Abstract
Accumulating evidence from the last decade has shown that many pharmaceutical excipients are not pharmacologically inert but instead have effects on metabolic enzymes and/or drug transporters. Hence, the absorption, distribution, metabolism, and elimination (ADME) of active pharmaceutical ingredients (APIs) may be altered due to the modulation of their metabolism and transport by excipients. The impact of excipients is a potential concern for Biopharmaceutics Classification System (BCS)-based biowaivers, particularly as the BCS-based biowaivers have been extended to class 3 drugs in certain dosage forms. The presence of different excipients or varying amounts of excipients between formulations may result in bio-inequivalence. The excipient impact may lead to significant variations in clinical outcomes as well. The aim of this paper is to review the recent findings of excipient effects on gastrointestinal (GI) absorption, focusing on their interactions with the metabolic enzymes and transporters in the GI tract. A wide range of commonly used excipients such as binders, diluents, fillers, solvents, and surfactants are discussed here. We summarized the reported effects of those excipients on GI tract phase I and phase II enzymes, uptake and efflux transporters, and relevant clinical significance. This information can enhance our understanding of excipient influence on drug absorption and is useful in designing pharmacokinetic studies and evaluating the resultant data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Oral administration of drugs is widely used due to its convenience, low cost, and high patient compliance (1). Oral bioavailability, therefore, is a key consideration for discovery and development of many new drugs for effective oral drug therapy (2). Poor oral bioavailability may result in therapeutic variability and diminished efficacy (1,3,4). In general, oral bioavailability is determined by several factors, including fraction of the dose absorbed from the gastrointestinal (GI) lumen to the enterocytes and fraction of the dose that escapes first-pass elimination that occurs in the GI tract and the liver. While the liver is generally considered to be the dominant site of drug metabolism, for a number of drugs, a significant amount of administered dose may be lost before reaching the liver because of the crucial role played by GI metabolic enzymes and uptake/efflux transporters during this process.
Metabolic reactions that occur intracellularly and extracellularly in the GI tract both contribute to first-pass elimination. The drug-metabolizing enzymes involved can be divided into luminal enzymes from intestinal and pancreatic secretions (e.g., peptidases and esterases), bacterial enzymes in the colon, and gut wall/mucosal enzymes (1). The role of luminal and bacterial enzymes in drug metabolism is very limited (5–7). The gut wall/mucosal enzymes are the primary enzymes responsible for the metabolism of most orally administered drugs. The gut wall/mucosal enzymes include alcohol dehydrogenase in the stomach mucosa and a variety of phase I (e.g., CYP3A4, CYP3A5, CYP1A1, CYP2D6, and CYP2E1) and phase II enzymes (e.g., glucuronosyl transferase, sulfotransferase, N-acetyl transferase, S-methyl transferase, thiopurine methyl transferase, and glutathione S-transferase) in the intestinal and colonic mucosa (1).
After oral administration, drug molecules can cross the luminal membrane by passive diffusion or active transport. Active uptake and efflux of drug substances are mediated by trans-membrane transporters of the enterocytes. These transporters include members of the adenosine triphosphate binding cassette (ABC) superfamily, such as P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance proteins (MRP1-6), and members of the solute carrier (SLC) superfamily, such as peptide transporter (PepT1), organic anion polypeptide transporters (OATP1A2 and OATP2B1), monocarboxylate transporters (MCT), and organic cation transporters (OCTN1 and OCTN2) (8). Some transporters are located on the apical membrane of the enterocytes for apical uptake or efflux, and others may be present on the basolateral membrane of the enterocytes for basolateral efflux (8). These intestinal efflux and uptake transporters may substantially influence the intestinal absorption of their respective drug substrates.
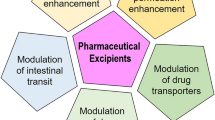
Pharmaceutical excipients are substances in the dosage forms other than the active pharmaceutical ingredient (API) or its prodrug. They play an important role in formulating a dosage form, which can ensure the safety and homogeneity of pharmaceutical products, facilitate disintegration, or enhance solubilization of the APIs (9). Commonly used pharmaceutical excipients based on their functions can be classified into several categories such as binders (e.g., glucose, povidone), fillers (e.g., lactose, mannitol, calcium phosphate, starch, sucrose), solvents/cosolvents (e.g., DMSO, ethanol, propylene glycol, polyethylene glycol), lubricants (e.g., stearic acid, magnesium stearate), and surfactants (e.g., Cremophor® EL, Tween® 20, Solutol® HS 15) (10,11). Pharmaceutical excipients are generally considered as inert vehicles in formulation and should not affect the API’s pharmacokinetics. However, emerging evidence has demonstrated that many excipients may alter the absorption and disposition properties of an incorporated drug and thus affect its therapeutic efficacy (10,12). This paper aims to review the effects of commonly used pharmaceutical excipients (Table I) on GI tract metabolic enzymes and transporters.
INTERACTIONS WITH INTESTINAL ENZYMES
The intestinal mucosa is the most important extrahepatic site of drug biotransformation, which contains both phase I and phase II enzymes (13). Although these enzymes are also present in the colonic mucosa, they are most active in the proximal small intestine.
Phase I CYP450 Enzymes
The most important enzymes involved in biotransformation of drugs in humans are the cytochrome P450s (CYPs), which are estimated to contribute to the metabolism of approximately 75% of all marketed drugs (14). More than 60 human CYP450 homologues have been identified (15), among which CYP3A4, CYP3A5, CYP2C9, CYP2C19, CYP1A2, CYP2E1, and CYP2D6 are the major enzymes responsible for drug biotransformation (16). In the intestine, CYP3A and CYP2C9 are the major CYP450s, accounting for 80% and 15% of total CYP450s detected, respectively (13). CYP1A1, CYP2C19, CYP2J2, and CYP2D6 are also present in the intestine (13). The other CYP450s such as CYP1A2, CYP2A6, CYP2B6, CYP2C8, and CYP2E1 are barely detected in the intestine (13).
CYP3A4
CYP3A4 isoenzyme is the most abundant CYP450, present at comparable levels in the liver and intestine (1). Immunohistochemical studies have found that CYP3A4 concentrations in the small intestinal are about 80–100% of that in the liver, and CYP3A4 enzyme activities prepared from human jejunal enterocytes are equal to or greater than those found in hepatic microsomes (17,18). The metabolism by CYP3A4 in the GI tract contributes to the poor oral bioavailability of many drugs that are substrates of the enzyme. Inhibition or induction of CYP3A4 activity thus can alter the absorption of drugs that are CYP3A4 substrates. It has been shown that not only drugs, herbal supplements, and food components but also some pharmaceutical excipients can affect the activity of CYP3A4 enzymes. Since the intestinal and hepatic CYP3A4 cDNA sequences are identical (19), compounds that affect liver CYP3A4 activity are assumed to affect intestinal CYP3A4 to a similar extent. Here, we summarize the excipients that were reported to interfere with CYP3A4 activity, tested either in liver or intestinal models (Table II).
