Abstract
New hydrazone derivatives were synthesized by the condensation of some selected heteroaromatic hydrazines with appropriate aromatic ketones at high temperature (100 °C). The structures of the synthesized compounds were established by elemental (CHN) and spectral (IR, 1HNMR, and Mass) analysis. The synthesized compounds were screened for their antibacterial (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Proteus mirabilis) activities, which reveal that all the compounds possess activity against all the tested organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrazones containing an azomethine –NHN=CH– group represent an important class of compounds which possess a wide range of biologic activities (Corey and Enders, 1976). For this reason, hydrazones derived from a diverse group of heteroaromatic scaffolds (Rollas and Küçükgüzel, 2007) have successfully been incorporated into a number of therapeutically useful drug candidates. For example, isoniazid (isonicotinoyl hydrazone) is a well known and potent antitubercular drug with very high in vivo inhibitory activity against Mycobacterium tuberculosis H37Rv (Sah and Peoples, 1954). In recent days, many newer hydrazone derivatives have also been known to possess antimicrobial (Vicini et al., 2002), anticonvulsant (Dimmock et al., 2000), analgesic, antiinflammatory, antiplatelet (Todeschini et al., 1998), antimalarial (Walcourt et al., 2004), and antitumor activities (Abadi et al., 2003; Imramovský et al., 2007).
It is believed that the azomethine (–NHN=CH–) moiety is essential for the bioactivity of hydrazones and/or hydrazone derivatives as reported in various literatures (Rollas and Küçükgüzel, 2007). A large number of heteroaromatic scaffolds possessing a wide range of biologic including antimicrobial activities have also been investigated (Mariappan et al., 2011; Pattan et al., 2006; Singh et al., 2010). In view of the above-mentioned facts, an assumption has been made to design some newer hydrazone derivatives using such bioactive heteroaromatic scaffolds as the basic structural unit. Some important pharmacophoric scaffolds which include 4-benzylidene-2-methyloxazol-5-one, 2-(4-aminophenyl)benzimidazole, 2-amino-4-phenylthiazole, and isonicotinoyl hydrazide were selected for the present study. We reported herein the synthesis and evaluation of the antibacterial activity of some new hydrazone derivatives.
Experimental
Chemicals and analysis
All chemicals and solvents of synthetic grade were procured from Sd Fine Chemicals Ltd., Mumbai, and used without further purification unless otherwise stated. Melting points (m.p.) were measured in open capillaries using an electric melting point apparatus and are uncorrected. The progress of reactions was constantly monitored by the silica gel-G TLC and spots were detected by exposure to iodine vapors. The λmax (nm) was recorded on an Elico SL 164 UV–Vis spectrophotometer. IR spectra (υ, cm−1) were obtained on the FT-IR Perkin Elmer Spectrum RX-I spectrometer using a KBR disk. 1HNMR spectra were recorded on a Bruker AC-F 400 FT-NMR spectrometer using acetone-d6 as the solvent and TMS as the internal reference standard (chemical shift δ, ppm). Mass spectra were obtained with the LC–MS Water 4000 ZQ instrument using electrospray ionization. Elemental analysis was performed on a Perkin Elmer 2400 Series II CHNS/O analyzer.
Two strains of Gram-positive bacteria, viz. Bacillus subtilis, Staphylococcus aureus; and four strains of Gram-negative bacteria, viz. Eschericia coli, Pseudomonas aeruginosa, Salmonella typhi, Proteus mirabilis, were used for the study. The cultures of bacteria were maintained in their appropriate agar slants at 4 °C throughout the study and used as stock cultures.
