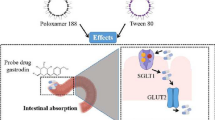
Abstract
Purpose
Despite no broad, direct evidence in humans, there is a potential concern that surfactants alter active or passive drug intestinal permeation to modulate oral drug absorption. The purpose of this study was to investigate the impact of the surfactant polysorbate 80 on active and passive intestinal drug absorption in humans.
Methods
The human (n = 12) pharmacokinetics (PK) of three probe substrates of intestinal absorption, valacyclovir, chenodeoxycholic acid (CDCA), and enalaprilat, were assessed. Endogenous bile acid levels were assessed as a secondary measure of transporter and microbiota impact.
Results
Polysorbate 80 did not inhibit peptide transporter 1 (PepT1)- or apical sodium bile acid transporter (ASBT)-mediated PK of valacyclovir and CDCA, respectively. Polysorbate 80 did not increase enalaprilat absorption. Modest increases in unconjugated secondary bile acid Cmax ratios suggest a potential alteration of the in vivo intestinal microbiota by polysorbate 80.
Conclusions
Polysorbate 80 did not alter intestinal membrane fluidity or cause intestinal membrane disruption. This finding supports regulatory relief of excipient restrictions for Biopharmaceutics Classification System-based biowaivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Excipients are inactive ingredients in drug products and have the potential to modulate drug absorption in undesirable ways (1). Such excipients have been conceived as “absorption-modifying excipients” (AMEs). AMEs in drug products have important regulatory implications during formulation changes (e.g., scale-up and post-approval modifications, formation of a new generic product) (2, 3). Specifically, the waiving of in vivo bioequivalence (BE) studies (i.e., biowaiver) for highly soluble drugs [i.e., Biopharmaceutics Classification System (BCS) Class I and III drugs] carries potential concerns. Hence, there are major limitations on quantitative and qualitative excipient changes for drug approval. For several common excipients, we have shown that such potential concerns did not have an in vivo impact in humans (4).
Despite no broad, direct evidence in humans, there is a potential concern that surfactants alter drug intestinal permeability to increase oral drug absorption (5). For example, the nonionic surfactant polysorbate 80 (also known as Tween 80 or polyoxyethylene 20 sorbitan monooleate), has been shown in Caco-2 cell monolayers (6, 7) and mice (8) to modulate intestinal permeability. Polysorbate 80 has been shown in vitro to inhibit intestinal transporters, specifically peptide transporter 1 (PepT1, SLC15A1) and P-glycoprotein (P-gp, MDR1, ABCB1) (1, 6, 7, 9). Transporters are membrane-bound proteins in the dynamic lipid bilayer. The fluidity of the lipid bilayer and specific lipids that are tightly bound to transporters can impact transporter function in vitro (10). Surfactants can alter membrane fluidity and hence presumably change the structure/conformation and function of transporters in vitro (11, 12). Such changes have been associated in vitro with the inhibition of PepT1 and P-gp (6, 9). Surfactants can also inhibit protein kinase C (PKC) in vitro and modulate the function of several transporters that are regulated by PKC-dependent pathways, such as P-gp and apical sodium bile acid transporter (ASBT, SLC10A2) (13, 14). Polysorbate 80 was also shown in vitro to disrupt cytochrome P450 3A4 (CYP3A4) enzyme activity (15, 16). Polysorbate 80 has been demonstrated ex vivo in humans to disrupt the composition and function of intestinal microbiota (17), which could damage the epithelial barrier and contribute to inflammation of the intestinal tract (18).
The primary objective of this study was to assess the effect of oral polysorbate 80 on the human (n=12) pharmacokinetics (PK) of three probe substrates of intestinal absorption: valacyclovir, chenodeoxycholic acid (CDCA; also known as chenodiol), and enalaprilat. Valacyclovir is a PepT1 substrate (19, 20). CDCA is an ASBT substate (21). PepT1 and ASBT are active transporters in the intestine. Enalaprilat was employed as a very low passive permeability marker (22). Endogenous bile acid levels were assessed as a secondary measure of transporter and microbiota impact. We hypothesized that polysorbate 80 will not inhibit PepT1-mediated and ASBT-mediated intestinal absorption of valacyclovir and CDCA, respectively, and not increase passive intestinal permeability of enalaprilat. We further hypothesized that polysorbate 80 will modify intestinal microbiota, leading to alterations in plasma secondary bile acids.
Plasma was assessed for changes in acyclovir (i.e., the active metabolite of valacyclovir), CDCA, enalaprilat, and endogenous bile acid PK, relative to placebo. Results showed that polysorbate 80 (400mg) BID had no impact on PepT1, ASBT, or passive intestinal membrane permeability. Polysorbate 80 increased the Cmax ratio of unconjugated secondary bile acids compared to placebo, whereas AUC was not impacted. Hence, polysorbate 80 may have modulated intestinal microbiota, leading to a higher Cmax of unconjugated secondary bile acids in the plasma.
