The antioxidant properties of dihydroquercetin (DHQ, taxifolin), its aminomethylated derivative (KH-14), and their inclusion complexes in cyclodextrin were compared. The results indicated that DHQ and KH-14 had comparable antioxidant activities. The antioxidant activities of DHQ and KH-14 were preserved upon formation of the inclusion complexes in cyclodextrin. Taxifolin, KH-14, and the water-soluble inclusion complex of KH-14 in cyclodextrin at a dose of 0.04 mmol/kg exhibited pronounced anti-inflammatory effects on the model of carrageenan-induced paw edema in mice that were equivalent to that of diclofenac at a dose of 0.02 mmol/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dihydroquercetin (taxifolin, DHQ) is a bioflavonoid with potent antioxidant activity. Also, DHQ and related compounds possess antitumor, angioprotective, neuroprotective, antiviral, and other properties [1,2,3,4].
DHQ and its derivatives are hydrophobic compounds so that alcohol or DMSO must be used to produce aqueous solutions. The lack of water-soluble forms of these compounds makes it very difficult to use them intravenously for therapeutic purposes.
Cyclodextrins are known to be capable of increasing the solubility and bioavailability of hydrophobic drugs, which is responsible for their broad use in pharmacology [5, 6].
The formation of inclusion complexes of DHQ and its derivatives with cyclodextrin significantly improved their water solubility [7] and enabled intravenous administration of them. However, this could also affect their antioxidant, anti-inflammatory, and other types of activity.
Therefore, the goal of the present work was to compare the antioxidant and anti-inflammatory activities of native DHQ, its aminomethylated derivative, and their inclusion complexes with cyclodextrin.
Experimental Chemical Part
Native DHQ and its aminomethylated derivative KH-14 (Fig. 1), which was synthesized at the Department of Organic Chemistry, Moscow Pedagogical State University, and water-soluble forms of these compounds, i.e., inclusion complexes with 2-hydroxypropyl-β-cyclodextrin (henceforth cyclodextrin or CD) [7, 8], were studied.
NMR spectra were recorded on a JNM-ECX400 spectrometer (100.5 MHz, Japan). Chemical shifts were given vs. TMS (internal standard). Elemental analyses used a Perkin-Elmer 2400 analyzer (USA).
Preparation of aminomethylated DHQ derivative KH-14. A mixture of formaldehyde (as paraformaldehyde, 0.3 g, 0.01 mol), racemic diisobutylamine (1.29 g, 0.01 mol), and EtOH (96%, 50 mL) was stirred at 60°C until fully homogeneous (~1.5 h); cooled to 20°C; treated with a solution of DHQ (3.5 g, 0.01 mol) in EtOH (10 mL); and stirred at 40°C for 1.5 h. The resulting precipitate was filtered off under vacuum; rinsed with EtOH, Et2O, and hexane; and dried in vacuo. Yield 2.49 g (56%), mp 205 – 206°C (dec.). Found, %: C 64.69; H 7.11; N 3.13. C24H31NO7. Calc., %: C 64.70; H 7.01; N 3.15. 13C NMR spectrum (DMSO-d6, δ, ppm): 83.89 (C2), 72.22 (C3), 196.98 (C4), 163.86 (C5), 101.13 (C6), 165.36 (C7), 94.87 (C8), 162.28 (C9), 101.55 (C10), 129.21 (C1′), 120.07 (C2′), 147.12 (C3′), 145.84 (C4′), 119.89 (C6′), 43.29 (C11′), 54.23 (C13–13′′), 28.42 (C14–14′′), 9.18 (C15–15′′), 19.76 (C16–16′′).
DHQ and its derivative KH-14 were encapsulated within the shell of a cyclic glucose oligomer, i.e., CD, according to the published method to form hydrophilic forms [7]. The products were water-soluble inclusion complexes with CD that were used for further studies.
Experimental Pharmacological Part
The ABTS test was used to evaluate the antioxidant activities of the studied compounds [9]. The ABTS test (decolorization method) is based on measuring the optical density of a solution of previously generated stable ABTS•+ cation-radicals at 734 nm (absorption maximum of ABTS•+).
The antioxidant activity (AOA), which was numerically equal to the inverse antioxidant concentration at which the optical density of the ABTS*+ solution decreased by 50%, was calculated from the results of the ABTS test. The control was a solution (3%) of DMSO, in which the studied compounds, which were primarily lipophilic compounds, were dissolved.
The anti-inflammatory activity (AIA) of the compounds was studied using a carrageenan-induced mouse-paw edema model [10]. The studied compounds were injected i.p. Experimental groups consisted of 6 – 8 outbred male white mice (40 – 42 g) that were kept under standard vivarium conditions and received a standard feed diet. DHQ, DHQ-CD, KH-14, and KH-14-CD were injected at equimolar doses of DHQ and KH-14 (0.16, 0.04, 0.02 mmol/kg). Diclofenac was injected at a dose of 0.02 mmol/kg, which corresponded to a daily dose of 75 mg for humans according to a dose conversion method [11, 12]. Control mice received normal saline.
