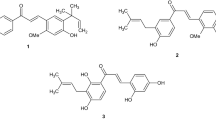
Newly designed chalconeimines were synthesized, characterized and evaluated for their in vitro antioxidant and antibacterial effectiveness. Results of antioxidant activity assay reveal that all the tested compounds possess good to moderate antioxidant activity which is lower in comparison to that of a standard drug (gallic acid). On the other hand, all the synthesized compounds were found to exhibit a considerably wider spectrum of antibacterial activity, but it was also narrower in comparison to that of a standard drug (ciprofloxacin). Elucidation of structure—activity relationships revealed that electron donating groups (-OH, -OCH3) contribute more to antioxidant potency, whereas electron withdrawing groups (-Cl) impart better antibacterial effectiveness. Moreover, results of drug-likeness studies indicate that a reasonable correlation exists between the drug-like properties and antioxidant activity of the synthesized chalconeimines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
In recent years, chalcones (1,3-diaryl-2-propen-1-ones) belonging to the class of natural flavonoids and related products have gained significant interest among medicinal chemists owing to their diverse biologic activities [1,2,3,4,5,6,7]. Structure – activity relationship (SAR) study indicated the biological potential of chalcone functional moiety (Ar-CO-CH=CH-Ar′), where the α,β-unsaturated keto group (enone function, -CO-CH=CH-) is considered to be the key pharmacophore of chalcones [1, 8]. On the other hand, imines, also known as Schiff′s bases, are widely found in various natural and synthetic compounds of medicinal interest and possess a wide array of biological activities [9, 10]. Literature reveals that the pharmacodynamic potential of bio-active imines is mainly due to the presence of pharmacophoric azomethine (>C=N) moiety [11, 12]. The conjugated chalcone-imine derivatives such as chalconeimines [13] and iminoflavones [14, 15] having enone-azomethine (-CH=CH-C=N-) pharmacophoric moiety have been reported as pharmacologically potent compounds.
Literature reports suggest that many chronic and degenerative diseases such as cancer, diabetes, cardiovascular diseases, neurodegenerative disorders, certain inflammatory diseases and bacterial infections are commonly associated with increased oxidative stress (OS) in humans [16, 17]. However, the bio-efficacy of existing natural (for example, plant polyphenols such as catechins, quercetin) and synthetic antioxidants (for example, BHA and BHT) is negligible as compared to the enzymatic (SOD, CAT and GPx) antioxidant defense system of human body [16, 18,19,20]. It is therefore required to develop new antioxidant molecules that would be effective yet safe in preventing and/or treating OS induced human disorders. The increasing incidence of bacterial infection caused by the rapid emergence of multi-drug resistant bacterial strains against majority of the currently available antibiotics has become a serious medical problem all over the world [21]. Therefore, the discovery and development of new antibacterial agents that would fight resistant bacterial infection with some new mechanisms of action is a topical task.
In view of the above facts, it was assumed that the rational design of new chalcone derivatives as chalconeimines would be an attractive approach to developing biologically active molecules. Accordingly, a series of structural analogs based on the conjugated scaffold of various chalcones and substituted chalcones coupled with a heteroaryl amine was designed with modifications in the aryl rings (ring B, Fig. 1) of the chalcone core. Newly designed chalconeimines were synthesized, characterized, and evaluated in vitro for their antioxidant and antibacterial properties. In the present work, the synthesized compounds were evaluated in silico for molecular properties assessment and drug-likeness prediction with the aim to assess their drug-likeness based on Lipinski′s rule of five with additional parameters for antioxidant activity.
2. EXPERIMENTAL
2.1. Chemicals, Instrumentation and Analytical Methods
All chemicals were obtained commercially from Merck Specialists Pvt. Ltd., Mumbai; S.D. Fine Chemicals Ltd., Mumbai; and Qualigens Fine Chemicals, Mumbai, and were used without further purification, unless otherwise stated. The solvents and reagents used in the biological study were of analytical grade. All reactions were performed in oven dried glass ware using synthetic grade chemicals. The progress of reactions was monitored by the silica gel-G thin layer chromatography (TLC) and the spots were visualized by iodine vapors.
