Abstract
A numerical investigation of a glow-type discharge in humid air with a water-cathode is reported. A complete block of chemical reactions that self-consistently describes the ionic composition of the plasma is considered. A water molar fraction up to 20% is examined. The electric field strength, emission discharge radius, as well as the OH (A → X) band emission in the positive column was also measured for discharge currents up to 155 mA. The model shows a non-thermal plasma with lower gas temperatures (around 3500 K) than those typically obtained in similar discharges but operating with metal electrodes in dry air. The gas temperature is almost unaffected by the discharge current. The vibrational relaxation through N2–H2O collisions is the main gas heating mechanism. The thermal diffusion due to enhanced thermal conductivity by water vapor is the primary cooling mechanism. The electron temperature is around 1 eV to ensure that the electron losses (mainly by dissociative recombination of NO+) are compensated by ionization phenomena. The NO+ is the dominant ion, mainly formed by electron-impact ionization of NO molecules. An electron number density close to 1019 m−3 is obtained. For the upper water fraction, the electron-impact ionization of O2 molecules, followed by a quick conversion to NO+, also plays a role. The concentration of OH is ~ 1022 m−3. A comparison between the model results and the experimental data suggests that the molar fraction of water in the plasma is around 20% for the conditions considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last two decades, a quite large number of investigations have been devoted to the study of electrical discharges in (or in contact with) liquids, primarily due to their wide range of technological applications [1,2,3,4,5]. These applications include but are not limited to, agriculture, disinfection, water decontamination, and environmental preservation [6,7,8]. These kinds of discharges can be classified into three main groups: (i) discharges in the liquid phase, (ii) discharges in the gaseous phase with one or both liquid electrodes, and (iii) discharges in multiple phases, such as bubbles or liquids in the form of spray or droplets [5].
The glow-type discharge with a liquid-cathode belongs to the second group. This specific discharge type shares similarities with low-pressure glow discharge between metallic electrodes [9, 10]. Numerous experimental investigations have been developed for atmospheric pressure glow discharges with liquid electrodes. Optical Emission Spectroscopy (OES) is commonly employed as an experimental diagnostic tool to estimate important discharge parameters such as electric field strength, electron density, and characteristic temperatures (see e.g., [1, 11,12,13]). However, the number of numerical models that have been developed to acquire information on plasma features, like neutral and ionic composition, water content, electron energy distribution function (EEDF), and heating and charge production mechanisms, are scarce [14,15,16,17].
The model developed in [14] focused on the plasma composition of a diffuse discharge in atmospheric pressure air ignited between two liquids (tap water) electrodes. The particle composition was calculated assuming that the plasma is in chemical equilibrium with electrons and heavy particles following two separated Maxwellian distributions, and with the population among different excited states following Boltzmann distributions; the corresponding temperatures being the parameters of the model. By comparison with experimental data, the ratios between these characteristic plasma temperatures, as well as the upper limit of the water vapor percentage in plasma were determined. The results show that the electrical neutrality is mainly due to NO+ and electrons for a water vapor percentage lower than 20% and to H3O+ for higher water vapor percentages. In [15], an experimental and theoretical study of a DC glow discharge in atmospheric pressure air with distilled water as cathode was presented. The EEDF and related electron features were obtained by means of the numerical solution of the Boltzmann equation for electrons. For these purposes, a water molar fraction up to 20% was considered. The electron density was estimated from the plasma conductivity for a discharge current density range of ~ 3.3–4.8 A/cm2. The results indicated that the EEDF essentially exhibits a non-Maxwellian behavior, especially in the high-energy electron range (referred to as the “tail” of EEDF), primarily due to the substantial impact of water vapor on this electron population, which considerably decreases the energy of electrons, due to the large cross section at low energies of the water molecules in comparison to other major components of humid air plasmas as nitrogen and oxygen molecules. On the other hand, the vibrational–translational relaxation was identified as the primary mechanism for gas heating. The measured gas temperature was almost unaffected by the discharge current in the range 20–50 mA. In [16], a zero-dimensional model of the plasma composition for the conditions considered in [15] was reported. The model solves the Boltzmann equation for electrons and considered the vibrational kinetics for ground states of N2, O2, H2O and NO. The electric field strength, averaged gas temperatures, and emission radii of the plasma column are the parameters of the model. The electron concentration is not calculated since the ionization mechanisms are unknown. It is estimated from plasma conductivity measurements. Water molar fraction up to 5% was considered. The numerical results indicate a high-vibrational temperature of N2 ground state, and that the main neutral species in the plasma are O2(a1Δ), O2(b1Σ), O(3P), NO, NO2, HNO3, H2O2, and OH. Numerous reactions involving charged particles (such as charge exchange, ionization, and recombination phenomena) were not considered. Because obtaining the ionic composition of the plasma was not one of the aims of the model. In [17], a numerical model was developed to determine the plasma composition over the surface of the electrolyte cathode. The model considers a glow discharge in humid air operating at a discharge current value of 25 mA. The gas temperature is fixed at 1000 K. Again, the electron density was estimated based on plasma conductivity and was not calculated. Its value was kept constant during the calculations. The authors reported that H2O+ is the main positive ion, independently of the water content. It is important to mention that the model did not consider the NO+ ion in the kinetic scheme. This revision shows that there is currently no consensus on the ionic composition nor the dominant ionization mechanisms in the positive column of humid air glow-type discharges with water cathode. This work is an attempt to address this issue.
