Abstract
Since the early days of medicine, chemical substances derived from animals, plants, and microorganisms have been used for treatment of various diseases. Among these, products derived from plants have dominated the pharmacopoeias for thousands of years, providing an inexhaustible source of medicinal resources. Croton L. is the second largest genus of Euphorbiaceae, comprising about 1300 species of trees, bushes, and herbs. Croton sphaerogynus Baill. belongs to the same clade of Croton cajucara Benth, used in traditional medicine. The aim of this study was to characterize the antioxidant activities of extracts and isolated flavonoids obtained from leaves of C. sphaerogynus. Chemical composition of leaf extracts and its fractions (flavonoid contents) and in vitro antioxidant properties were examined. Two flavonoids were isolated: quercetin 3-O-methyl ether and kaempferol 3-O-methyl ether. Antioxidant activity was higher, especially for fractions composed mainly by quercetin 3-O-methyl ether compared to crude extract and other fractions. For those fractions, EC50 values were found to be close to the ones presented by quercetin when they were analyzed for their radical scavenging activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the early days of medicine, chemical substances derived from animals, plants, and microorganisms have been used for treatment of various diseases (Schmidt et al. 2008; Dewick 2009). Among these, products derived from plants have dominated the pharmacopoeias for thousands of years, providing a source of medicinal resources (Ngo et al. 2013).
Euphorbiaceae is the third largest family of Angiosperms (Koehn and Carter 2005). In many parts of the world, species of this family are known to have medicinal or toxic properties. The second largest genus of Euphorbiaceae is Croton, comprising about 1300 species of trees, shrubs, and herbaceous plants. Croton species are distributed in tropical and subtropical regions of the new and the old world (Souza and Lorenzi 2005).
In many parts of Asia, Africa, and America, Croton species are used in traditional medicine. In Brazil, Croton species are used for treatment of a wide variety of ailments, such as hypercholesterolemia, obesity (C. cajucara Benth); inflammation, ulcers, leukemia (C. celtidifolius Baill.); anorexia (C. nepetifolius Baill.); wounds, intestinal inflammation (C. palanostigma Klotzsch.); rheumatism, cancer (C. urucurana Baill.); anorexia, gastrointestinal disorders, and also as a sweetener (C. zehntneri Pax & K. Hoffm.) (Salatino et al. 2007).
Many species of Croton produce red latex and are popularly known as “sangre-de-drago.” C. lechleri Müll. Arg., C. macrobothrys Baill., C. celtidifolius, C. palanostigma, and C. urucurana are among the species which produce red latex, all are native in Brazil. Latex composition was described with the presence of proanthocyanidins and some alkaloids, also presenting antibacterial (Chen et al. 1994) and antiproliferatve activity on leukemia cells (Rossi et al. 2003).
There are many studies focusing on the pharmacological effects of extracts and isolated substances produced by different plant parts, such as leaves and stems of Croton species. As an example, the water and ethanol extracts of C. schiedeanus Schltdl. presented antihypertensive activity (Guerrero et al. 2001, 2002a). Among the compounds isolated from Croton, trans-dehydrocrotonin has received increased attention on its pharmacological activity. This alkaloid obtained from C. cajucara was confirmed as hypoglycemic and hypolipidemic (Maciel et al. 2002), and anti-estrogenic and anti-tumor agent (Grynberg and Echevarria 1999). Diterpene plaunotol, isolated from C. sublyratus Kurz., potentiated the drug activity against Helicobacter pylori (Marshall et al. 1985) Goodwin et al. 1989 (which causes peptic ulcer) (Koga 2002) and showed anti-angiogenic activity (Kawai et al. 2005).
Croton is a challenge for phylogenetic studies. In 2011, a molecular phylogeny by van Ee and colleagues focused on the Croton species from the New World, dividing the genus into four subgenera (subgenus C. subg. Quadrilobi (Müll. Arg.) Pax in Engl. & Prantl, C. subg. Adenophylli (Griseb.) Riina, C. subg Geiseleria A. Gray, and C. subg Croton (exclusively of Old World) and describes 10 new sections, resulting in 31 sections that include all species of the New World (van Ee et al. 2011).
Croton section Cleodora (Klotzsch) Baill. comprises 18 species distributed in most or seasonally dry forest in tropical South America, Central America, and Mexico (van Ee et al. 2011). Species belonging to this section are commonly used for medicinal preparations by indigenous and traditional communities, in the treatment of wounds and prevention of infection (Salatino et al. 2007). The best-known species of this section is C. cajucara, widely distributed in the Amazon basin, and which has an extensive history of ethnobotanical uses (van Ee et al. 2011).
Croton sphaerogynus Baill. (Croton sect. Cleodora) is widely distributed in Brazilian seashore plains moist forest (“restinga forest”) (Caruzo et al. 2013), being native and endemic to Brazil. Taking into account the chemotaxonomic criteria, species belonging to Croton section Cleodora would present promising bioprospecting potential, since species of this section have been referenced as having bioactive substances.