Melting points (mp) were measured in open capillaries on an electrically heated melting point apparatus. UV-Vis spectra were recorded on Labindia UV-3000 UV-Vis spectrophotometer and the absorption band positions are reported as maximum wavelength (λmax , nm). Infrared (IR) absorption spectra were obtained on a Bruker Alpha FT-IR spectrometer using KBR disks and reported in terms of vibrational frequencies (, cm-1). The 1H and 13C NMR spectra were recorded on a Bruker AC-F 400 FT-NMR spectrometer at 400 and 100 MHz, respectively, with tetramethylsilane (TMS) as an internal standard and CDCl3 as a solvent. Chemical shifts (δ) are expressed in parts per million (ppm) relative to TMS (δ = 0.00 ppm). The 1H NMR data are presented in the following order: peak multiplicity (b, broad; s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet), number of protons (numerical integral value), coupling constants (J, in hertz). The mass spectra (MS) were recorded on an LC–MS Waters 4000 ZQ instrument using atmospheric pressure ionization (API-ES). The m/z values were recorded in the range of m/z = 150 – 1400 (in both the negative and positive ion modes), and the m/z values of the most intense quasimolecular ion [M+H]+ peaks (with relative intensities) in parentheses, are given followed by peaks corresponding to major fragment ions. Elemental analysis was performed on a Perkin Elmer 2400 Series II CHNS/O analyzer. In the study of antioxidant activity the absorbance readings were obtained using an ELICO CL157 colorimeter.
Chemical methods involving standard reaction procedures were employed for the synthesis of target compounds 5a – 5f. The structural assignment of obtained compounds was made on the basis of UV, FT-IR, 1H NMR, 13C NMR, and mass spectroscopy, and elemental analysis. Procedures used for the synthesis of target compounds and the related physicochemical, spectroscopic, and analytical data are presented below.
2.2. Synthesis of Target Compounds 5a – 5f
General procedure. Chalcone and substituted chalcones [23] 3a – 3f (0.01 mol) and 2-amino-4-phenylthiazole 4 [24] (0.01 mol) were dissolved in absolute ethanol (30 mL), 2 – 3 drops of conc. H2SO4 was added, and the mixture was refluxed for 6 – 12 h and kept in refrigerator overnight. The separated solid was collected by filtration, dried in air, and re-crystallized from absolute ethanol [14].
N-(1,3-Diphenylallylidene)-4-phenylthiazol-2-amine (5a): %Yield: 60.65%, color: yellow, mp: 178 – 180OC, Rf : 0.78 (EtOAc/CHCl3 = 1:1); UV spectrum (acetone), λmax, nm (abs): 370 (0.498); IR (KBr; , cm-1): 3083.58, 3052.94 (aromatic C-H str.), 3032.85, 3015.19 (vinylic C-H str., -CH=CH-), 1664.36 (>C=N- str., imine); 1H NMR (400 MHz, CDCl3 ; δ, ppm): 8.03 (d, 1H, Jtrans = 15.82 Hz, -CH=CH-), 7.95 (d, 1H, Jtrans = 15.80 Hz, =CH-Ar), 7.62 (s, 1H, C5-thiazolyl-H), 7.58 – 7.52 (m, 5H, Ar-H), 7.46 – 7.42 (m, 5H, Ar-H), 7.32 – 7.26 (m, 5H, Ar-H); 13C NMR (100 MHz, CDCl3 ; δ, ppm): 198.66 (>C=N), 168.26, 152.62, 148.20 (thiazolyl-C); 138.26, 134.86, 132.89, 128.52, (vinylic- & aromatic-CH); MS (API-ES), m/z (%): 367.12 (100), [M+H]+, 368.12 (28), 368.90 (6), 369.38 (4); CHN anal. for C24H18N2 S (366.48), calc. (%): C, 78.66, H, 4.95, N, 7.64, found (%): C, 79.00, H, 4.90, N, 7.78.
N-(3-(4-Chlorophenyl)-1-phenylallylidene)-4-phenylthiazol-2-amine (5b): %Yield: 82.20%, color: yellow, mp: 118 – 120°C, Rf : 0.72 (EtOAc/CHCl3 = 1:1); UV spectrum (acetone), λmax , nm (abs): 370 (0.301); IR (KBr; ν, cm-1): 3126.22, 3059.38 (aromatic C-H str.), 3024.85, 2922.19 (vinylic C-H str., -CH=CH-), 1658.22 (>C=N- str., imine), 1092.03 (aromatic C-Cl str.); 1H NMR (400 MHz, CDCl3; δ,ppm): 8.04 (d, 1H, Jtrans = 15.83 Hz, -CH=CH-), 7.94 (d, 1H, Jtrans = 15.85 Hz, = CH-Ar), 7.66 (s, 1H, C5-thiazolyl-H), 7.56 – 7.51 (m, 5H, Ar-H), 7.48 – 7.44 (m, 4H, Ar-H), 7.36 – 7.32 (m, 5H, Ar-H); 13C NMR (100 MHz, CDCl3 ; δ, ppm): 198.64 (>C=N), 168.40, 155.20, 149.52 (thiazolyl-C); 138.45, 134.39, 132.40, 126.06 (vinylic- & aromatic-CH); MS (API-ES), m/z (%): 401.01 (100), [M+H]+, 402.06 (29), 403.34 (9), 404.60 (2); CHN anal. for C24H17N2 SCl (400.92), calc. (%): C, 71.90, H, 4.27, N, 6.99, found (%): C, 70.62, H, 4.55, N, 7.82.