The surfactants Cremophor® EL, Cremophor® RH 40, Tween® 20, Tween® 80, Pluronic® F68, and PEG-40 stearate are the most studied excipients for their effects on the enzyme activities. Early in 2004, Bravo et al. have shown that both Cremophor® EL and Tween® 80 can significantly reduce the intrinsic clearance (Clint) of the CYP3A substrate midazolam in rat hepatocytes and microsomes (20). Later, using rat intestinal epithelial cell microsomes, Ren et al. found that four nonionic surfactants, Tween® 20, Cremophor® EL, PEG-40 stearate, and Pluronic® F68, inhibited CYP3A-mediated midazolam 1′-hydroxylation formation with IC50s of 317 μM (converted with a MW of 1228 g/mol), 250 μM (converted with a MW of 2500 g/mol), 6.8 mM (converted with a MW of 328 g/mol), and 750 μM (converted with a MW of 8400 g/mol), respectively (21). These surfactants also inhibited CYP3A activity when tested using isolated rat liver microsomes (21). When coadministered with midazolam in the rat, both Tween® 20 and Cremophor® EL increased the AUCtotal of midazolam by 1.4–2.3-fold (21). However, the other two surfactants, PEG-40 stearate and Pluronic® F68, decreased the AUCtotal of midazolam to 65–74% compared to the control (21), which indicated that other mechanisms might also get involved in vivo. The inhibitory effects of those surfactants on CYP3A4 were reported in other studies as well. In human liver microsomes, Cremophor® EL, Cremophor® RH 40, and Tween® 80 inhibited CYP3A4-mediated 6β-hydroxylation of testosterone with IC50s of 600, 800, and 400 μM, respectively (22). Cremophor® RH 40 inhibited CYP3A4 activity with an IC50 of 100 μM (converted with a MW of 2500 g/mol) using recombinant CYP3A4 protein (23). Pluronic® F68 inhibited CYP3A4 activity with an IC50 of 13 μM in human liver microsomes (24). In another study using human liver microsomes, PEG-40 stearate was shown to inhibit CYP3A4 activity with an IC50 of ~300 μM (25). Most recently, Martin et al. showed that Tween® 80 and Pluronic® F68 inhibited CYP3A4 activity with IC50s of 200 and 60 μM using the Vivid Cytochrome P450 Screening Kit (26).
Some other excipients have also been shown to inhibit CYP3A4 activity, though these findings have been reported only in single studies. Solutol® HS 15 [0.3% (w/v)] reduced Clint of midazolam mediated by CYP3A by 50% (20). Vitamin E TPGS and sucrose laurate inhibited CYP3A4-mediated 6β-hydroxylation of testosterone with IC50s ranging from 0.15 to 0.2 mM in human liver microsomes (22). Martin et al. showed that both sodium deoxycholate (NaDC) and dioctyl sodium sulfosuccinate (AOT) seemed to be potent inhibitors of CYP3A4 with IC50s of 10–60 μM while cetyltrimethylammonium bromide (CTAB) and polyvinylpyrrolidone K30 (PVP K30) were less potent with IC50s of 100–200 μM (26).
Other excipients as binders, fillers, solvents, or cosolvents may affect CYP3A activity as well. In intestinal LS174T cells, Tompkins et al. showed that hydroxypropylmethyl cellulose (HPMC), pregelatinized starch, crospovidone (X-PVP), and polyethylene glycol 3350 (PEG-3350) significantly decreased CYP3A4 mRNA expression up to 65% (27). In human primary hepatocytes, dicalcium phosphate dehydrate (DCP), pregelatinized starch, and Tween® 80 decreased CYP3A4 mRNA expression by 30%, 90%, and 70%, respectively (27).
The induction of CYP3A4 activity or expression by pharmaceutical excipients seemed to be rare based on the literature. Tompkins et al. examined 19 commonly used excipients and found that no excipient activated human PXR or practically induced CYP3A4 (27). The strongest induction of CYP3A4 mRNA (2.3-fold) was observed with polyethylene glycol 3350 (PEG-3350) after incubation with human primary hepatocytes but not in intestinal LS174T cells (27).
CYP3A5
CYP3A5 is more commonly expressed in the human intestine than in the liver (28). A majority of CYP3A4 substrates are also metabolized by CYP3A5 with comparable intrinsic clearance (29), suggesting the important role of CYP3A5 in drug metabolism.
Martin et al. using Cytochrome P450 Fluorescent Assay showed that Tween® 20, Tween® 80, cetyltrimethylammonium bromide (CTAB), sodium deoxycholate (NaDC), and Pluronic® F68 inhibited CYP3A5 activity with IC50s of 100–200 μM (26). The solvent polyethylene glycol (PEG, 1000 g/mol) inhibited CYP3A5 activity with an IC50 of 78 μM (26). Intestinal CYP3A5 does not appear to be inducible evaluated by the typical enzyme-inducing drugs (28,30,31), and no induction of CYP3A5 activity by pharmaceutical excipients has been reported so far.
CYP2C9
CYP2C9 contributes more than 10% of total immunoquantified CYP450 content in the human small intestine and is functionally active as measured by diclofenac 4-hydroxylation (13,32).
In human liver microsomes, it was shown that Tween® 80, Vitamin E TPGS, sucrose laurate, Cremophor® EL, and Cremophor® RH 40 inhibited CYP2C9-mediated 4-hydroxylation of diclofenac with IC50s ranging from 0.03 to 0.07 mM (22). Pluronic® F68, PEG (1000 g/mol) and Sisterna® 16 inhibited CYP2C9 activity with IC50s of 250–560 μM (26). PEG-40 stearate inhibited CYP2C9 activity with an IC50 of 30 μM, which was examined in human liver microsomes (25).
CYP2C19
CYP2C19 is present at 2% of the total intestinal CYP450s (13). PEG (1000 g/mol), Tween® 20, and Tween® 80 were shown to inhibit CYP2C19 activity moderately with IC50s of 120–230 μM, while NaDC and AOT were weaker inhibitors with IC50s of ~350 μM (26). More potent inhibition of CYP2C19 was noticed with PEG-40 stearate with an IC50 of 21 μM in human liver microsomes (25).
CYP2D6
CYP2D6 accounts for less than 1% of the intestinal CYP450s (13). NaCAP, vitamin E PEG, AOT, and Kollicoat® Protect were potent inhibitors of CYP2D6 with IC50s ranging from 40 to 100 μM (26). Solutol® HS 15, NaDC, polyethylene glycol hexadecyl ether (Brij 58), hyamine, and CTAB inhibited CYP2D6 activity moderately with IC50s of 100–300 μM (26). PEG 1000 inhibited CYP2D6 activity weakly with an IC50 of 410 μM (26). PEG-40 stearate inhibited CYP2D6 activity with an IC50 of 490 μM which was examined with human liver microsomes (25).
