An HPLC-MS/MS method for determining dabigatran in human blood serum was developed. Samples were prepared by precipitation of proteins using MeOH followed by centrifugation and dilution with deionized H2O. The developed method had a simple sample-preparation procedure, short analysis time, and wide analytical range (from 1 to 1,000 ng/mL). This method was suitable for routine bioanalytical studies, in particular, therapeutic drug monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
According to the World Health Organization (WHO), cardiovascular diseases (CVDs) are the leading global cause of death (31%) [1]. The total global incidence of atrial fibrillation (AF) associated with CVD is 1 – 2% [2,3,4,5]. Warfarin has been successfully used to treat a broad spectrum of CVDs. However, the risk of intracranial hemorrhaging and hemorrhages in patients taking warfarin is 1.3%, among which 0.3% is attributed to the former, because of the deficiencies of warfarin, e.g., narrow therapeutic window [substrate for various isoforms of cytochrome P450 (CYP) and variable protein binding] and drug and food interactions [4].
Novel oral anticoagulants (NOACs) are the most promising drug class for treating CVD associated with AF [2, 6]. The direct thrombin (factor IIa, FIIa) inhibitor dabigatran was registered in 2010 by the US FDA [7].
Dabigatran is claimed to have predictable pharmacokinetic parameters. However, its use poses the risk of developing adverse drug reactions (ADRs) due to age, gender, physiological features, or co-administration with P-glycoprotein (P-gp) inhibitors [7, 8]. Therapeutic drug monitoring (TDM) of dabigatran is advised in cases of bleeding, during the perioperative period, and with co-administration of P-gp inhibitors, a high body mass index, kidney dysfunctions, etc. [7, 9,10,11].
The prodrug dabigatran etexilate is hydrolyzed by liver and blood esterases (including liver carboxylesterase 1) and converted to the active compound, i.e., dabigatran (Fig. 1) [7, 8, 12]. Dabigatran is not metabolized by the CYP system. However, the prodrug (dabigatran etexilate) is a substrate for the protein-transporter P-gp, which is coded by the gene MDR1. Various genotypes of MDR1 are associated with the various activities of P-gp. This also affects the pharmacokinetic parameters. Correspondingly, P-gp inhibitors increase the maximum dabigatran blood-serum concentration [7, 8, 12]. Conjugation of dabigatran with glucuronic acid produces four pharmacologically active acylglucuronide isomers 1-O, 2-O, 3-O, and 4-O that make up <10% of the total blood-plasma content of dabigatran [7,8,9,10,11,12,13,14,15,16].
Dangerous ADRs can result from genetic polymorphism of MDR1 and drug interactions with carboxylesterase substrates and P-gp inhibitors, which increase the maximum blood-serum concentration of dabigatran [7,8,9,10,11,12,13,14,15,16,17,18]. Therefore, TDM is the optimum method for choosing individual dabigatran doses.
Liquid chromatography with mass-spectrometric detection has been used to determine dabigatran in human blood plasma [19,20,21]. Its common drawbacks are a narrow analytical range, excessive dilution of the analytes by a precipitant, lack of description of the used linear gradient [20, 21], or, e.g., sample preparation and chromatographic separation under conditions where the analyte is eluted as two separate bands [19]. Furthermore, HCl solution was used as a precipitant although it is highly corrosive for both chromatographic and mass-spectrometric equipment [20].
TDM is based on coagulation methods and is a rather effective method for selecting doses. However, this method is inferior in accuracy, sensitivity, and universality to HPLCMS/MS, which measures directly the human blood-serum concentration of dabigatran [22,23,24].
Therefore, the goal of the present work was to design a rapid and sensitive method with simple sample preparation for determination of dabigatran in human blood serum using HPLC-MS/MS.
Experimental Part
The investigation used Shimadzu (Japan) equipment and a Nexera LCMS-8040 system (QQQ).
The reagents MeCN (PanReac AppliChem, UHPLC Supergradient, ACS); MeOH (PanReac AppliChem, Gradientgrad for HPLC, ACS); formic acid (Fluka, for mass spectrometry); and deionized H2O (electrical resistance 18.2 MΩ∙cm) were used the work. Calibration solutions were prepared using dabigatran (Sigma-Aldrich) and promethazine hydrochloride (Sigma-Aldrich) that met USP requirements. The separation was performed over an XBridge C18 column (50 × 4.6 mm, 3.5 μm, 80 Å, Waters, USA) with a universal C18 precolumn (4 × 3.0 mm, Phenomenex, USA) with the temperature thermostatted at 40°C. The mobile phase consisted of eluent A (0.1 vol%, formic-acid/deionized-H2O) and eluent B (0.1 vol%, formic-acid/MeCN). The separation was performed with gradient elution. Table 1 lists the mobile-phase gradient compositions. The injected sample volume was 20 μL. Promethazine was used as an internal standard.
