Abstract
Some glycoside drugs can be transported through intestinal glucose transporters (IGTs). The surfactants used in oral drug preparations can affect the function of transporter proteins. This study aimed to investigate the effect of commonly used surfactants, Poloxamer 188 and Tween 80, on the drug transport capacity of IGTs. Previous studies have shown that gastrodin is the optimal drug substrate for IGTs. Gastrodin was used as a probe drug to evaluate the effect of these two surfactants on intestinal absorption in SD rats through pharmacokinetic and in situ single-pass intestinal perfusion. Then, the effects of the two surfactants on the expression of glucose transporters and tight-junction proteins were examined using RT-PCR and western blotting. Additionally, the effect of surfactants on intestinal permeability was evaluated through hematoxylin-eosin staining. The results found that all experimental for Poloxamer 188 (0.5%, 2.0% and 8.0%) and Tween 80 (0.1% and 2.0%) were not significantly different from those of the blank group. However, the AUC(0-∞) of gastrodin increased by approximately 32% when 0.5% Tween 80 was used. The changes in IGT expression correlated with the intestinal absorption of gastrodin. A significant increase in the expression of IGTs was observed at 0.5% Tween 80. In conclusion, Poloxamer 188 had minimal effect on the drug transport capacity of IGTs within the recommended limits of use. However, the expression of IGTs increased in response to 0.5% Tween 80, which significantly enhanced the drug transport capacity of IGTs. However, 0.1% and 2.0% Tween 80 had no significant effect.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose transporters are common transporters found in various tissues throughout the body. Its primary function is to transport nutrients, such as glucose, in and out of organs. Some glucoside-containing glycoside drugs, such as gastrodin, quercetin, isoquercetin, and salidroside, may also be transported through glucose transporters [1]. There are two main types of glucose transporters: sodium-dependent glucose transporters (SGLTs) and sodium-independent glucose transporters (GLUTs) [2, 3]. Among them, SGLT1 and GLUT2 are involved in the transmembrane absorption of glucose in the intestines [4, 5]. SGLT1 is located primarily on the brush border of intestinal epithelial cells, whereas GLUT2 is found mainly on the basolateral side of intestinal epithelial cells [6, 7]. Researchers have extensively studied the identification, activity, structure, function, and mechanism of glucose transporters [8,9,10,11]. However, research on the factors that influence drug transport via glucose transporters is limited, impeding the development and application of drug delivery strategies based on glucose transport pathways. Our research group has investigated the effects of biological factors, such as species, on the drug transport of intestinal glucose transporters (IGTs). We demonstrated that Sprague–Dawley rats are more suitable than Wistar rats for studying IGTs [12]. In addition to biological factors, the influence of excipients in drug formulations on the drug transport ability of IGTs is worth exploring. Excipients are often considered inert; however, this is not always the case. Excipients, such as surfactants, can also affect the function of transporter proteins [13].
Currently, studies have shown that surfactants can affect the activity of transport proteins, thereby affecting drug absorption [14,15,16]. Tween 80 has been found to affect organic cation-selective transporters, thereby influencing the absorption of metformin in the intestines [17]. In another study, Tween 80 was shown to affect transport in the MOCK II cell model by inhibiting efflux transport proteins, such as P-glycoprotein and MRP2 [18]. Recent studies have demonstrated that surfactants can also affect the expression of transporters. For example, Tween 80 can downregulate the expression of P-glycoprotein (P-gp) and play a positive role in P-gp-mediated transport [19]. In addition to the aforementioned P-glycoprotein (P-gp), there are three other efflux transporters: breast cancer resistance protein (BCRP), multidrug resistance-associated proteins (MRPs), and the bile salt export pump (BSEP) [20]. Currently, the focus of related research is mainly on efflux transporters, whereas studies on influx transporters are relatively scarce [21,22,23]. Efflux transporters play a critical role in drug excretion in the intestine, thus garnering increased attention in the study of drug absorption and detoxification mechanisms [24, 25]. In contrast to efflux transporters, influx transporters have complex functions and regulatory mechanisms in the intestine [26, 27]. The complexity of studying influx transporters has resulted in relatively few related studies. IGTs are commonly found as influx transporters in the intestine. However, there is no information regarding the effect of surfactants on the drug transport capacity of IGTs. Studying the effect of surfactants on the drug transport capacity of IGTs can deepen our understanding of the mechanisms by which surfactants regulate influx transporters.
Surfactants can be classified as ionic or nonionic. Owing to their high safety profile, nonionic surfactants are widely used in oral drug formulations [28, 29]. Commonly used nonionic surfactants in oral drug formulations include Poloxamer 188, Tween 80, Cremophor RH 40, PEG 400, and Tween 20 [18, 30]. In this study, Poloxamer 188 and Tween 80 were selected for investigation of their effects on the drug transport capacity of IGTs. The use of Poloxamer 188 in oral drug formulations can improve drug solubility and bioavailability while also providing gastrointestinal protection. This approach facilitates the easier absorption of orally administered drugs, enhances therapeutic efficacy, and improves patient convenience [31, 32]. Tween 80 can form micellar structures with drugs, thereby enhancing drug solubility in the gastrointestinal tract and promoting drug absorption. Additionally, Tween 80 can increase formulation stability and protect drugs from degradation and inactivation. It can form a protective layer, reducing the interaction between drugs and other components and thus improving formulation stability [33,34,35]. There is information from the FDA regarding the use of these two surfactants in oral drug formulations. In terms of dosage, the maximum safe dosage of Poloxamer 188 is not clear, with typical dosages ranging from 3 to 1800 mg. However, Tween 80 is slightly toxic, with an acceptable daily intake (ADI) of 25 mg/kg. By converting these dosages to equivalent doses for humans and rats, the maximum daily dosage of Poloxamer 188 in rats was estimated to be approximately 300 mg/kg, whereas the maximum safe dosage of Tween 80 is approximately 160 mg/kg. On the basis of these dosages, we designed low, medium, and high dosages. To facilitate oral gavage administration during the experiments, we further calculated the concentrations based on the dosage and stomach capacity of the rats. Thus, the concentrations selected for administration were Poloxamer 188 (0.5%, 2.0%, and 8.0% w/v) and Tween 80 (0.1%, 0.5%, and 2% w/v).