General procedure for the synthesis of 1-(4-phenylthiazol-2-yl)hydrazine, 9/1-(4-(benzimidazol-2-yl)phenyl)hydrazine, 15
5 g (0.0011 mol) of 2-amino-4-phenylthiazole (Pattan et al., 2006), 8/6 g (0.055 mol) of 2-(4-aminophenyl)benzimidazole (Mariappan et al., 2011), 14 were dissolved in 3.0 mL of conc. HCl and 5.0 mL of water in a 250-mL conical flask. The mixture was cooled to 0–5 °C in an ice bath with vigorous shaking. The hydrochloride salt of amine was separated as a fine crystalline precipitate. A solution of 3.3 g (0.048 mol) of NaNO2 in 10 mL of water was prepared in a 100-mL beaker, cooled to 0–5 °C, and added slowly into the flask for a period of 15 min. The reaction mixture was then stirred well for a period of at least 1 h. The hydrochloride salt of amine was dissolved as the very soluble diazonium chloride salt was formed. The completion of the reaction was checked by KI-starch paper as indicated by the formation of blue color due to presence of slight excess of free nitrous acid in the solution. The cold diazonium chloride solution was added slowly into cold tin (2 g)-conc. HCl (15 mL) mixture with frequent stirring of the mixture. The mixture was refluxed in a water bath for an additional 2 h with occasional shaking and then cooled in an ice bath. The separated solid was collected by filtration, dried in air, and recrystallized from ethanol (Furniss et al., 2007).
General procedure for the synthesis of hydrazones
A solution of appropriate hydrazine (0.02 mol in 24 mL of alcohol-water mixture) was added into the solution of appropriate aromatic ketone (0.02 mol in 15 mL of ethanol) contained in a 250-mL RBF (round bottom flask). The mixture was refluxed for 1.5 h, cooled to room temperature, and then in refrigerator for at least 1 h. The separated solid was poured into 50 mL of ice-cold water and the precipitated product was filtered under suction, washed thoroughly with cold water, and dried. The crude solid was recrystallized from the alcohol-water (1:1) mixture and left to dry overnight (Cocco et al., 1999).
Analytical and spectral data
(7a) Yield 76 %, R f 0.35, m.p. 158–162 °C. UV–Vis (acetone), λmax (nm): 412. IR (KBr), υ (cm−1): 3345 (N–H str., >NH); 3065 (Ar. C–H str.); 2960, 2854 (C–H str., υas & υs –CH3); 1678 (>C=N– str.); 1352, 1260 (C–N str.); 1046 (C–O–C str.). 1HNMR (acetone-d6), δ (ppm): 1.85 (s, 3H, CH3); 5.63 (bs, 1H, NH); 5.98 (s, 1H, >C=CH–C6H5); 6.46–6.62 (m, 5H, –C6H5), 7.21–7.30 (m, 5H, –C6H5). MS (ESI), m/z (%): 278.72 (100), (M+H)+. CHN anal. (C17H15N3O); calc. (%): C, 73.63; H, 5.45; N, 15.15; found (%): C, 73.55; H, 5.53; N, 15.00.
(7b) Yield 76 %, R f 0.37, m.p. 175–178 °C. UV–Vis (acetone), λmax (nm): 576. IR (KBr), υ (cm−1): 3348 (N–H str., >NH); 3068 (Ar. C–H str.); 2965, 2855 (C–H str., υas & υs –CH3); 1672 (>C=N– str.); 1528, 1378 ((N=O)2 str., υas & υs Ar. NO2); 1352, 1266 (C–N str.); 1032 (C–O–C str.). 1HNMR (acetone-d6), δ (ppm): 1.89 (s, 3H, CH3); 5.87 (bs, 1H, NH); 6.13 (s, 1H, >C=CH– C6H5)); 6.52–6.67 (m, 5H, –C6H5), 7.21–7.30 (m, 3H, –C6H3 (NO2)2). MS (ESI), m/z (%): 368.13 (100), (M+H)+. CHN anal. (C17H13N5O5); calc. (%): C, 55.59; H, 3.57; N, 19.07; found (%): C, 56.01; H, 3.56; N, 19.09.