N-(3-(4-Methoxyphenyl)-1-phenylallylidene)-4-phenylthiazol-2-amine (5c): %Yield: 82.32%, color: yellow, mp: 100 – 104°C, Rf : 0.79 (EtOAc/CHCl3 = 1:1); UV spectrum (acetone), λmax , nm (abs): 370 (0.490); IR (KBr; ν, cm-1): 3152.00, 3067.46 (aromatic C-H str.), 3035.29, 3015.46 (vinylic C-H str., -CH=CH-), 2964, 2889 (C-H str., CH3), 1657.45 (>C=N- str., imine), 1170.43, 1180.67 (-C-O-C str.); 1H NMR (400 MHz, CDCl3; δ, ppm): 8.08 (d, 1H, Jtrans = 15.57 Hz, -CH=CH-), 7.89 (d, 1H, Jtrans = 15.37 Hz, =CH-Ar), 7.62 (s, 1H, C5-thiazolyl-H), 7.58 – 7.54 (m, 5H, Ar-H), 7.46 – 7.42 (m, 4H, Ar-H), 7.38 – 7.33 (m, 5H, Ar-H), 3.86 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3; δ, ppm): 194.69 (>C=N), 168.20, 156.26, 149.22 (thiazolyl-C); 138.45, 134.40, 132.52, 128.36, 126.23 (vinylic- & aromatic-CH), 52.58 (OCH3); MS (API-ES), m/z (%): 397.96 (100), [M+H]+, 398.58 (28), 399.36 (12), 399.24 (8); CHN anal. for C25H20N2 OS (396.50), calc. (%): C, 71.90, H, 4.27, N, 6.99, found (%): C, 70.62, H, 4.55, N, 7.82.
N-(3-(2-Hydroxyphenyl)-1-phenylallylidene)-4-phenylthiazol-2-amine (5d): %Yield: 89.52%, color: pale yellow, mp: 186 – 190OC, Rf: 0.94 (EtOAc/CHCl3 = 1:1); UV spectrum (acetone), λmax , nm (abs): 380 (0.397); IR (KBr; ν,cm-1): 3430 (Phenolic O-H str.); 3158.34, 3080.71 (aromatic C-H str.), 3034.36, 3012.38 (vinylic C-H str., -CH=CH-), 1664.82 (>C=N- str., imine), 1280.96 (C-O str.); 1H NMR (400 MHz, CDCl3; δ, ppm): 8.12 (d, 1H, Jtrans = 15.68 Hz, -CH=CH-), 7.88 (d, 1H, Jtrans = 15.25 Hz, =CH-Ar), 7.64 (s, 1H, C5-thiazolyl-H), 7.58 – 7.55 (m, 5H, Ar-H), 7.46 – 7.43 (m, 4H, Ar-H), 7.39 – 7.36 (m, 5H, Ar-H), 5.32 (bs, 1H, OH); 13C NMR (100 MHz, CDCl3; δ, ppm): 192.47 (>C=N), 168.67, 156.45, 149.33 (thiazolyl-C); 138.38, 134.73, 132.55, 128.63, 126.34 (vinylic- & aromatic-CH); MS (API-ES), m/z (%): 383.27 (100), [M+H]+, 384.60 (40), 385.56 (30), 386.27 (12); CHN anal. for C24H18N2OS (382.48), calc. (%): C, 75.37, H, 4.74, N, 7.32, found (%): C, 77.28, H, 4.78, N, 8.20.
N-(3-(4-Hydroxy-3-methoxyphenyl)-1-phenylallylidene)-4-phenylthiazol-2-amine (5e): %Yield: 87.86%, color: buff, mp: 178 – 182OC, Rf: 0.96 (EtOAc/CHCl3 = 1:1); UV spectrum (acetone), λmax , nm (abs): 370 (0.99); IR (KBr; ν, cm-1): 3423 (Phenolic O-H str.); 3147.30, 3058.20 (aromatic C-H str.), 3033.20, 3011.10 (vinylic C-H str., -CH=CH-), 2966, 2862 (C-H str., CH3), 1665.37 (>C=N- str., imine), 1283.40 (>C-O str.); 1H NMR (400 MHz, CDCl3; δ, ppm): 8.26 (d, 1H, Jtrans = 16.23 Hz, -CH=CH-), 7.92 (d, 1H, Jtrans = 16.26 Hz, =CH-Ar), 7.64 (s, 1H, C5-thiazolyl-H), 7.59 – 7.54 (m, 5H, Ar-H), 7.48 – 7.42 (m, 3H, Ar-H), 7.38 – 7.33 (m, 5H, Ar-H), 5.38 (bs, 1H, OH), 3.75 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3; δ, ppm): 188.60 (>C=N), 167.52, 154.28, 148.49 (thiazolyl-C); 138.27, 134.36, 132.44, 128.08 (vinylic- & aromatic-CH), 52.60 (OCH3); MS (API-ES), m/z (%):413.14 (100), [M+H]+, 414.29 (38), 415.27 (26), 416.36 (8); CHN anal. for C25H20N2O2 S (412.50), calc. (%): C, 72.79 H, 4.89, N, 6.79, found (%): C, 73.30 H, 5.46, N, 6.88.
