed to protect the nutritional and organoleptic properties of the packaged foods and to make the transport process easier and faster.
One of the problems derived from food degradation is lipid oxidation and peroxidation. These two processes are natural chemical phenomena that affect the nutritional value of foods, and they are responsible for changes in foods flavor, odor, taste, and texture. Lipids, besides contributing to the nutritional value of foods, are essential to a healthy diet and grant organoleptic characteristics to foods (Gutiérrez 2000; Márquez-Ruiz et al. 2008). Foods with high lipid content are highly susceptible to oxidation and peroxidation. In the oxidation process, unsaturated fatty acids interact with the oxygen present in the matrix and in the atmosphere surrounding the food, leading to the formation of peroxide radicals. Once the peroxide radicals are formed, the peroxidation process starts, accelerating the oxidation of lipids (Ferrari 1998; McClements and Decker 2000; Frankel 2005). This natural process can be accelerated in the presence of radiation, oxygen, humidity, high temperature, among other factors (McClements and Decker 2000). Also, the final products of the lipid oxidation process may lead to cancer, heart disease, cellular mutation, and atherosclerosis (McClements and Decker 2000; Chanwitheesuk et al. 2005; Márquez-Ruiz et al. 2008). This is a major cause of degradation in high-fat content foods, and therefore, it is important to slow down or inhibit this oxidation process. One option is to add additives, such as antioxidants from synthetic or natural origin, directly to food to prevent oxidation, maintaining food quality, and extending the shelf-life of the product.
Antioxidants are compounds capable of slowing down or stop autoxidation of foods, increasing their shelf-life by delaying their natural or induced deterioration (André et al. 2010; Amorati et al. 2013). The most commonly used synthetic antioxidants in food are the BHT (butyl hydroxytoluene) and BHA (butyl hydroxyanisole) (André et al. 2010). The reduction or replacement of synthetic additives for natural additives has encouraged several studies towards the utilization of natural products such as EOs (Kelen and Tepe 2008; Bouaziz et al. 2009; Goñi et al. 2009; Rozman and Jersek 2009). However, the effects of lipid oxidation on proteins, pigments, and lipid degradation can be minimized using natural antioxidants into foods (Li et al. 2014). EOs present a considerable antioxidant capacity as can be seen in Table 5.
The antioxidant capacity of the EOs is directly related to their composition. Phenolic compounds, such as phenolic acids and flavonoids, are considered the major contributors for antioxidant capacity (Choi et al. 2000; Nuutila et al. 2003; Shan et al. 2005; Wei et al. 2014). Their strong antioxidant capacity may be also explained by the synergistic effect among active compounds (Kelen and Tepe 2008).
These compounds act by the inactivation of free radicals and preventing their formation which will avoid the formation of hydroperoxides. Antioxidants can compete with free radicals avoiding the propagation of oxidation reactions (Pokorny et al. 2001; Tian et al. 2013).
An antioxidant can play a completely different role or present a different performance depending on the reactive oxygen species (ROS) or target substrate. Therefore, when selecting the methods to measure antioxidant capacity, the choice of the substrate and its concentration is a very important criterion. For instance, to study the relative bioactivity of an antioxidant or mixture of antioxidants (e.g., plant extract or EO), the application of both aqueous and lipophilic phase systems is important (Patras et al. 2013).
In line with this, antioxidant effect of EOs shall always be evaluated by the combination of at least two or more different in vitro antioxidant capacity assays to obtain relevant data regarding their antioxidant activity (Table 4) as suggested by Patras et al. (2013). Antioxidants can act by different mechanisms like the ability to scavenge free radicals, as a hydrogen atom/electron donor or directly reacting with free radicals, and inhibiting the lipid peroxidation (Shahwar et al. 2012; Teixeira et al. 2012).
The antioxidant capacity can be measured using numerous in vitro assays such as DPPH• (2,2-diphenyl-1-picrylhydrazyl), ABTS•+(2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), FRAP (ferric-reducing antioxidant power), Rancimat test, ORAC (oxygen-radical antioxidant capacity), β-carotene bleaching, TBARS (thiobarbituric acid reactive species), hydroperoxides, and volatile oxidation products (Amorati et al. 2013).
FRAP, DPPH•, and ABTS•+ methods are the most commonly used (Bernaert et al. 2012; Teixeira et al. 2012; Zeng et al. 2016). DPPH• and ABTS•+ assays measure the scavenging ability of radicals, while FRAP measures the ability to reduce metals, non-radical species such as Fe3+ ions (Dudonné et al. 2009; Shahwar et al. 2012).
The DPPH• method is based in the capacity of a sample to capture the free radical DPPH at room temperature, which causes a decrease in the absorbance at a determined wavelength (515–520 nm) (Brand-Williams et al. 1995; Bondet et al. 1997; Mareček et al. 2017). Initially, the free radical DPPH presents a purple color by having a free electron. When the hydrogen radical is donated to the sample with antioxidant capacity, the purple turns into yellow by the formation of diphenyl-picryl-hydrazine. This method is considered fast and practical and presents a good stability (Arnao et al. 2001). Usually, the free radical DPPH is dissolved in ethanol at concentrations that can vary between 1 and 22.5 mM, but it can also be dissolved in methanol. For instance, in the method described by Moure et al. (2001), the free radical DPPH is diluted in methanol to form a solution of 14.2 μg ml−1(Moure et al. 2001). One of the disadvantages of the free radical DPPH scavenging method is that this method does not represent the reaction that occur naturally in foods. Another disadvantage of this method is that, in the literature, the amount of time that a sample is exposed to the DPPH• solution is highly variable, ranging between 20 and 60 or 90 min, the common choice being 30 min (Patras et al. 2013).
The ABTS•+ method is based on the capture of the ABTS+ cation. ABTS●+ is produced from a precursor, 2,2-azino-bis(3-ethylbenzotiazoline)-6-sulfonic acid, which is oxidized by the potassium persulfate in order to generate the radical. With the addition of an antioxidant, occurs the reduction of the ABTS●+ which promotes the loss of the coloration of the solution (Rufino et al. 2007). ABTS●+ is considered stable with high water solubility, and this method is considered fast, practical, and simple (Arnao et al. 2001). However, the low selectivity of ABTS●+ in the reaction with hydrogen donor atoms presents a disadvantage of this method (Rufino et al. 2007).
On the other hand, the FRAP method is based in the reduction of Fe+3 to Fe2+ in the presence of an antioxidant in acid conditions. The formed solution has a blue color with a maximum absorbance at 593 nm (Rufino et al. 2006). This method is considered fast and reproducible; however, besides being unable to measure all the antioxidants present in a complex food matrix, it requires an aqueous system, and it does not detect compounds that act by the donation of hydrogen atoms and can be reduced by other compounds leading to false results (Magalhães et al. 2008).
The Rancimat test, due to its easy application and reproducibility, is also a widely applied method that measures the oxidative stability of fats and oils (García-Moreno et al. 2013). The method is based on the measurement of the volatile products of the oxidation that are dissolved in distilled water (Velasco et al. 2009). In this assay, the sample of fat or oil is passed through a stream of purified air. Once the air contains the volatile organic acids produced during the oxidation of the oil or the fat sample, the air is used to oxygenize the deionized water, whose conductivity is being constantly monitored. The conductivity of the water, once mixed with the air, increases (AOCS 1994).
Boulanouar et al. (2013) compared the antioxidant capacity of Artemisia campestris L. EO by several methods such as TBARS, lecithin liposome oxidation assay, ferrous ion-chelating ability assay, and 5-lipoxygenaseinhibition assay. The results expressed as IC50 (mg mL−1) (concentration of extract able to prevent 50% lipid oxidation) were 0.849, 0.080, 0.182, and 0.090, respectively (Boulanouar et al. 2013).
ORAC method with results expressed as gTrolox/gEO (gT/gEO) was the selected method to analyze the antioxidant capacity of EOs by Bentayeb et al. (2014). Oregano (2.62 gT/gEO), clove (2.43 gT/gEO), and cinnamon (2.11 gT/gEO) EOs showed the highest antioxidant capacity, followed by red thyme (1.24 gT/gEO) and thyme (0.79 gT/gEO) EOs (Bentayeb et al. 2014).