(10a) Yield 68 %, R f 0.32, m.p. 137–140 °C. UV–Vis (acetone), λmax (nm): 427. IR (KBr), υ (cm−1): 3369 (N–H str., >NH); 3044 (Ar. C–H str.); 2958, 2854 (C–H str., υas & υs –CH3); 1668 (>C=N– str.); 1635, 1610 (C=C str., –C=C–Ar.); 1354, 1260 (C–N str.). 1HNMR (acetone-d6), δ (ppm): 1.87 (s, 3H, CH3); 5.50 (bs, 1H, NH); 5.86 (d, 1H, J = 8.2 Hz, =C(alkyl)–CH =); 6.12 (d, 1H, J = 8.6 Hz), =CH–C6H5); 6.22 (s, 1H, thiazolyl-H5); 7.12–7.29 (m, 5H, –C6H5); 7.32–7.42 (m, 5H, –C6H5). MS (ESI), m/z (%): 320.45 (100), (M+H)+. CHN anal. (C19H17N3S); calc. (%): C, 71.44; H, 5.36; N, 13.15; found (%): C, 70.98; H, 5.55; N, 13.47.
(10b) Yield 77 %, R f 0.36, m.p. 165–168 °C. UV–Vis (acetone), λmax (nm): 476. IR (KBr), υ (cm−1): 3336 (N–H str., >NH); 3048 (Ar. C–H str.); 1662 (>C=N– str.); 1639, 1602 (C=C str., –C=C–Ar.); 1354, 1260 (C–N str.). 1HNMR (acetone-d6), δ (ppm): 5.55 (bs, 1H, NH); 5.88 (d, 1H, J = 8.0 Hz, =C(aryl)–CH=); 6.17 (d, 1H, J = 8.3 Hz), =CH–C6H5); 6.25 (s, 1H, thiazolyl-H5); 7.08–7.12 (m, 5H, –C6H5); 7.26–7.32 (m, 5H, –C6H5); 7.56–7.62 (m, 5H, –C6H5). MS (ESI), m/z (%): 382.59 (100), (M+H)+. CHN anal. (C24H19N3S); calc. (%): C, 75.56; H, 5.02; N, 11.01; found (%): C, 75.83; H, 5.75; N, 10.99.
(16a) Yield 68 %, R f 0.35, m.p. 158–162 °C. UV–Vis (acetone), λmax (nm): 488. IR (KBr), υ (cm−1): 3358 (N–H str., >NH); 3069 (Ar. C–H str.); 2978, 2858(C–H str., υas & υs –CH3); 1668 (>C=N– str.); 1630, 1600 (C=C str., –C=C–Ar.); 1345, 1276 (C–N str.). 1HNMR (acetone-d6), δ (ppm): 1.97 (s, 3H, CH3); 5.00 (bs, 1H, –NH, benzimidazolyl-H1); 5.67 (bs, 1H, –NH–C6H5); 5.68 (d, 1H, J = 7.9 Hz, =C(alkyl)–CH=); 6.27 (d, 1H, J = 7.8 Hz), =CH–C6H5); 7.26–7.31 (m, 5H, –C6H5), 7.38–7.46 (m, 4H, –C6H4); 7.64–7.87 (m, 4H, benzimidazolyl-H4, H5, H6 & H7). MS (ESI), m/z (%): 353.03 (100), (M+H)+. CHN anal. (C23H20N4); calc. (%): C, 78.38; H, 5.72; N, 15.90; found (%): C, 78.73; H, 5.90; N, 15.01.
(16b) Yield 74 %, R f 0.37, m.p. 175–178 °C. UV–Vis (acetone), λmax (nm): 496. IR (KBr), υ (cm−1): 3366 (N–H str., >NH); 3073 (Ar. C–H str.); 1677 (>C=N– str.); 1632, 1598 (C=C str., –C=C–Ar.); 1343, 1286 (C–N str.). 1HNMR (acetone-d6), δ (ppm): 5.16 (bs, 1H, –NH, benzimidazolyl-H1); 5.66 (bs, 1H, –NH–C6H5); 5.98 (d, 1H, J = 8.3 Hz, =C(aryl)–CH=); 6.34 (d, 1H, J = 8.4 Hz), =CH–C6H5); 7.29–7.36 (m, 5H, –C6H5), 7.43–7.48 (m, 4H, –C6H4); 7.56–7.62 (m, 4H, benzimidazolyl-H4, H5, H6 & H7). MS (ESI), m/z (%): 415.21 (100), (M+H)+. CHN anal. (C28H22N4); calc. (%): C, 81.13; H, 5.35; N, 13.52; found (%): C, 82.03; H, 5.40; N, 13.74.