N-(1,5-Diphenylpenta-2,4-dienylidene)-4-phenylthiazol-2-amine (5f): %Yield: 86.98%, color: dark yellow, mp: 190 – 194OC, Rf : 0.88 (EtOAc/CHCl3 = 1:1); UV spectrum (acetone), λmax , nm (abs): 370 (0.160); IR (KBr; ν, cm-1): 3123.45, 3079.80 (aromatic C-H str.), 3032.54, 3009.43 (vinylic C-H str., -CH=CH-), 1658.40 (>C=N- str., imine); 1H NMR (400 MHz, CDCl3; δ, ppm): 8.22 (d, 1H, Jtrans = 16.42 Hz, -CH=CH-), 8.14 (t, 1H, Jtrans = 16.32 Hz, -CH=CH-CH=), 7.89 (t, 1H, Jtrans = 16.24 Hz, =CH-CH=CH-), 7.78 (d, 1H, Jtrans = 16.86 Hz, =CH-Ar), 7.62 (s, 1H, C5-thiazolyl-H), 7.56 – 7.52 (m, 5H, Ar-H), 7.48 – 7.44 (m, 5H, Ar-H), 7.40 – 7.36 (m, 5H, Ar-H); 13C NMR (100 MHz, CDCl3; δ, ppm): 192.47 (>C=N), 168.67, 156.45, 149.33 (thiazolyl-C); 138.25, 134.53, 132.60, 126.50 (vinylic- & aromatic-CH); MS (API-ES), m/z (%): 393.89 (100), [M+H]+, 393.07 (56), 394.48 (34), 395.02 (20); CHN anal. for C26H20N2 S (392.52), calc. (%):C, 79.56, H, 5.14, N, 7.14, found (%):C, 79.02, H, 6.27, N, 7.48.
2.3. Studying Antioxidant Activity
The in vitro antioxidant activity of the synthesized compounds 5a – 5e was determined by two methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and ferric reducing antioxidant power (FRAP) assay [12].
DPPH free radical scavenging assay. Methanolic DPPH solution (0.1 mmol) was added to 3.0 mL of test drug solutions (in methanol) of three different concentrations (50, 100 and 200 μg/mL). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. The absorbance was then measured at 517 nm in a colorimeter. Methanol was used as the blank, and the reaction mixture of DPPH and methanol served as control. Gallic acid (1.0, 2.5 and 5.0 μg/mL) was used as the reference standard drug. The percentage scavenging activity was calculated using the following formula:
where A0 is the absorbance of control (without test drug) and A1 is the absorbance of the test drug solution. All the tests were carried out in triplicate, and results were expressed as mean % inhibition ± SEM. The percentage scavenging activity was determined at various solution concentrations, and the results of test samples were compared with that of the standard drug, gallic acid. Data were subjected to statistical analysis by Student′s t-test using the SPSS Ver. 19.0 statistical software package. Results were considered as statistically significant for p ≤ 0.05.
Ferric reducing antioxidant power (FRAP) assay. 1.0 mL of test drug solution (in ethanol) of different concentrations (50, 100 and 200 μg/mL) was mixed with 2.5 mL of phosphate buffer (0.2M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was then incubated at 50°C for 20 min. 2.5 mL of 1% trichloroacetic acid (10%) was added to the reaction mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 solution (0.1%). The absorbance was measured at 700 nm using a colorimeter. Gallic acid was used as the reference standard. All the tests were performed in triplicate, and the results are expressed as mean ± SEM.
2.4. Studying Antibacterial Activity
Test strain and bacterial inoculums. Three strains of Gram positive bacteria, viz. Bacillus subtilis, Bacillus pumilus, Staphylococcus aureus and three strains of Gram negative bacteria, viz. Eschericia coli, Pseudomonas aeruginosa, Pseudomonas vulgaris were used in this study. The cultures of bacteria were maintained in their appropriate agar slants at 4°C throughout the study and used as stock cultures. Bacterial inoculums for each strain were prepared by transferring a loop full (0.005 mL) of stock culture into a clean and sterilized tube containing 4 – 5 mL nutrient broth medium. The broth cultures were then incubated at 37 ± 1(C for 18 h.