In this work, a zero-dimensional numerical model describing the elementary processes occurring within the positive column of a millisecond DC-pulsed humid air glow-type discharge with a water-cathode is reported. The model self-consistently calculate the ionic composition as well as the relevant mechanisms responsible for the charged particle balance in the discharge. The EEDF is obtained by solving the electron Boltzmann equation. A water molar fraction up to 20% is examined. Additionally, to validate the simulation results, measurements of the electric field strength, emission discharge radius, and gas temperature in the positive column of the discharge were carried out.
Experimental
Water-Cathode Glow-Type Discharge
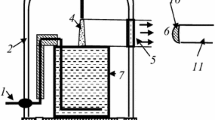
The discharge is ignited in a pin-to-water electrode configuration with an inter-electrode distance L = 12 mm. A cone-shaped tungsten-thoriated bar is the anode of the discharge while the distilled water contained in a 1–L grounded reservoir made of AISI 304 acts as the cathode (Fig. 1). To ignite the discharge, a variable autotransformer was used to adjust the discharge current I. This autotransformer was connected to the primary circuit of a high-voltage AC power transformer (25 kV, 50 Hz) with high dispersion reactance (95.3 ± 0.5 kΩ). This high impedance creates negative feedback between the discharge current and voltage, eliminating the necessity for external ballasts. Furthermore, a full-wave semiconductor rectifier bridge was used in the secondary circuit to fix the electrode polarity. The measurement of the discharge voltage V was conducted using a high-voltage probe (Tektronix P6015A, 1000X, 3 pF, 100 MΩ) connected to an oscilloscope (Tektronix TDS 2004C) with a sampling rate of 1 GS/s and an analog bandwidth of 70 MHz. Simultaneously, the discharge current I was inferred by employing a (low inductance) 100 Ω shunt resistor.
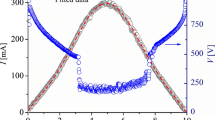
The voltage and current discharge waveforms are shown in Fig. 2. The current waveform shows an almost sinusoidal form with a peak value of about 155 mA (100 mA RMS value) as it is controlled by the high dispersion reactance of the transformer. Voltage signals for three different times measured from the discharge ignition (1, 30, and 300 s) are presented. At the beginning of each pulse, voltage spikes of about 4 kV are found, corresponding to the discharge ignition by a streamer-to-spark high-voltage transition [5]. Then, after the breakdown, the voltage drops to about 2 kV due to the high-impedance of the transformer and the discharge stabilizes to a glow-type discharge. The measured voltage includes not only the voltage drop in the gas gap but also the voltage drop in the electrode sheaths (mainly in the cathode) as well as in the resistive cathode (i.e., the distilled water). According to Fig. 2, the V–I characteristic curve has a positive slope immediately after the discharge is ignited (reaching a maximum value of about 4 kV for the peak current). This is due to the high voltage drop in the cathode due to the initial low electrical conductivity of the distilled water (5 µS/cm). However, as the time increases, the electrical conductivity of the water soon increases (to a value of about 50 µS/cm after 30 s, and about 180 µS/cm after 300 s), and the voltage drop in the liquid cathode decreases; thus, leading to the typically negative slope in the V–I characteristic of such discharge between metallic electrodes (e.g., [18]). The increase in the electrical conductivity of the water in contact with air discharges is always observed due to the ions formed in the liquid volume [19].