Croton sphaerogynus had already been described as a major producer of diterpenes (Motta et al. 2013; Santos et al. 2015). Motta et al. (2013) observed moderate antiproliferative activity of the leaf dichloromethane extract (mean Log GI50 = 0.86), a fraction rich on diterpenes. Santos et al. (2015) used maceration at room temperature to extract plant constituents and observed a similar composition of diterpenes to that described by Motta et al.’s study (2013), which used serial extraction of plant material in hexane, dichloromethane, and methanol. Both studies tested the antiproliferative activities of foliar extract and its fractions, but did not test isolated substances. Moreover, Santos et al. (2015) observed moderate antiproliferative activity of leaf methanol phase, a phase also rich on diterpenes (mean Log GI50 = 0.54), but when this methanol phase was fractioned, one fraction rich in flavonoids and absent of diterpenes also presented moderate antiproliferative activity (mean Log GI50 = 1.05), suggesting a possible synergistic combination of flavonoids and diterpenes to enhance the antiproliferative activity of the methanol phase.
Using the same extract described by Santos et al. (2015), the aim of this study was to characterize the antioxidant activity of the crude ethanol extract, their fractions, subfractions and isolated flavonoids from C. sphaerogynus, a Brazilian native plant species, in order to better characterize this species and its biological activity.
Materials and methods
Plant material
Croton sphaerogynus Baill. (Figs. 1–3), occurs in the States of Bahia, Rio de Janeiro, Espírito Santo and São Paulo. Most of its populations are from Brazilian seashore plains moist forest (“Restinga forest”). Diagnostic characteristics of this species are the shrub habit; globose, sessile, and brown extra-floral nectaries on the base of leaves; oval-lanceolate to oval leaves, cordate to sagittate, with the basal lobes sometimes overlapping; indumentum of stellate trichomes and inflorescences in spiciform thyrses, with proximal cymules bisexual and distal ones staminate (Caruzo et al. 2013).
A voucher specimen (LBM 65) was deposited in the herbarium Maria Eneyda P. K. Fidalgo (SP), São Paulo. Leaves, and stems of a population of C. sphaerogynus were collected in the municipality of Itanhaem, southern coast of State of São Paulo. Samples were dried with aeration at 40 °C for 1 week. Subsequently leaves were grounded for chemical analysis.
Extraction and fractionation
Extraction and fractionation procedures were performed according to Santos et al. (2015). Dried and powdered leaf material (1 kg) was extracted by maceration with EtOH for 7 days at room temperature. Crude EtOH extract (EE) was concentrated under reduced pressure, evaporated to dryness under a stream of nitrogen, and lyophilized, affording 70.62 g of crude EtOH extract (yield: 7 %). Part of EE (65 g) was solubilized in MeOH and partitioned using hexane. Hexane phase (HP) was concentrated under reduced pressure to yield 20 g (2 %) of hexane phase, while the MeOH phase (MP) was lyophilized to afford 15 g (yield: 1.5 %), based on dried and powdered leaf material. Part of MP (8 g) was fractionated over Sephadex LH-20 using MeOH as eluent, affording five fractions: F1 (2 g), F2 (0.119 g), F3 (0.090 g), F4 (0.353 g), and F5 (0.090 g). Fraction F4 was fractionated by preparative high-performance liquid chromatography (prep-HPLC1200) using a PrepHT XDB C-18 column (150 × 21.2 mm, 5 µm) affording subfractions F4.1 (1.8 mg) and F4.2 (1.3 mg). Solvent used was 85 % MeOH isocratic for 10 min. Solvent flow was 8 mL min−1, injection volume of 250 µL, and detection at 352 nm.
Hexane phase (HP), already known for its rich presence on diterpenes (Motta et al. 2013; Santos et al. 2015) and its absence on flavonoids, was not used to test the antioxidant activity.
Chemical composition
All lyophilized EE, MP, fractions (F1–F5) and subfractions (F4.1 and F4.2) were dissolved in MeOH (2 mg mL−1) and analyzed by high-performance liquid chromatography (HPLC, 1260 Agilent Technologies) using diode array detector (DAD) and a Zorbax-C18 column (150 × 4.6 mm, 3.5 µm) at 40 °C. Solvents used were 0.1 % acetic acid (AcOH) and acetonitrile (CH3CN), starting with 15 % of CH3CN (0–20 min), increasing to 100 % (20–25 min); isocratic (5 min); decreasing to 15 % (30–32 min); isocratic (3 min). Solvent flow rate was 1.5 mL min−1 (0–25 min), 1.0 mL min−1 (25–26 min), 1.5 mL min−1 (26–35 min); injection volume of 3 L, and detection at 352 and 280 nm. Quercetin and kaempferol at concentrations from 0.0006 up to 0.36 µg mL−1 were used to prepare calibration curves following the same analysis conditions. Results are expressed as quercetin equivalent milligrams (QE) or kampferol equivalent milligrams (KE) per gram of dry sample (mg g−1).