Phase II Enzymes
In phase II biotransformation, xenobiotics and their phase I metabolites undergo conjugation reactions such as glucuronidation, sulfation, methylation, acetylation, glutathione, and amino acid conjugation. The conjugations usually increase the hydrophilicity of xenobiotics and therefore facilitate their renal and biliary excretion (known as detoxification of xenobiotics). Most enzymes involved in phase II biotransformation are transferases such as UDP-glucuronosyltransferases (UGTs), sulfotransferases (SULTs), N-acetyltransferases (NATs), glutathione S-transferases (GSTs), and various methyltransferases. Compared with CYP450s, phase II enzymes have attracted much less attention because drug-drug interactions and excipient effects involving these enzymes are relatively rare. Studies of excipient effects on phase II transferases are very limited. Two recent reports revealed the inhibition effects of commonly used pharmaceutical excipients on UGT2B7 (33) and aryl sulfotransferase (34).
UGTs
UGT enzymes are responsible for the glucuronidation of many xenobiotics and endobiotics. The formation of glucuronide conjugates is the most important detoxication pathway of the phase II drug metabolism. Twenty-two human UGT proteins have been identified. Among them, UGT1A1, 1A3, 1A4, 1A6, 1A9, 2B7, and 2B15 enzymes are considered to be important drug-metabolizing UGTs in the human liver, while UGT1A7, UGT1A8, and UGT1A10 are mainly expressed in the GI tract (35).
UGT2B7, expressed in the liver, kidney, lower GI tract, and brain, is the major enzyme responsible for the glucuronidation of opioids, carboxylic nonsteroidal anti-inflammatory drugs, and anticarcinogens (36). Twenty commonly used pharmaceutical excipients were screened in vitro using human and rat liver microsomes to identify inhibitors of UGT2B7 (33). The results showed that at a concentration of 0.425% (w/v), several excipients inhibited UGT2B7 activity by over 50% (33). In order of decreasing potency, these were Tween® 20 > Cremophor® EL > Cremophor® RH > PEG400 > Tween® 80 > Solutol® H15. Two preservatives, propyl paraben and methyl paraben, almost completely inhibited UGT2B7 activities at a concentration of 0.425% (w/v). The IC50 values of a mixture of Tween® 20 and PEG 400 (1:1) on UGT2B7 inhibition were determined to be 0.00419% and 0.00415% (w/v) in human and rat liver microsomes, respectively. The combination of Tween® 20 and PEG 400 decreased the IC50 of Tween® 20 alone or PEG 400 alone by an order of magnitude, indicating a synergistic effect between Tween® 20 and PEG 400 on UGT2B7 inhibition (33).
The in vivo inhibition effects of Tween® 20 and PEG 400 mixture on UGT2B7 were assessed in rats as well (33). An oral dose of Tween®-PEG mixture (4 mg/kg Tween® 20 and 4 mg/kg PEG 400) in rats inhibited UGT2B7 by about 80% in the drug absorption phase. When the same dose of Tween®-PEG mixture was administered to the rat via intra-portal vein (i.p.v.), UGT2B7 activity was inhibited by 88%. Similar inhibition effects on UGT2B7 activity were observed in healthy volunteers when Tween®-PEG mixture (240 mg of each excipient) was orally administered (33). This study showed that the use of the Tween®-PEG mixture is an effective strategy to reduce the metabolism of UGT2B7 substrates and improve their bioavailability.
SULTs
SULTs are transferase enzymes that catalyze the transfer of a sulfo group from a donor molecule to an acceptor alcohol or amine (37). SULTs exhibit wide tissue distribution. For example, members of the SULT1A subfamily have been identified in the liver, brain, breast, intestine, jejunum, lung, adrenal gland, endometrium, placenta, kidney, and blood platelets (35). SULT2A1 is expressed in the liver, adrenal, duodenum, and fetal adrenal gland (35).
SULT1A1 is also named phenol sulfotransferase or aryl sulfotransferase, which is expressed in the liver and the intestine, and it is responsible for the sulfoconjugation of phenolic compounds (35). For example, β2 agonists are substrates of SULT1A1 and hence inhibition of SULT1A1 can increase the bioavailability of β2 agonists. In a report published in 2014 (34), 11 commonly used excipients were tested for inhibition activity on aryl sulfotransferase by an in vitro enzyme activity assay. Among the 11 tested excipients, Tween® 20, Tween® 80, Cremophor® EL, Cremophor® ELP, Cremophor® RH 40, Cremophor® RH 60, and sodium lauryl sulfate (SLS) inhibited the activity of aryl sulfotransferase. The inhibition constants, viz., Ki values, of Tween® 80, Cremophor® EL, and SLS were determined to be 6.43, 3.24, and 0.22 mg/mL, respectively. The study results indicated that co-administration of aryl sulfotransferase substrate with Tween® 80, Cremophor® EL, or SLS might be able to improve the bioavailability of aryl sulfotransferase substrates.
Summary of Interaction with Metabolic Enzymes
The reported interactions of phase I CYP450 enzymes (Tables II and III) and phase II enzymes with common pharmaceutical excipients were summarized. As the most abundant and important metabolic enzyme, CYP3A4 has been studied most extensively for its interaction with pharmaceutical excipients. More than 20 excipients have been shown to inhibit CYP3A4 function, either through suppression of gene expression at transcription and/or protein levels or via direct interference with enzyme activity. Some of these excipients have shown inhibitory effects on other CYP enzymes and phase II enzymes as well. The induction of enzyme activity was uncommon, with only one case of minor induction of CYP3A4 transcription reported (27). Generally, the IC50s of CYP inhibition by pharmaceutical excipients range from 10 to 500 μM, which indicates a much weaker inhibition compared to inhibition by many drugs whose IC50s are usually at a nanomolar level. Majority of the studies are limited to in vitro assays and the clinical outcomes remain to be further investigated.
INTERACTIONS WITH TRANSPORTERS
Transporters are membrane proteins that can facilitate the permeation of compounds and ions to cross biological membranes. They play an important role in enhancing or limiting the disposition of drugs in the plasma and target issues. Numerous preclinical and clinical studies have established the importance of transporters in drug disposition (38). Most transporters in humans and animals can be classified into two large superfamilies: the solute carrier (SLC) superfamily and the ATP-binding cassette (ABC) superfamily. The SLC superfamily mainly mediates the cellular influx of substrates, mostly endogenous molecules, through facilitated diffusion or active transport. ABC transporters are responsible for the efflux of substrates, normally xenobiotics, with energy derived from ATP hydrolysis (39,40). The transporters involved in drug disposition include the active SLC transporters such as organic anion-transporting polypeptide (OATP) 1B1, 1B3, and 2B1; SLC transporters that mediate facilitated diffusion such as organic cation transporter 1 (OCT1) and OCT2; SLC antiport transporters such as multi-antimicrobial extrusion protein 1 (MATE1) and MATE2K; and the ABC efflux transporters such as P-glycoprotein (P-gp), multidrug resistance protein 2 (MRP2), breast cancer resistance protein (BCRP), and bile salt export pump (BSEP) (41).