Prior to conducting relevant research, it is crucial to find a suitable drug probe to evaluate the drug transport capacity of IGTs. Glucose is a substrate for glucose transporters. However, endogenous glucose can interfere with the quantitative analysis of exogenous glucose, rendering the results unreliable [36, 37]. Therefore, glucose is not a suitable substrate for evaluating the drug-transport capacity of IGTs. Gastrodin is a phenolic glycoside and the main active ingredient of Gastrodia elata. Since its discovery in 1978, gastrodin has been extensively studied and is highly soluble in water (450 mg/mL) [38,39,40]. Generally, highly soluble drugs are difficult to absorb through passive diffusion by intestinal epithelial cells. For example, mannitol cannot pass through intestinal epithelial cells and is used as a tool to verify the integrity of the Caco-2 cell model [1]. However, compared to mannitol, gastrodin, which has a similar molecular weight and solubility, has an oral bioavailability of approximately 80% in rats. Among the known drugs transported through IGTs, gastrodin has the highest water solubility and highest oral bioavailability. Its absorption in the intestine does not occur via passive diffusion [40]. Our previous work also confirmed that gastrodin, which has a structure similar to that of glucose (Fig. 1), can be transported and absorbed through IGTs [12, 41]. Therefore, gastrodin could be used as a probe drug for studying the drug transport capacity of IGTs.
This study investigated the effect of Poloxamer 188 and Tween 80, which are commonly used surfactants, on the drug transport capacity of IGTs in Sprague–Dawley rats using gastrodin as a probe drug. The effects of the two surfactants on the intestinal absorption of gastrodin were studied through pharmacokinetic and in situ single-pass intestinal perfusion experiments. We investigated the regulatory effects of the two surfactants on the expression of intestinal glucose transporters (IGTs) in all segments of the intestine using RT‒PCR and western blotting. To evaluate the effect of surfactants on intestinal structure and intestinal tight junction proteins (IJTPs), we conducted hematoxylin–eosin staining, RT‒PCR, and western blotting. This procedure was used to exclude the influence of intestinal permeability, as the bioavailability of drugs is closely related to transporter activity and permeability. This study evaluated the effect of two commonly used surfactants, Poloxamer 188 and Tween 80, on the drug transport capacity of IGTs. This evaluation was based on the results of gastrointestinal absorption, IGT expression, and intestinal permeability. These findings provide valuable references for the rational selection and use of excipients in drug formulation design for transport through IGTs.
Materials and Methods
Materials
Gastrodin, Polysorbate 80, and Poloxamer 188 were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (China). Salidroside was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (China). HPLC-grade water and methanol were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (China). Calcium chloride (CaCl2), potassium chloride (KCl), magnesium sulfate (MgSO4), potassium phosphate monobasic (KH2PO4), sodium phosphate dibasic (Na2HPO4), HEPES, sodium bicarbonate (NaHCO3), and sodium chloride (NaCl) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (China).
Animals
Healthy male Sprague–Dawley rats (approximately 250 g, 8 weeks old) were purchased from the Southern Medical University Experimental Animal Center (China). Sprague–Dawley rats need to undergo a one-week adaptation period before the experiment. During this period, the rats have free access to standard chow and water. However, they should be fasted for 12 h prior to the start of the experiment, while still being allowed to drink freely. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Southern Medical University (SMUL2022104).
In Vivo Pharmacokinetic Study
Effect of Excipient Concentrations on Gastrodin Bioavailability
On the day of the experiment, each rat was weighed. Each group (n = 6) was given 8 mL/kg intra-gastrically of Tween 80 (0.1%, 0.5%, 2% w/v) and Poloxamer 188 (0.5%, 2.0%, 8.0% w/v) respectively. After 15 min, each group was intra-gastrically administered 200 mg/kg of gastrodin, and retro-orbital blood samples (350 μL) were collected after 2, 5, 10, 20, 30, 40, 60, 90, 120, 180, and 240 min in rats.
Preparation of Blood Samples
Approximately 500 µL of blood samples were taken and centrifuged at 8000 rpm for 10 min in heparinized centrifuge tubes. The supernatant was then collected as plasma samples. A 100 µg/mL solution of salidroside internal standard was prepared using methanol. Plasma samples, methanol, and the internal standard were mixed in centrifuge tubes in a ratio of 2:7:1. The mixed samples were further vortexed for 5 min and centrifuged at 13000 rpm for 30 min. The supernatant was taken and subjected to vacuum drying. The dried residue was dissolved in 30% methanol at a concentration 3.5 times that of the supernatant. The reconstituted solution was vortexed for 3 min to ensure thorough mixing. After centrifugation at 13,000 rpm for 30 min, 10 µL of the supernatant was taken. Finally, the supernatant was analyzed using an ultra-performance liquid chromatography (UPLC) system.
UPLC Analysis [12]
Chromatographic analysis was performed using the Agilent 1290 series HPLC system (Agilent Technologies, USA). Separation was conducted using a column (InfinityLab Poroshell 120 EC-C18, 3.0 × 100 mm, 2.7 μm) under the following chromatographic conditions: column temperature 25 °C; mobile phase A: 100% water; mobile phase B: 100% methanol; gradient: 3%–30% B at 0–2 min, 30%–70% B at 2–3 min, 70% B at 3–5 min, 70%–30% B at 5–5.5 min, 30%–5% B at 5.5–6 min, and 5%–3% B at 6–7 min; sample injection volume: 10 µL; flow rate: 0.3 mL/min; and detection wavelength: 220 nm.