EOs can present the same or higher antioxidant capacity than synthetic antioxidants. Bouaziz et al. (2009) demonstrated that sage EO exhibited a remarkably higher antioxidant capacity than BHT (Bouaziz et al. 2009). While Hertiacheirifolia L. EO from flowers showed a lower antioxidant capacity than BHT regarding DPPH• and β-carotene bleaching tests (Majouli et al. 2016).
Sage and rosemary EOs showed the highest antioxidant capacity according to DPPH•, TBARS, FRAP, and Rancimat tests, while clove and oregano EOs showed the lowest antioxidant capacity for the first three tests, followed by thyme. However, in Rancimat test, oregano and thyme had the highest antioxidant capacity. Clove presented a higher antioxidant capacity than BHT and ascorbic acid in the DPPH• and FRAP assays. In the ferrous iron chelating assay, all EOs presented better antioxidant capacity than BHT, and ascorbic acid, rosemary, and sage EOs were the most efficient and oregano showed the lowest antioxidant capacity (Viuda-Martos et al. 2010).
According to Singh et al. (2007), depending on the plant and the part of the plant used to produce the EO, the antioxidant capacity can vary considerably. Besides, cinnamon bark EO presented a higher antioxidant capacity in the DPPH• and hydroxyl• radical assays than cinnamon leaf EO. Among the constituents of cinnamon EO, an amount of 25 μL of eugenol showed a higher radical scavenging activity by DPPH• assay (92.9%) than the same amount of E-cinnamaldehyde (78.3%). It is possible that the radical scavenging activity might be mostly affected by the position of the phenolic hydroxyl group which is present in eugenol (Singh et al. 2007).
In the DPPH•assay, the free radical inhibition (%) for rosemary EO was determined to be 62.45%, whereas the values of their major constituents were 42.74%, 45.61%, and 46.21% (v/v) for 1,8-cineole, α-pinene, and β-pinene, respectively. In the β-carotene bleaching test, the concentrations providing 50% inhibition (IC50) were 2.04, 4.05, 2.28, and 2.56% (v/v) for the EO, 1,8-cineole, α-pinene, and β-pinene, respectively (Wang et al. 2008). This indicates that minor components contributed to the antioxidant capacity of rosemary EO.
The different units of expressing the results of the same method can complicate the comparison among EOs’ antioxidant capacity. There is a need for a standardized approach in order to generate meaningful data that can be compared among different sources (Amorati et al. 2013). According to Patras et al. (2013), expressing kinetic parameters as EC50 provides a more comprehensive evaluation of antioxidant activity.
The broad variety of potential antioxidant compounds can result in a wide variation of the antioxidant capacity of the EOs (Nuutila et al. 2003; Viuda-Martos et al. 2010).
Determination of Active Compounds: Analysis Methods
In order to be able to study the EOs, it is important to evaluate their components, but this can become a difficult task. Besides the numerous compounds present in each EO, most of them are present in minor quantities requiring methods with low detection limits. The separation of the compounds can be achieved by fractional distillation, GC, high-performance liquid chromatography (HPLC), MS, and nuclear magnetic resonance (NMR) spectroscopy (Nakatsu et al. 2000; Tisserand and Young 2014).
Fractional distillation is used to purify the EOs or to concentrate the fraction or fractions of the EO to be further evaluated. The main goal is to separate substances through the volatility difference among them. Apart from depending of the natural physico-chemical properties of the substance to be analyzed, it also depends on the pressure and temperature of the system. Generally, this method is used as pre-treatment or pre-purification of the sample and cannot achieve the necessary separation resolution to the study of the biological activities of EOs (Nakatsu et al. 2000; Silvestre et al. 2016). Chromatography is the most used technique when it comes to chemical separation of EO components. Several conditions used to quantify and identify the compounds present in the EO by chromatography are resumed in Table 6. Gas chromatography-mass spectrometry (GC-MS) is the most used technique in the separation of EOs (Tables 6, 7, and 8) because it can achieve the highest resolution and is the most suitable technique to analyze volatile compounds (Cooke et al. 2000; Nakatsu et al. 2000). In general, it was observed that most of the times, the analytical column used in GC is composed of 5% diphenyl and 95% dimethyl polydimethylsiloxane (Table 6).
Although GC is widely used, there are many studies using also LC. Ribeiro-Santos et al. (2017) identified major compounds of cinnamon and basil EOs by the two chromatographic techniques, and similar results regarding major components were found by GC (GC-MS) and LC (UHPLC—ultra-high-performance liquid chromatography).
Legal Aspects of the Use of EOs in Food
The use of EOs as flavoring agents is registered by the European Commission (EC) and by the FDA and they are classified as GRAS (under section 201 (s) and 409 of the Act, and FDA’s implementing regulations in 21 CFR 182.20) and approved in food additive status list (European Commission 2008; Food And Drug Administration (FDA) 2016).
The European Union has adopted a list of approved flavorings since October 1, 2012, which is periodically updated (EU Regulation No. 872/2012). This regulation prohibits the use of certain natural substances that are proved to be harmful for public health and lays down maximum levels for certain substances which may raise concern for human health. These natural substances are present in EOs.
Despite of being approved as a food additive, which involves a series of steps to guarantee food safety, there are concerns about the EOs potential for either allergic or skin irritation reactions. Not many reports are available to tackle this complex question and little is discussed about it.
EOs present a particular challenge because they are not only mixtures but also different batches, sources, and varieties which may contain different concentrations of potentially toxic constituents. When two or more EOs contain the same possible toxic constituent, or different constituents that exhibit the same type of toxicity, this should be taken into account when considering maximum safe dose. This could apply to skin irritants, allergens, phototoxins, neurotoxins, teratogens, carcinogens, and hepatotoxins.
In addition, oral reactions have been reported such as inflammation of the oral mucous membrane and inflammation of the lips which occurred due to any products applied to the mouth or lips containing EO (Unlu et al. 2010). The ingestion of higher doses of these natural compounds can induce serious problems of oral toxicity. It is necessary to find a balance between the effective EO dose and the risk of toxicity (Sánchez-González et al. 2010), since some EOs are found to be safe for human consumption at low concentrations (Raut and Karuppayil 2014).
Toxicity of EO depends on the frequency of use, on the susceptibility of the individual to potentially toxic substances which can vary considerably, and also on the concentrations used (Unlu et al. 2010; Raut and Karuppayil 2014). EO interactions can occur between one and more of their constituents, as well as between a component and drug or a food item (Unlu et al. 2010). Table 9 reports some toxic effects of the EOs or their constituents. Most of the toxic effects were observed in animal studies (adapted from Raut and Karuppayil 2014).
EOs may cause allergic reactions, such as dermatitis by their frequent use, as it happens in aromatherapy (Bleasel et al. 2002; Trattner et al. 2008). Various uncovered parts of the skin, in particular the scalp, neck, and hands, can be affected. Lavender, jasmine, rosewood, laurel, eucalyptus, and pomerance EOs have shown some allergic effects (Schaller and Korting 1995). Data of Information Network of Departments of Dermatology (IVDK) have reported a total of 637 cases of allergy to ylang-ylang, lemongrass, jasmine, sandalwood, and clove EOs in Germany between 2000 and 2008 (Uter et al. 2010).
A study carried out by Groot and Schmidt (2016) reported that nearly 80 EOs have caused contact allergy. In general, most of the reactions were caused by application of pure oils or high-concentration products.
Sometimes only a single major component isolated from the EO is analyzed, as in the study carried out by Audrain et al. (2014). In this study, limonene, which is constituent of the lime and orange EOs, and linalool, which is constituent of the basil and lavender EOs, have been associated with allergic contact dermatitis (Audrain et al. 2014).
Concluding Remarks
The deterioration by microorganisms and oxidation of lipids are the important causes of food loss. Therefore, control of the growth of microorganisms and lipid oxidation are necessary to increase food stability and to extend product shelf-life and consequently food safety and industry economic profitability.
Microbial and antioxidant in vitro assays were reported as analysis techniques suitable to study the potential biological activities of EOs. In fact, diffusion in agar and DPPH• assay were more frequently used assays to evaluate antimicrobial and antioxidant capacity, respectively. In general, volatile active agents of EOs were determined by gas chromatography. The identification and quantification of EO constituents are of great importance in order to assure their appropriate use. It was shown that most of the EOs have antimicrobial, antioxidant, or both properties, and their composition of EOs has generally wide variability, which greatly influences their biological activity. Therefore, the standardization of the EO manufacturing process is a way of ensuring their effectiveness and safety. Moreover, in spite of the potential of EOs, more studies such as clinical trials should evaluate their safety and possible side effects before considering their use for food or food packaging purposes.