ABC Transporters
ABC transporters belong to a superfamily of membrane proteins highly expressed at tissues/locations of drug distribution and elimination, including the blood-brain barrier, intestine, liver, kidney, and other related sites. They are very important in determining the bioavailability, distribution, and clearance of xenobiotics (42).
P-glycoprotein
P-glycoprotein (P-gp), also known as multidrug resistance protein 1 (MDR1) or ABC subfamily B member 1 (ABCB1), is an important transporter on the cell membrane that pumps many foreign substances out of cells. Highly expressed in intestinal epithelial cells and blood-brain barrier, P-gp can limit the bioavailability and systemic exposure of its substrates. Many preclinical and clinical studies have demonstrated that the inhibition of P-gp function could lead to increased drug or toxin exposure. As the most studied xenobiotic transporter, the interaction between pharmaceutical excipients and P-gp has been documented and many pharmaceutical excipients have been found to inhibit or at least attenuate its efflux function in the GI tract.
The Cremophor® class of pharmaceutical excipients has been identified as P-gp inhibitors (43). Cremophor® EL was shown to inhibit both P-gp and MRP2 activity in a Madin–Darby canine kidney epithelial (MDCK) cell model (44). Zhao et al. later reported that both Cremophor® EL- and Cremophor® RH 40-based self-microemulsifying drug delivery systems (SMEDDS) could enhance etoposide uptake in rats, which caused the C max of etoposide to increase by 28% and 33%, and the AUC0–24 h to increase by 40% and 65%, respectively (45). The increase of etoposide exposure was deemed to be resulting from P-gp inhibition (45).
For the poloxamer type surfactants, Pluronic® F68 decreased the efflux ratio of P-gp substrate celiprolol by twofold in Caco-2 cells and increased celiprolol transfer by 1.9–2.2-fold in intestinal everted sacs (24,46). Ma et al. further showed that in the rat Pluronic® F68 decreased the total body clearance of rifampicin by 1.8-fold and increased the AUC by 1.5-fold (46). Using rhodamine-123 as a substrate, Guan et al. demonstrated that Pluronic® P123 and Pluronic® F127 were able to decrease the efflux ratio by 6.8-fold and 1.6-fold in the human Caco-2 cell model, respectively (47). Pluronic® P123 and Pluronic® F127 also enhanced rhodamine-123 absorption by 6.6-fold and 2.7-fold in rat everted gut sac model, respectively (47). However, another study indicated that Pluronic® F127 did not enhance the cellular accumulation of nelfinavir in the MDCK/MDR1 cell model, which may be caused by the amphiphilic properties of the pluronic (48). Other poloxamers, such as Pluronic® P85 and Pluronic® PE 10300, were suggested to be P-gp inhibitors as well (44,48).
The polysorbates are another type of routinely used surfactant in pharmaceutical preparations. Both Tween® 20 and Tween® 80 have been identified as P-gp inhibitors, which enhanced the transfer of digoxin across the intestinal mucosa in the rat everted gut sac model by 2.1-fold and 1.8-fold, respectively (43,49). Hanke et al. studied the inhibition of P-gp function by Tween® 80 with an ABCB1-expressing MDCK cell model and found it resulted in a twofold increase of fluorescence substrate uptake (44). Later, in another study, Tween® 80 was found to enhance the bioavailability of etoposide in the rat by 2.5- fold (45). It also enhanced the permeability of etoposide by 2.7-fold in an in situ single-pass intestinal perfusion model (45). P-gp inhibition was found to play a critical role in those studies.
Vitamin E TPGS, a water-soluble non-toxic surfactant, has been characterized as an inhibitor of P-gp in Caco-2 and other cell models with an IC50 of ~0.006% (w/v) (50,51). It also increased the transport of celiprolol across the everted gut sacs by twofold (49), and enhanced the permeability of etoposide by 60% in an everted gut sac model (52). Later in vivo studies in the rat showed vitamin E TPGS to increase the permeability of paclitaxel, resulting in a 6.3-fold increase of bioavailability (53). Vitamin E TPGS also increased the bioavailability of colchicine by twofold (54). All those effects seem to be related with the inhibition of P-gp.
Some other surfactants have been found to be able to alter drug permeability and absorption by inhibiting P-gp as well. Hanke et al. reported that sucrose ester L-1695 could strongly inhibit P-gp activity, as demonstrated by a 6.3-fold increase of calcein-AM accumulation in a transfected MDCK cell model (44). Zhu et al. studied the effects of PEG-40 stearate on P-gp activity using an in vitro diffusion chamber system using rat intestinal segments and Caco-2 cell model (25). They found that PEG-40 stearate inhibited P-gp-mediated Rh123 efflux in a concentration-dependent manner, mainly by modulating substrate-stimulated P-gp ATPase activity (25). Li et al. illustrated the mechanism of mPEG-PLA on P-gp efflux activity with a Caco-2 model. This study confirmed that mPEG-PLA unimers were responsible for the P-gp inhibition effect and the inhibition potency was related to the structure property of mPEG-PLA (55). mPEG-PLA did not disrupt the Caco-2 cell plasma membrane fluidization, and it did not inhibit basal P-gp ATPase activity nor substrate-stimulated P-gp ATPase activity (55). It was concluded that ATP depletion could be the reason for P-gp inhibition by mPEG-PLA (55). Simon et al. found that phosphatidylcholine (PC) with saturated fatty acid residues of eight (8:0 PC) or ten carbon atoms (10:0 PC) could inhibit P-gp activity in both Caco-2 and MDCK-mdr1 cell models, using transport experiments and calcein accumulation assays (56). In the same study, phosphatidylcholine with two unsaturated docosahexaeonic acid residues (cis-22:6 PC) also inhibited P-gp function in cell models, but through a yet unknown mechanism (56). The surfactant polyoxyethyleneglycol dodecyl ether (Brij-35) was shown to enhance the absorption of bis (12)-hupyridone (B12H) by >70%, a substrate of both P-gp and MRP2 in the Caco-2 cell model (57).
Being widely used as a solvent, PEG 400 was found to interact with P-gp. Ashiru et al. revealed that PEG 400 stimulated the P-gp ATPase activity (58). In a Caco-2 monolayer cell model, PEG 400 decreased the efflux ratio of 3 H-ranitidine from 5.4 to 3.9 while the change mainly came from increased absorptive transport (58). PEG 300 showed similar activity in the same study while PEG 200 only showed negligible effects. However, Parsa et al. reported that PEG 400 had no effect on the small intestine transport of etoposide in a rat everted sac model (52).