MATERIALS AND METHODS
Materials
Polysorbate 80 (400mg) capsules were manufactured at the University of Maryland Good Manufacturing Practice (GMP) facility using Polysorbate 80, NF (Spectrum Chemical; New Brunswick, NJ). Placebo tablets were manufactured at the University of Maryland GMP facility using PROSOLV® EASYtab SP (JRS PHARMA; Weissenborn, Germany). Valacyclovir (500mg) tablets were obtained from Mylan Pharmaceuticals (Canonsburg, PA). CDCA (250mg) tablets (Chenodal; Lot #004949) were kindly supplied by Travere Therapeutics (San Diego, CA). Enalaprilat solution (2.5mg/2mL) was obtained from West-Ward Pharmaceuticals (Berkeley Heights, NJ). LC-MS/MS grade solvents were purchased from Fisher Scientific (Pittsburgh, PA). Acyclovir was obtained from United States Pharmacopeia (Rockville, MD) and enalaprilat was obtained from Sigma Aldrich (St. Louis, MO). Acyclovir-d4 was obtained from Toronto Research Chemicals (North York, ON, Canada) and enalaprilat-d5 was obtained from CDN Isotopes (Pointe-Claire, GC, Canada). Bile acid standards and stable isotope labeled standards were purchased from Sigma Aldrich (St. Louis, MO), Toronto Research Chemicals (North York, ON, Canada), Steraloids (Newport, RI), Cambridge Isotope Laboratories (Tewksbury, MA), or CDN Isotopes (Pointe-Claire, QC, Canada). ISOLUTE PLD+ phospholipid depletion columns were purchased from Biotage (Uppsala, Sweden).
Methods
Clinical Study
An open-label, randomized, single-dose, placebo-controlled, fasted, crossover PK study was conducted in n=12 healthy adult volunteers (ClinicalTrials.gov identifier: NCT04640571). The study was approved by the Institutional Review Board at University of Maryland, Baltimore and was conducted at the General Clinical Research Center at the University of Maryland. Informed consent was obtained from all participants in the study. Table SI (see Supplementary Information) describes participant demographics. All volunteers received polysorbate 80 and placebo with a minimum washout period of 10 days and maximum washout period of 28 days. In this study, polysorbate 80 (400mg) BID or placebo BID was administered orally for 6 days. Volunteers were given a study schedule and journal to report adverse events. On day 7, after a minimum of 10h overnight fast, a single dose of valacyclovir (500mg), CDCA (250mg), and enalaprilat (20mg) were administered orally with 240mL water. Cocktail drug doses were selected based on Food and Drug Administration (FDA) approved doses and known risks, as well as doses where valacyclovir is a PepT1 substrate, CDCA is an ASBT substate, and enalaprilat exhibits very low passive permeability (19,20,21,22). A final dose of polysorbate 80 (400mg) was included in the polysorbate 80 arm to assess for effects on the intestinal membrane and transporter inhibition. Pravastatin (80mg) was also administered concomitantly in a cocktail approach for a corresponding sub-study with a shared placebo arm; this separate and distinct sub-study assessed potential metformin impact. The four probes of the cocktail (i.e., valacyclovir, CDCA, enalaprilat, and pravastatin) were not expected to modulate one another (23,24,25,26,27,28). Nonetheless, cocktail was administered orally in the same fashion across all three arms to minimize the impact of potential interactions among the four probes in comparing polysorbate 80 arm to placebo arm.
The polysorbate 80 dosing design aimed to administer a large, allowable amount of polysorbate 80, where 418.37 mg of polysorbate 80 is present in an approved capsule product (29). Polysorbate 80 BID for 6 days, plus a morning dose at time of cocktail administration, aimed to allow for potential polysorbate 80 effects on the intestinal membrane and intestinal transporters (e.g., transporter inhibition and/or induction).
Water was not allowed 1h before and 1h after administration of probe cocktail. Participants were provided standardized lunch and a snack 4 and 7.5h after cocktail administration, respectively. Blood samples (~5cc, heparinized tubes) for PK analysis were drawn prior to cocktail administration and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 10h post-dose. The blood samples were centrifuged at >2000rpm at 4°C for 10min within 15min of collection to produce plasma. Harvested plasma aliquots were stored at −80°C until assayed. The plasma concentrations of acyclovir, CDCA, enalaprilat, and endogenous bile acids were quantified by mass spectrometry.
Computational Modeling
We conducted computational modeling to assess if CDCA or pravastatin were predicted inhibitors of PepT1, and if valacyclovir inhibits ASBT (Figure S1). Briefly, PepT1 inhibition activity for 223 compounds was obtained from the ChEMBL dataset (www.ebi.ac.uk/chembl). A Bayesian approach using Assay Central software was employed,(30, 31) using a PepT1 model threshold of 100µM. The model exhibited favorable precision, recall, and specificity. CDCA and pravastatin were each predicted to be non-inhibitors of PepT1. Similarly, ASBT inhibition activity for 251 compounds was obtained, and a model was developed using a threshold of 60nM. The model exhibited favorable precision, recall, and specificity. Valacyclovir was predicted to be a non-inhibitor of ASBT.