References
Aburjai, T., & Natsheh, F. M. (2003). Plants used in cosmetics. Phytotherapy Research, 17, 987–1000. doi:10.1002/ptr.1363.
Adrar, N., Oukil, N., & Bedjou, F. (2016). Antioxidant and antibacterial activities of Thymus numidicus and Salvia officinalis essential oils alone or in combination. Industrial Crops and Products, 88, 112–119. doi:10.1016/j.indcrop.2015.12.007.
Aguilar-González, A. E., Palou, E., & López-Malo, A. (2015). Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innovative Food Science and Emerging Technologies, 32, 181–185. doi:10.1016/j.ifset.2015.09.003.
Aidi Wannes, W., Mhamdi, B., Sriti, J., Ben Jemia, M., Ouchikh, O., Hamdaoui, G., et al. (2010). Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food and Chemical Toxicology, 48(5), 1362–1370. doi:10.1016/j.fct.2010.03.002.
Al Abbasy, D. W., Pathare, N., Al-Sabahi, J. N., & Khan, S. A. (2015). Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum Linn.) Asian Pacific Journal of Tropical Disease, 5(8), 645–649. doi:10.1016/S2222-1808(15)60905-7.
Ali, B., Al-Wabel, N. A., Shams, S., Ahamad, A., Khan, S. A., & Anwar, F. (2015). Essential oils used in aromatherapy: a systemic review. Asian Pacific Journal of Tropical Biomedicine, 5(8), 601–611. doi:10.1016/j.apjtb.2015.05.007.
Amorati, R., Foti, M. C., & Valgimigli, L. (2013). Antioxidant activity of essential oils. Journal of Agricultural and Food Chemistry, 61(46), 10835–10847. doi:10.1021/jf403496k.
André, C., Castanheira, I., Cruz, J. M., Paseiro, P., & Sanches-Silva, A. (2010). Analytical strategies to evaluate antioxidants in food: a review. Trends in Food Science & Technology, 21(5), 229–246. doi:10.1016/j.tifs.2009.12.003.
Ansorena, M. R., Zubeldía, F., & Marcovich, N. E. (2016). Active wheat gluten films obtained by thermoplastic processing. LWT-Food Science and Technology, 69, 47–54. doi:10.1016/j.lwt.2016.01.020.
Arnao, M. B., Cano, A., & Acosta, M. (2001). The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chemistry, 73(2), 239–244. doi:10.1016/S0308-8146(00)00324-1.
AOCS - O fficial methods and recommended practices of the American Oil Chemists’ Society (4th ed.). (1994). Standard Method Cd 12b-92. Champaign: AOCS Press.
Asdadi, A., Hamdouch, A., Oukacha, A., Moutaj, R., Gharby, S., Harhar, H., et al. (2015). Study on chemical analysis, antioxidant and in vitro antifungal activities of essential oil from wild Vitex agnus-castus L. seeds growing in area of Argan Tree of Morocco against clinical strains of Candida responsible for nosocomial infections. Journal de Mycologie Médicale/Journal of Medical Mycology, 25(4), e118–e127. doi:10.1016/j.mycmed.2015.10.005.
Asif, M., Yehya, A. H. S., Al-Mansoub, M. A., Revadigar, V., Ezzat, M. O., Khadeer Ahamed, M. B., et al. (2016). Anticancer attributes of Illicium verum essential oils against colon cancer. South African Journal of Botany, 103, 156–161. doi:10.1016/j.sajb.2015.08.017.
Atrea, I., Papavergou, A., Amvrosiadis, I., & Savvaidis, I. N. (2009). Combined effect of vacuum-packaging and oregano essential oil on the shelf-life of Mediterranean octopus (Octopus vulgaris) from the Aegean Sea stored at 4 °C. Food Microbiology, 26(2), 166–172. doi:10.1016/j.fm.2008.10.005.
Audrain, H., Kenward, C., Lovell, C. R., Green, C., Ormerod, A. D., Sansom, J., et al. (2014). Allergy to oxidized limonene and linalool is frequent in the U.K. British Journal of Dermatology, 171(2), 292–297. doi:10.1111/bjd.13037.
Avila-Sosa, R., Palou, E., Jiménez Munguía, M. T., Nevárez-Moorillón, G. V., Navarro Cruz, A. R., & López-Malo, A. (2012). Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. International Journal of Food Microbiology, 153(1–2), 66–72. doi:10.1016/j.ijfoodmicro.2011.10.017.
Azeredo, G. A., Stamford, T. L. M., Nunes, P. C., Gomes Neto, N. J., de Oliveira, M. E. G., & de Souza, E. L. (2011). Combined application of essential oils from Origanum vulgare L. and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Research International, 44(5), 1541–1548. doi:10.1016/j.foodres.2011.04.012.
Azevedo, A. N., Buarque, P. R., Cruz, E. M. O., Blank, A. F., Alves, P. B., Nunes, M. L., & Santana, L. C. L. D. A. (2014). Response surface methodology for optimisation of edible chitosan coating formulations incorporating essential oil against several foodborne pathogenic bacteria. Food Control, 43, 1–9. doi:10.1016/j.foodcont.2014.02.033.
Bastos, M. S. R., Da Silva Laurentino, L., Canuto, K. M., Mendes, L. G., Martins, C. M., Silva, S. M. F., et al. (2016). Physical and mechanical testing of essential oil-embedded cellulose ester films. Polymer Testing, 49, 156–161. doi:10.1016/j.polymertesting.2015.11.006.
Ben Ghnaya, A., Amri, I., Hanana, M., Gargouri, S., Jamoussi, B., Romane, A., & Hamrouni, L. (2016). Tetraclinis articulata (Vahl.) masters essential oil from Tunisia: chemical characterization and herbicidal and antifungal activities assessment. Industrial Crops and Products, 83, 113–117. doi:10.1016/j.indcrop.2015.12.026.
Bendahou, M., Muselli, A., Grignon-Dubois, M., Benyoucef, M., Desjobert, J.-M., Bernardini, A.-F., & Costa, J. (2008). Antimicrobial activity and chemical composition of Origanum glandulosum Desf. essential oil and extract obtained by microwave extraction: comparison with hydrodistillation. Food Chemistry, 106(1), 132–139. doi:10.1016/j.foodchem.2007.05.050.
Bentayeb, K., Vera, P., Rubio, C., & Nerín, C. (2014). The additive properties of oxygen radical absorbance capacity (ORAC) assay: the case of essential oils. Food Chemistry, 148, 204–208. doi:10.1016/j.foodchem.2013.10.037.
Bernaert, N., De Paepe, D., Bouten, C., De Clercq, H., Stewart, D., Van Bockstaele, E., et al. (2012). Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chemistry, 134(2), 669–677. doi:10.1016/j.foodchem.2012.02.159.
Bleasel, N., Tate, B., & Rademaker, M. (2002). Allergic contact dermatitis following exposure to essential oils. Australasian Journal of Dermatology, 43(3), 211–213. doi:10.1046/j.1440-0960.2002.00598.x.
Bondet, V., Brand-Williams, W., & Berset, C. (1997). Kinetics and mechanisms of antioxidant activity using the DPPHFree radical method. LWT - Food Science and Technology, 30(6), 609–615. doi:10.1006/fstl.1997.0240.
Botre, D. A., de Soares, N. F. F., Espitia, P. J. P., de Sousa, S., & Renhe, I. R. T. (2010). Avaliação de filme incorporado com óleo essencial de orégano para conservação de pizza pronta. Revista Ceres, 57(3), 283–291. doi:10.1590/S0034-737X2010000300001.
Bouaziz, M., Yangui, T., Sayadi, S., & Dhouib, A. (2009). Disinfectant properties of essential oils from Salvia officinalis L. cultivated in Tunisia. Food and Chemical Toxicology, 47(11), 2755–2760. doi:10.1016/j.fct.2009.08.005.
Boulanouar, B., Abdelaziz, G., Aazza, S., Gago, C., & Miguel, M. G. (2013). Antioxidant activities of eight Algerian plant extracts and two essential oils. Industrial Crops and Products, 46, 85–96. doi:10.1016/j.indcrop.2013.01.020.