Measurement of the OH (A → X) Band Emission in the Positive Column
OH (A2Σ+–X2Π, Δv = 0) band emission from the discharge was measured with a monochromator (Spectral Products DK480, f = 480 mm, f/7.8, 2400 l/mm grating) coupled to a CCD linear detector (Alphalas CCD-S3600-D-(UV), 3648 active pixels (16 bit-ADC), 200–1200 nm) with an exposure time of 10 ms. Moderately high-resolution spectra of the OH band were obtained for discharge current pulses of 70, 106, and 155 mA peak values (50, 75, and 100 mA RMS values, respectively) with a resolution of 0.055 nm FWHM. A UV high-quality-convex lens (focal length, f = 100 mm) was mounted facing the discharge, collecting the light emitted only from the positive column, and redirected (through a UV–Vis optical fiber) toward the entrance slit, of 25 µm width, of the spectrometer (Fig. 3). The time-averaged rotational temperature of the OH (A → X) transition in the wavelength range λ = 306–311 nm is obtained by the Boltzmann plot method using the MassiveOES software [20,21,22,23]. Ten emission spectra were averaged.
Measurement of the Emission Discharge Radius and the Electric Field Strength in the Positive Column
To measure the emission radius of the discharge at the vicinities of the middle of the positive column, a large number (~ 100) of visible photographs were taken for several discharge current pulses with different peak values. To achieve this, a UV-convex lens (f = 100 mm) was used for focusing each captured image onto the sensor of a CCD camera (Lumenera Lt225m, resolution 2048 × 1088 pixels), with an exposure time of 0.1 ms (Fig. 3). These images were saved in BMP format and digitized using an 8-bit gray-level frame grabber. Only the brightest images (corresponding to the passage of the discharge current by its peak value and having a similar pixel intensity distribution) were selected and analyzed. To eliminate any interference from background noise, a background image (with the discharge off) was subtracted to each image of the discharge. The discharge emission radius was then defined as the radial distance at which the pixel intensity dropped to 50% of the maximum (axial) intensity [24, 25]. In addition, as the discharge emission radius in atmospheric pressure air glow-type discharges is close twice smaller than the current-carrying radius [25], the current density at the positive column j corresponding to each peak current value is then calculated by assuming that the current-carrying radius is equal to the diameter of the luminous region. A similar approach was employed in [25, 26]. The results are presented in Fig. 4.
To determine the electric field strength (E) in the positive column, the discharge voltage was measured as a function of the interelectrode distance L for several current pulses having different peak values. To do this, the anode was moved using a micrometer to change L between 1 and 12 mm with an uncertainty of about ± 500 µm. The value of E is then determined from the slope of the fitting line. To ensure that the resistive voltage drop in the water is negligible small with respect to that in the gas gap, such measurements were carried out with an acidified cathode (i.e., for t > 300 s).
The electron number density for each single discharge current value is then inferred from the electrical conductivity of the plasma considering the experimentally measured average gas temperature. As the mobility of electrons depends largely on the amount of water vapor in the plasma (which is initially unknown), the electron number density is calculated for a humid air plasma containing a mole fraction of water up to 20%.
Plasma model
The model considers a fairly complete kinetic block consisting of 216 reactions in a mixture of water vapor with dry air ([N2]:[O2] = 4:1) with the participation of molecular species, N2(X1∑g+,v), N2(A3∑u+), N2(B3Πg), N2(a’1∑u−), N2(C3Πu), O2, O2(a1Δg), O2(b1∑g+), NO, H2O, OH, H2, N2O, NO2, NO3, O3, HO2, HNO2, HNO3 and HNO; atomic neutral species, N(4S), N(2D), N(2P), O(3P), O(1D), O(1S) and H(1S); positive ions, NO+, N2+, O2+, H2O+, H3O+, and O+; negative ions, O−, O2−, O3−, H− and OH−; and electrons (e). For the simulations, two different water molar fractions χ are considered, 2 and 20%. The reactions are presented in the Table 1 of the Appendix 1. The proposed kinetic model does not incorporate reactions involving species such as N4+, O4+, HN2+, and hydrated ions. At gas temperatures above 900 K, the N4+ and O4+ cluster ions are efficiently destroyed owing to their low dissociation energy [27]. Consequently, the absence of this cluster of ions interrupts the chain of hydrated ions production as O2+(H2O) and H3O+(H2O)n (the subscript n indicates the coordination number) [28].
The local balance of neutral, ions, and electrons, are obtained through the time-dependent continuity equations,
The subscript k denotes the k-th species, with [nk] representing its respective number density. D refers to the diffusion coefficient, τ represents the time scale of the convective flow, and Λ denotes the characteristic diffusion length, estimated from the discharge radius R inferred from optical experiments. The diffusion coefficients of the neutral species are corrected according to the gas temperature [29], considering the data reported in [30] for Tg = 300 K. The rate at which species k is produced or destroyed (negative in this case), because of reaction i, is denoted by the term S. The number density of the dominant species N2 is determined by the conservation of the pressure (p = 105 Pa). Simultaneously, the densities of O2 and H2O are calculated through the atoms conservation laws of N, O, and H, considering the initial gas composition. The dominant positive ion NO+ is determined by applying the plasma quasi-neutral condition.
