Abstract
Purpose
The aim of this work was to develop clarithromycin microparticles (CLARI-MP) and evaluate their aerodynamic behavior, safety in bronchial cells and anti-bacterial efficacy.
Methods
Microparticles containing clarithromycin were prepared as dry powder carrier for inhalation, using leucine and chitosan. CLARI-MP were deposited on Calu-3 grown at air-interface condition, using the pharmaceutical aerosol deposition device on cell cultures (PADDOCC). Deposition efficacy, transport across the cells and cytotoxicity were determined. Anti-antibacterial effect was evaluated against Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus.
Results
Microparticles were of spherical shape, smooth surface and size of about 765 nm. Aerosolization performance showed a fine particle fraction (FPF) of 73.3%, and a mass median aerodynamic diameter (MMAD) of 1.8 μm. Deposition on Calu-3 cells using the PADDOCC showed that 8.7 μg/cm2 of deposited powder were transported to the basolateral compartment after 24 h. The safety of this formulation is supported by the integrity of the cellular epithelial barrier and absence of toxicity, and the antimicrobial activity demonstrated for Gram positive and Gram negative bacteria.
Conclusions
The appropriate aerodynamic properties and the excellent deposition on Calu-3 cells indicate that clarithromycin microparticles are suitable for administration via pulmonary route and are efficient to inhibit bacteria proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Respiratory infections (RI) have been treated since long time with antibiotics. Despite of the great progress of antimicrobial agents in the last century, it is still a challenge to treat bacterial infection, mainly due to the development of resistance. Traditionally, the treatment of RI consists on high doses of single or combined antibiotics administered by oral or intravenous route, which induce undesirable side effects mainly due to high systemic bioavailability (1). To overcome this and improve the efficacy of RI therapy, the inhalation route has been used with pressurized metered dose inhalers (pMDIs), nebulizers and dry powder inhalers (DPI) (1–5). Inhalation route has several advantages including: rapid onset of action (3), high drug amount targeted to the lungs (2,6), lower systemic bioavailability leading to a decrease of both, side effects (7) and drug resistance build-up (8). However, DPI devices have some advantages over other methods of pulmonary drug delivery, such as the direct delivery of drug into the deep lungs utilizing the patient’s breathing.
Among several methods available to produce inhalable particles as DPI, spray drying is one of the most used. Spray drying is performed in a one-step process, and according to the materials and conditions used (9) it is possible to produce dry powder particles with specific and controlled characteristics (such as particle size, size distribution and particle surface morphology). The particles properties might have direct influence on the aerosol properties of DPI formulation and consequently on the DPI deposition in the respiratory tract (10).
Nevertheless, the safety and efficacy of new DPI formulations must always be tested to predict their effects on the body before clinical use. Moreover, deposition in vitro should considerer the physiological condition in vivo. Thereby Hein et al. developed a device called Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC), which allows studying the DPI deposition and absorption in pulmonary epithelial cells (11). This system avoids the cell damage caused by turbulences of air streams as shown for example with electrostatic precipitation (12). Moreover, the PADDOCC use the sedimentation process, that is the mainly deposition mechanism in the deep lung, while twin stage impinger (TSI), multistage liquid impinger (MSLI) and anderson cascade impactor (ACI) rely on deposition by impaction (5,13–15).
Tobramycin is the only antibiotic available in the market as DPI (16), but others antibiotics are under investigation to be also used as DPI for the treatment of RI; these include aminoglycosides, fluoroquinolones, glycopeptides, macrolides, monolactams and polypeptides (1,17). Other important product that is under clinical trials is the combination of two different antibiotics (fosfomycin and tobramycin); as inhalation solution this is recommended to provide a broad spectrum of activity to treat chronic infection in cystic fibrosis and to decrease the ototoxicity and nephrotoxicity side effects from the inhaled tobramycin, improving therefore the treatment (18).
Clarithromycin (CLARI) is a hydrophobic macrolide, which is known by its bacteriostatic action and the anti-inflammatory effect (19,20). Macrolides bind reversibly to domain V of 23S ribosomal RNA (rRNA) in the 50s subunit of the bacterial ribosome, inhibiting RNA-dependent protein synthesis (20). CLARI together with its active metabolite, 14-hydroxyclarithromycin, are responsible for enhanced antimicrobial activity against respiratory pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumoniae, Escherichia coli, Mycobacteria smegmatis and Haemophilus influenza (20). Despite been used to that broad pathogen spectrum, several problems are reported with CLARI treatment, mainly due to its low oral bioavailability (55%), side effects (gastrointestinal disturbs, hepatotoxicity and renal tubular degeneration) and pathogen resistance caused by the administration of high antibiotic doses (21,22). The administration of clarithromycin as DPI could improve the antimicrobial activity and overcome adverse effect, reaching thus a better patient compliance. Some works have been done towards to develop DPI-CLARI. Park and coworkers already developed a DPI-CLARI formulation with excellent aerosol performance (2). Moreover, Moghaddam et al. increased the yield and fine powder fraction (FPF) with the addition of leucine in a new PLGA-clarithromycin formulation (23). However, these papers did not test the formulations efficacy against respiratory pathogens and the safety in pulmonary cells. Recently, Haghi et al. developed clarithromycin as pMDIs intended for anti-inflammatory therapy, with beneficial effects on cell barrier integrity, regulation of anti-inflammatory cytokine (IL-8) and inhibition of mucus production after deposition of Clarithromycin at low concentration on Calu-3 cells (5). The administration of clarithromycin, a hydrophobic drug, as pMDIs or nebulizers needs to use solvents to solubilize it, while DPI would avoid solvent exposition.
Therefore the aim of this work was to explore clarithromycin-microparticles as DPI, using chitosan and L-leucine as carriers in order to improve the yield and FPF. For this purpose, we evaluated microparticle characteristics, in vitro aerosolization performance, in vitro deposition on Calu-3 cells using the Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) and their effect on the growth of Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus.