Quantification of Acyclovir and Enalaprilat
Drug concentrations in plasma were measured using a liquid chromatography - tandem mass spectrometry (LC-MS/MS) method. Acyclovir and enalaprilat were measured in human plasma using deuterated internal standard (IS) with extraction by simple acetonitrile-based protein precipitation. Briefly, acyclovir and acyclovir-d4 stock solutions were prepared in dimethyl sulfoxide (DMSO). Similarly, enalaprilat and enalaprilat-d5 stock solutions were prepared by independently weighing out reference material and dissolving in methanol. All stock solutions were stored at -20°C for the duration of the study. Fresh calibration standard samples were prepared at nine concentrations (5, 10, 50, 100, 500, 1000, 2000, 4000 and 5000ng/mL for acyclovir; and 0.2, 0.4, 1, 5, 10, 25, 100, 300, and 500ng/mL for enalaprilat) for each analytical batch. Quality control samples at four concentrations (15, 210, 2100 and 4200ng/mL for acyclovir; and 0.8, 8, 200 and 400ng/mL for enalaprilat) were used to assess assay reliability. All stock solutions were used within 2 months of preparation.
For extraction, 100µL of plasma was mixed with 25µL of internal standard solution (4000ng/mL of acyclovir-d4 and 700ng/mL of enalaprilat-d5) and were extracted with 500µL of acetonitrile. The samples were shaken for 3min and were centrifuged at 15000rpm for 10min at 5°C. Supernatant was collected and evaporated to dryness. The residue was reconstituted in 100µL of water and methanol (50:50, v/v) and a 2µL aliquot was injected.
LC-MS/MS was performed on a Waters H-Class ultraperformance liquid chromatography system coupled to a Waters TQ-XS tandem quadrupole mass spectrometer (Waters Corporation, Milford, MA, USA). The chromatographic separation was achieved on a Phenomenex (Torrance, CA, USA) Kinetex C18 column (2.1×100 mm, 2.6μm) at a column temperature of 45 ± 2°C. Acetonitrile was used as mobile phase A and 5 mM ammonium acetate with 0.1% formic acid was used as mobile phase B. The gradient program was performed over 6min, starting with 99.9% B, ramping to 80% B over 0.5min, continuing to ramp to 20% B over 2.0min, holding at 80% B for 1min and then equilibrating the column for 2.5min. Flow rate was 0.400mL/min. The mass spectrometer was operated in electrospray ionization (ESI) positive ion mode for the analysis with m/z transitions of 225.6 → 151.9 for acyclovir; 229.6 → 151.9 for acyclovir-d4; 348.8 → 206.1 for enalaprilat and 354.1 → 211.0 for enalaprilat-d5. Parameters were: capillary voltage 1.5kV; desolvation temperature 600°C; desolvation flow 1000L/hr; cone flow 150 L/hr; nebulizer 7Bar; collision gas flow 0.15mL/min; source temperature 150°C; dwell time 30msec; cone voltage: acyclovir 4V, acyclovir-d4 8V, enalaprilat 2V, and enalaprilat-d5 14V; collision energy: acyclovir 16V, acyclovir-d4 22V, enalaprilat 22V, and enalaprilat-d5 24V.
Analyte quantification was achieved in the concentration range of 5-5000ng/mL of acyclovir and 0.2-500ng/mL of enalaprilat in plasma. Batches were considered acceptable if standards and QCs met the acceptance criteria as detailed by the FDA Guidance for Bioanalytical Method Validation (32). All data was acquired using MassLynx (v 4.1). Standard regression was performed using TargetLynx (v 4.1) and all analytes used linear 1/x2 weighting. AUC was calculated between 0 and 10h using the trapezoidal rule.
Quantification of CDCA and Endogenous Bile Acids
Bile acids were quantified using a method previously described with minor adjustments (33). Briefly, stock solutions were prepared by independently weighing out reference material and dissolving in methanol. The concentration of the resulting stock solution considered salt form and purity from the certificate of analysis, as necessary. All stock solutions were stored at -20°C for the duration of the study. Fresh calibration standard samples were prepared at nine concentrations (1, 5, 10, 50, 100, 200, 500, 900, and 1000ng/mL) for each analytical batch. Quality control samples at three concentrations (15, 400, and 800ng/mL) were used to assess assay reliability. All stock solutions were used within 2 months of preparation. No significant decrease in response was noted and slope was consistent for all analytical runs.
For extraction, 100µL of plasma was loaded directly onto the ISOLUTE PLD+ column per manufacturer’s instructions and then 5µL of internal standard mixture (1000ng/mL stable isotope labeled bile acid cocktail) was added to each tube. Samples were then spiked with 400µL of acetonitrile and independently vortexed for 30s to precipitate proteins. Sample flow through was collected by applying approximately 4psi via positive pressure manifold. Eluent was dried down under nitrogen stream at 40°C and the samples were reconstituted with 50:50 water/acetonitrile with 0.01% formic acid. Samples were then transferred to glass autosampler vials with conical inserts for analysis.