MP was also submitted to high-performance liquid chromatography coupled mass spectrometry (HPLC–MS); Zorbax SB-C18 (150 × 4.6 mm, 5 µm). Solvents: 0.1 % acetic acid and acetonitrile starting with 15 % CH3CN (0–20 min), increasing to 100 % (20–25 min), isocratic (5 min), decreasing to 15 % (30–32 min) and isocratic (3 min). Flow 90 µL min−1, voltage 4000 V, nebulizer 27 psi, drying gas at 320 °C and flow of 7 L min−1. Constituents were identified by comparing the corresponding UV–Vis and ESI/MS–MS spectra with MS data from the literature.
For establishment of substitution patterns, F4.1 and F4.2 were submitted to UV–Vis absorption spectroscopy (240–600 nm) using methanol solution and ionization (KOH, NaOAc) or complexing (AlCl3, AlCl3/HCl, NaOAc/H3BO3) shift reagents (Markham 1982). Kaempferol and quercetin were identified by co-chromatography over cellulose thin layer chromatography (TLC) by comparison with authentic samples using BAW (n-butanol:acetic acid:water—4:1:5) and CAW (chloroform:acetic acid:water—30:15:2) and UV–Vis analysis (Markham 1982). Identification of sugar presence was carried out with chemical hydrolysis (1 N hydrochloric acid). Hydrolysis products were identified by co-chromatography over cellulose TLC by comparison with authentic samples using 15 % acetic acid.
Free radical scavenging activity determination using DPPH• (2,2-Diphenyl-1-picrylhydrazyl)
Free radical scavenging activities of all samples were determined according to the DPPH radical method modified from Furlan et al. (2015). DPPH solution in methanol (0.2 mM) was freshly prepared and 200 μL were mixed with 20 μL of each sample. Reaction mixture was incubated for 20 min at room temperature and in the dark. The decrease in absorbance was measured at 515 nm by an EPOCH microplate reader. As positive control, Trolox (6–200 μg mL−1) and quercetin (7.5–120 μg mL−1) were used. Methanol was used as negative control.
Free radical scavenging activity determination using ABTS• (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)
Scavenging activity of the ABTS radical followed the protocol described by Rufino et al. (2007) with modifications. The ABTS solution (3.84 mg mL−1) was prepared using ultrapure water and a solution of 2.6 mM potassium persulfate. For the formation of ABTS radical, the two solutions above were mixed in a ratio of 1:1. Mixture was maintained in the dark at room temperature for 12–16 h. At the time of the test, 1 mL of the ABTS• previously prepared was diluted in 30 mL of methanol. To each 20 µL of sample and controls were added 280 µL of the diluted solution of ABTS•. Microplate was incubated for 2 h at room temperature in the dark. The absorbance was detected at 734 nm by an microplate reader. Trolox (12–250 mg mL−1) and quercetin (15–120 mg mL−1) were used as positive controls. Methanol was used as negative control.
Ferric-reducing antioxidant potential (FRAP)
Ferric-reducing power of samples was determined according to a modified protocol from Furlan et al. (2015). FRAP solution was prepared on the day of analysis by mixing 25 mL of acetate buffer (0.3 M, pH 3.6), 2.5 mL of 10 mM TPTZ and 2.5 mL of 20 mM ferric chloride. FRAP solution (265 µL) was mixed with 10 µL of each sample and 25 µL of ultrapure water. After incubation of 30 min at 37 °C, the absorbance at 595 nm was detected by an microplate reader. Trolox (6–200 mg mL−1) and quercetin (7.2–180 mg mL−1) were used as positive control. Methanol was used as negative control.
β-carotene bleaching assay
Antioxidant activity by β-carotene bleaching assay was determined according to Duarte-Almeida et al. (2006), with modifications. The reactive mixture was prepared in 500 µL of dichloromethane: 21 µL of linoleic acid, 100 mg of Tween 40 and 210 µL of β-carotene solution in dichloromethane (2 mg mL−1). Solvent was evaporated under nitrogen until complete dryness and 32 mL of water previously saturated with oxygen, were vigorously mixed. Absorbance of the solution was measured by a microplate reader to ensure that was between 0.5 and 0.6 at 450 nm. Reaction solution (250 µL) was mixed to 10 µL of samples or standards. First reading was made immediately and the mixture was incubated at 45 °C. Readings were made at intervals of 15 min for 120 min. As positive control, Trolox (6–200 μg mL−1) and quercetin (7.5–120 μg mL−1) were used. Methanol was used as negative control.
Metal chelating activity
Ferric chelating activity was performed according to Min et al. (2011), by mixing 10 µL of each sample or control with 130 µL of 10 % acetate buffer, 10 µL of 1 mM ferrous ammonium sulfate, and 10 µL of 6.1 mM ferrozine solution (after 5 min). Microplate was kept at room temperature for 10 min, and absorbance at 562 nm was detected by an microplate reader. A methanolic solution of gallic acid (5–100 mg mL−1) was used as a positive control and methanol as negative control.