Pharmacokinetic Analysis
The pharmacokinetic parameters (Cmax, Tmax, and AUC0-∞) of gastrodin were estimated using DAS 2.0 software.
In Situ Single-Pass Intestinal Perfusion Experiments
Effect of Excipient Concentrations on Gastrodin Permeability Coefficient
In situ single-pass intestinal perfusion experiments were conducted using a perfusion pump (Model PHD 2000; Harvard Apparatus, USA). The perfusate contained 5.963 g/L HEPES, 1.164 g/L NaCl, 0.400 g/L KCl, 0.372 g/L NaHCO3, 0.171 g/L CaCl2, 0.098 g/L MgSO4, 0.060 g/L KH2PO4, 0.048 g/L Na2HPO4, and 50 μg/mL gastrodin. Tween 80 (0.1%, 0.5%, 2% w/v) and Poloxamer 188 (0.5%, 2.0%, 8.0% w/v) were added to the perfusate in each group. Rats (n = 6) in each group received a intraperitoneal injection of 2.8 mL/kg of 50% urethane. After anesthesia and fixation, the abdominal cavity was opened and the bile duct was severed. Short tubes were then inserted into the entry and exit points of the duodenum (D), jejunum (J), ileum (I), and colon (C). The intestines were flushed with isotonic saline to remove the contents. The inlet catheters of each intestinal segment were connected to the constant speed injectors of four 50 mL multi-channel infusion pumps. The catheters were insulated with a constant-temperature water bath to ensure that the drug solution infused into the animal intestines was maintained at 37 ℃. The drug perfusion solution was administered at a constant rate of 10 mL/h through the intestinal lumen. After 30 min, perfusate samples were collected every thirty minutes. The collected perfusate samples (350 μL) were mixed with 150 μL of methanol and vortexed for five minutes. After centrifugation at 13000 rpm for 30 min, 10 μL of the supernatant was taken for analysis using the UPLC system. The formula for calculating the effective permeability (Peff) is as follows:
In the formulas, Cin (μg/mL) and Cout (μg/mL) represent the concentrations of gastrodin at the inlet and outlet, respectively. Q represents the flow rate, L (cm) represents the length of the segment, and D represents the diffusion factor.
UPLC Analysis
Chromatographic analysis was performed using the Agilent 1290 series HPLC system (Agilent Technologies, USA). Separation was conducted using a column (InfinityLab Poroshell 120 EC-C18, 3.0 × 100 mm, 2.7 μm) under the following chromatographic conditions: column temperature 25 °C; mobile phase A: 100% water; mobile phase B: 100% methanol; gradient: 3%–30% B at 0–2 min, 30%–70% B at 2–3 min, 70% B at 3–5 min, 70%–30% B at 5–5.5 min, 30%–5% B at 5.5–6 min, and 5%–3% B at 6–7 min; sample injection volume: 10 µL; flow rate: 0.3 mL/min; and detection wavelength: 220 nm.
Effect of Excipient Concentrations on IGTs Expression and ITJPs Expression
Tissue Samples
In each group, a total of six Sprague–Dawley rats were intra-gastrically administered with Tween 80 (0.1%, 0.5%, 2% w/v) or Poloxamer 188 (0.5%, 2.0%, 8.0% w/v) at a dosage of 8 mL/kg. After a 15-min interval, tissue samples from the duodenum, jejunum, ileum, and colon were collected. These samples were then treated and stored at -80 °C until further use.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA (n = 6) was isolated and purified using the Foregene Animal Total RNA Isolation Kit according to the manufacturer’s instructions. The concentration of the extracted RNA was determined using a Synergy H1 instrument. Subsequently, the RNA was reverse-transcribed into cDNA using the HiScript Ш cDNA Synthesis Kit. RT-PCR reactions were performed on a Fast Real-time PCR System using the 2 × ChamQ Universal SYBR qPCR Master Mix. The specific oligonucleotide primers used in this study can be found in Table 1. The relative expression of each mRNA was calculated using the 2−ΔΔCt method [42].
Western Blotting
Tissue samples (n = 3) were homogenized using RIPA buffer obtained from Beyotime Biotechnology, China. The protein concentration was determined using a BCA assay kit from Meilunbio, China. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was conducted to separate the targeted transporter proteins. The proteins within the gel were then transferred onto polyvinylidene fluoride (PVDF) membranes obtained from Millipore, USA. The membranes were probed with primary antibodies, including β-actin (AF7018, Affinity Biosciences, USA), SGLT1 (PS08943M, Abmart, China), GLUT2 (A12307, ABclonal, China), claudin-1 (A21971, ABclonal, China), occludin (T55997S, Abmart, China), and ZO-1 (T56872S, Abmart, China). Subsequently, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (ab6721, Abcam, UK). The protein bands were visualized using an enhanced chemiluminescence method. To perform further statistical analysis, the relative expression of GLUT2 or SGLT1 was calculated by determining the ratio of SGLT1 or GLUT2 to β-actin.
Hematoxylin–eosin Staining
Tissue Samples
In each group, three Sprague–Dawley rats were intra-gastrically administered with Tween 80 (0.1%, 0.5%, 2% w/v) or Poloxamer 188 (0.5%, 2.0%, 8.0% w/v) at a dosage of 8 mL/kg. After a 15-min interval, tissue samples from the duodenum, jejunum, ileum, and colon were collected. These samples were then soaked in polyformaldehyde.
Effect of Excipient Concentrations on Intestinal Structure
The tissue samples soaked in polyformaldehyde were sent to Wuhan Baiqiandu Biotechnology Co., Ltd. (China) for hematoxylin–eosin staining. Upon receiving the feedback photos, the changes in the structure of the intestinal tissues were observed.