Bozin, B., Mimica-Dukic, N., Simin, N., & Anackov, G. (2006). Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. Journal of Agricultural and Food Chemistry, 54(5), 1822–1828. doi:10.1021/jf051922u.
Brahmi, F., Abdenour, A., Bruno, M., Silvia, P., Alessandra, P., Danilo, F., et al. (2016). Chemical composition and in vitro antimicrobial, insecticidal and antioxidant activities of the essential oils of Mentha pulegium L. and Mentha rotundifolia (L.) Huds growing in Algeria. Industrial Crops and Products, 88, 96–105. doi:10.1016/j.indcrop.2016.03.002.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30. doi:10.1016/S0023-6438(95)80008-5.
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology, 94(3), 223–253. doi:10.1016/j.ijfoodmicro.2004.03.022.
Cacho, J. I., Campillo, N., Viñas, P., & Hernández-Córdoba, M. (2016). Determination of synthetic phenolic antioxidants in edible oils using microvial insert large volume injection gas-chromatography. Food Chemistry, 200, 249–254. doi:10.1016/j.foodchem.2016.01.026.
Calogirou, A., Larsen, B. R., & Kotzias, D. (1999). Gas-phase terpene oxidation products: a review. Atmospheric Environment, 33(9), 1423–1439. doi:10.1016/S1352-2310(98)00277-5.
Centers for Disease Control and Prevention. (2015). Food Safety. http://www.cdc.gov/foodsafety/cdc-and-food-safety.html. Accessed 10 July 2016.
Chanwitheesuk, A., Teerawutgulrag, A., & Rakariyatham, N. (2005). Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chemistry, 92(3), 491–497. doi:10.1016/j.foodchem.2004.07.035.
Choi, H.-S., Song, H. S., Ukeda, H., & Sawamura, M. (2000). Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-diphenyl-2-picrylhydrazyl. Journal of Agricultural and Food Chemistry, 48(9), 4156–4161. doi:10.1021/jf000227d.
Choi, W. S., Singh, S., & Lee, Y. S. (2016). Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of “Formosa” plum (Prunus salicina L.) LWT-Food Science and Technology, 70, 213–222. doi:10.1016/j.lwt.2016.02.036.
Cooke, M., Poole, C. F., Wilson, I. D., & Adlard, E. R. (Eds.). (2000). Encyclopedia of separation science. Academic Press.
Dahham, S. S., Tabana, Y. M., Ahmed Hassan, L. E., Khadeer Ahamed, M. B., Abdul Majid, A. S., & Abdul Majid, A. M. S. (2016). In vitro antimetastatic activity of agarwood (Aquilaria crassna) essential oils against pancreatic cancer cells. Alexandria Journal of Medicine, 52(2), 141–150. doi:10.1016/j.ajme.2015.07.001.
Dhouioui, M., Boulila, A., Chaabane, H., Zina, M. S., & Casabianca, H. (2016). Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis Batt. (Aristolochiaceae) roots and its antimicrobial activity. Industrial Crops and Products, 83, 301–306. doi:10.1016/j.indcrop.2016.01.025.
Diaz, P., Jeong, S. C., Lee, S., Khoo, C., & Koyyalamudi, S. R. (2012). Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chinese Medicine, 7(1), 26. doi:10.1186/1749-8546-7-26.
Dimić, G., Kocić-Tanackov, S., Mojović, L., & Pejin, J. (2014). Antifungal activity of lemon essential oil, coriander and cinnamon extracts on foodborne molds in direct contact and the vapor phase. Journal of Food Processing and Preservation, 39(6), 1778–1787. doi:10.1111/jfpp.12410.
Djabou, N., Lorenzi, V., Guinoiseau, E., Andreani, S., Giuliani, M. C., Desjobert, J. M., et al. (2013). Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Control, 30(1), 354–363. doi:10.1016/j.foodcont.2012.06.025.
Duarte, A., Luís, Â., Oleastro, M., & Domingues, F. C. (2016). Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control, 61, 115–122. doi:10.1016/j.foodcont.2015.09.033.
Dudonné, S., Vitrac, X., Coutière, P., Woillez, M., & Mérillon, J.-M. (2009). Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest: comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP. Journal of Agricultural and Food Chemistry, 57, 1768–1774. doi:10.1021/jf803011r.
Dvaranauskaité, A., Venskutonis, P. R., Raynaud, C., Talou, T., Viškelis, P., & Sasnauskas, A. (2009). Variations in the essential oil composition in buds of six blackcurrant (Ribes nigrum L.) cultivars at various development phases. Food Chemistry, 114(2), 671–679. doi:10.1016/j.foodchem.2008.10.005.
Edris, A. E. (2007). Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytotherapy Research, 21(4), 308–323. doi:10.1002/ptr.2072.
Elzaawely, A., Xuan, T., Koyama, H., & Tawata, S. (2007). Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & amp; R.M. Sm. Food Chemistry, 104(4), 1648–1653. doi:10.1016/j.foodchem.2007.03.016.
Emiroğlu, Z. K., Yemiş, G. P., Coşkun, B. K., & Candoğan, K. (2010). Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Science, 86(2), 283–288. doi:10.1016/j.meatsci.2010.04.016.
Espitia, P. J. P., de Soares, N. F. F., Botti, L. C. M., de Melo, N. R., Pereira, O. L., & da Silva, W. A. (2012). Assessment of the efficiency of essential oils in the preservation of postharvest papaya in an antimicrobial packaging system. Brazilian Journal of Food Technology, 15(4), 333–342. doi:10.1590/S1981-67232012005000027.
European Commission. (2008). Regulation (EC) no. 1334/2008. Official Journal of the European Union, L 354/34(1334), 34–50 http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32008R1334.
Fernández-Pan, I., Carrión-Granda, X., & Maté, J. I. (2014). Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control, 36(1), 69–75. doi:10.1016/j.foodcont.2013.07.032.
Ferrari, C. K. b. (1998). Oxidação lipídica em alimentos e sistemas biológicos: mecanismos gerais e implicações nutricionais e patólogicas. Revista de Nutrição, 11(1), 3–14. doi:10.1590/S1415-52731998000100001.
Fisher, K., & Phillips, C. A. (2006). The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacterjejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. Journal of Applied Microbiology, 101(6), 1232–1240. doi:10.1111/j.1365-2672.2006.03035.x.
Food And Drug Administration (FDA). (2016). Code of Federal Regulations (CFR). Title 21: food and drugs. Chapter I—Food and Drug Administration, Department of Health and Human Services, subchapter B—food for human consumption (continued), part 182—substances generally recognized as safe (GRAS), subpart A—general provisions, subpart 182.20—essential oils, oleoresins, and natural extractives. Office of the Federal Register, Washington.
Frankel, E. N. (2005). Lipid oxidation. Oily press lipid library, 18(19), 1–22.
Gaio, I., Saggiorato, A. G., Treichel, H., Cichoski, A. J., Astolfi, V., Cardoso, R. I., et al. (2015a). Antibacterial activity of basil essential oil (Ocimum basilicum L.) in Italian-type sausage. Journal für Verbraucherschutz und Lebensmittelsicherheit, 10(4), 323–329. doi:10.1007/s00003-015-0936-x.
Gaio, I., Saggiorato, A. G., Treichel, H., Cichoski, A. J., Astolfi, V., Cardoso, R. I., et al. (2015b). Antibacterial activity of basil essential oil (Ocimum basilicum L.) in Italian-type sausage. Journal fur Verbraucherschutz und Lebensmittelsicherheit, 10(4), 323–329. doi:10.1007/s00003-015-0936-x.
García-Moreno, P. J., Pérez-Gálvez, R., Guadix, A., & Guadix, E. M. (2013). Influence of the parameters of the Rancimat test on the determination of the oxidative stability index of cod liver oil. LWT-Food Science and Technology, 51(1), 303–308. doi:10.1016/j.lwt.2012.11.002.
Ghods, A. A., Abforosh, N. H., Ghorbani, R., & Asgari, M. R. (2015). The effect of topical application of lavender essential oil on the intensity of pain caused by the insertion of dialysis needles in hemodialysis patients: a randomized clinical trial. Complementary Therapies in Medicine, 23(3), 325–330. doi:10.1016/j.ctim.2015.03.001.