Barta et al. studied the effects of two commonly used monoglycerides, 1-monoolein and 1-monostearin, on the activity and expression of P-gp in the Caco-2 cell model. The study found that the two monoglycerides did not change the influx (apical to basolateral), but decreased the efflux (basolaterial to apical) significantly by ~50% compared to control (59). Upon monoglyceride treatment, cells maintained normal P-gp expression, membrane fluidity, and nuclear membrane integrity. It was suggested that both monoglycerides were P-gp inhibitors (59).
The effects of other excipients on P-gp expression and activity have also been reported. Tompkins et al. found that in LS174T cells, pregelatinized starch decreased MDR1 mRNA expression by greater than 40% compared to vehicle (27). Meanwhile, HPMC also lowered the MDR1 expression, but the result was not statistically significant (27). Another study demonstrated that N-octyl-O-sulfate chitosan (NOSC) increased the intestinal absorption of etoposide, a P-gp substrate with low water solubility, in rat jejunum and ileum (60). In addition, NOSC increased etoposide cell uptake and decreased efflux in the Caco-2 cell model (60). Since NOSC did not disrupt the Caco-2 cell monolayer, these results indicated that NOSC could potentially inhibit P-gp and improve the absorption of P-gp substrates (60).
BCRP
Breast cancer resistance protein (BCRP, also known as ABCG2) is present at the apical membrane of the intestine, blood-testis barrier, blood-brain barrier, placenta, and membranes of hematopoietic progenitor and other stem cells (61). It has a protective function to reduce the absorption and increase the excretion of xenobiotics (61).
Yamagata et al. showed that Cremophor® EL, Tween® 20, Span 20, Pluronic® P85, and Polyoxyl 4 lauryl ether (Brij 30) increased the uptake of 3 H-mitoxantrone in BCRP-expressing cells (62). In the mouse, they found that Pluronic® P85 and Tween® 20 increased the AUC of topotecan after oral administration by 2.0- and 1.8-fold, respectively (63). The increased oral absorption was due to the inhibited intestinal BCRP function (63). Later in a BCRP-expressing MDCK cell model, Pluronic® P85 and Tween® 20 were found to inhibit BCRP-mediated efflux of mitoxantrone by 40% and 60%, respectively (64). The inhibition was reversible. Further investigation demonstrated that inhibition of BCRP by Pluronic® P85 was competitive and the inhibition mode by Tween® 20 was a combination of competitive and noncompetitive inhibition (64).
The induction of BCRP activity was reported in the presence of oleic acid (65). Aspenstrom-Fagerlund et al. found oleic acid (C18:1) increased the apical to basolateral transportation of 3 H-mitoxantrone by threefold in Caco-2 cells (65). It was also reported that oleic acid induced BCRP gene expression by 1.7-fold after 6 h exposure although the protein level did not increase (65).
MRP2
Multidrug resistance-associated protein 2 (MRP2, also known as ABCC2) is an ABC transporter expressed both in the liver and intestine (61,66).
Hanke et al. studied the effects of several nonionic surfactants on MRP2 function using MRP2-expressing MDCK II cell model (44). Cremophor® EL, vitamin E TPGS 1000, Cremophor® RH 40, and higher concentrations of Tween® 80 inhibited MRP2 activity, but Pluronic® PE 10300 and sucrose ester L-1695 only inhibited P-gp activity (44). Li et al. comprehensively studied the MRP2 inhibition effect of commonly used excipients with scutellarin as the probe in Caco-2 cells and MRP2-over-expressing Sf-9 cell membrane vesicles. The study found that among 15 excipients studied, 12 excipients decreased efflux ratio of scutellarin in Caco-2 cells (67). The surfactants that showed inhibition were Cremophor® EL (fourfold decrease), Cremophor® RH (threefold decrease), Pluronic® F68 (2.5-fold decrease), Labrasol® (twofold decrease) and Pluronic® F127 (twofold decrease). Other excipients that also decreased MRP-2 activity were Labrafac Lipophile® WL 1349 (1.5-fold decrease), Capmul® MCM (3-fold decrease), Maisine® 35-1 (3.5-fold decrease), PEG 400 (3.5-fold decrease), PEG 2000 (3.5-fold decrease), Transcutol® (2.2-fold decrease) and β-cyclodextrin (2.2-fold decrease) (67). Cremophor® EL and PEG 2000 also showed the strongest inhibition MRP-2-mediated scutellarin efflux in Sf-9 cell membrane vesicles (67). In conclusion, both Cremophor® EL and PEG 2000 directly inhibit MRP2-mediated efflux while other excipients may increase scutellarin accumulation via other mechanisms (67). Later, Cremophor® EL, Cremophor® RH, Pluronic® F127, Maisine® 35-1, and β-cyclodextrin were further shown to inhibit the scutellarin efflux ratio in a concentration-dependent manner (68). It also revealed that the combination of excipients, such as Cremophor® EL with Pluronic® F127 or PEG 2000, could decrease the efflux ratio more than using only one excipient (68).
The effects of monoglycerides on the efflux activity and protein expression of MRP2 were investigated (69). Three monoglycerides, 1-monoolein, 1-monoplamitin, and 1-monostearin, all significantly increased Rh123 accumulation by 22%, 19%, and 23%, respectively, and decreased E217βG efflux ratio by 62%, 44%, and 76%, respectively (69). The 1-monoolein- and 1-monoplamitin-treated Caco-2 cells also led to 35 and 19% downregulation of MRP2 protein expression while 1-monostearin had no effect on MRP2 expression (69).
Uptake Transporters
The organic anion transporter polypeptide (OATP; SLCO family) is a superfamily of uptake transporters involved in the cellular uptake of numerous endogenous and xenobiotic organic anions in various tissues, including the liver, kidney, brain, and intestine (70). Among the OATP transporters, OATP1A2, 2B1, 3A1, and 4A1 have been reported to be present in human intestine (70).
One study has examined the effects of pharmaceutical excipients on uptake transporters (71). In a stably transfected human embryonic kidney (HEK) cell model, PEG 400 inhibited the uptake of estrone-3-sulfate (E3S, IC50 = 0.14%) and taurocholate (TA, IC50 = 0.05%) by OATP1A2 while other OATPs were not influenced (71). Hydroxypropyl-β-cyclodextrin (HPCD) strongly inhibited the uptake of E217βG by OATP1B3 (IC50 = 0.001%) and mildly inhibited the uptake of E3S and TA by OATP1A2 and OATP2B1 (IC50 = 0.01% to 0.24%). Both Solutol HS 15 and Cremophor® EL inhibited the uptake of bromosulfophthalein (BSP) by OATP2B1, the uptake of estrogen conjugates by OATP1A2 and OATP2B1, and the uptake of taurocholate (TA) by OATP1A2 (IC50 between 0.0003 and 0.3%) (71). The inhibition of the intestinal uptake transporter activity by excipients may reduce of absorption of their substrate drugs.