Statistical Analysis
One-way ANOVA was performed using IBM SPSS Statistics 20. A statistically significant difference between the groups was determined when P < 0.05.
Results
Pharmacokinetics
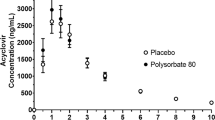
The time‒concentration curves of gastrodin after gavage differed for the two different surfactants (Fig. 2). In the Poloxamer 188 group, the areas under the drug-time curve (AUCs) for 0.5%, 2.0%, and 8.0% of the Sprague–Dawley rats were 12,664.12, 11,201.95, and 11,416.71 mg/L*min, respectively, while the AUC for the blank group was 10,471.05 mg/L*min (Table 2). No significant changes in the AUC were detected for different concentrations of Poloxamer 188 compared with those in the blank group. There were no significant differences in the Cmax or Tmax between the groups. These findings indicate that Poloxamer 188 did not have a significant effect on the absorption of gastrodin.
In the Tween 80 group, the AUC of the drug-time curve for the 0.1%, 0.5%, and 2.0% groups of Sprague–Dawley rats were 10,764.29, 14,096.24, and 9160.85 mg/L*min, respectively. The AUC for the blank group was 10,686.19 mg/L*min (Table 3). The 0.1% and 2.0% groups did not show any significant changes in absorption. Similar results were observed for Cmax, whereas Tmax did not significantly differ. The results indicated that, compared with no treatment, treatment with only 0.5% Tween 80 significantly increased the absorption of gastrodin in the intestine.
In Situ Single-Pass Intestinal Perfusion
The gastrointestinal tract is the main site where orally administered drugs are absorbed. The effective permeability (Peff) reflects the absorption of drugs in the gastrointestinal tract. In the Poloxamer 188 group, the Peff values in various intestinal segments for the blank group and the 0.5%, 2.0%, and 8.0% groups were D (1.23, 1.26, 1.18, 1.08 cm/s), J (1.01, 0.78, 0.78, 0.90 cm/s), I (0.71, 0.97, 0.89, 0.87 cm/s), and C (-0.29, -0.12, -0.26, -0.07 cm/s), respectively, with no significant differences compared to those of the blank group (Fig. 3a).
In the Tween 80 group, the Peff values in various intestinal segments for the blank group and the 0.1%, 0.5%, and 2.0% groups were D (1.13, 1.43, 1.93, 1.51 cm/s), J (1.09, 1.32, 2.14, 1.24 cm/s), I (0.91, 1.14, 1.81, 1.21 cm/s), and C (0.16, 0.20, 0.82, 0.07 cm/s), respectively (Fig. 3b). The Peff values for the 0.5% Tween 80 group showed an increasing trend in the various intestinal segments. The data analysis indicated that 0.5% Tween 80 can increase the absorption of gastrodin in various intestinal segments, but the difference was not significant. The 0.1% and 2.0% groups exhibited minimal changes.
Expression of IGTs
Transporter expression is a major factor influencing drug transport capacity. In the Poloxamer 188 group, there were no significant differences in the expression of IGTs between the groups and the blank group (Figs. 4a and 5a). In the Tween 80 group, only the 0.5% group showed significantly increased expression of IGTs compared to that in the blank group, whereas the 0.1% and 2.0% groups did not show significant changes (Figs. 4b and 5b). This finding is consistent with the in vivo pharmacokinetic results and unidirectional in situ intestinal perfusion experiments.
Hematoxylin–Eosin Staining
The oral absorption of drugs is closely related to intestinal permeability, which is associated with the integrity of the intestinal structure. This study also investigated the effect of Poloxamer 188 and Tween 80 on intestinal structure. No inflammation or disruption of the intestinal structure was observed in either the Poloxamer 188 or Tween 80 group (Fig. 6). It can be preliminarily concluded that Poloxamer 188 and Tween 80 have no significant effect on the intestinal structure. To confirm this, the expression of ITJPs (Claudin-1, Occludin, and ZO-1) in the intestine was examined.
Expression of ITJPs
Tight junctions are important membrane complexes between adjacent cells that act as barriers to epithelial tissues. The expression of tight junction proteins in all intestinal segments did not significantly differ among the Poloxamer 188 or Tween 80 groups (Figs. 7 and 8). This finding is consistent with the results of hematoxylin–eosin staining.
Discussion
Drug transporter capacity refers to the ability of an organism to transport drugs throughout the body. Drug transporters located in the intestinal epithelium typically mediate the transport of drugs into the intestines. These transporters facilitate the crossing of cell membranes, thereby influencing the intestinal absorption of drugs. However, there is a lack of clarity regarding how to systematically evaluate the drug transport capacity of intestinal transporters [43,44,45]. This paper introduces the concept of drug transport capacity and provides an answer to how to evaluate it systematically. In this study, the effect of the drug transport capacity of IGTs was evaluated based on the intestinal absorption of the probe drug, the expression level of IGTs, and the intestinal permeability. Currently, when studying drug absorption related to intestinal glucose transporters, glucose or labeled glucose is commonly used as a substrate [46,47,48]. As a result, owing to the restrictive nature of these substrates, most studies are limited to in vitro cell transport experiments and lack animal experiments [49,50,51]. This limitation arises from the lack of suitable probe drugs.
Glucose, as a substrate for IGTs, lacks a UV absorption chromophore, which makes quantitative detection inconvenient. One of the highlights of this study was the use of probe drug for IGTs that can be used in animal experiments. In terms of structure, the active ingredient in Gastrodia elata, known as gastrodin, is 4-hydroxybenzyl alcohol-β-D-glucoside. Its structure was similar to that of glucose. From a structural perspective, the 4-hydroxybenzyl alcohol moiety of gastrodin (MW: 124 g/mol) can be considered a UV absorption chromophore attached to glucose (MW: 180 g/mol) [52]. Moreover, gastrodin exhibited high stability. After oral administration, it predominantly exists in its native form in the bloodstream, which facilitates quantitative analysis [53,54,55]. Among the reported glycoside-active substances transported through IGTs, gastrodin has the highest oral bioavailability (approximately 80%) [41]. Our research group previously confirmed through in vivo and in vitro experiments that gastrodin can be rapidly absorbed through the intestinal glucose transport pathway [12, 40]. Therefore, gastrodin can be considered an optimal drug probe for evaluating the drug transport ability of IGTs.