Gniewosz, M., Kraśniewska, K., Woreta, M., & Kosakowska, O. (2013). Antimicrobial activity of a pullulan-caraway essential oil coating on reduction of food microorganisms and quality in fresh baby carrot. Journal of Food Science, 78(8), M1242–M1248. doi:10.1111/1750-3841.12217.
Gomes Neto, N. J., da Luz, I. S., Tavares, A. G., Honório, V. G., Magnani, M., & de Souza, E. L. (2012). Rosmarinus officinalis L. essential oil and its majority compound 1,8-cineole at sublethal amounts induce no direct and cross protection in Staphylococcus aureus ATCC 6538. Foodborne Pathogens and Disease, 9(12), 1071–1076. doi:10.1089/fpd.2012.1258.
Gómez-Estaca, J., López de Lacey, A., López-Caballero, M. E., Gómez-Guillén, M. C., & Montero, P. (2010). Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiology, 27(7), 889–896. doi:10.1016/j.fm.2010.05.012.
Goñi, P., López, P., Sánchez, C., Gómez-Lus, R., Becerril, R., & Nerín, C. (2009). Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chemistry, 116(4), 982–989. doi:10.1016/j.foodchem.2009.03.058.
Groot, A. C., & Schmidt, E. (2016). Essential oils. Part IV. Dermatitis, 27(4), 170–175. doi:10.1097/DER.0000000000000197.
Gutiérrez, J. B. (2000). Ciencia bromatológica: principios generales de los alimentos. Ediciones Díaz de Santos.
Haiyan, G., Lijuan, H., Shaoyu, L., Chen, Z., & Ashraf, M. A. (2016). Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi Journal of Biological Sciences, 23(4), 524–530. doi:10.1016/j.sjbs.2016.02.020.
Handa, S. S., Khanuja, S. P. S., Longo, G., & Rakesh, D. D. (Eds.). (2008). Extraction technologies for medicinal and aromatic plants. Trieste: ICS-UNIDO. doi:10.1021/np800144q.
Harkat-Madouri, L., Asma, B., Madani, K., Bey-Ould Si Said, Z., Rigou, P., Grenier, D., et al. (2015). Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Industrial Crops and Products, 78, 148–153. doi:10.1016/j.indcrop.2015.10.015.
Hill, L. E., Gomes, C., & Taylor, T. M. (2013). Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT-Food Science and Technology, 51(1), 86–93. doi:10.1016/j.lwt.2012.11.011.
Hossain, M. B., Brunton, N. P., Barry-Ryan, C., Martin-Diana, A., & Wilkinson, M. (2008). Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan Journal of Chemistry, 1(4), 751–756.
Hossain, M. A., Shah, M. D., Sang, S. V., & Sakari, M. (2012). Chemical composition and antibacterial properties of the essential oils and crude extracts of Merremia borneensis. Journal of King Saud University-Science, 24(3), 243–249. doi:10.1016/j.jksus.2011.03.006.
Hosseini, S. F., Rezaei, M., Zandi, M., & Farahmandghavi, F. (2016). Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chemistry, 194, 1266–1274. doi:10.1016/j.foodchem.2015.09.004.
Hussain, A. I., Anwar, F., Hussain Sherazi, S. T., & Przybylski, R. (2008). Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chemistry, 108(3), 986–995. doi:10.1016/j.foodchem.2007.12.010.
Hyun, J. E., Bae, Y. M., Yoon, J. H., & Lee, S. Y. (2015). Preservative effectiveness of essential oils in vapor phase combined with modified atmosphere packaging against spoilage bacteria on fresh cabbage. Food Control, 51, 307–313. doi:10.1016/j.foodcont.2014.11.030.
Imelouane, B., Amhamdi, H., Wathelet, J. P., Ankit, M., Khedid, K., Bachiri, E., & a. (2009). Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from Eastern Morocco. International Journal of Agriculture & Biology, 11(5), 205–208 http://www.partochemi.com/userfiles/uploads/GC-MS_Thymus_vulgaris_2012_01.pdf.
Jordán, M. J., Lax, V., Rota, M. C., Lorán, S., & Sotomayor, J. A. (2013). Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis L. Food Control, 30(2), 463–468. doi:10.1016/j.foodcont.2012.07.029.
Jrah Harzallah, H., Kouidhi, B., Flamini, G., Bakhrouf, A., & Mahjoub, T. (2011). Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chemistry, 129(4), 1469–1474. doi:10.1016/j.foodchem.2011.05.117.
Kelen, M., & Tepe, B. (2008). Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresource Technology, 99(10), 4096–4104. doi:10.1016/j.biortech.2007.09.002.
Khajeh, M., Yamini, Y., Bahramifar, N., Sefidkon, F., & Reza Pirmoradei, M. (2005). Comparison of essential oils compositions of Ferula assa-foetida obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chemistry, 91(4), 639–644. doi:10.1016/j.foodchem.2004.06.033.
Kpadonou Kpoviessi, B. G. H., Kpoviessi, S. D. S., Yayi Ladekan, E., Gbaguidi, F., Frédérich, M., Moudachirou, M., et al. (2014). In vitro antitrypanosomal and antiplasmodial activities of crude extracts and essential oils of Ocimum gratissimum Linn from Benin and influence of vegetative stage. Journal of Ethnopharmacology, 155(3), 1417–1423. doi:10.1016/j.jep.2014.07.014.
Kykkidou, S., Giatrakou, V., Papavergou, A., Kontominas, M. G., & Savvaidis, I. N. (2009). Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 °C. Food Chemistry, 115(1), 169–175. doi:10.1016/j.foodchem.2008.11.083.
Li, Y., Fabiano-Tixier, A.-S., & Chemat, F. (2014). Essential oils as reagents in green chemistry, 9–21. doi:10.1007/978-3-319-08449-7.
Llana-Ruíz-Cabello, M., Pichardo, S., Jiménez-Morillo, N. T., Bermúdez, J. M., Aucejo, S., González-Vila, F. J., et al. (2015). Molecular characterization of a bio-based active packaging containing Origanum vulgare L. essential oil using pyrolysis gas chromatography/mass spectrometry (Py-GC/MS). Journal of the Science of Food and Agriculture, n/a-n/a. doi:10.1002/jsfa.7502.
Longaray Delamare, A. P., Moschen-Pistorello, I. T., Artico, L., Atti-Serafini, L., & Echeverrigaray, S. (2007). Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chemistry, 100(2), 603–608. doi:10.1016/j.foodchem.2005.09.078.
Luís, Â., Duarte, A., Gominho, J., Domingues, F., & Duarte, A. P. (2016). Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Industrial Crops and Products, 79, 274–282. doi:10.1016/j.indcrop.2015.10.055.
Lv, F., Liang, H., Yuan, Q., & Li, C. (2011). In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Research International, 44(9), 3057–3064. doi:10.1016/j.foodres.2011.07.030.
Lv, J., Huang, H., Yu, L., Whent, M., Niu, Y., Shi, H., et al. (2012). Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chemistry, 132(3), 1442–1450. doi:10.1016/j.foodchem.2011.11.135.
Magalhães, L. M., Segundo, M. A., Reis, S., & Lima, J. L. F. C. (2008). Methodological aspects about in vitro evaluation of antioxidant properties. Analytica Chimica Acta, 613(1), 1–19. doi:10.1016/j.aca.2008.02.047.
Mahboubi, M., & Haghi, G. (2008). Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. Journal of Ethnopharmacology, 119(2), 325–327. doi:10.1016/j.jep.2008.07.023.
Majouli, K., Besbes Hlila, M., Hamdi, A., Flamini, G., Ben Jannet, H., & Kenani, A. (2016). Antioxidant activity and α-glucosidase inhibition by essential oils from Hertia cheirifolia (L.) Industrial Crops and Products, 82, 23–28. doi:10.1016/j.indcrop.2015.12.015.
Mareček, V., Mikyška, A., Hampel, D., Čejka, P., Neuwirthová, J., Malachová, A., & Cerkal, R. (2017). ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. Journal of Cereal Science, 73, 40–45. doi:10.1016/j.jcs.2016.11.004.
Márquez-Ruiz, G., García-Martínez, M. C., & Holgado, F. (2008). Changes and effects of dietary oxidized lipids in the gastrointestinal tract. Lipid Insights. Libertas Academica. doi:10.4137/LPI.S904.