Drug dilution. Test drug solutions were prepared in acetone. Ciprofloxacin Injection IP (200 mg/100 mL; Cadila Health care Ltd., Bhiwadi) was diluted with distilled water to obtain a concentration of 200 μg/mL and used as positive control. Acetone was used as negative control. The stock solution (1 mg/mL) of each test compound 5a – 5f was used to prepare a test solution of 200 μg/mL concentration.
Agar disk diffusion assay[21,25]. Nutrient agar medium was sterilized by autoclaving at 121(C (15 lb/sq. inch.) for 15 min. The flat-bottomed petri dishes (100 mm in diameter) were sterilized previously in hot-air oven at 160(C for about 1 h. Freshly prepared and cooled (45 – 50°C) molten agar medium of about 25 mL volume was inoculated with a standard inoculum (0.5 – 1.0 mL) aseptically in laminar air flow unit. The medium was then transferred aseptically into sterilized petri-plates to occupy a depth of about 4 mm. The plates were then left at room temperature to allow solidification. Under aseptic condition, four wells of 5 mm diameter were made in each plate using a sterile borer. Accurately 100 μL of test and standard solutions and vehicle were transferred to cups aseptically and labeled accordingly. The plates were left undisturbed for 1 h at room temperature to allow pre-incubation diffusion of the solution into the medium in order to minimize the effects of variation in time between the application of different solutions. After incubation of the plates at 37 ± 1(C for 24 h, diameters of the zones of complete inhibition surrounding each well were measured on a millimeter scale. The studies were performed in triplicate and mean ± SEM values (in mm) were determined. Data were subjected to statistical analysis by Student′s t-test using the SPSS Ver. 19 statistical software package, and differences were considered as statistically significant for p ≤ 0.05.
Drug-likeness prediction. The drug-likeness of synthesized compounds 5a – 5f was evaluated in silico using web based Molsoft (molsoft.com/molprop/) and Molinspiration Cheminformatics (www.molinspiration.com) academic software packages. The drug-likeness prediction score was calculated based on the following molecular descriptors: molecular weight, aqueous solubility (logS), octanol – water partition coefficient (logP), acceptor and donor hydrogen-bond count, polar surface area, and rotatable bonds. The drug-likeness score was calculated for all test compounds.
3. RESULTS AND DISCUSSION
In the present study, a series of new chalconeimines were synthesized, characterized, and screened for their antioxidant and antibacterial effectiveness.
3.1. Design and Synthesis
The design of target chalconeimines involved substitutions on two terminal phenyl rings connected via conjugated enone-imine moiety with different functional groups particularly at 2′-ortho and/or 4′-para positions of ring B of the parent chalcone moiety. The electronic character of substituents is the main motif in this design strategy. The synthesis route used to obtain the target compounds is depicted in Scheme 1. Chalconeimines 5a – 5f were synthesized by condensation reaction between chalcone/substituted chalcones 3a – 3f and 2-amino-4-phenylthiazole 4 in absolute ethanol as solvent in the presence of conc. H2SO4 as catalyst at about 100°C by one-step reaction mechanism involving nucleophilic addition.
3.2. Chemistry and Analysis
The purity of the synthesized compounds was confirmed by melting point determination and TLC measurements on silica gel G plates. All synthesized compounds 5a – 5f were obtained in good yields with high purity. The spectral data considered above are in close agreement with the proposed structures of synthesized compounds 5a – 5f.
All compounds dissolved in acetone exhibited prominent UV absorption maxima (with λmax in the region of 370 – 380 nm indicating the presence of strong chromophoric conjugatedchalcone-imine (CH=CH-CO-)- (>C=N-) functional moiety.
IR spectroscopy showed characteristic absorption bands of specific functional groups, which supported the proposed structures of synthesized compounds. The presence of two vinylic C-H bonds in 5b was manifested by weak bands at 3024.85 and 2922.19 cm-1. The characteristic imine moiety (>C=N-) was manifested by a sharp peak of medium intensity at 1657.45 cm-1 for compound 5c. The appearance of broad absorption peaks at 3430 and 3423 cm-1 confirmed the presence of phenolic –OH group in the structures of compounds 5d and 5e, respectively. The presence of >C=N- and C-O linkages in the structure of 5d was confirmed by the appearance of peaks at 1664.82 and 1280.96 cm-1, respectively.