LC-MS/MS was performed on a Waters I-Class ultraperformance liquid chromatography system coupled to a Waters TQ-XS tandem quadrupole mass spectrometer (Waters Corporation, Milford, MA, USA). Chromatographic separation was performed using 0.01% formic acid in water as mobile phase A and 0.01% formic acid in acetonitrile as mobile phase B. The column used was Acquity BEH C18 (150×2.1mm, 1.7µm;Waters Corporation, Milford, MA, USA) and the column temperature was set to 55°C. The gradient program was performed over 32min, starting with 25% B, ramping to 40% B over 12min, continuing to ramp to 75% B over 14min, followed by 100% B for 3min and then equilibrating the column for 4min. The flow rate was 0.350mL/min. Of note, the strong needle wash was changed from the original study to acetonitrile:2-propanol:methanol:formic acid/30:30:40:0.5/v:v:v:v in order to alleviate carry over from taurine conjugated bile acids. 3µL was injected for each sample. Tandem mass spectrometry analysis was carried out in ESI negative ion mode due to the acidic group on bile acids. Parameters were: capillary voltage 2.5kV; desolvation temperature 500°C; desolvation flow 800L/hr; cone flow 150L/hr; nebulizer 7Bar; collision gas flow 0.15mL/min; and source temperature 150°C.
Three scheduled periods were used to monitor the elution from the column: Period 1 (4-10min) monitored for glycoursodeoxycholic acid (GUDCA), glycocholic acid (GCA), tauroursodeoxycholic acid (TUDCA), and taurocholic acid (TCA); Period 2 (10-16min) monitored for ursodeoxycholic acid (UDCA), cholic acid (CA), glycochenodeoxycholic acid (GCDCA), glycodeoxycholic acid (GDCA), taurochenodeoxycholic acid (TCDCA), and taurodeoxycholic acid (TDCA); Period 3 (16-24min) monitored for lithocholic acid (LCA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA), glycolithocholic acid (GLCA), and taurolithocholic acid (TLCA). Mass transitions, cone voltage, and collision energy are provided in Table SII. Batches were considered acceptable if standards and QCs met the acceptance criteria as detailed by the FDA Guidance for Bioanalytical Method Validation (32). All data was acquired using MassLynx (v 4.1). Standard regression was performed using TargetLynx (v 4.1) and all analytes used linear 1/x2 weighting except for UDCA, which used quadratic 1/y2 weighting as recommended by Gu et al. (34). AUC was calculated between 0 and 10h using the trapezoidal rule.
Statistical Analysis
All numerical results were expressed as mean of three replicates ± SEM. Differences were determined to be statistically significant using Student’s t-test.
Non-compartmental analysis was used to calculate the PK parameters Cmax and AUC for drugs within cocktail (i.e., valacyclovir, baseline-corrected CDCA, enalaprilat, and pravastatin) and endogenous plasma bile acids. Valacyclovir is the pro-drug of the active moiety acyclovir. Differences between polysorbate 80 and placebo arms were considered statistically significant when p < 0.05 for acyclovir, CDCA, enalaprilat, and endogenous bile acids using an unpaired one-tailed Student’s t-test. Differences in Cmax and AUC point estimate ratios (i.e., means of the ratios of polysorbate 80/placebo) were considered statistically significant from a value of one when p < 0.05 for acyclovir, CDCA, enalaprilat, and endogenous bile acids, using a one sample, unpaired one-tailed Student’s t-test.
RESULTS
Participant Adverse Events
Polysorbate 80 (400mg) BID was well-tolerated among all participants with only two participants exhibiting adverse events. One participant had easy bowel movements on day 7 after cocktail administration and one participant exhibited an itchy rash following the polysorbate 80 round (i.e., during the washout period). In the placebo arm, one participant exhibited diarrhea on day 1, one participant exhibited lightheadedness and vision tunnelling following the pre-dose blood draw, and one participant had vasovagal syncope due to intravenous insertion. All adverse events resolved without sequalae.
Valacyclovir
Mean profiles of acyclovir in the polysorbate 80 and placebo arms are shown in Fig. 1. Polysorbate 80 had no effect on acyclovir plasma exposure, as hypothesized. Mean PK parameters of acyclovir after polysorbate 80 and placebo arms are shown in Table I. Polysorbate 80 had no effect on the Cmax (p=0.4) or AUC (p=0.4) of acyclovir compared to placebo. Acyclovir Cmax and AUC ratios for polysorbate 80 versus placebo were 1.10 and 1.03, respectively, and were not statistically different than unity (p=0.1 and 0.3, respectively).
Mean concentration versus time profile of acyclovir in n = 12 participants in the polysorbate 80 and placebo arms. Participants took 400 mg polysorbate 80 or placebo BID for 6 days. On day 7, participants were administered a single dose of 500 mg valacyclovir, 250 mg CDCA, and 20 mg enalaprilat, as well as 400 mg polysorbate 80 in polysorbate 80 arm. Polysorbate 80 had no effect on the plasma concentrations of acyclovir. Data are expressed as mean ± SEM.
CDCA
Mean profiles of CDCA in the polysorbate 80 and placebo arms are shown in Fig. 2. Polysorbate 80 did not alter CDCA plasma exposure, as hypothesized. Mean PK parameters of baseline-corrected CDCA after polysorbate 80 and placebo arms are shown in Table I. Polysorbate 80 had no effect on the Cmax (p=0.4) or AUC (p=0.3) of baseline-corrected CDCA compared to placebo. Baseline-corrected CDCA Cmax and AUC ratios for polysorbate 80 versus placebo were 1.12 and 1.10, respectively, and were not statistically different than unity (p=0.2 and 0.1, respectively).