Materials and Methods
Materials
Clarithromycin, L-leucine, ammonium acetate, amitriptyline, dimethyl sulfoxide (DMSO), sigmacote, sodium pyruvate and 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co. (St. Louis, USA). Ultrapure chitosan chloride Protasan UP CL113 with a molecular weight of 50–150 kDa and a degree of deacetylation between 75 and 90% was purchased from NovaMatrix (FMC Bio-Polymer, Drammen, Norway). Calu-3 cells (HTB-55) were obtained from the DSMZ GmbH (Braunschweig, Germany). Minimum essential medium (MEM) containing Earl’s Salts and L-glutamine was bought from Gibco (Life Technologies, Paisley, UK). Fetal calf serum (FCS) was bought from Lonza (Verviers, Belgium), and Nonessential amino acid (NEAA) solution from GE Healthcare (PAA laboratories, Pasching, Austria). Methanol, ethanol and all other chemicals and solvents used were analytical grade. Purified water was produced by Milli-Q water purification system (Merk Millipore, Billerica, USA).
CLARI Loaded Microparticles
Initially, 0.05 g of Leucine and 0.05 g of chitosan were solubilized in 25 mL of water at room temperature by controlled magnetic stirring. Separately, 0.05 g of CLARI was dissolved in 5 mL of ethanol. After both phases were completely dissolved, they were mixed under moderate magnetic stirring at room temperature during 15 min. The solution was atomized with the Nano Spray Dryer B-90 (Büchi Labortechnik AG, Switzerland). The equipment was maintained in open mode configuration using the following parameters: pump mode 2 with 80% of spray rate (20 mL/h), airflow of 120 ± 5 L/min, inlet temperature of 80°C and spray mesh with aperture size of 4.0 μm. The separation of Clarithromycin microparticles (CLARI-MP) from particle-collecting electrode was carried-out with a soft brush.
Spray-Dried Powder Characterization
Yield and Drug Content
The yield was determined through the related amount of powder obtained in the initial concentration of all materials used in the solution. The clarithromycin content was quantified by liquid chromatography - tandem mass spectrometry (LC-MS/MS) method as described above.
LC-MS/MS Method
Clarithromycin quantification was carried out using a LC-MS/MS technique based on a previously validated method (24). This system consists of a TSQ Quantum® Access MAX Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific, San Jose, USA) equipped with an Accela 1250 pump, Accela Autosampler, and the TSQ Quantum. CLARI separation was made with an Accucore RP-MS column (150 mm × 2.1 mm, 2.6 μm, Thermo Fisher Scientific, San Jose, USA) kept at 30°C. The isocratic mobile phase consisted of 70% of methanol containing 0.1% of formic acid (v/v) and 30% of ammonium acetate buffer (5 mM) containing 0.1% of formic acid (v/v) was eluted at a flow rate of 0.25 mL/min during 4 min. We used the following Mass Spectrometry conditions: heated electrospray ionization source (HESI-II), positive mode; sheath gas, nitrogen at a flow rate of 35 arbitrary units; collision gas, argon; vaporizer temperature, 300°C; ion transfer capillary temperature, 250°C; skimmer offset, 0 V; spray voltage, 4000 V. The mass spectrometer was operated in the high-resolution selected reaction-monitoring (H-SRM) mode. The precursor ion transition of CLARI at m/z 748.343 to ion at m/z 158.145 was selected with collision energy of 28 V; scan time, 0.1 s; scan width, m/z 0.02; and tube lens offset, 111 V. While, for amitriptyline, used as internal standard (IS), the precursor ion transition at m/z 278.5 to ion at m/z 233.150 was selected with collision energy of 14 V; scan time, 0.1 s; scan width, m/z 0.02; and tube lens offset, 91 V. The peak areas were integrated automatically using the software Thermo Xcalibur (v. 2.2, San Jose, USA) with the settings as follows: peak detection algorithm, ICIS; smoothing points, 5; baseline window, 80; area noise factor, 5; peak noise factor, 10.
Briefly, 100 μL of samples were extracted with 0.9 mL of methanol:water (1:1, v/v) containing the IS (500 ng/mL) under ultra sonication for 10 min. The samples were centrifuged (10,000 g, 10 min) and the supernatant (5 μL) was injected using partial loop injection mode in the LC-MS/MS system. The developed method was linear on the concentration range of 5–500 ng/mL (R 2 = 0.99).
Scanning Electron Microscopy
Morphology and particle size analysis of CLARI-MP and Clarithromycin raw material were evaluated with scanning electron microscopy (SEM, Zeiss EVO HD15, Oberkochen, Germany). Samples were added in aluminum stubs with carbon conductive double-sided tape and sputter-coated with a 10 nm layer of gold (Quorum Q150R ES, Ashford, UK). Analyses were conducted under high vacuum conditions at an acceleration voltage of 10 kV. Particle size distribution was analyzed using the software ImageJ (version 1.47v, National Institute of Health, USA) and it is expressed as the mean diameter ± standard deviation.
Differential Scanning Calorimetry (DSC)
Thermal analysis of pure raw materials, their physical mixture and the microparticle developed were performed using differential scanning calorimetry (DSC) TA Q100 (TA Instruments, New Castle, USA). The physical mixture was prepared using the same amount of each component and it was mixed with a pestle and a mortar. Approximately 5 mg of samples were placed on aluminum pans, hermetic sealed and heated from 20 to 350°C with a scan rate of 10°C/min under a nitrogen atmosphere of 10 mL/min. DSC heating curves were analyzed using Universal Analysis 2000 software (Version 4.5a, TA Instruments, New Castle, USA).
Dissolution
The dissolution of CLARI-MP was performed in a dissolutor Sotax AT6 (Sotax AG, Basel, Switzerland). Approximately 6 mg of the powder CLARI-MP (containing about 2 mg of CLARI) were filled in hypromellose capsule (size n° 3). In order to compare the dissolution of CLARI, it was also determined the dissolution of CLARI raw material (2 mg) with a paddle speed of 100 rpm. The sink conditions was maintained employing 1000 mL of phosphate buffer medium with pH = 7.4 and temperature of 37 ± 1°C. Samples (1 mL) were withdrawn at predetermined time points at 15, 30, 45, 60, 90 and 120 min. The samples (0.1 mL) were diluted with 0.9 mL of methanol:water (1:1) containing the IS and filtered through 0.45 μm and analyzed in the LC-MS/MS, as described before. Additionally, we used the statistical moment of Mean Dissolution Time (MDT) to compare the release rates from both free drug and CLARI-MP. MDT is commonly used to describe in vitro drug release profile for dissolution of controlled release products (25).