The 1HNMR spectra displayed various signals with characteristic chemical shift (δ, ppm) values corresponding to various protons present in the structure of synthesized compounds. Two vinylic protons (-CH=CH-) of the chalcone moiety in 5a were observed as two doublets at δ = 8.03 and 7.95 ppm. The geometry of each proton was assigned to be trans (E) as the coupling constant (J) values for these protons were 15.82 and 15.80 Hz, respectively. A singlet at δ = 7.62, 7.64 or 7.66 ppm was attributed to C5 proton of thiazolyl moiety present in the structure of synthesized compounds. A shift for the hydroxyl proton on the ring B of chalcone moiety in compounds 5d and 5e was manifested by a broad singlet peak at δ = 5.32 and 5.38 ppm, respectively. Sharp singlets at δ = 3.86 and 3.75 were assigned to methoxy protons (ring B) for compounds 5d and 5e respectively [26].
The mass spectra of compounds 5a – 5f exhibited prominent peaks of molecular ions [M+H]+ in accordance with the anticipated masses corresponding to their molecular formulas. The results of elemental analyses were within the acceptable limits of calculated values for all synthesized compounds.
3.3. Antioxidant Activity
The results of antioxidant activity screening (Table 1) showed that all tested compounds 5a – 5e exhibited moderate to good antioxidant activity. From tabulated data, it is evident that the activity (% scavenging or reducing power) increases with the concentration of test compound. All these compounds were equally active within some degree of variation at all the three test concentrations. However, the overall activity of compounds was comparatively lower than that of the standard drug, gallic acid. Among the synthesized drugs, compound 5d with hydroxy substituent and compound 5e with both substituted hydroxy and methoxy groups showed good activity, while the rest of synthesized analogs (compound 5a without any substituent or compound 5b with chloro group or compound 5c with only methoxy substituent) exhibited moderate radical scavenging activity.
It is clear from the obtained results that the variation in activity among the synthesized analogs might be related to the nature of substituents or substitution pattern in ring B of the parent chalcone moiety. The presence of para-OH group (5d) or ortho-3,4-(OH) (OCH3) groups (5e) is probably more important for increasing antioxidant activity, whereas the presence of para-OCH3 (5c) or para-Cl (5b) groups is likely to be les important for the activity. The SAR study clearly indicates that electron donating groups (-OH, -OCH3) are more contributing toward the antioxidant activity than does the electron withdrawing groups (-Cl). The antioxidant ability decreases in the following order: OH > OCH 3 > Cl > H. The presence of only methoxy group does not infer high activity, but it may potentiate the antioxidant activity when present in the hydroxylated ring, preferably in the ortho position with respect to hydroxy (para) substitution. The –OH group (at para position) alone significantly contributes to antioxidant activity (5d, 5e) since it is converted into reactive phenoxy radical after accepting electron from a reactive radical (ROS). A stable phenoxy radical imparts antioxidant activity by disrupting free radical chain mediated oxidation reactions in cellular components [18, 30]. The presence of electron withdrawing groups (-Cl) seems to decrease the antioxidant activity. Unsubstituted (5a) compound showed the least activity in this series.
Results of the present investigation are in accordance with previous reports [8] that various structural substitutions with electron donating/releasing groups (in A and B aryl rings) greatly influence the antioxidant potency of chalcone derivatives by activating/deactivating the molecule (via mesomeric effect) through delocalization of electrons over these rings for target (DNA, proteins, etc.) specific interaction of chalcone pharmacophoric moiety with the corresponding receptor. The lipophilicity of a molecule (due to the presence of bulky aryl fragments) is also important for lipoidal membrane permeation in order to get access to cellular targets. Finally, it can be concluded that the chalconearylthiazole conjugate having enone-azomethine pharmacophoric moiety (-CH=CH-C=N-) is a structural feature important for the antioxidant activity.
3.4. Antibacterial Activity
All synthesized compounds 5a – 5f were found active against selected strains of Gram- positive and Gram-negative bacteria at the test concentration. There was no inhibition of growth by vehicle control (acetone). Results presented in Table 2 clearly indicate that all compounds were equally active with little variation, but were less potent in comparison to the standard drug, ciprofloxacin. Although the antibacterial efficacy of these compounds was less significant, they possess considerably wider spectrum of activity. The results demonstrate that compounds 5b (4′-chloro) and 5c (4′-methoxy) exhibited somewhat higher inhibitory activity than compounds, 5a (unsubstituted), 5d (2′-hydroxy) and 5e (3′-methoxy, 4′-hydroxy), particularly against Gram-positive B. subtilis and B. pumilus and Gram-negative P. vulgaris bacterial strains. It was found that compound 5b was slightly more potent than compound 5c, and compound, 5f showed lowest activity in the series studied.