The balance equations that govern the average vibrational energy εV of the N2 molecule and the average translational energy of the neutral species (gas) are described as follows,
where εV is related to the vibrational temperature Tv by \({\varepsilon }_{V}=q \hslash \omega /\left[\text{exp} \left(q \hslash \omega /\left({k}_{B} {T}_{V}\right)\right) -1\right]\). Here, q represents the elementary charge, kB is the Boltzmann constant and \(\hslash\) ω denotes the vibrational quantum (= 0.29 eV) of the N2 molecule. The expression \({\varepsilon }_{V}({T}_{g})\) corresponds to the equilibrium vibrational energy value (i.e., for Tv = Tg) [29, 31]. The vibration–translation relaxation time (V–T), τVT, takes into account relevant V–T relaxation phenomena in water–nitrogen–oxygen mixture [29, 32, 33]. The rate of V–T due to N2–O collisions is taken from [34]. Water vapor strongly contributes to the acceleration of V–T relaxation of N2(X1∑g+, v) molecules: through N2–H2O vibration–vibration (V–V) collisions followed by the extremely fast V–T relaxation process of H2O–H2O [32, 33]; as well as the fast V–T relaxation process by N2–H2O collisions. The rate of V–T relaxation of N2(X1∑g+, v) molecules by collisions with H2O molecules is taken from [29]. σ is the electrical conductivity (= q [ne] µe). The coefficients \({\eta }_{V}\) and \({\eta }_{T}\) depict the quantity of electronic energy that is transferred into the vibrational and translational modes, respectively. The term QR denotes the fast-gas heating in chemical reactions [34,35,36] described with allowance for electron-impact predissociation, electron-ion recombination, quenching of excited species, and H2O dissociation. cpk is the specific heat of the heavy species k (= 5/2 kB, 7/2 kB, and 8/2 kB for atoms, diatomic molecules, and triatomic molecules, respectively). QCD (= 8 κ (Tg−T0)/Rt2) is the radial heat transport, assuming that the spatial variation of the gas temperature follows a radial parabolic profile [37]. κ is the translational component of the heavy particles thermal conductivity of humid air as a function of the molar fraction of water vapor [38, 39], Rt is the thermal radius(fixed to a value of 1 mm), and T0 (= 300 K) the ambient gas temperature. Finally, Hv is the water latent heat of vaporization, \({\dot{m}}_{{\text{H}}_{2}\text{O}}\) is the rate of water evaporation, and Vp is the volume of the plasma column.
The last term on the right-hand side of Eqs. (1) and (2), as well as the fifth term in Eq. (3), account for the axial (vertical) transport resulting from the weak longitudinal flow effects by natural convection [9, 40]. The convective transport time \(\tau\) was estimated using a simple approximation of the convective flow velocity according to Bernoulli’s equation [25, 40, 41].
Experimental studies on liquid-cathode glow discharge at atmospheric pressure have shown that the liquid is effectively transferred to the plasma column through the formation of droplets and fine plumes, mainly by ion sputtering [42,43,44]. In the plasma column, the water droplets quickly vaporize resulting in plasma cooling. This cooling mechanism due to the increase in water evaporation with increasing discharge current is considered through the last term on the right-hand side in Eq. (3). The time-averaged evaporation rate was measured from the mass loss of liquid over several discharge cycles as,
Under the considered conditions, the time-averaged evaporation rate was 3.4 ± 0.2 mg/s.
Then, by assuming that the instantaneous evaporation rate has a quadratic dependence on the discharge current [43, 45], the instantaneous and average evaporation rates can be correlated as,
where \(\widehat{I}\) is the peak value of the discharge current pulse. The Eq. (5) is an approximation based on experiments conducted under DC conditions. However, it is expected that this relationship will be maintained under the conditions considered because the current variation timescale (10 ms) is slow enough as compared to the characteristic timescales of the plasma processes. This point will be discussed in the next section.