Aerodynamic Particle Analysis
The aerodynamic particle size distribution of CLARI-MP was determined with the next-generation pharmaceutical impactor (NGI) (Copley Scientific, Nottingham, UK), set at a flow rate of 60 L/min from a pump (HCP5 High Capacity Pump, Copley Scientific, Nottingham, UK) with the critical flow controller TPK (Copley Scientific, UK). The equipment was previously calibrated with a flow meter DFM3 (Copley Scientific, Nottingham, UK). The NGI was fitted with the USP throat and its appropriate pre-separator. The effective cut-off diameters of stages 1, 2, 3, 4, 5, 6 and 7 at 60 L/min flow rate were 8.06, 4.46, 2.82, 1.66, 0.94, 0.55 and 0.34 μm, respectively. Before the experiment, the NGI collection cups were coated with Sigmacote in order to avoid bias from particle bounce and re-entrainment within the impactor. CLARI-MP powder was accurately weighed (about 6 mg) into size no 3 hypromellose capsules and loaded into a HandiHaler® dry powder inhaler (Boehringer, Ingelheim, Germany). After the capsule was perforated, the powder was released into the NGI for 4 s at 60 L/min. The particles retained in the inhalation device, mouthpiece adaptor, throat device, pre-separator and each stage from one to eight were removed with 1:1 (methanol–water) solution and collected into volumetric flasks. The samples (0.1 mL) were diluted with 0.9 mL of methanol:water (1:1) containing the IS, filtered through 0.45 μm and injected in the LC-MS/MS for analysis. The emitted dose (ED) was calculated from the proportion of drug that entered in the NGI compared to the total drug present in each capsule. The fine particle fraction (FPF), which is the percentage of particles with aerodynamic diameter (dae) lower than 5 μm, was determined as the fraction of nominal dose deposited in the NGI between the stage 2 (dae < 4.46 μm) and the filter.
Particle Size Distribution
The mass median aerodynamic diameter (MMAD) and the geometric standard deviation (GSD) were determined with the NGI data based on the cumulative particle size distribution functions. MMAD represents the average aerodynamic particle size of inhaled particles and is defined as the diameter above and below which dispose 50% of the mass of the particles. While for GSD, a dimensionless number is equal to the ratio between the MMAD with either 84% or 16% of the diameter size distribution. The GSD, together with the MMAD, describe the particle size distribution of the formulation.
In Vitro Deposition Study
Cell Culture
Calu-3 cells (HTB-55) were used between passage 30 and 42. The cells were cultivated in 75 cm2 flasks in MEM supplemented with Earl’s Salts, L-glutamine, 10% fetal calf serum (FCS), 1% non-essential amino acid (NEAA), 50 mM sodium pyruvate, 100-units/mL penicillin and 100 μg/mL streptomycin. The cells were incubated at 37°C, 5% CO2 and 95% humidity. Once reached confluence Calu-3 were detached with Trypsin and seeded in 12 well Snapwells polyester inserts (1.12 cm2, 0.4 μm pore size, Corning Costar, Lowell, MA, USA) at 1.33 × 105 cells/cm2 concentration. After 48 h, the cells were cultivated at air-liquid interfaced (ALI) by removing the apical medium and feeding only the basolateral part with 1.0 mL of complete MEM. ALI cultures were grown for 12–14 days, in order to differentiate and perform tight epithelial barrier (26).
Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC)
Deposition experiments were performed with PADDOCC, as previously described by Hein et al. (11,27). Briefly, it consists of an airflow control unit (Akita®, Activaero, Gemünden/Wohra, Germany), an aerosolization unit and a deposition unit, which are all connected by silicon tubes. The PADDOCC (without the air flow control unit) was accommodated in an incubator at 37°C and the following airflow parameters were used: dispersion impulse of 60 L/min for 0.2 s and a ventilation flow of 6 L/min for 2.0 s.
CLARI Deposition
To evaluate the total deposition of clarithromycin, a dry powder capsule with approximately 6 mg of CLARI-MP was aerosolized with the PADDOCC and deposited in sampling wells (i.e. without cells) with 0.5 mL of methanol:water (1:1). After three aerosolization–deposition cycles of 10 min, each well of the sampling unit was washed with 0.5 mL of methanol:water (1:1) and quantified by LC-MS/MS analytic method, as previously described.
Permeability
Firstly, the Snapwells® were placed into the sampling wells of the PADDOCC with 0.5 mL of MEM at 37°C in the basolateral compartment and were pre-equilibrated for 15 min. Then, the system was set up for three deposition cycles resulting in 30 min for aerosolization and deposition process. After this period, Snapwells® containing the cells were placed in a cell culture plate, and 1.5 mL of cell culture medium was added in the basolateral compartment. The cells were incubated at 37°C, 5% CO2 and 95% humidity with a rotational shaker at 150 rpm. Samples of 100 μL were acquired each 30 min during 4 h and at 24 h from the basolateral compartment to evaluate the formulation permeability across Calu-3. To analyze drug concentration, samples were extracted with methanol:water (1:1) containing the IS, centrifuged at 10,000 g for 10 min at 12°C and the supernatants quantified by LC-MS/MS analysis. After the permeability experiments, TEER values were measured to verify the integrity of cell monolayer as mentioned below.
TEER Values
Transepithelial electrical resistance (TEER) was measured before and after the permeability experiment to monitor cellular barrier integrity using a Volt-Ohm Meter (EVOM with STX-2 chopstick electrodes, World Precision Instruments, Berlin, Germany). Briefly, pre-warmed cell culture medium was added in the apical (0.5 mL) and in the basolateral compartment (1.5 mL) before TEER measurement and incubated for 30 min. The electrical resistance of insert membranes without cells (120 Ω) was subtracted from all samples, and the resulted value multiplied by the area of the inserts (1.12 cm2).