Variation in antibacterial activity among the synthesized compounds seems to be related to different substitution patterns in aryl components of the core chalcone moiety. The nature of substitution is an additional contributing factor, which further modulates the activity of parent chalcone scaffold. The elucidated SAR suggests that electron-withdrawing and electron-donating groups impart different electronic environments to the molecules, depending upon the number and position of substituent(s) present in the aryl ring of the chalcone moiety, which in turn influences the biological properties of compounds. The presence of electron withdrawing group like 4′-chloro (5b) in ring B of the chalcone moiety has greater contributing effect to the antibacterial activity than do the electron donating groups like 4′-methoxy (5c) and 4′-hydroxy (5d). The activity of the unsubstituted compound (5a) was almost equal to that of compound 5e containing two electron releasing groups (4′-hydroxy and 3′-methoxy). The results are consistent with previous reports [8] in that electron withdrawing groups in chalcones contribute more activity than do electron donating group. The electron withdrawing or donating ability of groups causes delocalization of electrons over bulky aromatic rings of the molecule either by inductive or mesomeric effect [31], which in turn increases the lipophilicity of the molecule thus favoring permeation through the lipoid layer of bacterial membranes [12]. From the above considerations, it can be concluded that conjugated chalcone-arylthiazole scaffold having pharmacophoric enone-azomethine (-CH=CH-C=N-) moiety is an important structural requirement for the antibacterial activity of synthesized chalconeimines.
3.5. Drug-Likeness Assessment
The predicted drug-like properties and drug-likeness score of synthesized compounds 5a – 5f are presented in Table 3. All these compounds showed moderate drug-like characteristics based on Lipinski′s rule of five with additional parameters such as logS and PSA. Drug-likeness prediction evaluates the acceptability of derivatives as drug molecules based on Lipinski′s rule of five [32]. Poor absorption or permeation of a ligand is more likely in the presence of molecular weight >500, logPo/w > 5, HBA > 10, HBD > 5, and RotB >5. The assessment of drug-likeness score indicates the suitability of derivatives as drug-like molecules.
The values of all calculated drug-like properties are within the adopted range except for logP parameter. The octanol-water partition coefficient (logP) is greater than 5 (within the range of 6.27 – 7.36) for all compounds 5a – 5f. None of their molecular weights exceeds 500 Da. They possess HBAs less than or equal to 5 and have HBD values not exceeding 1. The number of rotatable bonds and the PSA values of all compounds are also in the permissible range. Furthermore, drug-likeness scores of compounds 5a – 5f ranges from –0.53 to +0.06. However, since all the synthesized compounds showed favorable drug-like properties, a reasonable correlation can be drawn between the calculated drug-like properties and their in vitro antioxidant activity profile. LogPo/w is a direct indicator of lipophilicity of drug substances. The higher the value of logP, the better the membrane permeability. It is essential for a molecule to have sufficient lipophilicity for optimal bioavailability and drug action. Sufficient aqueous solubility (logS) is important for optimal bioavailability of drugs. Hydrogen bond acceptor and donor groups are also of paramount importance for transport property and bioavailability of drug molecules. Molecular PSA is a useful parameter for drug transport properties. Practically, a compound with all these drug-like properties in the desired range appears to exhibit high level of therapeutic potency [22].
Higher antioxidant activity of compounds 5d and 5e might be because of their favorable hydrophilic behavior. Polar properties such as HBD and HBA groups and PSA have therefore more activity contributing effect than lipophilic property (logP). It can now be attributed that compounds 5d and 5e having PSA of 45.48 and 54.72, respectively, readily dissociate to give reactive ion species (phenoxy radical), which in turn is responsible for the antioxidant action. Drug-likeness score of compound 5e (0.06) supports the above considerations. Finally, it can be concluded that the conjugated chalcone-arylthiazole scaffold possess antioxidant as well as antibacterial potential. Different structural substitutions (electron withdrawing or releasing groups) in the aryl ring (ring B) of chalcone moiety further modulate the bio-efficacy of chalconeimines.
The newly synthesized chalconeimines can be used as lead molecules for further structural modifications in order to achieve various series of structural analogs with a spectrum of pharmacological activity. Modifications can be carried out in the aryl rings (rings A and B) of the chalcone moiety and/or in the heteroaryl system with a multitude of substitutions preferably with electron donating or withdrawing groups at different positions. From another point of view, rational design of various structural derivatives of chalcone and/or flavonoid analogs with diverse substitution pattern can be made in quest for the development of new chalcone-based bioactive leads and/drug molecules. However, preliminary in silico studies (molecular docking and ADMET) of such structural derivatives of chalcones are required for their drug-likeness optimization and bioactivity prediction.