Enalaprilat
Mean profiles of enalaprilat in the polysorbate 80 and placebo arms are shown in Fig. 3. Polysorbate 80 had no effect on enalaprilat plasma exposure, as hypothesized. Mean PK parameters of enalaprilat after polysorbate 80 and placebo arms are shown in Table I. Polysorbate 80 had no effect on the Cmax (p=0.3) or AUC (p=0.4) of enalaprilat compared to placebo. Enalaprilat Cmax and AUC ratios for polysorbate 80 versus placebo were 1.02 and 1.10, respectively, and were not statistically different than unity (p=0.5 and 0.4, respectively).
Endogenous Bile Acids
Profiles of all 15 bile acids in the polysorbate 80 and placebo arms are shown in Figure S2. Mean PK parameters of each bile acid is summarized in Table II. In comparing PK metrics from polysorbate 80 arm versus placebo arm, polysorbate 80 did not have a statistically significant effect on the Cmax or AUC of any bile acid (p>0.1 and 0.2, respectively). Average Cmax ratios (polysorbate 80/placebo) of unconjugated bile acids CA, DCA, LCA, and UDCA were statistically different than unity (p=0.04, 0.02, 0.03, and 0.04, respectively). Meanwhile, Cmax ratios of the other 11 bile acids were not statistically significant. Average AUC ratio of GCA was statistically different than unity (p=0.03), while all other bile acids were not statistically significant.
Other Cocktail Probes
Mean PK parameters from the other drug cocktail probe (i.e., pravastatin in a separate sub-study) after polysorbate 80 and placebo arms are shown in Table SIII. There was no statistically significant effect of polysorbate 80 on pravastatin Cmax, Cmax ratio, AUC, and AUC ratio (p=0.3, 0.08, 0.2, and 0.07, respectively). Mean profile of pravastatin in the polysorbate 80 and placebo arms is shown in Figure S3. Polysorbate 80 had no effect on pravastatin plasma exposure.
DISCUSSION
Potential Drug-Excipient Interaction
Polysorbate 80 capsules contained 400mg of polysorbate 80 and were administered BID for 6 consecutive days to allow for potential modulation of intestinal membrane fluidity and intestinal membrane disruption. On the next day, cocktail (i.e., valacyclovir, CDCA, and enalaprilat, along with pravastatin for a separate sub-study that shares the placebo arm) was administered with an additional 400mg dose of polysorbate 80 to allow for potential direct transporter inhibition.
Valacyclovir is an orally administered prototypical PepT1 targeted prodrug designed to increase the intestinal absorption of the antiviral agent acyclovir. The bioavailability of orally administered acyclovir is 10-20%, however, oral bioavailability increases by 3- to 5-fold to 54% when administered as valacyclovir (26, 35, 36). Specifically, intestinal PepT1 accounts for 90% of the intestinal uptake of valacyclovir (19). A potential concern is that the absorption of the PepT1 substrate valacyclovir may be reduced by polysorbate 80 though membrane fluidity modulation or direct PepT1 inhibition. Valacyclovir is rapidly converted to acyclovir, an organic cation transporter 1 (OCT1) substrate, with dose-linear PK. Pravastatin, CDCA, and enalaprilat were not expected to impact valacyclovir or acyclovir PK.
CDCA, an orally administered drug used to dissolve gallstones, is also a native bile acid substrate that significantly relies on intestinal reabsorption during enterohepatic circulation by intestinal ASBT (21, 37, 38). In healthy subjects, the enterohepatic recirculation system has high capacity to transport CDCA and bile acids. Valacyclovir is not an inhibitor of ASBT since it does not inhibit taurocholate uptake in vitro with up to 600µM valacyclovir in hASBT-COS cells (39). Pravastatin is not a potent inhibitor of ASBT (Ki = 1360 ± 360µM) (40). Valacyclovir is not expected to be an inhibitor of ASBT, whereas pravastatin and CDCA are not expected to inhibit PepT1. Valacyclovir, enalaprilat, and pravastatin are not expected to impact CDCA PK. A potential concern is that the absorption of CDCA may be impacted by polysorbate 80. However, results indicate that polysorbate 80 did not inhibit PepT1- or ASBT-mediated PK of valacyclovir and CDCA, respectively.
Enalaprilat, due to its high solubility and low membrane permeability, is poorly absorbed after oral administration (i.e., 3-12%) (22, 41, 42). In a human jejunal perfusion study, the permeability of enalaprilat was low, 0.20 x 10-4cm/s (43). Therefore, enalaprilat is typically orally administered as the mono-acid prodrug enalapril, which is 60-70% absorbed after oral administration (44). Enalaprilat, and not enalapril, is administered intravenously due to enalaprilat’s high solubility. Enalaprilat is a weak multidrug resistance protein 4 (MRP4) substrate. Enalaprilat is not an inhibitor of ASBT or PepT1 (45, 46). Valacyclovir, pravastatin, and CDCA and are not expected to impact enalaprilat PK. A potential concern is that polysorbate 80 may increase passive intestinal permeability (e.g., disrupt membrane integrity) and increase enalaprilat absorption. However, results indicate that polysorbate 80 did not module intestinal membrane fluidity or cause intestinal membrane disruption.