(17a) Yield 69 %, R f 0.38, m.p. 132–136 °C. UV–Vis (acetone), λmax (nm): 457. IR (KBr), υ (cm−1): 3376 (N–H str., >NH); 3089 (Ar. C–H str.); 2974, 2866 (C–H str., υas & υs –CH3); 1665 (>C=N– str.); 1626, 1590 (C=C str., –C=C–Ar.); 1358, 1278 (C–N str.). 1HNMR (acetone-d6), δ (ppm): 1.86 (s, 3H, CH3); 5.40 (bs, 1H, NH); 5.74 (d, 1H, J = 8.0 Hz, =C(alkyl)–CH=); 6.26 (d, 1H, J = 8.2 Hz, =CH–C6H5); 7.26–7.32 (m, 5H, –C6H5), 7.44–7.49 (m, 4H, –C6H4). MS (ESI), m/z (%): 237.15 (100), (M+H)+. CHN anal. (C16H16N2); calc. (%): C, 81.32; H, 6.82; N, 11.85; found (%): C, 81.79; H, 6.00; N, 11.69.
(17b) Yield 74 %, R f 0.42, m.p. 165–168 °C. UV–Vis (acetone), λmax (nm): 588. IR (KBr), υ (cm−1): 3378 (N–H str., >NH); 3075 (Ar. C–H str.); 1658 (>C=N– str.); 1604, 1568, 1498, 1425 (C=C str., Ar. ring); 1545, 1389 ((N=O)2 str., υas & υs Ar. NO2); 1366, 1274 (C–N str.). 1HNMR (acetone-d6), δ (ppm): 5.46 (bs, 1H, NH); 5.77 (d, 1H, J = 8.6 Hz, =C(aryl)–CH=); 6.46 (d, 1H, J = 7.9 Hz), =CH–C6H5); 7.35–7.42 (m, 5H, –C6H5), 7.57–7.64 (m, 4H, –C6H4). MS (ESI), m/z (%): 266.73 (100), (M+H)+. CHN anal. (C21H18N2); calc. (%): C, 84.53; H, 6.08; N, 9.39; found (%): C, 85.06; H, 6.38; N, 9.72.
(19a) Yield 75 %, R f 0.43, m.p. 158–160 °C. UV–Vis (acetone), λmax (nm): 488. IR (KBr), υ (cm−1): 3342 (N–H str., >NH); 3057 (Ar. C–H str.); 2965, 2853 (C–H str., υas & υs –CH3); 1720 (>C=O str., –CONH–); 1625, 1600 (C=C str., –C=C–Ar.); 1682 (>C=N– str.). 1HNMR (acetone-d6), δ (ppm): 1.90 (s, 3H, CH3); 5.68 (bs, 1H, NH); 1.86 (s, 3H, CH3); 5.40 (bs, 1H, NH); 5.88 (d, 1H, J = 7.4 Hz, =C(alkyl)–CH=); 6.34 (d, 1H, J = 7.9 Hz), =CH-C6H5); 7.35–7.41 (m, 5H, –C6H5), 7.52–7.59 (m, 4H, –C6H4). MS (ESI), m/z (%): 266.32 (100), (M+H)+. CHN anal. (C21H18N2); calc. (%): C, 72.43; H, 5.70; N, 15.84; found (%): C, 72.62; H, 5.81; N, 15.17.
(19b) Yield 78 %, R f 0.46, m.p. 178–180 °C. UV–Vis (acetone), λmax (nm): 476. IR (KBr), υ (cm−1): 3347 (N–H str., >NH); 3065 (Ar. C–H str.); 1725 (>C=O str., –CONH–); 1687 (>C=N– str.); 1626, 1492, 1476, 1448 (C=C str., Ar. ring). 1HNMR (acetone-d6), δ (ppm): 5.73 (bs, 1H, NH); 5.83 (d, 1H, J = 7.3 Hz, =C(aryl)–CH=); 6.38 (d, 1H, J = 7.1 Hz), =CH–C6H5); 7.33–7.438 (m, 5H, –C6H5), 7.44–7.49 (m, 4H, –C6H4). MS (ESI), m/z (%): 328.13 (100), (M+H)+. CHN anal. (C21H17N3O); calc. (%): C, 77.04; H, 5.23; N, 12.84; found (%): C, 77.79; H, 5.51; N, 11.68.