Martucci, J. F., Gende, L. B., Neira, L. M., & Ruseckaite, R. A. (2015). Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Industrial Crops and Products, 71, 205–213. doi:10.1016/j.indcrop.2015.03.079.
McClements, D. J., & Decker, E. A. (2000). Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. Journal of Food Science, 65(8), 1270–1282. doi:10.1111/j.1365-2621.2000.tb10596.x.
de Melo, A. A. M., Geraldine, R. M., Silveira, M. F. A., Torres, M. C. L., Rezende, C. S. M. E., Fernandes, T. H., & de Oliveira, A. N. (2012). Microbiological quality and other characteristics of refrigerated chicken meat in contact with cellulose acetate-based film incorporated with rosemary essential oil. Brazilian Journal of Microbiology, 43(4), 1419–1427. doi:10.1590/S1517-83822012000400025.
Moghaddam, M., & Farhadi, N. (2015). Influence of environmental and genetic factors on resin yield, essential oil content and chemical composition of Ferula assa-foetida L. populations. Journal of Applied Research on Medicinal and Aromatic Plants, 2(3), 69–76. doi:10.1016/j.jarmap.2015.04.001.
Morelli, C. L., Mahrous, M., Belgacem, M. N., Branciforti, M. C., Bretas, R. E. S., & Bras, J. (2015). Natural copaiba oil as antibacterial agent for bio-based active packaging. Industrial Crops and Products, 70, 134–141. doi:10.1016/j.indcrop.2015.03.036.
Mostafa, D. M., Kassem, A. A., Asfour, M. H., Al Okbi, S. Y., Mohamed, D. A., & Hamed, T. E. S. (2015). Transdermal cumin essential oil nanoemulsions with potent antioxidant and hepatoprotective activities: in vitro and in vivo evaluation. Journal of Molecular Liquids, 212, 6–15. doi:10.1016/j.molliq.2015.08.047.
Moure, A., Franco, D., Sineiro, J., Dominguez, H., Núñez, M. J., & Lema, J. M. (2001). Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Research International, 34(2–3), 103–109. doi:10.1016/S0963-9969(00)00136-8.
Muhammad, N., Barkatullah, Ibrar, M., Khan, H., Saeed, M., Khan, A. Z., & Kaleem, W. A. (2013). In vivo screening of essential oils of Skimmia laureola leaves for antinociceptive and antipyretic activity. Asian Pacific Journal of Tropical Biomedicine, 3(3), 202–206. doi:10.1016/S2221-1691(13)60050-7.
Muriel-Galet, V., Cran, M. J., Bigger, S. W., Hernández-Muñoz, P., & Gavara, R. (2015). Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. Journal of Food Engineering, 149, 9–16. doi:10.1016/j.jfoodeng.2014.10.007.
Naik, M. I., Fomda, B. A., Jaykumar, E., & Bhat, J. A. (2010). Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacteria. Asian Pacific Journal of Tropical Medicine, 3(7), 535–538. doi:10.1016/S1995-7645(10)60129-0.
Nakatsu, T., Lupo, A. T., Chinn, J. W., & Kang, R. K. L. (2000). Biological activity of essential oils and their constituents (part B). In Atta-ur-Rahman (Ed.), Studies in natural products chemistry (Vol. 21, pp. 571–631). Amsterdam: Elsevier B.V.
Negi, P. S. (2012). Plant extracts for the control of bacterial growth: efficacy, stability and safety issues for food application. International Journal of Food Microbiology, 156(1), 7–17. doi:10.1016/j.ijfoodmicro.2012.03.006.
Nikolic, M., Glamoclija, J., Ferreira, I. C. F. R., Calhelha, R. C., Fernandes, Â., Markovic, T., et al. (2014). Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Industrial Crops and Products, 52, 183–190. doi:10.1016/j.indcrop.2013.10.006.
Nuutila, A. M., Puupponen-Pimiä, R., Aarni, M., & Oksman-Caldentey, K. M. (2003). Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chemistry, 81(4), 485–493. doi:10.1016/S0308-8146(02)00476-4.
Ocaña-Fuentes, A., Arranz-Gutiérrez, E., Señorans, F. J., & Reglero, G. (2010). Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food and Chemical Toxicology, 48(6), 1568–1575. doi:10.1016/j.fct.2010.03.026.
Ojeda-Sana, A. M., van Baren, C. M., Elechosa, M. A., Juárez, M. A., & Moreno, S. (2013). New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control, 31(1), 189–195. doi:10.1016/j.foodcont.2012.09.022.
Olagnier, D., Costes, P., Berry, A., Linas, M.-D., Urrutigoity, M., Dechy-Cabaretb, O., & Benoit-Vical, F. (2007). Modifications of the chemical structure of terpenes in antiplasmodial and antifungal drug research. Bioorganic & Medicinal Chemistry Letters, 17(22), 6075–6078. doi:10.1016/j.bmcl.2007.09.056.
Ooi, L. S. M., Li, Y., Kam, S.-L., Wang, H., Wong, E. Y. L., & Ooi, V. E. C. (2006). Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. The American Journal of Chinese Medicine, 34(3), 511–522. doi:10.1142/S0192415X06004041.
Organização Mundial de Saúde, O. (2015). Food safety. Fact sheet N°399. http://www.who.int/mediacentre/factsheets/fs399/en/. Accessed 24 August 2016.
Otero, V., Becerril, R., Santos, J. A., Rodríguez-Calleja, J. M., Nerín, C., & García-López, M. L. (2014). Evaluation of two antimicrobial packaging films against Escherichia coli O157: H7 strains invitro and during storage of a Spanish ripened sheep cheese (Zamorano). Food Control, 42, 296–302. doi:10.1016/j.foodcont.2014.02.022.
Oussalah, M., Caillet, S., Salmiéri, S., Saucier, L., & Lacroix, M. (2004). Antimicrobial and antioxidant effects of milk protein-based film containing essential oils for the preservation of whole beef muscle. Journal of Agricultural and Food Chemistry, 52(18), 5598–5605. doi:10.1021/jf049389q.
Oussalah, M., Caillet, S., Salmiéri, S., Saucier, L., & Lacroix, M. (2007). Antimicrobial effects of alginate-based films containing essential oils on Listeria monocytogenes and Salmonella typhimurium present in bologna and ham. Journal of Food Protection, 70(4), 901–908 http://www.ncbi.nlm.nih.gov/pubmed/17477259.
Patras, A., Yuan, Y. V., Costa, H. S., & Sanches-Silva, A. (2013). Antioxidant activity of phytochemicals. In B. K. Tiwari, N. P. Brunton, & C. S. Brennan (Eds.), Handbook of plant phytochemicals: sources, stability and extraction (pp. 452–472). Hoboken: John Wiley & Sons, Ltd..
Pavela, R. (2015). Essential oils for the development of eco-friendly mosquito larvicides: a review. Industrial Crops and Products, 76, 174–187. doi:10.1016/j.indcrop.2015.06.050.
Pavela, R., Žabka, M., Bednář, J., Tříska, J., & Vrchotová, N. (2016). New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.) Industrial Crops and Products, 83, 275–282. doi:10.1016/j.indcrop.2015.11.090.
Pei, R. S., Zhou, F., Ji, B. P., & Xu, J. (2009). Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. Journal of Food Science, 74(7), 379–383. doi:10.1111/j.1750-3841.2009.01287.x.
Perdones, A., Escriche, I., Chiralt, A., & Vargas, M. (2016). Effect of chitosan-lemon essential oil coatings on volatile profile of strawberries during storage. Food Chemistry, 197, 979–986. doi:10.1016/j.foodchem.2015.11.054.
Periasamy, V. S., Athinarayanan, J., & Alshatwi, A. A. (2016). Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrasonics Sonochemistry, 31, 449–455. doi:10.1016/j.ultsonch.2016.01.035.
Petretto, G. L., Fancello, F., Zara, S., Foddai, M., Mangia, N. P., Sanna, M. L., et al. (2014). Antimicrobial activity against beneficial microorganisms and chemical composition of essential oil of Mentha suaveolens ssp. insularis grown in Sardinia. Journal of Food Science, 79(3), M369–M377. doi:10.1111/1750-3841.12343.