Summary of Interaction with Transporters
The efflux transporters particularly P-gp, BCRP, and MRP2 have been mostly studied for the interactions with pharmaceutical excipients. Inhibition of these transporters by excipients can result in a more than fivefold decrease of efflux, which may lead to a significant increase of exposure for some drugs (Table IV). The inhibition on uptake transporters present in the intestine like OATPs has been investigated as well. Overall, for a drug whose absorption is influenced substantially by an active transporter, caution should be taken when formulating it with certain excipients.
CLINICAL SIGNIFICANCE
The inhibition or induction effects of pharmaceutical excipients on metabolic enzymes and/or drug transporters may result in significant pharmacokinetic differences in the clinic. However, pharmaceutical excipients can affect the pharmacokinetics of drugs via other mechanisms as well. The functions of excipients include facilitating disintegration or enhancing solubilization, which can enhance absorption and oral bioavailability of drugs. In addition, pharmaceutical excipients can alter the composition and characteristics of gastrointestinal (GI) fluid (72,73), modulate GI motility and transit time (74), and change drug permeability (75) in the clinic. Thus, the net alteration of drug absorption and disposition is usually the result of an interplay of a variety of mechanisms.
One of the most studied excipients for its effect on drug pharmacokinetics in the clinic is PEG 400. PEG 400 can enhance the solubility of poorly water-soluble drugs and increase bulk fluid volume in the intestine, and it can also stimulate GI motility, shorten small intestine transit time, and modulate P-gp activity. One study showed that PEG 400 reduced absolute bioavailability of ranitidine by 31% (76), and another study showed that the effect of PEG 400 was concentration-dependent, with low concentrations of PEG 400 enhancing ranitidine absorption while high concentrations of PEG 400 significantly decreasing absorption (77). Most recently, PEG 400 was reported to enhance the bioavailability of ranitidine up to 63% only in male subjects but not in females (74). Therefore, the mechanism of PEG 400 on drug absorption is the combination of various factors including solubility enhancement, transit effects, gender differences of fluid volumes, and efflux and influx transporters, which can hardly be predicted from in vitro assays.
Cremophor® EL is another excipient that has been investigated in the clinic. Tomaru et al. studied Cremophor® EL on the inhibition of intestinal CYP3A and P-gp in healthy Japanese subjects (78). It was found that Cremophor® EL (720 mg) significantly decreased the C max and AUC of saquinavir, a substrate of CYP3A and P-gp (78). In contrast, Cremophor® EL (1440 mg) significantly increased the C max (1.3-fold) and AUC0–24 (1.6-fold) of another P-gp substrate fexofenadine (78). Further investigation has shown that saquinavir, a hydrophobic compound, was extensively entrapped into the Cremophor® EL micelles and thus there were less free molecules available for absorption (78). Therefore, although excipients like Cremophor® EL can inhibit P-gp, its effect on the absorption also depends on other factors and should be assessed case-by-case.
Vaithianathan et al. carried out a clinical study to assess the impact of larger than conventional amounts of 14 commonly used excipients on absorption of Biopharmaceutics Classification System (BCS) class 3 drugs, using cimetidine and acyclovir as BCS class 3 model drugs, in three separate four-way crossover bioequivalence (BE) studies (n = 24 each) in healthy human volunteers (79). Twelve common excipients, sodium lauryl sulfate (SLS), corn starch, sodium starch glycolate, colloidal silicon dioxide, dibasic calcium phosphate, crospovidone, lactose, povidone, stearic acid, pregelatinized starch, croscarmellose sodium, and magnesium stearate, were found not able to impact BCS class 3 drug absorption in humans (79). Thus, the author concluded that these excipients in a test product may not necessarily be qualitatively the same or quantitatively very similar to those in the reference product for a BCS class 3 biowaiver, as long as the amount used does not exceed what has been studied in the article (79). Sorbitol was shown to decrease the extent of ranitidine and cimetidine absorption (79,80). HPMC and microcrystalline cellulose lowered cimetidine and acyclovir absorption, which meant that BCS class 3 biowaivers required these excipients to be qualitatively the same and quantitatively very similar to the reference (79).
CONCLUSIONS
Pharmaceutical excipients are essential components of pharmaceutical products and historically considered to be pharmacologically inert. However, increasing evidence has shown that they can alter the pharmacokinetics of active pharmaceutical ingredients thorough various mechanisms. Sometimes, this alteration can lead to clinically significant consequences. In this paper, we reviewed recent findings regarding the effects of pharmaceutical excipients on GI tract absorption, primarily focusing on the interaction of excipients with phase I and II metabolic enzymes, and efflux and uptake transporters present in the GI tract. We have found that more than 60 excipients were shown to interfere with metabolic enzyme and/or transporters based on in vitro or preclinical assay results. Nevertheless, only a few of them have been assessed in humans. Retrospective interpretation of clinical data suggests that the observed results may be determined by various factors, on a case-by-case basis. The effects of pharmaceutical excipients on drug absorption may be influenced by excipient concentrations, the physicochemical properties of the API, and interplay of other concurrent mechanisms. There seems to be a lack of a robust approach that can integrate the in vitro and preclinical data to predict the pharmacokinetic alteration in humans. Further research efforts are warranted to shed light on solving this issue.
References
Gavhane YN, Yadav AV. Loss of orally administered drugs in GI tract. Saudi Pharm J SPJ: Off Publ Saudi Pharm Soc. 2012;20(4):331–44.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Bardelmeijer HA, van Tellingen O, Schellens JH, Beijnen JH. The oral route for the administration of cytotoxic drugs: strategies to increase the efficiency and consistency of drug delivery. Investig New Drugs. 2000;18(3):231–41.
Katsura T, Inui K. Intestinal absorption of drugs mediated by drug transporters: mechanisms and regulation. Drug Metab Pharmacokinet. 2003;18(1):1–15.
Lee V, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Adv Drug Deliv Rev. 1989;4(2).
Crauste-Manciet S, Decroix M, Farinotti R, Chaumeil J. Cefpodoxime-proxetil hydrolysis and food effects in the intestinal lumen before absorption: in vitro comparison of rabbit and human material. Int J Pharm. 1997;157(2):153–61.
Canny GO, McCormick BA. Bacteria in the intestine, helpful residents or enemies from within? Infect Immun. 2008;76(8):3360–73.
Ayman El-Kattan MV. Oral absorption, intestinal metabolism and human oral bioavailability. Paxton J, editor: InTech; 2012.
Chaudhari SP, Patil PS. Pharmaceutical excipients: a review. Int J Adv Pharmacy Biol Chem. 2012;1(1):21–34.
Buggins TR, Dickinson PA, Taylor G. The effects of pharmaceutical excipients on drug disposition. Adv Drug Deliv Rev. 2007;59(15):1482–503.
Shilpa P, Chaudhari PSP. Pharmaceutical excipients: a review. Int J Adv Pharmacy Biol Chem. 2012;1(1):21–34.