Potential Disruption of Intestinal Microbiota
Polysorbate 80 has been shown in an ex vivo human model to disrupt the composition and function of intestinal microbiota (17). Bile acid composition and gut bacteria are tightly coupled, such that bile acids are metabolized by intestinal microbiota and are also regulators of the intestinal microbiota. The primary bile acids CDCA and CA are synthesized exclusively in the liver and are conjugated to glycine or taurine to be secreted into bile and stored in the gallbladder. Following a meal, bile acids are released into the small intestine to emulsify dietary fats and enhance lipid absorption. Most bile acids (>95% daily) are reabsorbed in the terminal ileum and returned to the liver via the portal circulation. Unconjugated BAs that are not reabsorbed undergo bacterial biotransformation to form secondary bile acids, DCA and LCA, from CA and CDCA, respectively. CDCA is also transformed to the secondary bile acid UDCA. These secondary bile acids can also be absorbed and undergo enterohepatic circulation (37, 47). Modest increases in the Cmax ratio of the unconjugated secondary bile acids DCA, LCA, and UDCA was observed, without a change in their AUC ratios. While the 15 bile acids were not broadly modulated, there was a potential alteration of the in vivo intestinal microbiota by 400mg polysorbate 80 BID.
Study Limitations
BE was not assessed. Rather, the study employed a drug-drug interaction study design, involving n=12 crossed-over healthy volunteers. Multiple drug probes were evaluated, such that statistics suffer from the general multiple comparisons problem. However, with a view of detecting polysorbate 80 effect on any of the drug probes, a critical p-value of 0.05 was applied, without correcting for multiple comparisons to maintain a low 5% type 1 error rate. Nevertheless, polysorbate 80 was not detected to impact valacyclovir, CDCA, or enalaprilat absorption.
Implications for BCS-based Biowaivers and Next Steps
BCS-based biowaivers beneficially streamline the development of new and generic drug products by reducing the need for in vivo BE assessment when not needed. Biowaivers also provide regulatory relief for scale-up and post-approval changes. From a public health perspective, biowaivers eliminate the risk of exposing participants to investigative clinical research, provides economic relief for drug sponsors by decreasing drug development time and cost, and reduces level of regulatory review burden (48).
Previously, many regulatory agencies and organizations around the world, such as the US Food and Drug Administration (FDA), World Health Organization (WHO), and the European Medicines Agency (EMA) had implemented BCS-based biowaiver systems. A harmonized M9 BCS guidance was adopted in 2019 by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
Prior to M9 BCS guidance, the FDA issued an updated BCS guidance in 2017 that exemplified polysorbate 80 as a surfactant that may be an AME. Similarly, M9 BCS guidance cautions that surfactants may impact drug permeability and references sodium lauryl sulfate [SLS; also known as sodium dodecyl sulphate (SDS)]. M9 BCS is more conservative about excipient changes than prior guidance (2).
Results here for polysorbate 80 follow results of SLS. Previously, multiple in vitro and in situ studies showed SLS increased drug permeability (49,50,51,52,53). For example, SLS has been shown in a rat jejunal perfusion model to increase the absorption of enalaprilat by 8- to 9-fold (53). However, Vaithianathan et al. found that 25mg SLS had no significant impact on the human bioavailabilities of cimetidine nor acyclovir (4). Interestingly, M9 BCS cautions SLS as an excipient with potential to modulate permeability. Like SLS, polysorbate is a surfactant, and multiple in vitro and in situ studies showed polysorbate 80 increased drug permeability (6,7,8). However, like SLS previously, polysorbate 80 (400mg BID and at co-administration) showed no impact on drug absorption in humans.
CONCLUSION
Polysorbate 80 (400mg) BID was well-tolerated among all participants. In polysorbate 80 arm, one participant had easy bowel movements on day 7 after cocktail administration. Results indicate that polysorbate 80 does not impact active or passive intestinal drug absorption in humans. Polysorbate 80 did not inhibit PepT1- or ASBT-mediated PK of valacyclovir and CDCA, respectively. Polysorbate 80 did not increase intestinal absorption of enalaprilat, a low permeability probe. Modest increases in Cmax ratios – but not AUC ratios - of unconjugated secondary bile acids suggest a potential alteration of the in vivo intestinal microbiota composition by polysorbate 80. These findings support reducing excipient restrictions of BCS-based biowaivers.
Abbreviations
- AME:
-
Absorption-modifying excipient
- ASBT:
-
Apical sodium bile acid transporter
- BCS:
-
Biopharmaceutics Classification System
- BE:
-
Bioequivalence
- BID:
-
Twice a day
- CDCA:
-
Chenodeoxycholic acid
- CYP:
-
Cytochrome P450
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- GMP:
-
Good Manufacturing Practice
- ICH:
-
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- Ki :
-
Inhibitory constant
- MRP4:
-
Multidrug resistance protein 4
- OCT1:
-
Organic cation transporter 1
- PepT1:
-
Peptide transporter 1
- P-gp:
-
P-glycoprotein
- PK:
-
Pharmacokinetics
- PKC:
-
Protein kinase C
- WHO:
-
World Health Organization
References
Zhang W, Li Y, Zou P, Wu M, Zhang Z, Zhang T. The Effects of Pharmaceutical Excipients on Gastrointestinal Tract Metabolic Enzymes and Transporters-an Update. AAPS J. 2016;18(4):830–43.