Determination of oxygen radical absorbance capacity (ORAC)
ORAC assay was adapted from the protocols proposed by Min et al. (2011). Sodium fluorescein was dissolved in phosphate buffer solution (PBS) (75 mM, pH 7.0) to obtain a stock solution of 4.0 µM. The fluorescein working solution (8 nM) was freshly prepared in PBS, and 150 µL of this was mixed with 25 µL of each sample (in PBS) at different concentrations. The reaction mixtures were incubated for 30 min at 37 °C, and 25 µL of 75 mM and AAPH (2,2′-Azobis(2-methylpropionamidine) dihydrochloride) solution (152 mM) were added to the wells. As positive controls, a PBS Trolox solution was plotted (6.25–50 mM). A blank with PBS was run with each assay. The fluorescence (excitation = 485 nm; emission = 520 nm) was registered 120 times with a delay of 60 s between repeats using a microplate reader. The antioxidant capacity was based on the calculation of the area under the curve (AUC), using the following formula:
where f 0 is the initial fluorescence reading at 0 min, and f 1 is the fluorescence reading at time 1. The net AUC was obtained by subtracting the AUC of the blank from the AUC of the sample. The final ORAC values were calculated by using a regression equation between Trolox concentrations and the net AUC.
For all antioxidant assays, sample antioxidant potential was expressed as milligram per gram of dry extract equivalents to positive control (Trolox, quercetin, or galic acid) and effective concentration of each sample to achieve 50 % of the antioxidant activity (EC50 µg mL−1).
Results and discussion
Croton sphaerogynus had already been described as a major producer of diterpenes (Motta et al. 2013; Santos et al. 2015). According to Santos et al. (2015), maceration at room temperature resulted in a similar composition of diterpenes described by Motta et al. (2013). Using the same extract used by Santos et al. (2015), partition using MeOH:Hexane was not efficient to eliminate all diterpenes from methanol phase (MP) and from F1. Column chromatography using Sephadex was efficient to eliminate this class of compounds from fractions F2, F3, F4 and F5. As well as, subfractions F4.1 and F4.2 did not present diterpenes. Studies on diterpenes characterization and their relative amounts on each sample were recently published by Santos et al. (2015). Flavonoids’ compositions of EE, MP, F1-F5, F4.1, and F4.2 are listed in Table 1.
Twenty flavonoids were detected, mainly quercetin and kaempferol derivatives, but no flavone was detected. F1, F2, and F3 exhibited similar flavonol composition, with the presence of more than five constituents (Table 2). F4 showed a composition of four compounds: isorhamnetin monoglycoside (compound 9), quercitrin (compound 14), quercetin 3-O-methyl ether (compound 17), and kaempferol 3-O-methyl ether (compound 19), while F5 was composed by one single flavonoid, quercetin 3-O-methyl ether (compound 17) (data also presented by Santos et al. 2015).
Fractions F5, F4.1, and F4.2 were subjected to ionizing and complexing reactions for their structural elucidation. Using Markham (1982), it was possible to further characterize the degree of hydroxylation of the flavonoid molecules: substance 17 as quercetin 3-O-methyl ether and substance 19 as kaempferol 3-O-methyl ether. In addition, comparison of spectroscopic data to those from the literature (Krenn et al. 2003; Bertelli et al. 2012) allowed the confirmation of compound 17 as 5,7,3′,4′-tetrahydroxy-3-methoxy-flavonol (quercetin 3-O-methyl ether).
Leaves of C. sphaerogynus using maceration in ethanol as extraction method resulted in 0.06 % of substances equivalent to kaempferol and 0.03 % of substances equivalent to quercetin. C. sphaerogynus yielded 0.09 % of flavonoids related to dry mass of leaves. Table 2 shows the content of flavonoids detected in each sample analyzed.
The presence of flavonols in many species of Croton had already been reported by several authors: Savietto (2011) studying C. dichrous Müll. Arg., C. erythroxyloides Baill., C. myrianthus Müll. Arg., and C. splendidus Mart.; Matos (2011) using C. betulaster Müll. Arg., C. glutinosus Müll. Arg., C. pycnocephalus Müll. Arg., C. montevidensis Spreng., C. hemiargyreus Müll. Arg., C. antisyphiliticus Mart., and C. grandivelum Baill.; Athayde (2013) studying C. echinocarpus Baill. and C. vulnerarius Baill.; and Soares (2013) studying C. pallidulus Baill. var. pallidulus Baill.. On comparing the flavonoid composition of C. sphaerogynus to those presented by the above studies, it can be observed the common presence of rutin and a higher proportion of flavonols instead of flavones. However, unlike C. dichrous, C. erythroxyloides, C. myrianthus, C. pallidulus var. pallidulus, C. echinocarpus, and C. vulnerarius, C. sphaerogynus did not present the acylated flavonoid tiliroside (kaempferol p-coumaroyl).