Cell Viability
Cell viability of CLARI-MP was determined by MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. After 24 h of aerosolization and deposition process using the PADDOCC system, the cells were washed with PBS and incubated with 5 mg/mL MTT solution in PBS pH 7.4. After 4 h incubation, the cells were lysed with DMSO and the formation of blue formazan crystals evaluated by measuring absorbance at 550 nm with the plate reader Infinite M200 PRO (Tecan, Grödig, Austria). Untreated cells and cells incubated with Triton X-100 1% were used as negative and positive controls, respectively.
Antibacterial Studies
Minimal Inhibitory Concentration (MIC) Assays
MIC50 values were determined in Pseudomonas aeruginosa PA14, Escherichia coli K12 and Staphylococcus aureus subsp. aureus (Newman strain) and were performed as described recently (28), with slight modifications. Stock test formulations of 2 mg/mL were prepared in water while CLARI was dissolved in DMSO (maximal DMSO concentration in the experiment: 1%). Final concentrations of the test formulations were obtained ranging from 0.02 to 20 μg/mL. The optical density (OD) was determined after incubate the formulations for 18 h at 37°C and 50 rpm (200 rpm for P. aeruginosa). Samples were read in 96 well plates (Sarstedt, Nümbrecht, Germany) using a FLUOStar Omega (BMG labtech, Ortenberg, Germany). Given MIC50 values are the mean of three independent experiments and are defined as the concentration of compounds that reduced the OD600 by 50%. Additionally, bacteria growth was obtained by the difference between the final OD and the OD in the beginning of the experiment. For the calculation, the OD from CONTROL (untreated samples) was considered as 100% and the OD from each experimental group was calculated related to the CONTROL (Fig. S2).
Bacteria Scanning Electron Microscopy
The interactions between the bacteria (S. aureus or P. aeruginosa) with CLARI-MP or CLARI were visualized with SEM. For that the samples were previously fixed with 1.0% glutaraldehyde in 0.2 M Hepes buffer (pH 7.4), dehydrated in a graded ethanol series (from 30 to 100%) and finally dried by evaporation with hexamethyldisilazane. The specimens were mounted on stubs and sputtered with gold to a layer thickness of 10 nm (Quorum Q150R ES, Ashford, UK) and observed at 5 kV under high vacuum conditions on a Zeiss EVO HD15 SEM (Zeiss, Oberkochen, Germany).
Data Analysis
The experiments values were expressed as mean ± standard deviation (S.D.). Data were subjected to statistical analysis using the software package Prism 6 (GraphPad Prism, San Diego, USA). The significance were analyzed by t-test or Two-way ANOVA with post hoc Tukey. Difference was considered statistically significant when p < 0.05.
Results
Microparticles Characterization
CLARI-loaded microparticles as DPI were developed and evaluated regarding cell interaction and anti-microbial activity. A DPI formulation containing clarithromycin microparticles was produced by spray drying technique using the Nano Spray Dryer Buchi B-90®, with a yield of 60.7 ± 2.3%. The obtained powder had a drug loading of 36.0 ± 1.4% (m/m). Microparticles were nearly spherical, showed a smooth surface with some holes and were not agglomerated (Fig. 1a). Particle size ranged from approximately 0.3 to 2.5 μm with an average of 765 ± 101 nm (Fig. 1b) which is suitable for deposition in the deeper regions of the lungs (29). This size is much smaller than clarithromycin raw material (Fig. S1), which was about 35 μm and not suitable for aerosolization.
The interaction between different components in the microparticles was evaluated by DSC. Analysis was carried out for CLARI-MP, its physical mixture (PM) and raw materials. As showed in Fig. 2, clarithromycin melting and decomposition temperature were respectively, 227 and 280°C. When the drug was mixed with chitosan and leucine (PM), an early onset of endothermic peak (214.8°C) was observed. Furthermore, the onset of endothermic peak of CLARI-MP sample was even lower (184.5°C).
Dissolution Study
The dissolution rate of CLARI-MP obtained by Nano Spray Dryer B-90® was compared with CLARI raw material (Fig. 3). The release profiles were expressed as percentage of drug release versus time. After 30 min, more than 90% of CLARI was released in the dissolution medium, while for such release of CLARI-MP this was observed only after 60 min. The MDT values obtained were 0.22 ± 0.06 h and 0.66 ± 0.15 h for CLARI and CLARI-MP, respectively. Assessing the release through this independent model, it was possible to show the controlled release of Clarithromycin from the microparticles.
Aerodynamic Behavior
The aerodynamic performance of microparticles is an important parameter for particle deposition in the lung. Aerosolization studies using NGI were conducted at a flow of 60 L/min during 4 s (Fig. 4). The ED obtained from hypromellose capsules and dry powder inhaler was 87.5 ± 4.4%. Moreover, the percentage of particles that could be deposited in the deep lung was measured by the FPF. For particles deposited between the stages 2 and the filter (lower than 4.46 μm) the FPF was 73.3 ± 2.3%. In addition, the MMAD and the GSD of CLARI-MP were 1.81 ± 0.14 μm and 2.59 ± 0.08, respectively.
Aerosol performance of clarithromycin microparticles using the Next Generator Impactor (NGI) at a flow of 60 L/min for 4 s. C, T, PS and F represent the percentage of particles retained in the capsule, throat, pre-separator and filter stages, respectively. Each point represents the mean ± S.D. (n = 4, from 2 independent experiments).
In Vitro CLARI-MP Transport
CLARI-MP deposition was carried out in the PADDOCC with capsules of CLARI-MP, deposited in three cycles of 10 min each, using aerosolization chambers without cells. CLARI-MP were uniform and reproducible deposited at concentration of 16.2 ± 6.9 μg/cm2 of the aerosolized dose. Moreover, the total amount collected in all the three wells per capsule corresponded to 2.37 ± 0.84% of the aerosolized dose CLARI-MP deposition. Thereafter, CLARI-MP deposition was evaluated on Calu-3 monolayers previously grown on Snapwell filter inserts. For that, aliquots of basolateral medium were collected during 24 h (Fig. 5a). The results show that CLARI-MP transport across the monolayer increased progressively in two different rates, one faster until 120 min and other slower reaching 8.7 ± 1.7 μg/cm2 after 24 h.
