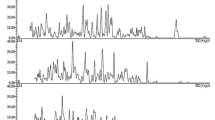
Two different literature methods were used to compare the experimental efficacy and accuracy of dabigatran assays in blood of 30 patients with knee replacements. Blood plasma was collected from patients who underwent anticoagulant therapy and were administered the medicine at a dose of 220 mg. Residual and peak dabigatran concentrations were determined by HPLC-MS and HPLC-MS/MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Peroral anticoagulants are a new class of medicines that appeared recently and are used in pharmacology to prevent thromboembolism [1,2,3,4,5,6].
Three methods are used to estimate the anticoagulant concentration in patient blood for drug monitoring to prevent bleeding and other serious complications. They are:
Rapid analysis of human blood for anticoagulant content in clinics, hospitals, and polyclinics often uses coagulometers to monitor the drug concentration in blood of patients on anticoagulants. However, the measurement is made indirectly using activated partial thromboplastin and prothrombin time, ecarin clotting time, etc. [7,8,9].
Chromatographic methods are most common and convenient for drug monitoring and solving pharmacokinetic problems [10,11,12,13]. The present work used two developed and validated methods for quantitative determination of dabigatran in human blood plasma [14, 16] that corresponded to existing documentation for selectivity, accuracy, linearity, precision, stability, matrix effect, carryover, and recovery [17,18,19]. Data from patients with knee arthroplasty who took the drug for prevention were analyzed. Samples (60) were taken from patients (30) and compared to determine the anticoagulants in blood. The most efficient method for estimating the pharmacokinetic parameters and drug monitoring was determined.
The goal of the present study was to compare the efficiency of methods used for quantitative determination of dabigatran in patient blood plasma and to choose the optimal method for therapeutic drug monitoring and solving pharmacokinetic problems.
Experimental Part
The study included 30 patients with knee replacements. In the post-operative period, they took dabigatran at a dose of 220 mg. Two samples were taken twice from each patient and were divided into two groups, i.e., group 1 before administration and group 2, 3 h after administration of the drug.
Quantitative analysis used two different HPLC methods with mass-spectrometric detection.
Data from the two methods were statistically processed using Kolmogorov—Smirnov and Wilcoxon tests and SPSS Statistics software.
Table 1 lists the equipment used to determine the drug in blood plasma by the two methods.
Calibrators and quality-control (QC) samples in method 1 were prepared using (Table 1):
matrix solution of dabigatran (1 mg/mL) in DMSO;
internal standard (IS) of deuterated dabigatran [M + 4] (1 mg/mL);
human blood plasma from patients.
Chemical reagents used for sample preparation and preparation of standards and QC samples included:
N2 (99%);
Ar (99.95%);
MeCN for HPLC (99.9%; LabScan, Poland);
ultrapure H2O from a Simplicity® UV system (with a UV lamp; Merck Millipore, Germany);
formic acid for HPLC (50%; Fluka, Switzerland);
CH2Cl2(dichloromethane; chemical pure; Khimmed,RF).
Method 2 used the following to prepare calibrators and QC samples (Table 1):
matrix solution of dabigatran (1 mg/mL) in MeOH – DMSO (9:1);
IS of deuterated dabigatran [M + 4] (1 mg/mL); human blood plasma from patients.
Chemical reagents used for sample preparation and preparation of calibrators and QC samples included:
N2(98%);
N2(99.99%);
DMSO (99.9%; Panreac);
HCl (37%, Panreac);
MeOH (99.8%; Fisher Scientific);
Ammonium acetate (Merck);
formic acid (85%; Acros Organics);
ultrapure H2O from a Millipore Direct-Q 5 UV system.
Reference standards
Standard solutions for method 1 were prepared by successive dilution of matrix solutions in H2O. QC standard solution was prepared by diluting stock solution in H2O to concentration 0.5 ng/mL.
Standard solutions of analytes and IS were stored in a refrigerator at 4°C for 1 week.
Standard solutions of dabigatran and its deuterated analog for method 2 were prepared using starting matrix solutions of the compounds in MeOH – DMSO (9:1). The concentration of dabigatran and its deuterated standard in the matrix solutions was 10 _g/mL. An IS working solution at concentration 50 ng/mL was prepared by successive dilution of matrix solution in MeOH and 0.1% HCl (9:1).