Furlan et al. (2015), studying nine Argentinian species of Croton, identified the presence of tiliroside in all samples, but Croton species studied were from sections Adenophylli, Barhamia, Lamprocroton, and Pedicellati. This acylated flavonol was also detected by Matos (2011) when investigating species from Croton sections Barhamia, Argentini, and Cleodora, being C. hemiargyreus (Croton sect. Cleodora) the species with less expressive amount of this flavonoid. Maciel et al. (2002), studying C. cajucara, also from Croton sect. Cleodora, reported the presence of kaempferol derivatives, mainly kaempferol 3,4,7-thymethyl ether, but the complete absence of tiliroside. Although tiliroside could be a taxonomic marker for Croton, these studies suggest differences in flavonoid composition among Croton sections.
Flavonoids highly methoxylated were also described as frequent in Croton species. For C. schiedeanus and C. cajucara were reported quercetin 3,7-dimethyl ether, rutesin, artemetin, and methoxylated kaempferol derivatives (Guerrero et al. 2002b; Maciel et al. 2002; Salatino et al. 2007).
Several in vitro methods are used to determine the antioxidant capacity of a substance. Currently used are the ones based on power metal reduction, for example, FRAP, or based on anti-radicalar capacity (ABTS•, DPPH•), on quantifying products formed during lipid peroxidation (oxidation of β-carotene), and on ORAC (Sanchez-Moreno 2002; Sánchez-Burgos et al. 2013; Roby et al. 2013; Thatoi et al. 2014).
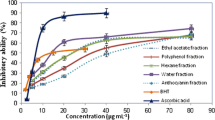
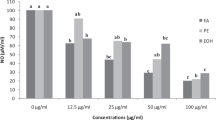
Antioxidant potentials of samples are shown in Table 3. Both tests of radical scavenging capacity (DPPH• and ABTS•) yielded similar results with F3, F4, and F5 being the most active samples. F4 was the sample that showed the highest content of ferric-reducing capacity (1000 mg g−1 TE) followed by F5 (813.97 mg g−1 TE). For metal chelating activity, F4.2 presented the highest chelating potential (229.01 mg g−1 GAE) followed by F4 (136.53 mg g−1 GAE). For β-carotene bleaching assay, Trolox calibration curves showed no linearity between concentration versus intensity of the response, and hence, values of Trolox equivalent were not calculated. For ORAC, F4.1 presented the highest absorbance capacity (3536.14 µM g−1 TE) followed by F4.2 (2976.66 µM g−1 TE).
According to Table 3, most fractions presented higher antioxidant activities than ethanolic extract (EE) and methanol phase (MF). This result indicates the antioxidant activities of the samples are strongly related to flavonoid composition. F1 showed lower antioxidant activity compared to F2 and F3, presenting also lower contents of flavonoids. F2 and F3 had relatively similar contents and compositions of flavonols and also presented similar antioxidant capacity. F4 was the most active sample in almost tests (except for metal chelating activity and ORAC), suggesting compound 17 (quercetin 3-O-methyl ether) and 19 (kaempferol 3-O-methyl ether) as responsible for the antioxidant properties of this fraction. F5, composed exclusively by quercetin 3-O-methyl ether showed very similar results to F4, but not higher.
These results suggest that antioxidant capacity seems to be directly related to a synergy between quercetin derivatives in F4. Synergistic interactions among chemical substances may potentiate the effect of an extract, or also can interfere with its action (Lila and Raskin 2005). Besides the composition, contents of those flavonols in both F4 and F5 are probably related to their activities, since F4 has contents of quercetin derivatives approximately twice higher than F5 (Table 2).
F4 was the most active fraction in DPPH•, β-carotene, and FRAP assays, and its fractionation was an attempt to isolate compounds 14 and 19 (Table 2) and test their antioxidant potential when isolated. Analyzing results from F4.2, composed mainly by kaempferol 3-O-methyl ether, the antioxidant activity by radical scavenging capacity (DPPH• and ABTS•), and ferric-reducing capacity was low when compared to fractions rich on quercetin derivatives. The relationship between function and structure of the flavonoid is the key to the various biological activities performed by those substances. A study performed by van Acker et al. (1996) correlated the structural features of flavonoids with their antioxidant activities and suggested flavonols exhibiting a catechol group in the B-ring (two vicinal hydroxyl groups) as having higher antioxidant activity. Antioxidant activity of quercetin derivatives has already been reported by Heim et al. (2002) who attributed the higher radical scavenging activity to quercetin aglycone, followed by O-methylated and O-glycosylated derivatives.
Furthermore, the combination of the catechol group with a double bond between C2–C3 plus a hydroxyl group in position 3 enhances the antioxidant capacity of a substance, for example, the flavonol quercetin (van Acker et al. 1996). The low antioxidant capacity of F4.2 corroborates with the proposal of van Acker et al. (1996): flavonol without a catechol group, such as derivatives of kaempferol, has lower antioxidant activity.