Deposition of clarithromycin microparticles (CLARI-MPs) on Calu-3 monolayers using the Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) system. (a) Transport of clarithromycin during 24 h demonstrating two different rates, one faster until 120 min and other slower until the end of experiment. (n = 15, from 2 independent experiments); (b) TEER values of Calu-3 monolayers before and after 24 h of deposition of CLARI-MP were similar to CONTROL; (c) Cell viability of Calu-3 using MTT assay after 24 h of deposition of CLARI-MP in the PADDOCC shows that microparticles did not induce toxic effects; Cells without any treatment were used as CONTROL. No significant difference was observed between the groups (t-test, p > 0.05; n = 9 from 2 independent experiments, mean ± S.D.).
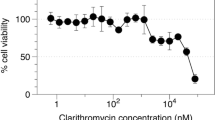
Epithelial barrier property was also evaluated by TEER measurements before and after 24 h of the deposition of CLARI-MP (Fig. 5b). Before exposition in the PADDOCC, the TEER of Calu-3 cells remained around 300 Ω.cm2, dropped slightly to 266.67 ± 28.46 Ω.cm2 and were not significant different from the untreated monolayers (248 ± 7 Ω.cm2). CLARI-MP incubated for 24 h in Calu-3 did not induce toxic effect, as assessed with MTT assay (Fig. 5c).
Antimicrobial Activity of CLARI-MP
The antimicrobial activity of CLARI-MP was tested in relevant respiratory pathogens, either Gram negative (Pseudomonas aeruginosa and Escherichia coli) or Gram positive (Staphylococcus aureus). The results showed that the concentration of CLARI-MP needed to reduce 50% of bacterial growth was 20 μg/mL for P. aeruginosa, 6 μg/mL for E. coli and 1 μg/mL for S. aureus. These concentrations were similar to those observed in the MIC50 for the free drug (Fig. 6a). Moreover, drug-free microparticles (Blank-MP) did not show any antibacterial activity against all strains tested (Fig. S2). Therefore, the action of CLARI-MP was exclusively from the clarithromycin present in the microparticles. To further characterize the interaction between bacteria and microparticles the samples were processed by SEM (Fig. 6b–g). Untreated P. aeruginosa and S. aureus (Fig. 6b, e, respectively) showed a normal size and their respective typical morphologies: rod-shaped and round (cocci). After the treatment with free drug (CLARI) as well as CLARI-MP, we observed altered morphology in the bacterial population, with a melting-like network (Fig. 6c–d, f–g). For S. aureus we observed apoptosis-like structures, e.g. cytoplasmic blebs (Fig. 6f, g).
(a) Minimum inhibitory concentration 50% (MIC50) of clarithromycin solution (CLARI) and clarithromycin microparticles (CLARI-MP) against P. aeruginosa, E. coli and S. aureus; t-test did not show any significant differences between the two formulations for either bacteria (p > 0.05; n = 3 independent experiments). Scanning electron micrographs (SEM) of P. aeruginosa (b–d) and S. aureus (e–g) untreated (b, e), treated with free drug (c, f) or CLARI-MP (d, g). Examples of melting-like network and apoptosis-like structures are shown with black and white arrows, respectively.
Discussion
Drug administration via oral route may suffer from reduced bioavailability due to the pre-systemic metabolism during the passage through gut wall and liver. Therefore delivering antibiotic to the lungs by inhalation could decrease adverse effects since lower systemic drug concentrations are reached compared to oral delivery. However after the deposition in the lungs, the drug may be eliminated by clearance systems, such as mucociliarity clearance in the upper airways. Therefore, the use of carriers systems loaded with an active compound show as promise strategy to overcome clearance in the lungs. Moreover such approach could also have a better anti-infective efficacy, since the particle shall reach directly the infection site, and release the active compound. Here we developed dry powder microparticles intended for pulmonary application, constituted by chitosan and leucine, loaded with the hydrophobic antibiotic clarithromycin. With this formulation we aim to improve the FPF of the particles to be aerolized into the small airways. Thus inhalable CLARI-MP were prepared by means of spray drying, and safety on lung cells and their efficacy against gram positive and gram negative bacteria were determined.
Chitosan, a cationic polysaccharide, has the advantage to be non-toxic and biodegradable, and has already been considered safe for the pulmonary route in animal models (30–33). Chitosan administration via inhalation as vaccine carriers (31) or gene carrier for treatment of lung metastasis did not induce evident toxicity (34). In addition, the use of chitosan in our formulation avoided particle agglomeration and increased particle deposition in the electrostatic particles collector. Chitosan is also reported to have an excellent mucoadhesive property, which is important to extend the lung residence time (35,36). In assisting to the dry process of the particles, leucine can improve powder dispersibility and aerodynamic behavior due to its ability to form hollow particles with low particle density (29,37). Higher concentrations of this essential amino acid were previously reported to increase the FPF of formulations comparing to lactose, the most-used excipient in antibiotic DPI formulations, without induction of apparent cytotoxicity (23,37,38). Furthermore, leucine was also used in a Phase I study to treat drug-resistant tuberculosis using inhaled dry powder formulation of Capreomycin, without induce toxicity (39).
Clarithromycin, a hydrophobic antibiotic with broad spectrum, is widely used against respiratory infections (18). Considering that CLARI has poor solubility in water (22), one could expected that the formation of CLARI-MP as dry powder could improve the antimicrobial activity, since it would enable the inhalation with maximum bioavailability and target delivery to the cells. However further experiments should be performed to address this issue. New clarithromycin microparticles or nanoparticles have already been described to achieve improved delivery to the lungs; however in most of the cases only exploring the pharmaceutical development (2,4,6,23), without demonstrating some biological properties of such formulations, which is essential to predict both efficacy and safety regarding their clinical application in vivo. While antimicrobial activity has been demonstrated for other CLARI formulations (40,41), no tests regarding potential toxicity on mammalian cells were performed.