Pharmacokinetic and in situ single-pass intestinal perfusion experiments showed that Poloxamer 188 did not significantly affect the intestinal absorption of gastrodin compared to that in the control group, even when the concentration increased from 0.5% to 8%. According to the literature, Poloxamer 188 does not affect the absorption of orally administered drugs [56]. Subsequently, the expression of intestinal glucose transporters (IGTs) in all intestinal segments was quantified by RT‒PCR and western blotting. The results showed no significant difference in the expression of IGTs among the three concentrations of Poloxamer 188. These findings are consistent with the intestinal absorption of gastrodin. Hematoxylin–eosin staining was performed on intestinal tissue slices from rats that were administered high, medium, or low concentration of Poloxamer 188 via gastric lavage. The results showed that the intestinal structures, including the villi, were intact, with no apparent damage. To further verify these findings, we conducted RT‒PCR and western blotting to test the expression of tight junction proteins in the rat intestines. Consistent with the results of hematoxylin–eosin staining, the present study showed that the three concentrations of Poloxamer 188 did not significantly affect the expression of tight junction proteins in the rat intestines. Therefore, the possibility of Poloxamer 188 damaging intestinal structures and thereby increasing permeability can be ruled out. These results indicated that the three concentrations of Poloxamer 188 did not affect drug absorption by influencing intestinal permeability. Shah et al. reported that Poloxamer 188 enhanced the solubility of compounds without increasing intestinal permeability [57]. Additionally, Fischer et al. discovered that a concentration of approximately 20% Poloxamer 188 did not have any adverse effects on cell viability or monolayer integrity [58]. Furthermore, there is no established Acceptable Daily Intake (ADI) for Poloxamer 188 in the FDA. This result may be attributed to the low toxicity of Poloxamer 188 at safe dosage levels, which implies that it has a minimal effect on drug transport capacity [59]. Therefore, Poloxamer 188 had little effect on the drug transport capacity of IGTs within the limits of safe use.
In comparison with Poloxamer 188, Tween 80 affects the drug transport capacity of IGTs. In vivo pharmacokinetic experiments demonstrated that the presence of 0.5% Tween 80 resulted in an approximately 32% increase in the AUC(0-∞) of gastrodin, which was significantly different from that of the control group. However, when the concentration of Tween 80 increased to 2.0%, the oral bioavailability of gastrodin decreased. In single-pass intestinal perfusion experiments, no significant differences in gastrodin absorption were observed among the three Tween 80 concentrations in any of the intestinal segments. Although no statistically significant differences were found, the absorption of gastrodin in each intestinal segment was enhanced by 0.5% Tween 80. It can be inferred that 0.5% Tween 80 enhances the intestinal absorption of gastrodin when administered orally. This finding aligns with the conclusions drawn from the pharmacokinetic experiments. Following previous in vivo pharmacokinetic and in situ single-pass intestinal perfusion experiments, we focused on the expression levels of IGTs. The results showed that the expression of IGTs was promoted by 0.5% Tween 80, while no significant differences were observed in the groups treated with 0.1% or 2.0% Tween 80. We also excluded the influence of Tween 80 on intestinal permeability using hematoxylin–eosin staining, RT‒PCR, and Western blotting. In conclusion, the drug transport capacity of IGTs was enhanced by 0.5% Tween 80 because of the increased expression of IGTs. However, 0.1% and 2.0% Tween 80 did not significantly affect the drug transport capacity of the IGTs. Theoretically, the effect of surfactants on drug absorption is generally proportional to their concentration or gradual saturation. However, this was not the phenomenon observed in the experimental results, but there was a tendency to increase and then decrease. A similar phenomenon was observed in a study by Mai et al. on the sex-specific effects of orally administered drugs on excipients [60]. In addition, it has been suggested that the dose-dependent effect of Tween 80 on P-gp function can be explained by its significant influence within a certain range of concentrations but is negative at high concentration [61]. It has been shown in the literature that there are activation and inhibition regions in transporters [62]. For the results of this experiment, this may be due to the fact that the activating and inhibitory regions were not contacted at low concentration, whereas at moderate concentration the surfactant may bind to the activation region of the transporter and promote its function. However, at high concentration, surfactants can cause membrane loosening, leading to contact with the inhibitory region and subsequent membrane inhibition Thus, there would be a tendency for the concentration to increase from low to medium concentration, but due to the inhibitory effect of high concentration, these findings highlight that medium concentration enhance the drug transport capacity of intestinal glucose transporters. Additionally, studies have shown that Tween 80 does not cause damage to the intestinal membrane [33, 63]. Clearly, different surfactant concentrations may have varying effects on the drug transport capacity of IGTs.
In this study, Poloxamer 188 and Tween 80 did not negatively affect the drug transport capacity of IGTs. This may be because the surfactant concentrations used in this study were within the safe range and did not exceed the maximum or commonly used dose range. The safety of surfactants is determined by conducting toxicity assessment experiments to determine their toxicity in animals or cells. These experiments, which typically include acute toxicity experiments and subchronic/chronic toxicity experiments, assess the potential hazard of the surfactant to the whole organism by observing behavioral and physiological indicators and histopathological changes in animals [64]. However, relatively few studies on specific intestinal proteins, especially those related to intestinal transporters, exist [65, 66]. Therefore, for further studies, concentration experiments beyond a reasonable range can be carried out to determine whether different phenomena occur, such as protein inactivation. Additionally, experiments can be conducted by refining the concentration gradient to obtain more comprehensive data. Such studies will help to provide a more accurate reference for the design of drug formulations transported via IGTs to optimize drug uptake and understand the transport properties. To obtain more information about the effects of IGTs on drug transport capacity, further studies are needed to fill the knowledge gaps of existing studies.