Goole J, Lindley DJ, Roth W, Carl SM, Amighi K, Kauffmann JM, et al. The effects of excipients on transporter mediated absorption. Int J Pharm. 2010;393(1–2):17–31.
Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos Biol Fate Chem. 2006;34(5):880–6.
Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360(9340):1155–62.
Lewis DF. 57 varieties: the human cytochromes P450. Pharmacogenomics. 2004;5(3):305–18.
Yan Z, Caldwell GW. Metabolism profiling, and cytochrome P450 inhibition & induction in drug discovery. Curr Top Med Chem. 2001;1(5):403–25.
Mawe GM, Gardette R, D’Agostaro L, Role LW. Development of synaptic transmission at autonomic synapses in vitro revealed by cytochrome oxidase histochemistry. J Neurobiol. 1990;21(4):578–91.
Labroo RB, Paine MF, Thummel KE, Kharasch ED. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos Biol Fate Chem. 1997;25(9):1072–80.
Lown KS, Ghosh M, Watkins PB. Sequences of intestinal and hepatic cytochrome P450 3A4 cDNAs are identical. Drug Metab Dispos Biol Fate Chem. 1998;26(2):185–7.
Bravo Gonzalez RC, Huwyler J, Boess F, Walter I, Bittner B. In vitro investigation on the impact of the surface-active excipients Cremophor EL, Tween 80 and Solutol HS 15 on the metabolism of midazolam. Biopharm Drug Dispos. 2004;25(1):37–49.
Ren X, Mao X, Cao L, Xue K, Si L, Qiu J, et al. Nonionic surfactants are strong inhibitors of cytochrome P450 3A biotransformation activity in vitro and in vivo. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2009;36(4–5):401–11.
Christiansen A, Backensfeld T, Denner K, Weitschies W. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2011;78(1):166–72.
Ren X, Mao X, Si L, Cao L, Xiong H, Qiu J, et al. Pharmaceutical excipients inhibit cytochrome P450 activity in cell free systems and after systemic administration. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2008;70(1):279–88.
Huang J, Si L, Jiang L, Fan Z, Qiu J, Li G. Effect of pluronic F68 block copolymer on P-glycoprotein transport and CYP3A4 metabolism. Int J Pharm. 2008;356(1–2):351–3.
Zhu S, Huang R, Hong M, Jiang Y, Hu Z, Liu C, et al. Effects of polyoxyethylene (40) stearate on the activity of P-glycoprotein and cytochrome P450. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2009;37(5):573–80.
Martin P, Giardiello M, McDonald TO, Rannard SP, Owen A. Mediation of in vitro cytochrome p450 activity by common pharmaceutical excipients. Mol Pharm. 2013;10(7):2739–48.
Tompkins L, Lynch C, Haidar S, Polli J, Wang H. Effects of commonly used excipients on the expression of CYP3A4 in colon and liver cells. Pharm Res. 2010;27(8):1703–12.
Wrighton SA, Ring BJ, Watkins PB, VandenBranden M. Identification of a polymorphically expressed member of the human cytochrome P-450III family. Mol Pharmacol. 1989;36(1):97–105.
Soars MG, Grime K, Riley RJ. Comparative analysis of substrate and inhibitor interactions with CYP3A4 and CYP3A5. Xenobiotica; the fate of foreign compounds in biological systems. 2006;36(4):287–99.
Wrighton SA, Brian WR, Sari MA, Iwasaki M, Guengerich FP, Raucy JL, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol Pharmacol. 1990;38(2):207–13.
Schuetz EG, Schuetz JD, Strom SC, Thompson MT, Fisher RA, Molowa DT, et al. Regulation of human liver cytochromes P-450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology. 1993;18(5):1254–62.
Wolf KK, Paine MF, Watkins PB. Metabolic barrier of the gastrointestinal tract. Gastrointestinal Toxicology. 10. Second ed 2010. p 53–75.
Wang HJ, Hsiong CH, Ho ST, Lin MJ, Shih TY, Huang PW, et al. Commonly used excipients modulate UDP-glucuronosyltransferase 2B7 activity to improve nalbuphine oral bioavailability in humans. Pharm Res. 2014;31(7):1676–88.
Ro J, Kim H, Shim BH, Kim I, Kim JT, Kim H, et al. In vitro metabolic modulation of aryl sulfotransferases by pharmaceutical excipients. B Korean Chem Soc. 2014;35(8):2577–80.
Jancova P, Anzenbacher P, Anzenbacherova E. Phase Ii drug metabolizing enzymes. Biomed Pap. 2010;154(2):103–16.
Barre L, Fournel-Gigleux S, Finel M, Netter P, Magdalou J, Ouzzine M. Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7—identification of a critical aromatic amino acid residue at position 33. FEBS J. 2007;274(5):1256–64.
Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y, et al. Structure and function of sulfotransferases. Arch Biochem Biophys. 2001;390(2):149–57.
Kusuhara H, Sugiyama Y. Pharmacokinetic modeling of the hepatobiliary transport mediated by cooperation of uptake and efflux transporters. Drug Metab Rev. 2010;42(3):539–50.
Zhang EY, Knipp GT, Ekins S, Swaan PW. Structural biology and function of solute transporters: implications for identifying and designing substrates. Drug Metab Rev. 2002;34(4):709–50.
Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov Today. 2008;13(9–10):379–93.
Peters SA. Physiologically based pharmacokinetic (PBPK) modeling and simulations: principles, methods, and applications in the pharmaceutical industry. Hoboken: Wiley; 2011. xvii, 430 p.
Locher KP. Review. Structure and mechanism of ATP-binding cassette transporters. Philos Trans R Soc Lond Ser B Biol Sci. 2009;364(1514):239–45.
Chen ML. Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv Drug Deliv Rev. 2008;60(6):768–77.
Hanke U, May K, Rozehnal V, Nagel S, Siegmund W, Weitschies W. Commonly used nonionic surfactants interact differently with the human efflux transporters ABCB1 (p-glycoprotein) and ABCC2 (MRP2). Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2010;76(2):260–8.
Zhao G, Huang J, Xue K, Si L, Li G. Enhanced intestinal absorption of etoposide by self-microemulsifying drug delivery systems: roles of P-glycoprotein and cytochrome P450 3A inhibition. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2013;50(3–4):429–39.
Ma L, Wei Y, Zhou Y, Ma X, Wu X. Effects of Pluronic F68 and Labrasol on the intestinal absorption and pharmacokinetics of rifampicin in rats. Arch Pharm Res. 2011;34(11):1939–43.
Guan Y, Huang J, Zuo L, Xu J, Si L, Qiu J, et al. Effect of pluronic P123 and F127 block copolymer on P-glycoprotein transport and CYP3A metabolism. Arch Pharm Res. 2011;34(10):1719–28.
Shaik N, Giri N, Elmquist WF. Investigation of the micellar effect of pluronic P85 on P-glycoprotein inhibition: cell accumulation and equilibrium dialysis studies. J Pharm Sci. 2009;98(11):4170–90.