The local-field approximation was used to calculate the transport and reaction coefficients of electrons (i.e., as a function of reduced electric field E/N, where N is the neutral numerical density), employing a Boltzmann solver [46]. This approach is typically satisfied in molecular plasmas at atmospheric pressure (e.g., [16, 47, 48]). The transport and reaction coefficients of electrons are calculated for a humid air plasma containing a mole fraction of water up to 20%. The cross-section data are taken from the Morgan database [49]. The ‘effective’ electron temperature, Te, was found from the mean electron energy (= 3/2 kB Te). The parameters ηT and ηV were calculated as a function of the E/N values with the help of the Boltzmann solver [46]. In Eq. (3), the parameter ηT takes into account the energy transferred from electrons to the gas heating through elastic collisions, rotational excitation of the N2 and O2 molecules, the vibrational excitation of the H2O(010) state followed by the fast V–T relaxation in H2O(010)–H2O collisions, and the vibrational excitation of O2 molecules. The model also considers the impact of super-elastic collisions with vibrationally excited molecules on the EEDF, which has a strong influence on high-threshold electron-impact reactions, such as electronic excitation, dissociation, and ionization. To achieve this, the Boltzmann equation for electrons was solved setting the excitation temperature to the vibrational temperature and the transition energy to the first vibrational threshold (0.29 eV) of the N2 molecule.
The increases in the thermal dissociation rate of N2 under non-equilibrium conditions were considered through the Losev-β model [50, 51]. The electric field strength in the discharge column is calculated from Ohm’s law,
as a function of the experimentally inferred j values (Fig. 4). Discharge current density values for I < 50 mA were extrapolated by fitting the experimental data.
Balance Eqs. (1), (2), and (3) are solved numerically by a finite-difference explicit second-order Runge–Kutta predictor–corrector method [52]. To provide an accurate numerical integration, a short time-step of 1.0 × 10−10 s is used due to the stiffness of the problem, related to the wide range of timescales associated with various plasma processes. Appropriate initial conditions were used. The used initial conditions did not have a significant impact on the calculations results. Achieving a dynamic equilibrium required integrating the equations over several discharge periods, so that the densities of the species converge within an error of about 10−3 to their equilibrium values.
Results and Discussion
The presented results correspond to the millisecond DC-pulsed (rectified AC) discharge conditions according to the experimental arrangement shown in Fig. 1. Glow-type discharges in humid air with water cathode have been studied in several experiments (e.g., [3, 12, 13, 15, 16, 25, 53,54,55,56,57,58]). In most of these experiments the discharges were operated with DC currents, except for the experiment [57] operated at 60 Hz AC current. However, a direct comparison between the calculations and the literature data is still possible because the discharge current timescale (10 ms) is much larger than the characteristic timescales of the heavy particles processes in the discharge column. Consider for instance the timescale for diffusive losses of gas particles from a discharge column of radius R: tD ≡ Λ2/D, where D is the diffusion coefficient of the neutral particles (which is proportional to Tg3/2/p) and Λ ≡ R/2.4 is the characteristic diffusion length for a cylindrical volume [9]. D ~ 7 × 10−4 m2/s for air molecules at Tg ~ 3000 K and R is about 1 mm. Under the conditions considered it follows that tD is less than or equal to 0.3 ms, much shorter than the discharge current timescale (10 ms). To validate this statement, simulations were carried under DC conditions for several current values. The results indicate that the variations in gas and vibrational temperatures were about 1%; while for electron temperature and plasma number density the differences were around 4%; when compared to the millisecond DC-pulsed simulations. Note also that these results add consistency to the approximation (5), obtained by comparison with experiments conducted under DC conditions.
The time-averaged experimental spectrum of the OH(A–X) transition for a discharge current pulse having a peak value of 155 mA is presented in Fig. 5A. The corresponding Boltzmann plot up to the rotational quantum number J = 16.5 is shown in Fig. 5B. Note that the Boltzmann plot has a unique slope, indicating that the OH(A) rotational population follows a Boltzmann distribution under the conditions considered [1, 11]. Assuming that the rotational temperature of OH is in equilibrium with the translational (gas) temperature [11], a time-averaged gas temperature ⟨Tg⟩ = 3440 ± 460 K is obtained. Spectral fitting is not used to determine the gas temperature since this method does not exclude high rotational quantum numbers that may be overpopulated, leading to an overestimation of the gas temperature [1, 11]. For the other discharge current pulses having peak values of 70 and 106 mA, the Boltzmann plot shows 3530 ± 720 K and 3550 ± 840 K, respectively. These results are in agreement with previous investigations on DC glow-type discharge with water-cathode [12, 13] showing that the gas temperature measured by OES does not depend on the current discharge in the range 10–100 mA.