However, metal chelating activity assay revealed F4.2 as the fraction with the highest potential. It is not common that kaempferol derivatives present high metal chelating activity. Some studies had positively related the presence of hydroxyl groups to the metal chelating activity (Mira et al. 2002; Kumar and Pandey 2013a, b; Kumar et al. 2013), and it is known that kaempferol has one hydroxyl group lesser than quercetin. In addition, kaempferol derivative in F4.2 has a methoxyl replacing the hydroxyl group at C3, which would further reduce its activity. However, this subfraction has the presence of two quercetin derivatives closer in abundance to F5 (composed exclusively of quercetin 3-O-methyl ether). Presence of quercetin derivatives could explain the metal chelating activity of this sample.
Rather the other tests, measuring the oxygen radical absorbance capacity (ORAC), F4.1 showed increased activity. This fraction is composed mostly by compound 14 (quercitrin). Kim and Shim (2014), tested the hypothesis that methanol extract of Houttuynia cordata Thunb (MEH) and its targeted bioactive components including rutin, quercitrin, and chlorogenic acid can be effective in reducing reactive oxygen species (ROS) caused by nicotine and promoting conversion of nicotine to cotinine in HepG2 cell. Compared to nicotine only, pretreatments of MEH, rutin, and quercitrin were revealed to effectively inhibit ROS production in HepG2 cell by up to 9, 7.4, and 14 %, respectively. Boligon et al. (2012) demonstrated that quercitrin showed protective effects against damages caused by hydrogen peroxide in human lymphocytes, possibly by decreasing ROS.
Antioxidants can be synthesized in vivo or, for animals, obtained through diet. The most well-known exogenous antioxidants coming from dietary are vitamins A, C, and E and polyphenolics (Tripathi et al. 2007). Antioxidant activities of phenolic compounds, such as flavonoids, tannins, and phenylpropanoids are characterized by the redox property assigned to them, which can play an important role neutralizing reactive oxygen species (ROS) (Zheng and Wang 2001; Martin et al. 2013; Kumar and Pandey 2013a).
Medical effectiveness of many flavonoids is well established. Quercetin, for example, is the subject of many studies reporting antibacterial, hepatoprotective, anti-inflammatory, antitumor, and antiviral activities, and for all of those activities, the presence of a free hydroxyl group in C3, as well as, two vicinal hydroxyl groups in B-ring, seem to be important to enhance the flavonoid activity (van Acker et al. 1996; Kumar and Pandey 2013a).
Table 4 shows the effective concentration of each sample to achieve 50 % of the antioxidant activity (EC50). For some samples, a linear correlation between concentration and antioxidant activity was not observed, and EC50 was not calculated. EC50 reveals the real antioxidant activity of a sample, once it takes into account the dose–response curve.
F4 showed the lowest EC50 (µg mL−1) in DPPH•, β-carotene and FRAP assays, as expected. In metal chelating activity and ORAC, F4.2 exhibited the highest activity and F5 was the most active fraction in ABTS•. Comparing EC50 of the standards used in this study (Table 4), quercetin had higher antioxidant activity than Trolox. However, quercetin showed lower metal chelating activity compared with gallic acid. In almost tests, F4 and F5 presented EC50 values close to that presented by quercetin. Both fractions are mainly composed by the flavonol quercetin 3-O-methyl ether (substance 17).
There are many methods to assess the antioxidant capacity of a plant extract. Thus, it becomes difficult to choose the most appropriate method in order to avoid incorrect interpretation of results. A comparison of different studies is also difficult, and because of this, it is preferred to carry out a battery of assays, analyzing different mechanisms of antioxidant action and comparing them to synthetic antioxidant standards (such as Trolox, BHT, among others). According to Oliveira et al. (2009), in vitro methods must be developed carefully, taking into account the use of oxidants and targets of biological significance, such as proteins, triacylglycerol, and cellular models, as closest as possible to in vivo conditions. Furthermore, in vivo tests are necessary for confirmation of the observed activity and potential side effects of an extract or substance.
In conclusion, this research suggested C. sphaerogynus as promising to use in the exploration of bioactive substances, especially for antioxidant applications. Fractions showed EC50 values close to those presented by commercial standards as Trolox and quercetin. Further investigation and research is needed regarding the biological in vitro and particularly in vivo activities of extracts from C. sphaerogynus.