Pilar Santamarina, M., Roselló, J., Giménez, S., & Amparo Blázquez, M. (2016). Commercial Laurus nobilis L. and Syzygium aromaticum L. Merr. & Perry essential oils against post-harvest phytopathogenic fungi on rice. LWT-Food Science and Technology, 65, 325–332. doi:10.1016/j.lwt.2015.08.040.
Pires, C., Ramos, C., Teixeira, B., Batista, I., Nunes, M. L., & Marques, A. (2013). Hake proteins edible films incorporated with essential oils: physical, mechanical, antioxidant and antibacterial properties. Food Hydrocolloids, 30(1), 224–231. doi:10.1016/j.foodhyd.2012.05.019.
Pokorny, J., Yanishlieva, N., & Gordon, M. H. (Eds.). (2001). Antioxidants in food: practical applications. CRC Press.
Ponce, A. G., Fritz, R., Del Valle, C. & Roura, S. I. (2003). Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT- Food Science and Technology, 36, 679–68. doi:10.1016/S0023-6438(03)00088-4.
Ponce, A. G., Del Valle, C. E. & Roura, S. I. (2004). Natural essential oils as reducing agents of peroxidase activity in leafy vegetables. LWT- Food Science and Technology, 37, 199–204. doi:10.1016/j.lwt.2003.07.005.
Porres-Martínez, M., González-Burgos, E., Accame, M. E. C., & Gómez-Serranillos, M. P. (2013). Phytochemical composition, antioxidant and cytoprotective activities of essential oil of Salvia lavandulifolia Vahl. Food Research International, 54(1), 523–531. doi:10.1016/j.foodres.2013.07.029.
Prabuseenivasan, S., Jayakumar, M., & Ignacimuthu, S. (2006). In vitro antibacterial activity of some plant essential oils. BMC Complementary and Alternative Medicine, 6, 39. doi:10.1186/1472-6882-6-39.
Prakash, B., Singh, P., Kedia, A., & Dubey, N. K. (2012). Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Research International, 49(1), 201–208. doi:10.1016/j.foodres.2012.08.020.
Proestos, C., Sereli, D., & Komaitis, M. (2006). Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chemistry, 95(1), 44–52. doi:10.1016/j.foodchem.2004.12.016.
Raeisi, M., Tajik, H., Aliakbarlu, J., Mirhosseini, S. H., & Hosseini, S. M. H. (2015). Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT-Food Science and Technology, 64(2), 898–904. doi:10.1016/j.lwt.2015.06.010.
Raut, J. S., & Karuppayil, S. M. (2014). A status review on the medicinal properties of essential oils. Industrial Crops and Products, 62, 250–264. doi:10.1016/j.indcrop.2014.05.055.
Razzaghi-Abyaneh, M., Shams-Ghahfarokhi, M., Yoshinari, T., Rezaee, M.-B., Jaimand, K., Nagasawa, H., & Sakuda, S. (2008). Inhibitory effects of Satureja hortensis L. essential oil on growth and aflatoxin production by Aspergillus parasiticus. International Journal of Food Microbiology, 123(3), 228–233. doi:10.1016/j.ijfoodmicro.2008.02.003.
Riahi, L., Chograni, H., Elferchichi, M., Zaouali, Y., Zoghlami, N., & Mliki, A. (2013). Variations in Tunisian wormwood essential oil profiles and phenolic contents between leaves and flowers and their effects on antioxidant activities. Industrial Crops and Products, 46, 290–296. doi:10.1016/j.indcrop.2013.01.036.
Ribeiro-Santos, R., Carvalho-Costa, D., Cavaleiro, C., Costa, H. S., Albuquerque, T. G., Castilho, M. C., et al. (2015). A novel insight on an ancient aromatic plant: the rosemary (Rosmarinus officinalis L.) Trends in Food Science and Technology, 45(2), 355–368. doi:10.1016/j.tifs.2015.07.015.
Ribeiro-Santos, R., Andrade, M., de Melo, N. R., dos Santos, F. R., de Neves, I. A., de Carvalho, M. G., & Sanches-Silva, A. (2017). Biological activities and major components determination in essential oils intended for a biodegradable food packaging. Industrial Crops and Products, 97, 201–210. doi:10.1016/j.indcrop.2016.12.006.
Romano, C. S., Abadi, K., Repetto, V., Vojnov, A. A., & Moreno, S. (2009). Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives. Food Chemistry, 115(2), 456–461. doi:10.1016/j.foodchem.2008.12.029.
Ross, Z. M., Gara, E. A. O., Hill, D. J., & Sleightholme, H. V. (2001). Antimicrobial Properties of Garlic Oil against Human Enteric Bacteria : Evaluation of Methodologies and Comparisons with Garlic Oil Sulfides and Garlic Powder, 67(1), 475–480. doi:10.1128/AEM.67.1.475.
Rozman, T., & Jersek, B. (2009). Antimicrobial activity of rosemary extracts (Rosmarinus officinalis L.) against different species of Listeria. Acta agriculturae Slovenica, 93(1), 51–58. doi:10.2478/v10014-009-0007-z.
Rufino, M. S.., Alve, R. E., Brito, E. S., Morais, S. M., Sampaio, C. G., Pérez-Jiménez, J., & Saura-Calixto, F. D. (2006). Metodologia científica: determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Embrapa-Comunicado Técnico, 125.
Rufino, M. S.., Alve, R. E., Brito, E. S., Morais, S. M., Sampaio, C. G., Pérez-Jiménez, J., & Saura-Calixto, F. D. (2007). Metodologia científica: determinação da atividade antioxidante total em frutas pela captura do radical livre ABTS●+. Comunicado Técnico, 128.
Salgado, P. R., López-Caballero, M. E., Gómez-Guillén, M. C., Mauri, A. N., & Montero, M. P. (2013). Sunflower protein films incorporated with clove essential oil have potential application for the preservation of fish patties. Food Hydrocolloids, 33(1), 74–84. doi:10.1016/j.foodhyd.2013.02.008.
Sanches-Silva, A., Rodríguez-Bernaldo de Quirós, A., López-Hernández, J., & Paseiro-Losada, P. (2004). Determination of hexanal as indicator of the lipidic oxidation state in potato crisps using gas chromatography and high-performance liquid chromatography. Journal of Chromatography A, 1046(1–2), 75–81. doi:10.1016/j.chroma.2004.06.101.
Sánchez Aldana, D., Andrade-Ochoa, S., Aguilar, C. N., Contreras-Esquivel, J. C., & Nevárez-Moorillón, G. V. (2015). Antibacterial activity of pectic-based edible films incorporated with Mexican lime essential oil. Food Control, 50, 907–912. doi:10.1016/j.foodcont.2014.10.044.
Sánchez-González, L., Cháfer, M., Chiralt, A., & González-Martínez, C. (2010). Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydrate Polymers, 82(2), 277–283. doi:10.1016/j.carbpol.2010.04.047.
Santos, T. G., Dognini, J., Begnini, I. M., Rebelo, R. A., Verdi, M., de Gasper, A. L., & Dalmarco, E. M. (2013). Chemical characterization of essential oils from Drimys angustifoliaMiers (Winteraceae) and antibacterial activity of their major compounds. Journal of the Brazilian Chemical Society, 24(1), 164–170. doi:10.1590/S0103-50532013000100020.
Schaller, M., & Korting, H. C. (1995). Allergie airborne contact dermatitis from essential oils used in aromatherapy. Clinical and Experimental Dermatology, 20(2), 143–145. doi:10.1111/j.1365-2230.1995.tb02719.x.
Šegvić Klarić, M., Kosalec, I., Mastelić, J., Piecková, E., & Pepeljnak, S. (2007). Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Letters in Applied Microbiology, 44(1), 36–42. doi:10.1111/j.1472-765X.2006.02032.x.
Shahwar, D., Raza, M. A., Bukhari, S., & Bukhari, G. (2012). Ferric reducing antioxidant power of essential oils extracted from Eucalyptus and Curcuma species. Asian Pacific Journal of Tropical Biomedicine, 2(3), S1633–S1636. doi:10.1016/S2221-1691(12)60467-5.
Shan, B., Cai, Y. Z., Sun, M., & Corke, H. (2005). Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry, 53(20), 7749–7759. doi:10.1021/jf051513y.
Silvestre, W. P., Agostini, F., Muniz, L. A. R., & Pauletti, G. F. (2016). Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. Journal of Food Engineering, 178, 90–94. doi:10.1016/j.jfoodeng.2016.01.011.