Conclusion
In this study, we investigated the effects of the commonly used surfactants Poloxamer 188 and Tween 80 on the drug transport capacity of IGTs in Sprague–Dawley rats using gastrodin as a probe drug. The results indicated that Poloxamer 188, at high (8.0%), medium (2.0%), and low (0.5%) concentrations, did not significantly affect the intestinal absorption of gastrodin or the expression of IGTs. Additionally, none of the three concentrations of Poloxamer 188 affected intestinal permeability. However, compared with the control treatment, Tween 80 at a medium concentration of 0.5% significantly increased the oral bioavailability of gastrodin. In contrast, low concentration of 0.1% and high concentration of 2.0% Tween 80 did not significantly affect the oral bioavailability of gastrodin. The expression of IGTs was significantly increased with medium concentration of Tween 80 but not with low or high concentrations in all intestinal segments. Furthermore, none of the three Tween 80 concentrations had any effect on intestinal permeability. In summary, Poloxamer 188 had no effect on the drug transport capacity of IGTs at concentrations of 0.5%, 2.0%, or 8.0% within safe usage limits. However, the expression of IGTs increased in the presence of 0.5% Tween 80, resulting in a significant improvement in the drug transport capacity of the IGTs. In contrast, 0.1% and 2.0% Tween 80 had no significant effects on the serum ALB concentration. This study emphasizes the importance of considering the type and concentration of surfactants in pharmaceutical formulations. Additional research is required to understand the mechanisms underlying the effect of surfactants on the drug transport capacity of IGTs. Such studies can offer valuable insights into the rational use of drugs absorbed through the intestinal glucose transport pathway and the design of oral drug delivery systems based on this pathway. Therefore, it is crucial to improve our understanding of surfactants and to conduct thorough screening when designing drug formulations for transport through IGTs.
References
Wang X, Guo K, Huang B, Lin Z, Cai Z. Role of glucose transporters in drug Membrane Transport. Curr Drug Metab. 2020;21:947–58.
Stuart CA, Yin D, Howell MEA, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol-Endocrinol Metab. 2006;291:E1067–73.
Gyimesi G, Pujol-Giménez J, Kanai Y, Hediger MA. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflüg Arch - Eur J Physiol. 2020;472:1177–206.
Abbasi NN, Purslow PP, Tosh SM, Bakovic M. Oat β-glucan depresses SGLT1- and GLUT2-mediated glucose transport in intestinal epithelial cells (IEC-6). Nutr Res. 2016;36:541–52.
Wongon M, Limpeanchob N. Artocarpus lacucha Extract and Oxyresveratrol Inhibit Glucose Transporters in Human Intestinal Caco-2 Cells. Planta Med. 2021;87:709–15.
Stümpel F, Burcelin R, Jungermann K, Thorens B. Normal kinetics of intestinal glucose absorption in the absence of GLUT2: Evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci. 2001;98:11330–5.
Wallner EI, Wada J, Tramonti G, Lin S, Kanwar YS. Status of glucose transporters in the mammalian kidney and renal development. Ren Fail. 2001;23:301–10.
Shah K, DeSilva S, Abbruscato T. The Role of Glucose Transporters in Brain Disease: Diabetes and Alzheimer’s Disease. Int J Mol Sci. 2012;13:12629–55.
Głuchowska K, Pliszka M, Szablewski L. Expression of glucose transporters in human neurodegenerative diseases. Biochem Biophys Res Commun. 2021;540:8–15.
Karim S, Liaskou E, Fear J, Garg A, Reynolds G, Claridge L, et al. Dysregulated hepatic expression of glucose transporters in chronic disease: contribution of semicarbazide-sensitive amine oxidase to hepatic glucose uptake. Am J Physiol-Gastrointest Liver Physiol. 2014;307:G1180–90.
Szablewski L. Distribution of glucose transporters in renal diseases. J Biomed Sci. 2017;24:64.
Huang B, Lin Z, Chen Z, Chen J, Shi B, Jia J, et al. Strain differences in the drug transport capacity of intestinal glucose transporters in Sprague-Dawley versus Wistar rats, C57BL/6J versus Kunming mice. Int J Pharm. 2023;640:123000.
Patel R, Barker J, ElShaer A. Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. Int J Mol Sci. 2020;21:8224.
Otter M, Oswald S, Siegmund W, Keiser M. Effects of frequently used pharmaceutical excipients on the organic cation transporters 1–3 and peptide transporters 1/2 stably expressed in MDCKII cells. Eur J Pharm Biopharm. 2017;112:187–95.
Hodaei D, Baradaran B, Valizadeh H, Zakeri-Milani P. Effects of polyethylene glycols on intestinal efflux pump expression and activity in Caco-2 cells. Braz J Pharm Sci. 2015;51:745–53.
Ma R, Li G, Wang X, Bi Y, Zhang Y. Inhibitory effect of sixteen pharmaceutical excipients on six major organic cation and anion uptake transporters. Xenobiotica. 2021;51:95–104.
Ruan Y, Li X, You L, Chen J, Shen Y, Zhang J, et al. Effect of Pharmaceutical Excipients on Intestinal Absorption of Metformin via Organic Cation-Selective Transporters. Mol Pharm. 2021;18:2198–207.
Hanke U, May K, Rozehnal V, Nagel S, Siegmund W, Weitschies W. Commonly used nonionic surfactants interact differently with the human efflux transporters ABCB1 (p-glycoprotein) and ABCC2 (MRP2). Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Pharm Verfahrenstechnik EV. 2010;76:260–8.