After optimizing the processing parameters and excipients, a CLARI-MP powder formulation was obtained in high yields with excellent aerodynamic properties and without suffering incrustations at the piezoelectric nozzle as reported in other studies using the Nano Spray Dryer B-90® (42). We obtained a higher yield (more than 60%) in the process compared with other spray drying processes (lower than 50%) (43), mainly grace to the electrostatic particle collector. The effect of the electrostatic potential difference causes an increase in the particle attraction compared to the more common cyclone collector when cationic ions are presented in the formulation (43).
Thermal analysis of the particles performed with DSC is a useful tool to verify interactions between formulation components. We observed clarithromycin melting temperature of 227°C, as reported also by others authors (44). Importantly, we observed a shift of the onset temperature for the physical mixture, and even to a greater extend for CLARI-MP, compared to the free drug, suggesting some thermodynamic interaction between the components of this formulation, as the drug incorporation into the microparticles (45).
So far there are no agreements regarding the most appropriate dissolution method for dry powder inhalation products, as previously reviewed by Riley and coworkers (46). Dissolution studies can be performed with advanced apparatus like paddle-over-disk dissolution, flow-through cell dissolution, or diffusion cell apparatus. In this work we have used a simpler dissolution apparatus that works with similar efficacy as those previous one, to verify the difference between microparticles dissolution as well as the raw material. The dissolution test showed a slight delay (~3×) of clarithromycin release from microparticles compared to the raw material, similar to other aerosol powder formulations containing different hydrophobic drugs (47,48) and apparently suitable for pulmonary administration. The fast dissolution rate after 60 min could be an advantage to overcome clearance mechanism and optimize drug local activity.
Before deposition of CLARI-MP to pulmonary epithelial cells, we evaluated the aerodynamic behavior of aerosol particles that could be generated using hypromellose capsules and a HandiHaler® by collecting them in a NGI device. The ED of the formulation (about 87%) accomplished the European pharmacopoeia specification, which should be higher than 75% of the loaded dose. In addition, more than 75% of particles were deposited in stages 2 – 7 (FPF). This high FPF value of DPI formulation is a valuable indication of good aerosol performance when applied in vivo since it is greater than 40%, as recommended for antibiotic products (29,49) and it is 55% higher than the FPF obtained for clarithromycin pMDI (4). Moreover, the MMAD (about 1.9 μm) is in the range required for the deposition in deep lung (29).
The PADDOCC had been previously developed for reproducible-metered deposition of aerosol powders on pulmonary epithelial cell cultures under physiological conditions, allowing a simulation of complex processes of aerosol deposition and absorption in vivo, with the advantage of controlled in vitro condition. In this study, we used the bronchial epithelial cell line Calu-3 grown at air-liquid interface conditions due to their known barrier properties and easy accessibility (26,50). Hein et al. 2011 showed that the deposited amount of drug is directly proportional of the capsule dose using the PADDOCC (11). Previous studies showed a low deposition fraction (about 1.5%) for budesonide and salbutamol sulfate using the PADDOCC and modified twin stage impinger, respectively (11,15). We obtained a uniform CLARI-MP deposition of approximately 2.4% of total amount in the capsule. This value may appear to be low compared to the FPF (73.3 ± 2.3%) obtained by NGI for these particles. However, our deposition result is higher than other deposition studies (5,11,15). In addition, the recovery of drug obtained after administered ≈ 2 mg of clarithromycin was about 16.2 μg/cm2. Considering that the cells are seeded in an area of 3.36 cm2 we could speculate that the drug recover in our system correspond to 0.168 ng/cm2 in the lungs (100 m2), assuming the same rate of deposition (2.4%).
After 24 h of CLARI-MP deposition, about 50% of CLARI were transported across the Calu-3 epithelial barrier, suggesting that there might be also a substantial systemic absorption after aerosol delivery of such microparticles. This might have advantages as CLARI also has some anti-inflammatory and immune modulatory effects, according to a recent paper where the compound was applied as solution-based pressurized metered dose inhalers (pMDIs) (5). However the administration of clarithromycin as pMDIs or nebulizers requires the use of solvents in order to solubilize it; in case of CLARI-MP as DPI no solvent exposition is required. Our results also show that the epithelial barrier function was not influenced by the deposition of particles, as indicated by the absence of any significant effect on TEER over 24 h. Additionally CLARI-MP did not induce toxic effect on Calu-3 cells, which further endorses the safety of these particles for further in vivo tests, which would allow the evaluation of efficacy and tolerance of such formulation.
Finally the efficacy of CLARI-MP was tested on extracellular Gram positive (S. aureus) and Gram negative (P. aeruginosa and E. coli) bacteria. From the in vitro evaluation our formulation maintained the same antimicrobial efficacy (MIC) as observed for the free drug solubilized in DMSO. SEM results suggest that there were even a higher proportion of bacteria fragments compared to the treatment with free drug. A similar pattern was found for P. aeruginosa and E. coli strains, which could make these extracellular bacteria more susceptible to phagocytosis and degradation by macrophages.
Conclusion
In this paper we described a new dry powder inhaler formulation containing clarithromycin with aerodynamic particle properties suitable to reach the deep lung with safety and efficacy to kill bacteria. The formulation was easily achieved by spray drying technique in merely one step. Regarding the treatment of respiratory infections we could show a new drug delivery system efficient against both gram positive and negative bacteria, as Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus. Importantly, we also showed an efficient deposition of clarithromycin particles on bronchial cells (Calu-3) without inducing apparent toxicity. This technology could pave the way to better DPI formulations of CLARI and other anti-infective drugs in the future.
Abbreviations
- Blank-MP:
-
Blank microparticles
- CLARI:
-
Clarithromycin
- CLARI-MP:
-
Clarithromycin microparticles
- DPI:
-
Dry powder inhalers
- DSC:
-
Differential scanning calorimetry
- ED:
-
Emitted dose
- FPF:
-
Fine particle fraction
- IS:
-
Internal standard
- LC-MS/MS:
-
Liquid chromatography - tandem mass spectrometry
- MIC:
-
Minimal inhibitory concentration
- MMAD:
-
Mass median aerodynamic diameter
- NGI:
-
Next-generation pharmaceutical impactor
- PADDOCC:
-
Pharmaceutical aerosol deposition device on cell cultures
- PM:
-
Physical mixture
- RI:
-
Respiratory infection
- SEM:
-
Scanning electron microscopy
- TEER:
-
Transepithelial electrical resistance
References
Cipolla D, Chan H-K. Inhaled antibiotics to treat lung infection. Pharm Pat Anal. 2013;2(5):647–63.