Statistical analysis used the Mann—Whitney criterion. Differences were considered statistically significant for significance level p < 0.05.
Results and Discussion
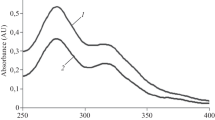
Concentration curves were plotted and AOA was calculated using the results of the ABTS test for DHQ, KH-14, DHQ-CD, and KH-14-CD (Fig. 2).
In general, the effects of the studied compounds on the optical densities of ABTS•+ solutions did not differ statistically significantly. An analysis of the results showed that the AOA of DHQ, KH-14, DHQ-CD, and KH-14-CD were of the same order of magnitude in the range from 0.074 to 0.100 μM–1. Thus, modification of the native form of DHQ and its aminomethylated derivative KH-14 into water-soluble forms by encapsulation in CD did not reduce their AOA.
The AOA of the DHQ derivatives could have been mediated by their anti-inflammatory properties.
The AIA of DHQ, KH-14, DHQ-CD, and KH-14-CD was studied using a mouse-paw carrageenan-induced edema model.
Figure 3 shows the experimental results from the studies of the AIA.
According to the results, all studied compounds possessed AIA in the used model. DHQ-CD had the lowest AIA. Injection of it lowered the size of the carrageenan-induced edema by 21%. KH-14-CD decreased the size of the edema by 46%; KH-14, 43%; and DHQ, 52%. Thus, DHQ, KH-14, and KH-14-CD at a dose of 0.16 mmol/kg possessed AIA that was comparable to that of diclofenac at a therapeutic dose.
According to the results, the potent AIA of DHQ, KH-14, and KH-14-CD was comparable to that of diclofenac at a therapeutic dose and was preserved upon decreasing the dose to 0.04 mmol/kg (12.5 mg/kg recalculated for DHQ).
A correlation analysis of the relationship between the AIA and AOA of the studied compounds was performed (Table 1).
The correlation analysis did not reveal a statistically significant correlation between the AIA and AOA. This result could be indicative of additional (besides antioxidant) mechanisms for manifestation of AIA and other pharmacological effects of plant flavonoid quercetin derivatives [13, 14].
Thus, the AOA and AIA of inclusion complexes of DHQ and its aminomethylated derivative (KH-14) with a cyclodextrin shell that increased their water-solubility [7] were preserved. This allowed them to be recommended for further studies to design injectable drug forms.
References
P. B. Tsydendambaev, B. S. Khyshikteuv, and S. M. Nikolaev, Byull. Vost.-Sib. Nauchn. Tsentra Sib. Otd. Ross. Akad. Med. Nauk, 52(6), 229 – 233 (2006).
V. V. Knyazev, V. S. Rogovskii, E. D. Sveshchnikova, et al., Khim.-farm. Zh., 52(3), 7 – 20 (2018); Pharm. Chem. J., 52(3), 205 – 208 (2018).
A. Garcia-Lafuente, E. Guillamon, A. Villares, et al., Inflammation Res., 58(9), 537 – 552 (2009).
Dihydroquercetin: Instruction, Use [in Russian]; http://rlsnet.ru/mnn_index_id_2487.htm.
A. Rashed, A. Kumar, and V. Sravanthi, Rev. Sci. Pharm., 76, 567 – 598 (2008).
V. P. Zinchenko, Yu. S. Tarakhovskii, and G. E. Bronnikov, Biofizika, 56(3), 433 – 438 (2011).
A. M. Koroteev, G. Z. Kariev, et al., RU Pat. 2,396,077, Aug. 10, 2010; Ref. Zh. Khim., A61P9 / 14 (2009).
V. S. Rogovskii, T. M. Arzamasova, M. A. Rozenfel′d, et al., Khim.-farm. Zh., 47(6), 10 – 13 (2013); Pharm. Chem. J., 47(6), 295 – 298 (2013).
R. Re, N. Pellegrini, A. Proteggente, et al., Free Radical Biol. Med., 26, 1231 – 1237 (1999).
C. Morris, Methods Mol. Biol., 225, 115 – 121 (2003).
E. J. Freireich, E. A. Gehan, et al., Cancer Chemother. Res., 50(4), 219 – 244 (1966).
D. Yu. Safronov, Vestn. Mosk. Gos. Obl. Univ., Ser. Estestv. Nauki, 3, 82 – 85 (2011).
V. S. Rogovskii, Author’s Abstract of a Candidate Dissertation in Medical Sciences, Moscow (2014).
V. A. Kurkin, K. N. Sazanova, E. N. Zaitseva, et al., Khim.- farm. Zh., 54(8), 10 – 12 (2020); Pharm. Chem. J., 54(8), 797 – 799 (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 8, pp. 25 – 27, August, 2021.
Rights and permissions
About this article
Cite this article
Rogovskii, V.S., Matyushin, A.I., Koroteev, A.M. et al. Antioxidant and Anti-Inflammatory Activities of Dihydroquercetin, its Aminomethylated Derivative, and their Inclusion Complexes with Cyclodextrin. Pharm Chem J 55, 778–780 (2021). https://doi.org/10.1007/s11094-021-02493-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02493-y