Metry M, Polli JE. Evaluation of Excipient Risk in BCS Class I and III Biowaivers. AAPS J. 2022;24(1):20.
Food and Drug Administration (FDA). Guidance for Industry: M9 Biopharmaceutics Classification System-Based Biowaivers. Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). May 2021.
Vaithianathan S, Haidar SH, Zhang X, Jiang W, Avon C, Dowling TC, Shao C, Kane M, Hoag SW, Flasar MH, Ting TY, Polli JE. Effect of Common Excipients on the Oral Drug Absorption of Biopharmaceutics Classification System Class 3 Drugs Cimetidine and Acyclovir. J Pharm Sci. 2016;105(2):996–1005.
Food and Drug Administration (FDA). Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Center for Drug Evaluation and Research (CDER). 2017
Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16(4–5):237–46.
Rege BD, Yu LX, Hussain AS, Polli JE. Effect of common excipients on Caco-2 transport of low-permeability drugs. J Pharm Sci. 2001;90(11):1776–86.
Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–6.
Otter M, Oswald S, Siegmund W, Keiser M. Effects of frequently used pharmaceutical excipients on the organic cation transporters 1–3 and peptide transporters 1/2 stably expressed in MDCKII cells. Eur J Pharm Biopharm. 2017;112:187–95.
Stieger B, Steiger J, Locher KP. Membrane lipids and transporter function. Biochim Biophys Acta (BBA) - Mol Basis of Dis. 2021;1867(5):166079.
Dudeja PK, Anderson KM, Harris JS, Buckingham L, Coon JS. Reversal of Multidrug-Resistance Phenotype by Surfactants: Relationship to Membrane Lipid Fluidity. Arch Biochem Biophys. 1995;319(1):309–15.
Woodcock DM, Linsenmeyer ME, Chojnowski G, Kriegler AB, Nink V, Webster LK, Sawyer WH. Reversal of multidrug resistance by surfactants. Br J Cancer. 1992;66(1):62–8.
Sarwar Z, Annaba F, Dwivedi A, Saksena S, Gill RK, Alrefai WA. Modulation of ileal apical Na+-dependent bile acid transporter ASBT by protein kinase C. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G532-538.
Zhao F-K, Chuang LF, Israel M, Chuang RY. Cremophor EL, a widely used parenteral vehicle, is a potent inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159(3):1359–67.
Tompkins L, Lynch C, Haidar S, Polli J, Wang H. Effects of commonly used excipients on the expression of CYP3A4 in colon and liver cells. Pharm Res. 2010;27(8):1703–12.
Mountfield RJ, Senepin S, Schleimer M, Walter I, Bittner B. Potential inhibitory effects of formulation ingredients on intestinal cytochrome P450. Int J Pharm. 2000;211(1):89–92.
Naimi S, Viennois E, Gewirtz AT, Chassaing B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome. 2021;9(1):66.
Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue barriers. 2018;6(3):1539595–1539595.
Yang B, Smith DE. Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab Dispos. 2013;41(3):608–14.
Ganapathy ME, Huang W, Wang H, Ganapathy V, Leibach FH. Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998;246(2):470–5.
Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb Exp Pharmacol. 2011;201:169–203.
Kubo SH, Cody RJ. Clinical pharmacokinetics of the angiotensin converting enzyme inhibitors. A review Clin Pharmacokinet. 1985;10(5):377–91.
Pravachol [package insert]. Princeton, NJ: Bristol-Myers Squibb. 2016.
Chenodal [package insert]. San Diego, CA: Retrophin, Inc. 2020.
Enalaprilat Injection [package insert]. Lake Forest, IL: Hospira, Inc. 2018.
Valtrex (valacyclovir) [package insert]. Research Triangle Park, NC: GlaxoSmithKline. 2008.
IBM Watson Health. Drug interactions: Pravastatin, Chenodiol, Enalaprilat, & Valacyclovir. In.: IBM Micromedex: Drug Interaction Checking [Electronic version]. Retrieved March 8, 2022, from https://www.micromedexsolutions.com/; 2022.
Pravastatin, Chenodiol, Enalaprilat, & Valacyclovir. In.: Lexicomp: Interactions [Electronic version]. Hudson, Ohio: Wolters Kluwer UpToDate, Inc., 2022. Retrieved March 8, 2022, from http://online.lexi.com; 2022.
Food and Drug Administration (FDA). Inactive Ingredient Database (IID). Center for Drug Evaluation and Research (CDER). 2022.
Sandoval PJ, Zorn KM, Clark AM, Ekins S, Wright SH. Assessment of Substrate-Dependent Ligand Interactions at the Organic Cation Transporter OCT2 Using Six Model Substrates. Mol Pharmacol. 2018;94(3):1057–68.
Russo DP, Zorn KM, Clark AM, Zhu H, Ekins S. Comparing Multiple Machine Learning Algorithms and Metrics for Estrogen Receptor Binding Prediction. Mol Pharm. 2018;15(10):4361–70.
U.S. Food and Drug Administration. Bioanalytical Method Validation. Guidance for Industry (2018).
Shiffka SJ, Jones JW, Li L, Farese AM, MacVittie TJ, Wang H, Swaan PW, Kane MA. Quantification of common and planar bile acids in tissues and cultured cells. J Lipid Res. 2020;61(11):1524–35.