Akhtar N, Ahad A, Khar RK, Jaggi M, Aqil M, Iqbal Z, et al. The emerging role of P-glycoprotein inhibitors in drug delivery: a patent review. Expert Opin Ther Pat. 2011;21:561–76.
Nigam SK. What do drug transporters really do? Nat Rev Drug Discov. 2015;14:29–44.
Soodvilai S, Soodvilai S, Chatsudthipong V, Ngawhirunpat T, Rojanarata T, Opanasopit P. Interaction of pharmaceutical excipients with organic cation transporters. Int J Pharm. 2017;520:14–20.
Engel A, Oswald S, Siegmund W, Keiser M. Pharmaceutical Excipients Influence the Function of Human Uptake Transporting Proteins. Mol Pharm. 2012;9:2577–81.
Cao S, Zhang M, Yuan M, Yang D, Zhao M, Zhang S, et al. The pharmaceutical excipient PEG400 affect the absorption of baicalein in Caco-2 monolayer model by interacting with UDP-glucuronosyltransferases and efflux transport proteins. Pharmacol Res Perspect. 2022;10:e00928.
Hashimoto Y, Michiba K, Maeda K, Kusuhara H. Quantitative prediction of pharmacokinetic properties of drugs in humans: Recent advance in in vitro models to predict the impact of efflux transporters in the small intestine and blood-brain barrier. J Pharmacol Sci. 2022;148:142–51.
Taylor EM. The impact of efflux transporters in the brain on the development of drugs for CNS disorders. Clin Pharmacokinet. 2002;41:81–92.
Darney K, Turco L, Buratti FM, Di Consiglio E, Vichi S, Roudot AC, et al. Human variability in influx and efflux transporters in relation to uncertainty factors for chemical risk assessment. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2020;140:111305.
Nakanishi T, Tamai I. Interaction of Drug or Food with Drug Transporters in Intestine and Liver. Curr Drug Metab. 2015;16:753–64.
Ren X, Mao X, Cao L, Xue K, Si L, Qiu J, et al. Nonionic surfactants are strong inhibitors of cytochrome P450 3A biotransformation activity in vitro and in vivo. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2009;36:401–11.
Al-Ali AAA, Nielsen RB, Steffansen B, Holm R, Nielsen CU. Nonionic surfactants modulate the transport activity of ATP-binding cassette (ABC) transporters and solute carriers (SLC): Relevance to oral drug absorption. Int J Pharm. 2019;566:410–33.
Mitra A, Fadda HM. Effect of surfactants, gastric emptying, and dosage form on supersaturation of dipyridamole in an in vitro model simulating the stomach and duodenum. Mol Pharm. 2014;11:2835–44.
Bollenbach L, Buske J, Mäder K, Garidel P. Poloxamer 188 as surfactant in biological formulations - An alternative for polysorbate 20/80? Int J Pharm. 2022;620:121706.
Paschen CA, Klemm D, Graf T, Kopf R, Pinto C, Müller C, et al. Simultaneous quantification of polysorbate 20 and poloxamer 188 in biopharmaceutical formulations using evaporative light scattering detection. J Pharm Biomed Anal. 2021;192:113640.
Metry M, Krug SA, Karra VK, Ekins S, Hoag SW, Kane MA, et al. Lack of an Effect of Polysorbate 80 on Intestinal Drug Permeability in Humans. Pharm Res. 2022;39:1881–90.
Kaur H, Ghosh S, Kumar P, Basu B, Nagpal K. Ellagic acid-loaded, tween 80-coated, chitosan nanoparticles as a promising therapeutic approach against breast cancer: In-vitro and in-vivo study. Life Sci. 2021;284:119927.
Zhu Z, Wen Y, Yi J, Cao Y, Liu F, McClements DJ. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J Colloid Interface Sci. 2019;536:80–7.
Machin PA, Tsonou E, Hornigold DC, Welch HCE. Rho Family GTPases and Rho GEFs in Glucose Homeostasis. Cells. 2021;10:915.
Hu L, Xia X, Zong Y, Gu Y, Wei L, Yin J. Calorie Restriction Enhanced Glycogen Metabolism to Compensate for Lipid Insufficiency. Mol Nutr Food Res. 2022;66:e2200182.
Cai Z, Hou S, Li Y, Zhao B, Yang Z, Xu S, et al. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16:178–84.
Cai Z, Song X, Sun F, Yang Z, Hou S, Liu Z. Formulation and evaluation of in situ gelling systems for intranasal administration of gastrodin. AAPS PharmSciTech. 2011;12:1102–9.
Cai Z, Huang J, Luo H, Lei X, Yang Z, Mai Y, et al. Role of glucose transporters in the intestinal absorption of gastrodin, a highly water-soluble drug with good oral bioavailability. J Drug Target. 2013;21:574–80.
Guo K, Wang X, Huang B, Wu X, Shen S, Lin Z, et al. Comparative study on the intestinal absorption of three gastrodin analogues via the glucose transport pathway. Eur J Pharm Sci. 2021;163:105839.
Valic MS, Halim M, Schimmer P, Zheng G. Guidelines for the experimental design of pharmacokinetic studies with nanomaterials in preclinical animal models. J Control Release Off J Control Release Soc. 2020;323:83–101.
Ferraris RP, Choe J-Y, Patel CR. Intestinal Absorption of Fructose. Annu Rev Nutr. 2018;38:41–67.
Xu X, Wang P, Wang B, Wang M, Wang S, Liu Z, et al. Glucose absorption regulation and mechanism of the compounds in Lilium lancifolium Thunb on Caco-2 cells. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2021;149:112010.
Zhao T, Yang S-B, Chen G-H, Xu Y-H, Xu Y-C, Luo Z. Dietary Glucose Increases Glucose Absorption and Lipid Deposition via SGLT1/2 Signaling and Acetylated ChREBP in the Intestine and Isolated Intestinal Epithelial Cells of Yellow Catfish. J Nutr. 2020;150:1790–8.