Antibacterial screening
The antibacterial activity of the synthesized compounds was evaluated against six different strains of Gram-positive and Gram-negative bacteria by the agar diffusion method (Collee et al., 1989; Hewitt, 2004). The test solutions of each compound were prepared in two different concentrations, viz. 100 and 50 μg/mL using DMF as solvent. A freshly prepared and cooled (45–50 °C) medium of about 25 mL was inoculated aseptically with each standardized inoculum (0.5–1.0 mL) in a laminar air flow unit. The medium was then poured into a previously sterilized petri plate to occupy a depth of 4 mm. The plates were left at room temperature to allow solidification. A sterilized disk (6-mm diameter) of Whatman filter paper No.2 impregnated with DMF was used as the negative control. Under aseptic condition, empty sterilized disks were impregnated with the test drug solutions of two different doses (50 and 100 μg/mL) and with vehicle control DMF. After solvent evaporation, the dried disks were placed on the surface of the agar medium and the plates were left undisturbed for an hour at room temperature for pre-incubation diffusion to minimize the effects of variation in time between the applications of different solution.
Ciprofloxacin injection I.P. (200 mg/100 mL) was diluted to 50 μg/mL with normal saline and used as the positive control. After incubation of the plates at 37 ± 1 °C for 24 h, the diameters of the zones of complete inhibition surrounding each of the disk were measured including the diameter of the disk with a centimeter scale. The average zone of inhibition was obtained from three replicates of the test/standard compounds.
Results and discussion
The present study reports the in vitro antibacterial activity of some newly synthesized hydrazone derivatives. New hydrazone derivatives were synthesized by condensation of various aromatic/heteroaromatic hydrazines with the appropriate ketone at high temperature, preferably 100 °C. Hydrazones were formed by the replacement of the oxygen of carbonyl compounds (Scheme 1) with the –NHNH2 (hydrazinoyl) group of hydrazines. The synthesis of these hydrazones is depicted in Schemes 2, 3, 4, 5 and 6. The yields of all the compounds were found to be satisfactory. Hydrazones were obtained as pale yellow-, orange-, or red-colored products. The purity of the compounds was ascertained by melting point determinations and TLC (R f value). Furthermore, the results of elemental analyses were within the acceptable limits of the calculated values as presented in the analytical and spectral data subsection.
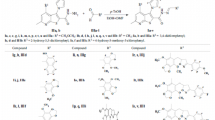
Synthesis of (4-benzylidene-2-methyloxazol-5(4H)-ylidene)-2-substituted hydrazone; (4)-N-acetyl glycine, (5)-4-benzylidene-2-methyloxazol-5-one, yield-85 %, m.p.-148–150 °C; (6a)-phenyl hydrazine hydrochloride, (6b)-2,4-DNPH, (7a)-(4-benzylidene-2-methyloxazol-5(4H)-ylidene)-2-phenylhydrazone, (7b)-(4-benzylidene-2-methyloxazol-5(4H)-ylidene)-2-(2,4-dinitrophenyl)phenylhydrazone
Synthesis of 1-Substituted-(4-phenylthiazol-2-yl)hydrazone; NH2CSNH2- thiourea, (8)-2-amino-4-phenylthiazole, yield- 85 %, m.p.-122–124 °C, (9)-1-(4-phenylthiazol-2-yl)hydrazine, yield- 75 %, m.p.-128–130 °C, (10a)-1-(4-phenylbut-3-en-2-ylidene)-(4-phenylthiazol-2-yl)hydrazone, (10b)-1-(1,3-diphenylallylidene)-(4-phenylthiazol-2-yl) hydrazone
Synthesis of 1-Substituted-(4-(1H-benzimidazol-2-yl)phenyl)hydrazone; (12)-o-phenylenediamine, (13)-PABA, (14)-2-(4-aminophenyl)benzimidazole, yield- 78 %, m.p.-140–142 °C, (15)-1-(4-(1H-benzimidazol-2-yl)phenyl)hydrazine, yield- 65 %, m.p.-155–158 °C, (16a)-1-(4-phenylbut-3-en-2-ylidene)-(4-(1H-benzimidazol-2-yl)phenyl)hydrazone, (16b)-1-(1,3-diphenylallylidene)-(4-(1H-benzimidazol-2-yl)phenyl)hydrazone