Park C-W, Li X, Vogt FG, Hayes Jr D, Zwischenberger JB, Park E-S, et al. Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols. Int J Pharm. 2013;455(1–2):374–92.
John SP, Peter RB. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74.
Saadat A, Zhu B, Haghi M, King G, Colombo G, Young PM, et al. The formulation, chemical and physical characterisation of clarithromycin-based macrolide solution pressurised metered dose inhaler. J Pharm Pharmacol. 2014;66(5):639–45.
Haghi M, Saadat A, Zhu B, Colombo G, King G, Young PM, Traini D. Immunomodulatory effects of a low-dose clarithromycin-based macrolide solution pressurised metered dose inhaler. Pharm Res. 2014:1–10.
Pilcer G, Rosiere R, Traina K, Sebti T, Vanderbist F, Amighi K. New co-spray-dried tobramycin nanoparticles-clarithromycin inhaled powder systems for lung infection therapy in cystic fibrosis patients. J Pharm Sci. 2013;102(6):1836–46.
Roa WH, Azarmi S, Al-Hallak MHDK, Finlay WH, Magliocco AM, Löbenberg R. Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J Control Release. 2011;150(1):49–55.
Adi H, Young PM, Chan H-K, Stewart P, Agus H, Traini D. Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci. 2008;97(8):3356–66.
Nandiyanto ABD, Okuyama K. Progress in developing spray-drying methods for the production of controlled morphology particles: from the nanometer to submicrometer size ranges. Adv Powder Technol. 2011;22(1):1–19.
Dimer FA, Durli TL, Fontana MC, Pohlmann AR, Beck RCR, Guterres SS. Piezoelectric atomizing spray-dryer to convert liquids to dry powders: operational parameters and formulation characteristics. In: Tran HT, Pillai G, editors. Advances in nanotechnology & applications - volume IV: CreateSpace Independent Publishing Platform; 2012. p. 105–16.
Hein S, Bur M, Schaefer UF, Lehr C-M. A new Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) to evaluate pulmonary drug absorption for metered dose dry powder formulations. Eur J Pharm Biopharm. 2011;77(1):132–8.
de Bruijne K, Ebersviller S, Sexton KG, Lake S, Leith D, Goodman R, et al. Design and testing of Electrostatic Aerosol in vitro Exposure System (EAVES): an alternative exposure system for particles. Inhal Toxicol. 2009;21(2):91–101.
Bur M, Rothen-Rutishauser B, Huwer H, Lehr C-M. A novel cell compatible impingement system to study in vitro drug absorption from dry powder aerosol formulations. Eur J Pharm Biopharm. 2009;72(2):350–7.
Haghi M, Traini D, Young P. In vitro cell integrated impactor deposition methodology for the study of aerodynamically relevant size fractions from commercial pressurised metered dose inhalers. Pharm Res. 2014;31(7):1779–87.
Haghi M, Traini D, Bebawy M, Young PM. Deposition, diffusion and transport mechanism of dry powder microparticulate salbutamol, at the respiratory epithelia. Mol Pharm. 2012;9(6):1717–26.
Hoppentocht M, Hagedoorn P, Frijlink HW, de Boer AH. Technological and practical challenges of dry powder inhalers and formulations. Adv Drug Deliv Rev. 2014;75:18–31.
Zarogoulidis P, Kioumis I, Ritzoulis C, Petridis D, Darwiche K, Porpodis K, et al. New insights in the production of aerosol antibiotics. Evaluation of the optimal aerosol production system for ampicillin-sulbactam, meropenem, ceftazidime, cefepime and piperacillin-tazobactam. Int J Pharm. 2013;455(1–2):182–8.
Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, et al. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. Am J Respir Crit Care Med. 2012;185(2):171–8.
David SR, Bergstrom RF, Bruner VL, Mitchell MI. Pharmacokinetics and pharmacodynamics of IM olanzapine. Schizophr Res. 2002;53(3):183.
Zuckerman JM, Qamar F, Bono BR. Review of macrolides (Azithromycin, Clarithromycin), Ketolids (Telithromycin) and Glycylcyclines (Tigecycline). Med Clin N Am. 2011;95(4):761–91.
Bermudez LE, Nash K, Petrofsky M, Young LS, Inderlied CB. Clarithromycin-resistant mycobacterium avium is still susceptible to treatment with clarithromycin and is virulent in mice. Antimicrob Agents Chemother. 2000;44(10):2619–22.
Global Alliance for TB Drug Development. Clarithromycin. Tuberculosis. 2008;88(2):92–5.
Moghaddam PH, Ramezani V, Esfandi E, Vatanara A, Nabi-Meibodi M, Darabi M, et al. Development of a nano–micro carrier system for sustained pulmonary delivery of clarithromycin. Powder Technol. 2013;239:478–83.
Shin J, Pauly DF, Johnson JA, Frye RF. Simplified method for determination of clarithromycin in human plasma using protein precipitation in a 96-well format and liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2008;871(1):130–4.
Podczeck F. Comparison of in vitro dissolution profiles by calculating mean dissolution time (MDT) or mean residence time (MRT). Int J Pharm. 1993;97(1–3):93–100.
Haghi M, Young PM, Traini D, Jaiswal R, Gong J, Bebawy M. Time- and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev Ind Pharm. 2010;36(10):1207–14.
Hein S, Bur M, Kolb T, Muellinger B, Schaefer UF, Lehr CM. The Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) in vitro system: design and experimental protocol. Altern Lab Anim. 2010;38(4):285–95.
Sahner JH, Groh M, Negri M, Haupenthal J, Hartmann RW. Novel small molecule inhibitors targeting the “switch region” of bacterial RNAP: structure-based optimization of a virtual screening hit. Eur J Med Chem. 2013;65:223–31.