Gu H, Liu G, Wang J, Aubry A-F, Arnold ME. Selecting the Correct Weighting Factors for Linear and Quadratic Calibration Curves with Least-Squares Regression Algorithm in Bioanalytical LC-MS/MS Assays and Impacts of Using Incorrect Weighting Factors on Curve Stability, Data Quality, and Assay Performance. Anal Chem. 2014;86(18):8959–66.
Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39(12):2759–64.
Zovirax (acyclovir) [package insert]. Research Triangle Park, NC: GlaxoSmithKline. 2005.
Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50(12):2340–57.
Balakrishnan A, Wring SA, Polli JE. Interaction of native bile acids with human apical sodium-dependent bile acid transporter (hASBT): influence of steroidal hydroxylation pattern and C-24 conjugation. Pharm Res. 2006;23(7):1451–9.
Tolle-Sander S, Lentz KA, Maeda DY, Coop A, Polli JE. Increased acyclovir oral bioavailability via a bile acid conjugate. Mol Pharm. 2004;1(1):40–8.
Zheng X, Ekins S, Raufman JP, Polli JE. Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol Pharm. 2009;6(5):1591–603.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Verbeeck RK, Kanfer I, Lobenberg R, Abrahamsson B, Cristofoletti R, Groot DW, Langguth P, Polli JE, Parr A, Shah VP, Mehta M, Dressman JB. Biowaiver Monographs for Immediate-Release Solid Oral Dosage Forms: Enalapril. J Pharm Sci. 2017;106(8):1933–43.
Lennernäs H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37(10–11):1015–51.
Swaan PW, Stehouwer MC, Tukker JJ. Molecular mechanism for the relative binding affinity to the intestinal peptide carrier. Comparison of three ACE-inhibitors: enalapril, enalaprilat, and lisinopril. Biochim Biophys Acta. 1995;1236(1):31–38.
Balakrishnan A, Wring SA, Coop A, Polli JE. Influence of charge and steric bulk in the C-24 region on the interaction of bile acids with human apical sodium-dependent bile acid transporter. Mol Pharm. 2006;3(3):282–92.
Lin CJ, Akarawut W, Smith DE. Competitive inhibition of glycylsarcosine transport by enalapril in rabbit renal brush border membrane vesicles: interaction of ACE inhibitors with high-affinity H+/peptide symporter. Pharm Res. 1999;16(5):609–15.
Poland JC, Flynn CR. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology. 2021;36(4):235–45.
Cook JA, Davit BM, Polli JE. Impact of Biopharmaceutics Classification System-Based Biowaivers. Mol Pharm. 2010;7(5):1539–44.
García-Arieta A. Interactions between active pharmaceutical ingredients and excipients affecting bioavailability: impact on bioequivalence. Eur J Pharm Sci. 2014;65:89–97.
van Os S, Relleke M, Piniella PM. Lack of bioequivalence between generic risperidone oral solution and originator risperidone tablets. Int J Clin Pharmacol Ther. 2007;45(5):293–9.
Sjögren E, Abrahamsson B, Augustijns P, Becker D, Bolger MB, Brewster M, Brouwers J, Flanagan T, Harwood M, Heinen C, Holm R, Juretschke HP, Kubbinga M, Lindahl A, Lukacova V, Münster U, Neuhoff S, Nguyen MA, Peer A, Reppas C, Hodjegan AR, Tannergren C, Weitschies W, Wilson C, Zane P, Lennernäs H, Langguth P. In vivo methods for drug absorption - comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur J Pharm Sci. 2014;57:99–151.
Parr A, Hidalgo IJ, Bode C, Brown W, Yazdanian M, Gonzalez MA, Sagawa K, Miller K, Jiang W, Stippler ES. The Effect of Excipients on the Permeability of BCS Class III Compounds and Implications for Biowaivers. Pharm Res. 2016;33(1):167–76.
Dahlgren D, Roos C, Lundqvist A, Tannergren C, Langguth P, Sjöblom M, Sjögren E, Lennernäs H. Preclinical Effect of Absorption Modifying Excipients on Rat Intestinal Transport of Model Compounds and the Mucosal Barrier Marker 51Cr-EDTA. Mol Pharm. 2017;14(12):4243–51.
ACKNOWLEDGMENTS AND DISCLOSURES
Ms. Kimberley Zorn and Dr. Alex Clark are acknowledged for assistance with computational models. All authors declared no competing interests for this work.
Funding
This work was funded by the generosity of Marilyn Shangraw. JEP is the Ralph F. Shangraw Endowed Professor in Industrial Pharmacy and Pharmaceutics. We acknowledge the support of the University of Maryland, Baltimore, Institute for Clinical & Translational Research (ICTR) and the National Center for Advancing Translational Sciences (NCATS) Clinical Translational Science Award (CTSA) grant number 1UL1TR003098. The machine learning work was supported by National Institutes of Health National Institute of General Medical Sciences grant R44GM122196. Additional support was provided by the University of Maryland School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Metry, M., Krug, S.A., Karra, V.K. et al. Lack of an Effect of Polysorbate 80 on Intestinal Drug Permeability in Humans. Pharm Res 39, 1881–1890 (2022). https://doi.org/10.1007/s11095-022-03312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03312-z