References
R. Pingaew, A. Saekee, P. Mandi, et al., Eur. J. Med. Chem., 85, 65 – 76 (2014).
S. Maayan, N. Ohad, K. Soliman, et al., Bioorg. Med. Chem.,13(2), 433 – 441 (2005).
K. Maria, H. Dimitra, and G. Maria, Med. Chem.,4, 586 – 596 (2008).
S. Sinha, B. Medhi, and R. Sehgal, J. Mod. Med. Chem.,1, 64 – 77 (2013).
A. Detsi, M. Majdalani, C. A. Kontogiorgis, and D. Hadjipavlou-Litina, Bioorg. Med. Chem.,20, 8073–8085 (2009).
B. Ngameni, V. Kuete, P. Ambassa, et al., Med. Chem.,3(3), 233 – 237 (2013).
H. Zhang, J. Liu, J. Sun, et al., Bioorg. Med. Chem.,20 3212 – 3218 (2012).
H. Iqbal, V. Prabhakar, A. Sangith, and B. Chandrika, Med. Chem. Res.,23, 4383 – 4394 (2014).
M. Rudrapal, R. Siva Satyanandam, T. Sri Swaroopini, et al., Med. Chem. Res.,22 (6), 2840 – 2846 (2013).
C. M. da Silva, D. L. da Silva, L. V. Modolo, et al., J. Adv. Res.,2, 1 – 8 (2011).
M. Rudrapal and B. De, Int. Res. J. Pure App. Chem.,3(3), 232 – 249 (2013).
A. A. Al-Amiery, A. D. H. Kadhum, M. Shamel, et al., Med. Chem. Res.,23, 236 – 242 (2014).
N. A. Ghanwate, A. W. Raut, and A. G. Doshi, Oriental J. Chem.,24(2), 733 – 736 (2008).
S. G. Patil, P. S. Utale, S. B. Gholse, et al., J. Chem. Pharm. Res., 4(1), 501 – 507 (2012).
S. Patil, P. Utale, S. Gholse, et al., Asian J. Biochem. Pharm. Res.,3(2), 114 – 122 (2013).
S. Das, I. Mitra, S. Batuta, et al., Bioorg. Med. Chem.,24, 5050 – 5054 (2014).
M. Liang, X. Yu, L. Cong, et al., Bioorg. Med. Chem.,21, 6763–6770 (2013).
M. S. Brewer, Compr. Rev. Food Sci. Food Safety,10, 221 – 242 (2011).
B. Uttara, A. V. Singh, P. Zamboni, and R. T. Mahajan, Curr. Neuropharmacol.,7, 65 – 74 (2009).
E. A. Shalaby and M. M. Sanaa Shanab, Afr. J. Pharm. Pharmacol.,7(10), 528 – 539 (2013).
M. Rudrapal, T. Mariya Babu, R. Ravi Chandra, et al., Anti-Infect. Agents,12 (2), 198 – 205 (2014).
E. H. Kerns and L. Di, Drug-like Properties: Concepts, Structure Design and Methods, Academic Press, New York (2008), pp. 7 – 9.
B. S. Furniss, A. J. Hannaford, P. W. G. Smith, and A. R. Tatchell, Vogel′xs Text Book of Practical Organic Chemistry, John Willey & Sons, Inc., New York (1989), pp. 256.
S. R. Pattan, M. S. Ali, J. S. Pattan, et al., Indian J. Chem.45B, 1929 – 1932 (2006).
J. G. Collee, J. P. Duguid, M. G. Fraser, B. P. Marmion and M. McCartney, Practical Medical Microbiology, Churchill Livingstone, London (1989), pp. 346.
R. M. Silverstein and F. X. Webster, Spectrometric Identification of Organic Compounds, John Wiley and Sons, Inc., New York (1963), pp. 203, 213.
A. Crozier, I. B. Jaganath, and M. N. Clifford, Nat. Prod. Rep.,26, 1001 – 1043 (2009).
R. Tsao, Nutrients, 2, 1231 – 1246 (2010).
A. Kunwar and K. I. Priyadarshini, J. Med. Allied Sci.,1(2), 53 – 60 (2011).
B. Kim, O. Kwang-Joong, J. Chun, and K. Hwang, Bull. Korean Chem. Soc.29(6), 1125 – 1130 (2008).
K. Parikh and D. Joshi, Med. Chem. Res.,22, 3688 – 3697 (2013).
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney, Adv. Drug Deliv. Rev.,23, 3 – 26 (1997).
Acknowledgements
The authors express their sincere thanks to Dr. K. Ravishankar, Principal, Sri Sai Aditya Institute of Pharmaceutical Sciences and Research, Surampalem, E. G. Dist., Andhra Pradesh (India) for his generous help and co-operation in studying out the antioxidant activity of synthesized compounds. The authors also wish to thank Mr. N. Sathish Reddy, Vice-Chairman, Aditya Educational Institutions, Surampalem, E. G. Dist., Andhra Pradesh (India) for providing necessary laboratory facilities to carry out the work.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rudrapal, M., Sowmya, M.P.K. Design, Synthesis, Drug-Likeness Studies and Bio-Evaluation of Some New Chalconeimines. Pharm Chem J 53, 814–821 (2019). https://doi.org/10.1007/s11094-019-02084-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02084-y