Cornaire G, Woodley J, Hermann P, Cloarec A, Arellano C, Houin G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int J Pharm. 2004;278(1):119–31.
Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm Res. 1999;16(10):1550–6.
Bogman K, Erne-Brand F, Alsenz J, Drewe J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J Pharm Sci. 2003;92(6):1250–61.
Parsa A, Saadati R, Abbasian Z, Azad Aramaki S, Dadashzadeh S. Enhanced permeability of etoposide across everted sacs of rat small intestine by vitamin E-TPGS. Iran J Pharm Res IJPR. 2013;12(Suppl):37–46.
Varma MV, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Europ J Pharm Sci Off J Eur Fed Pharm Sci. 2005;25(4–5):445–53.
Bittner B, Guenzi A, Fullhardt P, Zuercher G, Gonzalez RC, Mountfield RJ. Improvement of the bioavailability of colchicine in rats by co-administration of D-alpha-tocopherol polyethylene glycol 1000 succinate and a polyethoxylated derivative of 12-hydroxy-stearic acid. Arzneimittelforschung. 2002;52(9):684–8.
Li W, Li X, Gao Y, Zhou Y, Ma S, Zhao Y, et al. Inhibition mechanism of P-glycoprotein mediated efflux by mPEG-PLA and influence of PLA chain length on P-glycoprotein inhibition activity. Mol Pharm. 2014;11(1):71–80.
Simon S, Schubert R. Inhibitory effect of phospholipids on P-glycoprotein: cellular studies in Caco-2, MDCKII mdr1 and MDCKII wildtype cells and P-gp ATPase activity measurements. Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids. 2012;1821(9):1211–23.
Yu H, Hu YQ, Ip FC, Zuo Z, Han YF, Ip NY. Intestinal transport of bis(12)-hupyridone in Caco-2 cells and its improved permeability by the surfactant Brij-35. Biopharm Drug Dispos. 2011;32(3):140–50.
Ashiru-Oredope DAI, Patel N, Forbes B, Patel R, Basit AW. The effect of polyoxyethylene polymers on the transport of ranitidine in Caco-2 cell monolayers. Int J Pharm. 2011;409(1–2):164–8.
Barta CA, Sachs-Barrable K, Feng F, Wasan KM. Effects of monoglycerides on P-glycoprotein: modulation of the activity and expression in Caco-2 cell monolayers. Mol Pharm. 2008;5(5):863–75.
Mo R, Xiao Y, Sun M, Zhang C, Ping Q. Enhancing effect of N-octyl-O-sulfate chitosan on etoposide absorption. Int J Pharm. 2011;409(1–2):38–45.
Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61(1):14–25.
Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Effect of excipients on breast cancer resistance protein substrate uptake activity. J Control Release Off J Control Release Soc. 2007;124(1–2):1–5.
Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab Dispos Biol Fate Chem. 2007;35(7):1142–8.
Yamagata T, Morishita M, Kusuhara H, Takayama K, Benameur H, Sugiyama Y. Characterization of the inhibition of breast cancer resistance protein-mediated efflux of mitoxantrone by pharmaceutical excipients. Int J Pharm. 2009;370(1–2):216–9.
Aspenstrom-Fagerlund B, Tallkvist J, Ilback NG, Glynn AW. Oleic acid decreases BCRP mediated efflux of mitoxantrone in Caco-2 cell monolayers. Food Chem Toxicol Int J Published Br Ind Biol Res Assoc. 2012;50(10):3635–45.
Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52(12):1788–95.
Li L, Yi T, Lam CW. Interactions between human multidrug resistance related protein (MRP2; ABCC2) and excipients commonly used in self-emulsifying drug delivery systems (SEDDS). Int J Pharm. 2013;447(1–2):192–8.
Li L, Yi T, Lam CW. Inhibition of human efflux transporter ABCC2 (MRP2) by self-emulsifying drug delivery system: influences of concentration and combination of excipients. J Pharm Pharm Sci Publ Can Soc Pharm Sci Soc Can Sci Pharm. 2014;17(4):447–60.
Jia JX, Wasan KM. Effects of monoglycerides on rhodamine 123 accumulation, estradiol 17 beta-D-glucuronide bidirectional transport and MRP2 protein expression within Caco-2 cells. J Pharm Pharm Sci Publ Can Soc Pharm Sci Soc Can Sci Pharm. 2008;11(3):45–62.
Tamai I. Oral drug delivery utilizing intestinal OATP transporters. Adv Drug Deliv Rev. 2012;64(6):508–14.
Engel A, Oswald S, Siegmund W, Keiser M. Pharmaceutical excipients influence the function of human uptake transporting proteins. Mol Pharm. 2012;9(9):2577–81.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Houston JB, Levy H. Effect of various alcohols on intestinal net water flux and theophylline absorption in rats. J Pharm Sci. 1975;64.
Ashiru DA, Patel R, Basit AW. Polyethylene glycol 400 enhances the bioavailability of a BCS class III drug (ranitidine) in male subjects but not females. Pharm Res. 2008;25(10):2327–33.
Stavchansky S. Scientific perspectives on extending the provision for waivers of in vivo bioavailability and bioequivalence studies for drug products containing high solubility-low permeability drugs (BCS-Class 3). AAPS J. 2008;10(2):300–5.
Basit AW, Podczeck F, Newton JM, Waddington WA, Ell PJ, Lacey LF. Influence of polyethylene glycol 400 on the gastrointestinal absorption of ranitidine. Pharm Res. 2002;19(9):1368–74.
Schulze JD, Waddington WA, Eli PJ, Parsons GE, Coffin MD, Basit AW. Concentration-dependent effects of polyethylene glycol 400 on gastrointestinal transit and drug absorption. Pharm Res. 2003;20(12):1984–8.
Tomaru A, Takeda-Morishita M, Maeda K, Banba H, Takayama K, Kumagai Y, et al. Effects of Cremophor EL on the absorption of orally administered saquinavir and fexofenadine in healthy subjects. Drug Metab Pharmacokinet. 2015;30(3):221–6.
Vaithianathan S, Haidar SH, Zhang X, Jiang W, Avon C, Dowling TC, et al. Effect of common excipients on the oral drug absorption of biopharmaceutics classification system class 3 drugs cimetidine and acyclovir. J Pharm Sci. 2015:n/a-n/a.
Chen ML, Straughn AB, Sadrieh N, Meyer M, Faustino PJ, Ciavarella AB, et al. A modern view of excipient effects on bioequivalence: case study of sorbitol. Pharm Res. 2007;24(1):73–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editor: Peng Zou
Wenpeng Zhang and Yanyan Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, W., Li, Y., Zou, P. et al. The Effects of Pharmaceutical Excipients on Gastrointestinal Tract Metabolic Enzymes and Transporters—an Update. AAPS J 18, 830–843 (2016). https://doi.org/10.1208/s12248-016-9928-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9928-8