Electrospray ionization (ESI) in positive-ion mode was used. The mass-spectrometric detection conditions for selecting the protonated molecular ions (472.15 m/z for dabigatran and 285.10 m/z for promethazine) as the precursor ions were determined by total ion current scanning (scan+) (Fig. 2). Then, daughter ions were scanned (product scan+) to select fragment ions from the precursors that could be used further for multiple reaction monitoring (MRM) (Fig. 3). Table 2 lists the detection parameters in MRM+ mode that were selected experimentally.
Table 2 presents the detection parameters in MRM+ mode and the impact energies that were selected experimentally.
Sample preparation consisted of precipitating blood-serum proteins using MeOH. For this, a serum sample [200 μL, or 180 μL of intact human blood serum with added dabigatran working standard solution (20 μL)] was treated with internal standard solution (5 mL, promethazine solution, 150 ng/mL) and MeOH (400 μL); shaken for 15 sec on a laboratory vortex stirrer; and centrifuged for 15 min at 14,000 rpm. The supernatant (500 μL) was transferred into chromatographic vials, treated with deionized H2O (200 μL), and shaken for 15 sec on a laboratory vortex stirrer. The vials were placed into the chromatograph autosampler.
The step for diluting the sample with a weak solvent had to be introduced during sample preparation so that dabigatran would elute as a single chromatographic band. An additional centrifuge step was not needed after adding the weak solvent. Shaking the sample on the vortex stirrer was sufficient.
Method validation was conducted according to guideline requirements [25].
Selectivity was assessed by analyzing six samples of intact human blood serum from various sources, samples of intact human blood serum with added dabigatran working standard solutions to produce concentrations in the range 1 – 1,000 ng/mL, and a promethazine internal standard solution up to a working concentration of 3.75 ng/mL in blood serum.
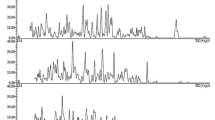
Chromatograms of intact human blood serum lacked peaks at retention times corresponding to dabigatran and promethazine (Figs. 4 and 5).
A calibration curve was constructed by analyzing 11 samples of intact human blood serum with added dabigatran working standard solutions to produce concentrations of 1, 5, 10, 25, 50, 75, 100, 250, 350, 500, and 1,000 ng/mL. Figure 6 shows the obtained calibration curve and correlation coefficient.
Accuracy and precision were estimated by analyzing samples of intact human blood serum with added dabigatran standard solutions to produce concentrations of 1, 5, 500, and 1,000 ng/mL. Three rounds including up to five samples for each concentration were analyzed. The intra-day and interday accuracy and precision were determined. Tables 3 and 4 present the relative standard deviations (RSDs, %) and relative errors (Es, %).
The resulting RSDs (precision) and Es (accuracy) met the norms [<20% for the lower limit of quantitation (lLOQ) and <15% for other concentrations].
The matrix factor (Mf) was calculated to evaluate the influence of the biological matrix on the mass-spectrometric detector signal intensity. The Mf was the ratio of the peak areas of dabigatran and promethazine in the chromatogram of blood serum with added standard solutions after protein precipitation (A) to the peak areas of dabigatran and promethazine in the chromatogram of a sample with intact blood serum replaced by an equal volume of H2O and with added dabigatran and promethazine standard solutions (B). The matrix effect was determined for dabigatran at 1 and 1,000 ng/mL and promethazine, 3.75 ng/mL. The Mf normalized to the internal standard was calculated as the ratio of dabigatran Mf values to those of promethazine (internal standard). The coefficient of variation (CV, %) of Mf normalized to the internal standard should be <15%. Table 5 presents the results.
The degrees of extraction of dabigatran and promethazine from blood serum during sample preparation were calculated as the ratio of the peak area of the studied compound in the chromatogram of blood serum with added standard solutions before protein precipitation (C) to the peak area of the studied compound in the chromatogram of blood serum with added standard solutions after protein precipitation (A). The degree of extraction was determined at dabigatran concentrations 1 and 1,000 ng/mL and promethazine, 3.75 ng/mL. Tables 6 and 7 present the results.
The lLOQ of the method was taken as the minimum dabigatran concentration in human blood serum at which dabigatran could be determined with RSD and E values <20% in the linear range. The lLOQ of the method was 1 ng/mL for dabigatran (Fig. 7). The signal-to-noise ratio was <10.4.
The starting and working standard solutions of dabigatran were confirmed to be stable during storage for 6 months at –30°C. Prepared samples were demonstrated to be stable for 24 h during storage in the chromatograph autosampler at 4°C.