Singh, G., Maurya, S., DeLampasona, M. P., & Catalan, C. A. N. (2007). A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food and Chemical Toxicology, 45(9), 1650–1661. doi:10.1016/j.fct.2007.02.031.
Skandamis, P. N., & Nychas, G.-J. E. (2002). Preservation of fresh meat with active and modified atmosphere packaging conditions. International Journal of Food Microbiology, 79(1–2), 35–45. doi:10.1016/S0168-1605(02)00177-0.
Souza, A. C., Goto, G. E. O., Mainardi, J. A., Coelho, A. C. V., & Tadini, C. C. (2013). Cassava starch composite films incorporated with cinnamon essential oil: antimicrobial activity, microstructure, mechanical and barrier properties. LWT-Food Science and Technology, 54(2), 346–352. doi:10.1016/j.lwt.2013.06.017.
Stojkovic, D., Glamoclija, J., Ciric, A., Nikolic, M., Ristic, M., Siljegovic, J., & Sokovic, M. (2013). Investigation on antibacterial synergism of Origanum vulgare and Thymus vulgaris essential oils. Archives of Biological Sciences, 65(2), 639–643. doi:10.2298/ABS1302639S.
Takala, P. N., Vu, K. D., Salmieri, S., Khan, R. A., & Lacroix, M. (2013). Antibacterial effect of biodegradable active packaging on the growth of Escherichia coli, Salmonella typhimurium and Listeria monocytogenes in fresh broccoli stored at 4 °C. LWT-Food Science and Technology, 53(2), 499–506. doi:10.1016/j.lwt.2013.02.024.
Teixeira, B., Marques, A., Ramos, C., Batista, I., Serrano, C., Matos, O., et al. (2012). European pennyroyal (Mentha pulegium) from Portugal: chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Industrial Crops and Products, 36(1), 81–87. doi:10.1016/j.indcrop.2011.08.011.
Teixeira, B., Marques, A., Ramos, C., Neng, N. R., Nogueira, J. M. F., Saraiva, J. A., & Nunes, M. L. (2013). Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Industrial Crops and Products, 43(1), 587–595. doi:10.1016/j.indcrop.2012.07.069.
Tian, F., Decker, E. A., & Goddard, J. M. (2013). Controlling lipid oxidation via a biomimetic iron chelating active packaging material. Journal of Agricultural and Food Chemistry, 61(50), 12397–12404. doi:10.1021/jf4041832.
Tisserand, R., & Young, R. (2014). Essential oil safety—a guide for health care professionals (Second edi ed.). Churchill Livingstone: Elsevier http://arxiv.org/abs/1011.1669.
Tongnuanchan, P., Benjakul, S., & Prodpran, T. (2013). Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. Journal of Food Engineering, 117(3), 350–360. doi:10.1016/j.jfoodeng.2013.03.005.
Trattner, A., David, M., & Lazarov, A. (2008). Occupational contact dermatitis due to essential oils. Contact Dermatitis, 58(5), 282–284. doi:10.1111/j.1600-0536.2007.01275.x.
Tung, Y.-T., Chua, M.-T., Wang, S.-Y., & Chang, S.-T. (2008). Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresource Technology, 99(9), 3908–3913. doi:10.1016/j.biortech.2007.07.050.
Ud-Daula, A. F. M. S., Demirci, F., Abu Salim, K., Demirci, B., Lim, L. B. L., Baser, K. H. C., & Ahmad, N. (2016). Chemical composition, antioxidant and antimicrobial activities of essential oils from leaves, aerial stems, basal stems, and rhizomes of Etlingera fimbriobracteata (K. Schum.) R.M.Sm. Industrial Crops and Products, 84, 189–198. doi:10.1016/j.indcrop.2015.12.034.
Unlu, M., Ergene, E., Unlu, G. V., Zeytinoglu, H. S., & Vural, N. (2010). Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food and Chemical Toxicology, 48(11), 3274–3280. doi:10.1016/j.fct.2010.09.001.
Uter, W., Schmidt, E., Geier, J., Lessmann, H., Schnuch, A., & Frosch, P. (2010). Contact allergy to essential oils: current patch test results (2000–2008) from the Information Network of Departments of Dermatology (IVDK)*. Contact Dermatitis, 63(5), 277–283. doi:10.1111/j.1600-0536.2010.01768.x.
Velasco, J., Dobarganes, C., Holgado, F., & Márquez-Ruiz, G. (2009). A follow-up oxidation study in dried micro encapsulated oils under the accelerated conditions of the Rancimat test. Food Research International, 42(1), 56–62. doi:10.1016/j.foodres.2008.08.012.
Velluti, A., Sanchis, V., Ramos, A. J., Egido, J., & Marín, S. (2003). Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. International Journal of Food Microbiology, 89(2–3), 145–154. doi:10.1016/S0168-1605(03)00116-8.
Viteri Jumbo, L. O., Faroni, L. R. A., Oliveira, E. E., Pimentel, M. A., & Silva, G. N. (2014). Potential use of clove and cinnamon essential oils to control the bean weevil, Acanthoscelides obtectus Say, in small storage units. Industrial Crops and Products, 56, 27–34. doi:10.1016/j.indcrop.2014.02.038.
Viuda-Martos, M., Ruiz Navajas, Y., Sánchez Zapata, E., Fernández-López, J., & Pérez-Álvarez, J. A. (2010). Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour and Fragrance Journal, 25(1), 13–19. doi:10.1002/ffj.1951.
Viuda-Martos, M., Mohamady, M. A., Fernández-López, J., Abd ElRazik, K. A., Omer, E. A., Pérez-Alvarez, J. A., & Sendra, E. (2011). In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control, 22(11), 1715–1722. doi:10.1016/j.foodcont.2011.04.003.
Volpe, M. G., Siano, F., Paolucci, M., Sacco, A., Sorrentino, A., Malinconico, M., & Varricchio, E. (2015). Active edible coating effectiveness in shelf-life enhancement of trout (Oncorhynchus mykiss) fillets. LWT-Food Science and Technology, 60(1), 615–622. doi:10.1016/j.lwt.2014.08.048.
Wang, W., Wu, N., Zu, Y. G., & Fu, Y. J. (2008). Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chemistry, 108(3), 1019–1022. doi:10.1016/j.foodchem.2007.11.046.
Wei, S.-D., Chen, H., Yan, T., Lin, Y.-M., & Zhou, H.-C. (2014). Identification of antioxidant components and fatty acid profiles of the leaves and fruits from Averrhoa carambola. LWT-Food Science and Technology, 55(1), 278–285. doi:10.1016/j.lwt.2013.08.013.
Ye, C.-L., Dai, D.-H., & Hu, W.-L. (2013). Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.) Food Control, 30(1), 48–53. doi:10.1016/j.foodcont.2012.07.033.
Yen, H. F., Hsieh, C. T., Hsieh, T. J., Chang, F. R., & Wang, C. K. (2015). In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. Journal of Food and Drug Analysis, 23(1), 124–129. doi:10.1016/j.jfda.2014.02.004.
Zeng, Q., Zhao, J., Wang, J., Zhang, X., & Jiang, J. (2016). Comparative extraction processes, volatile compounds analysis and antioxidant activities of essential oils from Cirsium japonicum Fisch. ex DC and Cirsium setosum (Willd.) M. Bieb. LWT-Food Science and Technology, 68, 595–605. doi:10.1016/j.lwt.2016.01.017.
Zinoviadou, K. G., Koutsoumanis, K. P., & Biliaderis, C. G. (2009). Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Science, 82(3), 338–345. doi:10.1016/j.meatsci.2009.02.004.
Acknowledgements
This work was supported by the research project “Development of an Edible Film Based on Whey Protein with Antioxidant and Antimicrobial Activity Using Essential Oils” (2012DAN807) funded by the National Institute of Health Dr. Ricardo Jorge, I.P. (Lisbon, Portugal). Regiane Ribeiro dos Santos (BEX 8754/14-4) is grateful for her research grant funded by Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) and Department of Food Technology, Institute of Technology, Rural Federal University of Rio de Janeiro, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro-Santos, R., Andrade, M., Sanches-Silva, A. et al. Essential Oils for Food Application: Natural Substances with Established Biological Activities. Food Bioprocess Technol 11, 43–71 (2018). https://doi.org/10.1007/s11947-017-1948-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1948-6