Figure 6 shows the simulated gas temperature Tg, the vibrational temperature Tv of the N2(X1∑g+,v) molecules, and the electron temperature Te versus the discharge current for water molar fractions of χ = 2 and 20%. It can be observed that the plasma is in thermal non-equilibrium, with a significant departure between Te and Tg. The primary process of gas heating is V–T energy relaxation, which occurs mostly due to collisions between N2 and H2O molecules. Accordingly, the departure between Tv and Tg decreases markedly with the increase in water content in the discharge. Despite this increase in the V–T energy relaxation with increasing plasma water content, the gas temperature does not increase appreciably (rather decreases slightly) due to the rise in the thermal conductivity of the plasma caused by the increase in the water content. The cooling of the gas by water droplets evaporation is not important, reaching < 10% of the total heat losses, being higher than the losses caused by the H2O dissociation via reaction (R64) with an energy consumption of 5.11 eV per dissociation event. Due to the values of the mean electron energy (of about 1 eV), the electron power is mainly transferred to the vibrational modes (ηV ~ 0.9) of N2 molecules. It is remarkably that the simulated values of both Tg and Tv are almost independent of the discharge current for χ = 20%. It should also be noted that the gas temperature is lower than those typically obtained in similar discharges but operating with metal electrodes in dry air (see e.g., [18, 31]). The experimental values of Tg measured by OES in this work, as well as those for Tg and Tv reported in the literature for this kind of discharge [12, 13, 15, 53,54,55] are also shown in Fig. 6. Experimental vibrational temperature data was derived from the radiative intensity distribution of the N2(C3Πu → B3Πg). According to numerical estimations the vibrational temperature of the (C3 Πu,v) exited state are similar to that of the lower levels of the (X1 Σg+,v) state in this type of discharges [15]. It is observed that the best agreement between the simulations and the experimental is achieved for χ = 20%.
The electron temperature is almost unaffected by the discharge current due to minimal variation in the reduced electric field, showing a decrease with increasing plasma water content. Experimental data of the electron excitation temperature of hydrogen atoms Texel (with values around 0.5–0.6 eV) are also shown in Fig. 6 [13, 25]. It is observed that the experimental values of Texel and simulated Te values show some discrepancy. The reported Texel measurements were obtained from the intensity ratio of the Hα over Hβ lines (with excitation energies of about of 12–13 eV), assuming a Maxwellian EEDF and a Boltzmann distribution for the population of excited states of hydrogen. However, the method may be doubtful because the EEDF is essentially non-Maxwellian under the conditions considered. The water vapor content decreases the number of electrons in the high energy ‘tail’ of the distribution [15, 25]. Consequently, high-level populations of hydrogen atoms may differ from the Boltzmann distribution. Furthermore, it is not possible to generate Boltzmann plots for the determination of Texel and, consequently, verify the assumption of the Boltzmann distribution due to the small intensity of the hydrogen emission and the superposition with other nitrogen emission lines. As a result, the evaluation of the excitation temperature of these hydrogen levels will be underestimated. This may be the reason explaining the discrepancies between the simulated electron temperature and the reported values of the hydrogen excitation temperature. It is worth to noting that the calculated Te values ensure that electron losses (essentially by dissociative recombination) are compensated by electron-impact ionization collisions with neutral particles with ionization energies close to or greater than 10 eV in the positive column of the discharge; thus, ~ 1 eV seems a realistic value for Te in this kind of discharges in contact with liquids (see the review [4] and references therein, as well as [59]).
Figure 7 shows the number density of several neutral species versus the discharge current for χ = 2% and 20%. The main mechanism of the production and destruction of NO molecules for χ = 2% can be described by the following reactions:
The thermal dissociation of nitroxyl (HNO) is the main mechanism of the production of NO molecules, through (R107), while the destruction process is governed by the formation of HNO by (R85). The production of H(1S) atoms is mainly governed by the reaction (R100) (which in turn is the dominant destruction mechanism of O(3P) atoms and OH molecules); while its destruction is mainly due to reaction (R81) for I < 100 mA, being replaced by reaction (R85) for higher discharge currents. In addition, the reaction (R81) is the dominant mechanism of production of O(3P) atoms and OH molecules. The production of OH by thermal dissociation of HNO in collision with O(3P) atoms (R107) becomes also comparable to the reaction (R81) for I > 100 mA. With the increment in the water content, the neutral kinetics are strongly affected, producing a reduction in the population of NO molecules and an increase in the hydrogen-containing species. The main reactions scheme for χ = 20% can be described by:
The concentration of OH molecules increases about one order of magnitude due to the thermal dissociation of H2O molecules in collisions with H(1S) atoms via (R92), which also serves as the primary destruction pathway for H(1S) atoms. The increasing water content also enhances the production of H2 molecules, which increases two orders of magnitude, through (R92). The production of H(1S) is mainly governed via (R100) for I < 70 mA, and via (106) for higher current values. The increase in the concentration of H(1S) atoms decrease in turn the NO molecules concentration via thermal recombination (R85), which exceeds the production of NO molecules through (R98). The reduction in the concentration of NO (with a low ionization energy of 9.27 eV) in turn induces a change in the ionization kinetics with increasing water content.