Application of the developed method. The proposed method is currently being used at I. V. Davydovsky Municipal Clinical Hospital for pharmacokinetic studies aimed at optimizing pharmacotherapy using dabigatran for CVD, i.e., prevention of venous thrombosis, infarct, and systemic thromboembolism and reduction of cardiovascular death in patients with AF; treatment and prevention of deep-vein thrombosis and/or pulmonary artery thromboembolism; prevention of lethal outcomes, etc. Table 8 and Figs. 8 – 10 show examples of the use of the method for TDM.
Thus, a method for determining dabigatran in human blood serum using HPLC-MS/MS was developed. Dilution of the sample with deionized H2O after centrifugation avoided elution of dabigatran as two chromatographic bands and increased the accuracy and precision of the results. This method was convenient for routine bioanalytical investigations, in particular, for TDM.
References
WHO. Cardiovascular Diseases. Informational Bulletin No. 317, January, 2015; http: //www.who.int/mediacentre/factsheets/fs317/ru/[http: //www.who.int/mediacentre/factsheets/fs317/en/].
S. Stewart, C. L. Hart, D. J. Hole, and J. J. V. McMurray, Heart, 86(5), 516 – 521 (2001).
A. S. Go, E. M. Hylek, K. A. Phillips, et al., J. Am. Med. Assoc., 285(18), 2370 – 2375 (2001).
Atrial Fibrillation Investigators, “Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized clinical trials,” Arch. Intern. Med., 154, 1449 – 1457 (1994).
P. Kirchhof, S. Benussi, D. Kotecha, et al., Eur. Heart J., 37(38), 2893 – 2962 (2016).
L. Wallentin, S. Yusuf, M. D. Ezekowitz, et al., Lancet, 376(9745), 975 – 983 (2010).
Pradaxa Prescribing Information; http: //www.docs.boehringer-ingelheim. com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf (accessed Nov. 10, 2007).
S. Blech, T. Ebner, E. Ludwig-Schwellinger, et al., Drug Metab. Dispos., 36(2), 386 – 399 (2008).
W. Ageno, A. S. Gallus, A. Wittkowsky, et al., Chest, 141(2), e44S-88S (2012).
M. V. Huisman, G. Y. Lip, H. C. Diener, et al., Thromb. Haemostasis, 107(5), 838 (2012).
A. J. Camm, G. Y. Lip, R. De Caterina, et al., Eur. Heart J., 33(21), 2719 – 2747 (2012).
K. H. Liesenfeld, T. Lehr, C. Dansirikul, et al., J. Thromb. Haemostasis, 9(11), 2168 – 2175 (2011).
G. Pare, N. Eriksson, T. Lehr, et al., Circulation, 127(13), 1404 – 1412 (2013).
T. Ebner, K. Wagner, and W. Wienen, Drug Metab. Dispos., 38(9), 1567 – 1575 (2010).
F. Scaglione, Clin. Pharmacokinet., 52(2), 69 – 82 (2013).
T. Hellwig and M. Gulseth, Ann. Pharmacother., 47(11), 1478 – 1487 (2013).
Z. Y. Hu, R. B. Parker, and V. L. Herring, Anal. Bioanal. Chem., 405(5), 1695 – 1704 (2013).
P. A. Reilly, C. A. Conrad, R. A. Faaij, et al., Eur. Heart. J., 32(1), 6 (2011).
X. Delavenne, J. Moracchini, S. Laporte, et al., J. Pharm. Biomed. Anal., 58, 152 – 156 (2012).
M. Korostelev, K. Bihan, L. Ferreol, et al., J. Pharm. Biomed. Anal., 100, 230 – 235 (2014).
J. P. Antovic, M. Skeppholm, J. Eintrei, et al., Eur. J. Clin. Pharmacol., 69(11), 1875 – 1881 (2013).
J. Douxfils, L. Pochet, S. Lessire, et al., TrAC, Trends Anal. Chem., 84, 41 – 50 (2016).
J. Douxfils, J. M. Dogne, F. Mullier, et al., Thromb. Haemostasis, 110(3), 543 – 549 (2013).
J. Douxfils, H. Mani, V. Minet, et al., BioMed Res. Int., 2015, Article ID 345138 (2015).
A. N. Mironov (ed.), Guideline for Drug Review, Vol. 1 [in Russian], Grifi K, Moscow (2013).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 12, pp. 57 – 64, December, 2017.
Rights and permissions
About this article
Cite this article
Rodina, T.A., Mel’nikov, E.S., Aksenov, A.A. et al. HPLC-MS/MS Method for Determining Dabigatran in Human Blood Serum. Pharm Chem J 51, 1129–1137 (2018). https://doi.org/10.1007/s11094-018-1753-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1753-1