References
Athayde NR (2013) Perfil químico e atividades biológicas de Croton echinocarpus Müll. Arg. e Croton vulnerarius Baill.. Dissertation, Universidade de São Paulo, São Paulo
Bertelli D, Papotti G, Bortolotti L, Marcazzan GL, Plessi M (2012) 1H-NMR simultaneous identification of health-relevant compounds in propolis extracts. Phytochem Anal 23:260–266. doi:10.1002/pca.1352
Boligon AA, Janovik V, Frohlich JK, Spader TB, Froeder ALF, Alves SH, Athayde ML (2012) Antimicrobial and cytotoxic activities of leaves, twigs and stem bark of Scutia buxifolia Reissek. Nat Prod Res 26:939–944. doi:10.1080/14786419.2010.535151
Caruzo M, Cordeiro I, Berry P, Riina R (2013) A new species of Croton section Cleodora (Euphorbiaceae s.s.) from Minas Gerais, Brazil. Phytotaxa 3:27–33
Chen Z, Cai Y, Phillipson J (1994) Studies on the anti-tumour, anti-bacterial, and wound-healing properties of dragon’s blood. Planta Med 60:541–545
Dewick PM (2009) Medicinal natural products: a biosynthetic approach. Wiley, New York
Duarte-Almeida JM, Santos RJ, Genovese MI, Lajolo FM (2006) Avaliação da atividade antioxidante utilizando sistema β-caroteno/ácido linoleico e método de sequestro de radicais DPPH. Ciênc Tecnol Aliment 26:446–452
Furlan CM, Santos KP, Sedano-Partida MD, Motta LB, Santos DYA, Salatino MLF, Negri G, Berry PE, van Ee BW, Salatino A (2015) Flavonoids and antioxidant potential of nine Argentinian species of Croton (Euphorbiaceae). Braz J Bot 38:693–702. doi:10.1007/s40415-014-0115-9
Goodwin CS, Armstrong JA, Chilvers T, Peters M, Collins MD, Sly L, McConnel W, Harper WES (1989) Transfer of Campylobacter pylori and Campylobacter mustelae to Helicobacter gen. nov. as Helicobacter pylori comb. nov. and Helicobacter mustelae comb. nov., respectively. Int J Syst Bacteriol 39:397–405
Grynberg N, Echevarria A (1999) Anti-tumour activity of two 19-nor-clerodane diterpenes, trans-dehydrocrotonin and trans-crotonin, from Croton cajucara. Planta Med 65:687–689
Guerrero MF, Carrón R, Martín ML, San Román L, Reguero MT (2001) Antihypertensive and vasorelaxant effects of aqueous extract from Croton schiedeanus Schlecht in rats. J Ethnopharmacol 75:33–36
Guerrero MF, Puebla P, Carrón R, Martín ML, Arteaga L, Román LS (2002a) Assessment of the antihypertensive and vasodilator effects of ethanolic extracts of some Colombian medicinal plants. J Ethnopharmacol 80:37–42
Guerrero MF, Puebla P, Carrón R, Martín ML, San Román L (2002b) Quercetin 3,7-dimethyl ether: a vasorelaxant flavonoid isolated from Croton schiedeanus Schlecht. J Pharma Pharmacol 54:1373–1378
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584. doi:10.1016/S0955-2863(02)00208-5
Kawai K, Tsuno N, Kitayama J (2005) Anti-angiogenic properties of plaunotol. Anticancer Drugs 16:401–407
Kim KM, Shim SM (2014) Nicotine detoxification of rutin, quercitrin, and chlorogenic acid isolated from Houttuynia cordata by reducing reactive oxygen species and inducing conversion from nicotine to cotinine. J Korean Soc Appl Biol Chem 57:503–509. doi:10.1007/s13765-014-4182-1
Koehn FE, Carter GT (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–220
Koga T (2002) Effect of plaunotol in combination with clarithromycin or amoxicillin on Helicobacter pylori in vitro and in vivo. J Antimicrob Chemother 50:133–136. doi:10.1093/jac/dkf094
Krenn L, Miron A, Pemp E, Petr U, Kopp B (2003) Flavonoids from Achillea nobilis L. Z Naturforsch 58:11–16. doi:10.1515/znc-2003-1-202
Kumar S, Pandey AK (2013a) Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. doi:10.1155/2013/162750
Kumar S, Pandey AK (2013b) Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Vegetos 26:301–307. doi:10.5958/j.2229-4473.26.1.043
Kumar S, Mishra A, Pandey AK (2013) Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Altern Med 13:120. doi:10.1186/1472-6882-13-120
Lila M, Raskin I (2005) Health related Interactions of Phytochemicals. J Food Sci 70:20–27. doi:10.1111/j.1365-2621.2005.tb09054.x
Maciel M, Pinto A, Veiga V (2002) Plantas medicinais: a necessidade de estudos multidisciplinares. Quím Nova 25:429–438
Markham K (1982) Techniques of flavonoid identification. Academic Press, New York
Marshall BJ, Royce H, Annear DI, Goodwin CS, Pearman JW, Warren JR, Armstrong JA (1984) Original isolation of Campylobacter pyloridis from human gastric mucosa. Microbios Lett 25:83–88
Martin C, Zhang Y, Tonelli C, Petroni K (2013) Plants, diet, and health. Annu Rev Plant Biol 64:19–46. doi:10.1146/annurev-arplant-050312-120142d