Hoe S, Ivey J, Boraey M, Shamsaddini-Shahrbabak A, Javaheri E, Matinkhoo S, et al. Use of a fundamental approach to spray-drying formulation design to facilitate the development of multi-component dry powder aerosols for respiratory drug delivery. Pharm Res. 2014;31(2):449–65.
Grenha A, Al-Qadi S, Seijo B, Remuñán-López C. The potential of chitosan for pulmonary drug delivery. J Drug Delivery Sci Technol. 2010;20(1):33–43.
Chu BY, Kobiasi MA, Zeng W, Mainwaring D, Jackson DC. Chitosan-based particles as biocompatible delivery vehicles for peptide and protein-based vaccines. Procedia Vaccinol. 2012;6:74–9.
Lee C, Choi JS, Kim I, Oh KT, Lee ES, Park E-S, et al. Long-acting inhalable chitosan-coated poly(lactic-co-glycolic acid) nanoparticles containing hydrophobically modified exendin-4 for treating type 2 diabetes. Int J Nanomedicine. 2013;8:2975–83.
Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Del Rev. 2010;62(1):3–11.
Okamoto H, Shiraki K, Yasuda R, Danjo K, Watanabe Y. Chitosan–interferon-β gene complex powder for inhalation treatment of lung metastasis in mice. J Control Release. 2011;150(2):187–95.
Sogias IA, Williams AC, Khutoryanskiy VV. Why is Chitosan Mucoadhesive? Biomacromolecules. 2008;9(7):1837–42.
Dhawan S, Singla AK, Sinha VR. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech. 2004;5(4):122–8.
Feng AL, Boraey MA, Gwin MA, Finlay PR, Kuehl PJ, Vehring R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int J Pharm. 2011;409(1–2):156–63.
Aquino RP, Prota L, Auriemma G, Santoro A, Mencherini T, Colombo G, et al. Dry powder inhalers of gentamicin and leucine: formulation parameters, aerosol performance and in vitro toxicity on CuFi1 cells. Int J Pharm. 2012;426(1–2):100–7.
Dharmadhikari AS, Kabadi M, Gerety B, Hickey AJ, Fourie PB, Nardell E. Phase I, single-dose, dose-escalating study of inhaled dry powder capreomycin: a new approach to therapy of drug-resistant tuberculosis. Antimicrob Agents Chemother. 2013;57(6):2613–9.
Mohammadi G, Nokhodchi A, Barzegar-Jalali M, Lotfipour F, Adibkia K, Ehyaei N, et al. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf B. 2011;88(1):39–44.
Valizadeh H, Mohammadi G, Ehyaei R, Milani M, Azhdarzadeh M, Zakeri-Milani P, et al. Antibacterial activity of clarithromycin loaded PLGA nanoparticles. Pharmazie. 2012;67(1):63–8.
Schmid K, Arpagaus C, Friess W. Evaluation of the Nano Spray Dryer B-90 for pharmaceutical applications. Pharm Dev Technol. 2011;16(4):287–94.
Durli TL, Dimer FA, Fontana MC, Pohlmann AR, Beck RC, Guterres SS. Innovative approach to produce submicron drug particles by vibrational atomization spray drying: influence of the type of solvent and surfactant. Drug Dev Ind Pharm. 2013.
Gomez-Burgaz M, Torrado G, Torrado S. Characterization and superficial transformations on mini-matrices made of interpolymer complexes of chitosan and carboxymethylcellulose during in vitro clarithromycin release. Eur J Pharm Biopharm. 2009;73(1):130–9.
Chiu MH, Prenner EJ. Differential scanning calorimetry: an invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J Pharm Bioall Sci. 2011;3(1):39–59.
Riley T, Christopher D, Arp J, Casazza A, Colombani A, Cooper A, et al. Challenges with developing in vitro dissolution tests for orally inhaled products (OIPs). AAPS PharmSciTech. 2012;13(3):978–89.
Beck-Broichsitter M, Schweiger C, Schmehl T, Gessler T, Seeger W, Kissel T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J Control Release. 2012;158(2):329–35.
Lee SH, Teo J, Heng D, Zhao Y, Ng WK, Chan HK, et al. Steroid-decorated antibiotic microparticles for inhaled anti-infective therapy. J Pharm Sci. 2014;103(4):1115–25.
Belotti S, Rossi A, Colombo P, Bettini R, Rekkas D, Politis S, et al. Spray dried amikacin powder for inhalation in cystic fibrosis patients: a quality by design approach for product construction. Int J Pharm. 2014;471(1–2):507–15.
Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res. 2006;23(7):1482–90.
ACKNOWLEDGMENTS AND DISCLOSURES
Frantiescoli Dimer is thankful to the Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) program “Ciência sem Fronteiras” project number BEX 18215/12-2. The authors kindly thank Simone Amann for bacteria experiments, Marius Hittinger for NGI and PADDOCC experiments and Dr. Chiara Rossi for technical support of LC-MS/MS and SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig S1
Scanning electron micrograph of Clarithromycin raw material. (JPEG 416 kb)
Fig S2
Bacteria growth after 18 h of treatment with microparticles without clarithromycin (BLANK-MP), clarithromycin solution (CLARI) and clarithromycin microparticles (CLARI-MP) compared to CONTROL against P. aeruginosa (A), E. coli (B) and S. aureus (C). The percentage was calculated from final experiment optical density subtracted from initial optical density compared to CONTROL. The data show 3 independent experiments (means ± SD). * Different from CONTROL: p < 0.05; ** Different from CONTROL: p < 0.01; *** Different from CONTROL: p < 0.001; ### Different from CLARI: p < 0.001. (JPEG 325 kb)
Rights and permissions
About this article
Cite this article
Dimer, F., de Souza Carvalho-Wodarz, C., Haupenthal, J. et al. Inhalable Clarithromycin Microparticles for Treatment of Respiratory Infections. Pharm Res 32, 3850–3861 (2015). https://doi.org/10.1007/s11095-015-1745-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1745-8