Sample preparation
Table 2 presents data for extraction of dabigatran from blood plasma by the two methods.
Chromatographic analysis conditions
Table 3 presents the dabigatran chromatographic separation conditions for the two methods.
Detection conditions
Table 4 presents the dabigatran detection conditions for the two methods.
Results and Discussion
The dabigatran concentration before its administration for method 1 was Max = 112.71 ng/mL; Min = 9.61 ng/mL; average deviation = 22.10 ng/mL; mean = 32.54 ng/mL. After its administration, the values were Max = 792.43 ng/mL; Min = 35.94 ng/mL; average deviation = 169.89 ng/mL; mean = 227.63 ng/mL. Table 5 presents the pharmacokinetic characteristics for the two methods.
A check for normal distributions of dabigatran concentrations before and after administration of capsules used the Kolmogorov—Smirnov one-sample test. Table 6 presents the results.
The asymptotic significance p < 0.05 indicated that the concentrations had a normal distribution. Statistically significant differences between concentrations obtained using the different analytical methods were found using the Wilcoxon criterion (Table 7).
The asymptotic significance p < 0.05 suggested that difference between medians of dabigatran concentration using the different analytical methods were not statistically significant.
Thus, the results obtained using the two methods wer statistically indistinguishable from each other so that either of the developed methods could be used.
References
V. T. Vavilova, Med. Sovet, No. 12, 44 – 47 (2015).
I. N. D’yakov, Remedium, No. 11, 42 – 43 (2014).
“Russian clinical recommendations for diagnosis, treatment, and prevention of complications from venous thromboembolism,” Flebologiya, 9(4), 1 – 52 (2015).
N. G. Khorev, A. P. Momot, and D. A. Zaloznyi, Tromb., Gemostaz Reol., No. 4(44), 31 – 47 (2010).
A. Dabi and A. P. Koutrouvelis, “Reversal strategies for intracranial hemorrhage related to direct oral anticoagulant medications,” Crit. Care Res. Pract., URL: https://doi.org/10.1155/2018/4907164.
A. Bromlcy and A. Plitt, J. Cardiol. Ther., 7(1), 1 – 13(2018).
Mosaad Almegren, Vasc. Health Risk Manage., 13, 287 – 292 (2017).
G. Lippi and E. Favaloro, Clin. Chem. Lab. Med., 53(2), 1 – 13 (2014).
V. Taune, M. Skeppholm, A. Agren, et al., J. Thromb. Haemostasis, 16(12), 2462 – 2470 (2018).
J. Stangier, K. Rathgen, H. Stahle, et al., Br. J. Clin. Pharmacol. B, 64(3), 292 – 303 (2007).
M. Korostelev, K. Bihan, L. Ferreol, et al., J. Pharm. Biomed. Anal., 100, 230 – 235 (2014).
J. Kuhn, T. Gripp, T. Flieder, et al., PLoS One, 10(12), 1 – 19 (2015).
E. M. H. Schmitz, K. Boonen, D. J. A. van den Heuvel, et al., J. Thromb. Haemostasis., 12, 1636 – 1646 (2014).
D. A. Sychev, A. N. Levanov, T. N. Shelekhova, et al., Pharmacogenomics Pers. Med., 11, 127 – 137 (2018).
J. Harenberg, S. Kraemer, S. Du, et al., Semin. Thromb. Hemostasis, 40(1), 129 – 134 (2014).
X. Delavenne, J. Moracchini, S. Laporte, et al., J. Pharm. Biomed. Anal., 58, 152 – 156 (2012).
Handbook for Drug Review [in Russian], Vol. 1, Grif i K, Moscow, 2014.
Guideline on Validation of Bioanalytical Methods (Draft), European Medicines Agency, Committee for Medicinal Products for Human Use, London, 2009.
Bioanalytical Method Validation, Guidance for Industry, U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evolution and Research (CDER). U. S. Government Printing Office,Washington, 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 53, No. 8, pp. 59 – 63, August, 2019.
Rights and permissions
About this article
Cite this article
Kozlov, A.V., Smirnov, V.V., Sychev, D.A. et al. Comparison of Quantitative Analytical Techniques for Dabigatran in Blood Plasma of Humans with Knee Replacements. Pharm Chem J 53, 771–774 (2019). https://doi.org/10.1007/s11094-019-02077-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02077-x