Müller U, Stübl F, Schwarzinger B, Sandner G, Iken M, Himmelsbach M, et al. In Vitro and In Vivo Inhibition of Intestinal Glucose Transport by Guava (Psidium Guajava) Extracts. Mol Nutr Food Res. 2018;62:e1701012.
Peixoto JAB, Andrade N, Machado S, Costa ASG, Oliveira MBPP, Martel F, et al. Green/Roasted Coffee and Silverskin Extracts Inhibit Sugar Absorption by Human Intestinal Epithelial (Caco-2) Cells by Decreasing GLUT2 Gene Expression. Foods Basel Switz. 2022;11:3902.
Schreck K, Melzig MF. Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells. Mol Basel Switz. 2018;23:2544.
Lee Y, Lim Y, Kwon O. Selected Phytochemicals and Culinary Plant Extracts Inhibit Fructose Uptake in Caco-2 Cells. Mol Basel Switz. 2015;20:17393–404.
Peixoto JAB, Andrade N, Machado S, Costa ASG, Puga H, Oliveira MBPP, et al. Valorizing Coffee Silverskin Based on Its Phytochemicals and Antidiabetic Potential: From Lab to a Pilot Scale. Foods Basel Switz. 2022;11:1671.
Satsu H, Awara S, Unno T, Shimizu M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci Biotechnol Biochem. 2018;82:636–46.
Jia Y, Shen J, Li X, Xie H, Wang J, Luo J, et al. Identification and analysis of gastrodin and its five metabolites using ultra fast liquid chromatography electrospray ionization tandem mass spectrometry to investigate influence of multiple-dose and food. J Chromatogr A. 2014;1358:110–6.
Jia Y, Li X, Xie H, Shen J, Luo J, Wang J, et al. Analysis and pharmacokinetics studies of gastrodin and p-hydroxybenzyl alcohol in dogs using ultra fast liquid chromatography-tandem mass spectrometry method. J Pharm Biomed Anal. 2014;99:83–8.
Lin L-C, Chen Y-F, Tsai T-R, Tsai T-H. Analysis of brain distribution and biliary excretion of a nutrient supplement, gastrodin, in rat. Anal Chim Acta. 2007;590:173–9.
Tang C, Wang L, Liu X, Cheng M, Qu Y, Xiao H. Comparative pharmacokinetics of gastrodin in rats after intragastric administration of free gastrodin, parishin and Gastrodia elata extract. J Ethnopharmacol. 2015;176:49–54.
Bogman K, Zysset Y, Degen L, Hopfgartner G, Gutmann H, Alsenz J, et al. P-glycoprotein and surfactants: effect on intestinal talinolol absorption. Clin Pharmacol Ther. 2005;77:24–32.
Shah D, Paruchury S, Matta M, Chowan G, Subramanian M, Saxena A, et al. A systematic evaluation of solubility enhancing excipients to enable the generation of permeability data for poorly soluble compounds in Caco-2 model. Drug Metab Lett. 2014;8:109–18.
Fischer SM, Brandl M, Fricker G. Effect of the non-ionic surfactant Poloxamer 188 on passive permeability of poorly soluble drugs across Caco-2 cell monolayers. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Pharm Verfahrenstechnik EV. 2011;79:416–22.
Maher S, Geoghegan C, Brayden DJ. Safety of surfactant excipients in oral drug formulations. Adv Drug Deliv Rev. 2023;202:115086.
Mai Y, Madla CM, Shao H, Qin Y, Merchant HA, Murdan S, et al. Sex-specific effects of excipients on oral drug bioavailability. Int J Pharm. 2022;629:122365.
Seelig A, Gerebtzoff G. Enhancement of drug absorption by noncharged detergents through membrane and P-glycoprotein binding. Expert Opin Drug Metab Toxicol. 2006;2:733–52.
Li-Blatter X, Nervi P, Seelig A. Detergents as intrinsic P-glycoprotein substrates and inhibitors. Biochim Biophys Acta. 2009;1788:2335–44.
Zhou Y, Li W, Chen L, Ma S, Ping L, Yang Z. Enhancement of intestinal absorption of akebia saponin D by borneol and probenecid in situ and in vitro. Environ Toxicol Pharmacol. 2010;29:229–34.
Seifert SA, Buckley N, Chan B, Chan TYK, Cumpston K, Kirschner RI, et al. Clinical Toxicology Review Metrics and Expert Reviewers, 2021. Clin Toxicol Phila Pa. 2022;60:e1-3.
Chassaing B, Compher C, Bonhomme B, Liu Q, Tian Y, Walters W, et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology. 2022;162:743–56.
Slaughter RJ, Watts M, Vale JA, Grieve JR, Schep LJ. The clinical toxicology of sodium hypochlorite. Clin Toxicol Phila Pa. 2019;57:303–11.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82173755, No. 81973268) and Natural Science Foundation of Guangdong Province (No. 2024A1515010462).
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by Jiasheng Chen, ZhenZhen Chen, and Zheng Cai. Jiasheng Chen, ZhenZhen Chen, Wentao Wang, and Liyang Wang conducted the experiments and analyzed the data. Jiaqi Zheng also contributed to part of the experiments. Shiqiong Wu, Yuru Pan and Sai Li provided valuable suggestions for writing the manuscript. Jiasheng Chen and ZhenZhen Chen wrote and reviewed the manuscript. The research was supervised by Jie Zhao and Zheng Cai, who also reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Chen, Z., Wang, W. et al. Effects of Commonly used Surfactants, Poloxamer 188 and Tween 80, on the Drug Transport Capacity of Intestinal Glucose Transporters. AAPS PharmSciTech 25, 163 (2024). https://doi.org/10.1208/s12249-024-02881-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02881-z