The UV–Vis spectra of the synthesized compounds showed characteristic absorption maxima (λmax) of longer wavelength, indicating the presence of strong chromophoric groups such as oxazolyl, –NO2 (7a, 7b); thiazolyl (10a, 10b); benzimidazolyl (16a, 16b); conjugated alkene with aryl (17a and 17b); and pyridyl (19a and 19b) moieties in the structure of the synthesized compounds. The structure of the compounds was supported by the infrared spectral data which showed characteristic absorption bands for specific functional groups like >NH, (3345 cm−1, 7a); aryl-CONH (1720 cm−1 , 19a); >C=N– (1662 cm−1, 10b); conjugated –C=C– with aryl ring (1630, 1600 cm−1, 16a); aromatic (N=O)2 (1545, 1389 cm−1, 17b); C–N (1343, 1286 cm−1, 16b)., C–O–C (1032 cm−1, 7b), etc. (Silverstein and Webster, 2005). The assignment of protons is fully supported by their characteristic chemical shift values for –CH3 (sharp singlet at δ 1.85, 7a); –NH of benzimidazolyl-H1 (broad singlet at δ 5.16, 16a); =C(Me)–CH= (doublet at δ 5.74, J = 7.9 Hz, 17a); >NH of =N–NH–Ar. (broad sharp singlet at δ 5.87, 7b); –NH of –CONH (broad sharp singlet at δ 5.73, 19b); =CH–C6H5 (doublet at δ 6.12, J = 8.6 Hz, 10a); and thiazole-H5 (sharp singlet at δ 6.12, 10b) groups present in the anticipated structure of the synthesized compounds (Silverstein and Webster, 2005). The prominent molecular ion peaks, [M+H]+, are in accordance with the anticipated mass of the synthesized compound (Silverstein and Webster, 2005).
All the synthesized compounds were found to be active against all the tested strains of Gram-positive and Gram-negative bacteria at two different tested doses, viz. 50 and 100 μg/mL. There was no inhibition of growth of vehicle control (DMF). The results depicted in Table 1 clearly indicate that all the compounds were equally active with some degree of variations, but were less potent when compared with the standard drug, ciprofloxacin. No significant difference was found in activities between the higher and lower dose of compounds.
Among the synthesized compounds, 7a showed the highest activity (14.0 mm and 16.0 mm at 50 and 100 μg/mL, respectively) which, however, was considerably less than that of the standard drug, ciprofloxacin (18 mm at 50 μg/mL), particularly against S. aureus. In contrast, the activity of the other compounds listed in Table 1 was less than that of compound 7a. The activity of 10a, for instance, was found to be 6.5 mm and 9.5 mm at 50 and 100 μg/mL, respectively, against S. aureus. Results clearly reveal that all the new hydrazones containing various heteroaromatic scaffolds possess antibacterial activity against all the tested strains. From the results, it could be assumed that heteroaromatic scaffolds are linked with the hydrazinoyl functional moiety; =N–NH– is an important structural prerequisite for the bioactivity of the prepared hydrazones. Furthermore, it has been observed that 7a possess more enhanced activity than the rest of the compounds, which indicates that 4-benzylidene-2-methyloxazol-5-one scaffold linked with unsubstituted phenyl hydrazinoyl substituent has a better contributing effect toward the activity of the compound than the substituted one (2,4-dinitrophenyl-, 7b) or any other substituents present in the rest of the compounds.
Conclusion
The present study involves the synthesis of some new hydrazone derivatives and the evaluation of their antibacterial activity against both Gram-positive and Gram-negative bacteria. The structural assignments of all the compounds were made on the basis of UV–Vis, IR, NMR, Mass spectroscopic data, and elemental analysis. All the synthesized compounds exhibited some degree of in vitro antibacterial activity at the tested doses which, however, was considerably less than that of the standard drug, ciprofloxacin. From our present investigation, it can be concluded that the selected heteroaromatic scaffolds when linked with the =N–NH– fragment become an important structural prerequisite for the bioactivity of the prepared hydrazones.