The loss and production of O(3P) atoms are also influenced by the water content. The impact of production reactions (R81) and (R93), as well as destruction reactions (R100) and (R106), becomes comparable, resulting in a slight reduction in the concentration of these atoms. The concentrations of OH and O(3P) radicals are in the range 1021–1023 m−3, according to reported experimental data [4, 56]. In particular, the best agreement between the simulations and the OH experimental data [56] is again achieved for χ = 20%. As indicated in [16], the formation of the OH is not the electron-impact dissociation of the H2O molecules via (R10), but the thermal dissociation of O2 via (R81) for the lower water content, and the thermal dissociation of H2O via (R92) when the molar fraction of water rises. The concentration of other oxygen–hydrogen particles like HO2 radical and H2O2 are not important under the relatively hot gas conditions considered. As expected, the numerical density of ozone is very low because it is thermally destroyed by (R90) under hot gas conditions as indicated in [16]. The main excited particle (not shown) is the O2(a1Δg) metastable. Independently of the water molar fraction, the formation is governed by the electron-impact excitation (R16), which is favored due to its low excitation energy (= 0.98 eV), and the destruction occurs mainly through quenching reactions with NO molecules via reaction (R114). The impact on the plasma dynamics of oxygen-excited atoms with the increment in the water molar fraction is weak because of the efficient quenching of the O(1D) and O(1S) states by water molecules via (R159) and (R165), respectively.
The mechanisms involved in electron production and loss versus the discharge current for χ = 2% and 20%, are depicted in Fig. 8. It is observed that the dominant process for electron production in the positive column is the electron-impact ionization of the NO molecule (R1), regardless of the plasma water content (up to 20%). On the other hand, the electron-ion recombination of NO+ (R31) is the dominant mechanism contributing to electron loss. However, as the plasma water content increases the concentration of the NO molecules in the plasma column decreases (see Fig. 7), mainly due to the formation of HNO by the reaction (R85). The consequent deficit in the NO+/electron pair production for χ = 20% is compensated by the electron-impact ionization of O2 via (R3),
followed by the rapid charge-exchange through (R206). Besides, as the level of water rises, the attachment cross-section for H2O becomes greater than the O2. Consequently, the water dissociative attachment process with a high energy threshold of 4.36 eV (R171) speeds up compared to the oxygen dissociative attachment reaction (R169), partially compensated by the detachment reaction (R192). Under the relatively cold gas conditions simulated, the associative ionization reaction in collisions between N(4S) and O(3P) atoms via (R20), which typically rules the charge production in glow-type discharges operating in atmospheric pressure dry at currents levels of the order of 100 mA (Tg > 4000–4500 K [18, 31]), does not play any significant role in the charge production.
The simulated the electric field strength as well as the reduced electric field in the positive column as a function of discharge current for χ = 2% and 20% are depicted in Fig. 9. Experimental data obtained in this work as well as available in the literature [3, 15] are also presented. The measured electric field strength in the positive column ~ 70–100 V/mm is usually higher than that for metallic cathodes (e.g., [60]). As quoted before, this is because H2O molecules have cross-sections considerable larger than those of N2 or O2 at low electron energies. A good agreement between the simulated and experimental results is observed. It is also observed that the simulated reduced electric field increases from ~ 35 to 40 Td when χ increases from 2 to 20% (1 Td ≡ 10−21 Vm2). This is because the ionization energy of O2 molecules (12.1 eV) is larger than that of NO molecules, whose concentration decreases with increasing water content in the plasma, as discussed above.