Matos LMM (2011) Química de espécies nativas de Croton L. (Euphorbiaceae). Dissertation, Universidade de São Paulo, São Paulo
Min B, McClung AM, Chen M-H (2011) Phytochemicals and antioxidant capacities in rice brans of different color. J Food Sci 76:117–126. doi:10.1111/j.1750-3841.2010.01929.x
Mira L, Tereza Fernandez M, Santos M, Rocha R, Helena Florêncio M, Jennings KR (2002) Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res 36:1199–1208
Motta LB, Furlan CM, Santos DYAC, Salatino MLF, Negri G, de Carvalho JE, Monteiro PA, Ruiz ALTG, Caruzo MB, Salatino A (2013) Antiproliferative activity and constituents of leaf extracts of Croton sphaerogynus Baill. (Euphorbiaceae). Ind Crop Prod 50:661–665. doi:10.1016/j.indcrop.2013.08.008
Ngo LT, Okogun JI, Folk WR (2013) 21st Century Natural Product Research and Drug Development and Traditional Medicines. Nat Prod Rep 30:584–592. doi:10.1039/c3np20120a
Oliveira A, de Valentim I, Goulart M (2009) Fontes vegetais naturais de antioxidantes. Quím Nova 32:689–702
Roby MHH, Sarhan MA, Selim KAH, Khalel KI (2013) Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme Thymus vulgaris L., sage Salvia officinalis L. and marjoram Origanum majorana L. extracts. Ind Crop Prod 43:827–831. doi:10.1016/j.indcrop.2012.08.029
Rossi D, Bruni R, Bianchi N, Chiarabelli C, Gambari R, Medici A, Lista A, Paganetto G (2003) Evaluation of the mutagenic, antimutagenic and antiproliferative potential of Croton lechleri (Muell. Arg.) latex. Phytomedicine 10:139–144. doi:10.1078/094471103321659843
Rufino MM, Alves RE, Brito ES, Morais SM, Sampaio CG, Pérez-Jiménez J, Saura-Calixto FD (2007) Metodologia Científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre ABTS. Comunicado técnico 128, Embrapa, Fortaleza
Salatino A, Salatino M, Negri G (2007) Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J Braz Chem Soc. doi:10.1590/S0103-50532007000100002
Sánchez-Burgos JA, Ramírez-Mares MV, Larrosa MM, Gallegos-Infante JA, González-Laredo RF, Medina-Torres L, Rocha-Guzmán NE (2013) Antioxidant, antimicrobial, antitopoisomerase and gastroprotective effect of herbal infusions from four Quercus species. Ind Crop Prod 42:57–62. doi:10.1016/j.indcrop.2012.05.017
Sanchez-Moreno C (2002) Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int 8(121–137):1082. doi:10.1106/01302026770
Santos KP, Motta LB, Santos DYAC, Salatino ML, Salatino A, Ferreira MJP, Lago JHG, Ruiz ALTG, Carvalho JE, Furlan CM (2015) Antiproliferative activity of flavonoids from Croton sphaerogynus Baill (Euphorbiaceae). BioMed Res Int. doi:10.1155/2015/212809
Savietto JP (2011) Análise fitoquímica e atividade antiproliferativa de espécies nativas de Croton L. (Euphorbiaceae). Dissertation, Universidade de São Paulo, São Paulo
Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I (2008) A natural history of botanical therapeutics. Metabolism. doi:10.1016/j.metabol.2008.03.001
Soares SA (2013) Isolamento biomonitorado de substâncias ativas de Croton pallidulus var. pallidulus (Euphorbiaceae). Dissertation, Universidade de São Paulo, São Paulo
Souza VC, Lorenzi H (2005) Botânica sistemática: guia ilustrado para identificação das famílias de angiospermas da flora brasileira, baseado em APG II. Instituto Plantarum de Estudos da Flora, Nova Odessa
Thatoi N, Patra JK, Das SK (2014) Free radical scavenging and antioxidant potential of mangrove plants: a review. Acta Physiol Plant 36:561–579
Tripathi R, Mohan H, Kamat JP (2007) Modulation of oxidative damage by natural products. Food Chem 100:81–90. doi:10.1016/j.foodchem.2005.09.012
van Acker SA, van Den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van Der Vijgh WJ, Bast A (1996) Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med 20:331–342. doi:10.1016/0891-5849(95)02047-0
van Ee BW, Riina R, Berry PE (2011) A revised infrageneric classification and molecular phylogeny of New World Croton (Euphorbiaceae). Taxon 60:791–823
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49:5165–5170. doi:10.1021/jf010697n
Acknowledgments
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support (FAPESP 2012/10079-0); KPS thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a Master’s research grant; IC and CMF are fellow researchers of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, K.P., Sedano-Partida, M.D., Motta, L.B. et al. Antioxidant activity of flavonoids from Croton sphaerogynus Baill.. Braz. J. Bot 39, 1021–1030 (2016). https://doi.org/10.1007/s40415-016-0302-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0302-y