References
Abadi AH, Eissa AAH, Hassan GS (2003) Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem Pharm Bull 51:838–844
Cocco MT, Congiu C, Onnis V, Pusceddo MC, Schivo ML, De Logu A (1999) Synthesis and antimycobacterial activity of some isonicotinoyl hydrazones. Eur J Med Chem 34:1071–1076
Collee JG, Duguid JP, Fraser MG, Marmion BP, McCartney M (1989) Practical medical microbiology, 13th edn. Churchill Livingstone, London
Corey EJ, Enders D (1976) Application of N,N-dimethylhydrazones to synthesis. Use in efficient, positionally and stereochemically selective C–C bond formation; oxidative hydrolysis to carbonyl compounds. Tetrahedron Lett 17:11–14
Dimmock JR, Vashishtha SC, Stables JP (2000) Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur J Med Chem 35:241–248
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (2007) Vogel’s text book of practical organic chemistry, 5th edn. Wiley Inc., New York
Hewitt W (2004) The agar diffusion assay-its quantative basis & choice of experimental design. Microbiological assay for pharmaceutical analysis: a rational approach. Interpharm/CRC, New York, pp 183–205
Imramovský A, Polanc S, Vinšová J, Kočevar M, Jampílek J, Rečková Z, Kaustová J (2007) A new modification of anti-tubercular active molecules. Bioorg Med Chem 15:2551–2559
Mariappan G, Bhuyan NR, Kumar P, Kumar D, Murali K (2011) Synthesis and biological evaluation of Mannich bases of benzimidazole derivatives. Indian J Chem 50B:1216–1219
Pattan SR, Ali MS, Pattan JS, Purohit SS, Reddy VVK, Nataraj BR (2006) Synthesis and microbiological evaluation of 2-acetanilido-4-arylthiazole derivatives. Indian J Chem 45B:1929–1932
Rollas S, Küçükgüzel SG (2007) Biological activities of hydrazone derivatives. Molecules 12:1910–1939
Sah PPT, Peoples SA (1954) Isonicotinyl hydrazones as antitubercular agents and derivatives for identification of aldehydes and ketones. J Am Pharm Assoc 43:513–524
Silverstein RM, Webster FX (2005) Spectrometric Identification of Organic Compounds, 6th edn. Wiley Inc., New York
Singh I, Kaur H, Kumar S, Lata S, Kumar A, Kumar A (2010) Synthesis and antibacterial activity of 3-chloro-4-(substitutedphenyl)azetidinonyl/thiazolidinonyl-4-(3-acetanilido) oxa/thiazoles. Int J Pharm Sci Res 1(2):148–168
Todeschini AR, Miranda AL, Silva CM, Parrini SC, Barreiro EJ (1998) Synthesis and evaluation of analgesic, antiinflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur J Med Chem 33:189–199
Vicini P, Zani Franca, Cozzini P, Doytchinova I (2002) Synthesis and antiproliferative activity of benzo[d]isothiazole hydrazones. Eur J Med Chem 37:553–564
Walcourt A, Loyevsky M, Lovejoy DB, Gordeuk VR, Richardson DR (2004) Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int J Biochem Cell Biol 36:401–407
Acknowledgments
The authors express their sincere thanks to the Principal, the Aditya Institute of Pharmaceutical Sciences and Research, Surampalem, E. G. Dist., Andhra Pradesh, India, for his help and cooperation toward completion of this research work. The authors also wish to thank Mr. N. Satish Reddy, Vice-Chairman, the Aditya Educational Institutions, Surampalem, E. G. Dist., Andhra Pradesh, India, for providing laboratory facilities and financial support to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rudrapal, M., Satyanandam, R.S., Swaroopini, T.S. et al. Synthesis and antibacterial activity of some new hydrazones. Med Chem Res 22, 2840–2846 (2013). https://doi.org/10.1007/s00044-012-0278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0278-5