Figure 10 shows the number density of the main ions and electrons as a function of discharge current for χ = 2 and 20%. It is observed that the electron number density grows as the discharge current increases, reaching approximately 1019 m−3 disregarding the water molar fraction. The plasma electrical neutrality is mainly due to NO+ and electrons. This agrees with [14], which shows that electrical neutrality is mainly due to NO+ and electrons when the water molar fraction is lower than 35%. However, the number densities of OH− and H3O+ increase close to one order of magnitude as the water molar fraction increases. The number density of OH− is relatively high because they are produced by ion-exchange reactions (R196 for χ = 2%, and R198 for χ = 20%), but it is still small as compared to the number density of electrons. The experimental electron density data obtained in this work as well as relevant available experimental data are also included for comparison. Most of the available experimental data on electron density have been obtained from plasma conductivity (e.g., [15, 16, 57, 58]) or measuring the Stark broadening of the Hβ Balmer line emission (e.g., [13]). Although some dispersion between the values of the electron number density obtained from the plasma conductivity can be observed (which may be mainly attributed to the fact that the emission and conduction radii of the discharge may be not equal, depending on the experimental conditions) the simulation results show the correct order. On the other hand, the number density value reported in [13] is more than one order of magnitude larger than the simulated values. However, this density corresponds to the electron density in the cathode region and not in the positive column (since the Hβ Balmer emission is practically only observable in the cathode region [13]). Note that a higher electron density can be expected near the cathode of the discharge due to its filamentary structure [13, 54].
Conclusions
A numerical investigation of a millisecond DC-pulsed glow-type discharge in atmospheric pressure air with a water cathode was reported. A zero-dimensional model with a complete block of chemical reactions that self-consistently describes the gas heating mechanisms and ionic composition in the plasma was considered. A water molar fraction up to 20% was examined. Moreover, to validate the model, the electric field strength, emission discharge radius, as well as the OH (A → X) band emission in the positive column were measured for discharge currents up to 155 mA. A comparison between the model results and the experimental data suggests that the fraction of water in the plasma is around 20% for the conditions considered. For such water content in the plasma, the simulation shows that:
-
An electron temperature ~ 1 eV seems a realistic value in this kind of discharge in contact with liquid. The NO+ is the dominant ion, mainly formed by electron-impact ionization of NO molecules. The electron-impact ionization of O2 molecules, followed by a quick conversion to NO+, also plays a role. The associative ionization in atomic collisions does not play any significant role due to the rather low temperature of the gas. Consequently, the electric field strength in the plasma is quite large. The electron number density is close to 1019 m−3, showing good agreement with those experimentally inferred from plasma conductivity.
-
The main neutral species in the positive column of the discharge are NO molecules as well as O(3P) and OH radicals. The concentration of OH radicals is of the order of 1022 m−3.
-
The humid air plasma is in thermal non-equilibrium. The gas temperature remains remarkably constant with a value around 3500 K, somewhat lower than those typically obtained in similar discharges but operating with metal electrodes in dry air. The simulated results agree with the obtained experimental data.
Data Availability
No datasets were generated or analysed during the current study.
References
Kovačević VV, Sretenović GB, Obradović BM, Kuraica MM (2022) Low-temperature plasmas in contact with liquids—a review of recent progress and challenges. J Phys D Appl Phys 55:473002. https://doi.org/10.1088/1361-6463/ac8a56
Adamovich I, Agarwal S, Ahedo E et al (2022) The 2022 plasma roadmap: low temperature plasma science and technology. J Phys D Appl Phys 55:373001. https://doi.org/10.1088/1361-6463/ac5e1c
Rybkin VV, Shutov DA (2017) Atmospheric-pressure electric discharge as an instrument of chemical activation of water solutions. Plasma Phys Rep 43:1089–1113. https://doi.org/10.1134/S1063780X17110071
Bruggeman PJ, Kushner MJ, Locke BR et al (2016) Plasma–liquid interactions: a review and roadmap. Plasma Sour Sci Technol 25:053002. https://doi.org/10.1088/0963-0252/25/5/053002
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys 42:053001. https://doi.org/10.1088/0022-3727/42/5/053001
Wong KS, Chew NSL, Low M, Tan MK (2023) Plasma-Activated water: physicochemical properties, generation techniques, and applications. Processes 11:2213. https://doi.org/10.3390/pr11072213
Konchekov EM, Gusein-zade N, Burmistrov DE et al (2023) Advancements in plasma agriculture: a review of recent studies. Int J Mol Sci 24:15093. https://doi.org/10.3390/ijms242015093
Barjasteh A, Lamichhane P, Dehghani Z et al (2023) Recent Progress of non-thermal atmospheric pressure plasma for seed germination and plant development: current scenario and future landscape. J Plant Growth Regul 42:5417–5432. https://doi.org/10.1007/s00344-023-10979-0
Raizer YP (1991) Gas Discharge Physics. Springer-Verlag, Berlin, Heidelberg
Mezei P, Cserfalvi T (2007) Electrolyte cathode atmospheric glow discharges for direct solution analysis. Appl Spectrosc Rev 42:573–604. https://doi.org/10.1080/05704920701624451
Bruggeman PJ, Sadeghi N, Schram DC, Linss V (2014) Gas temperature determination from rotational lines in non-equilibrium plasmas: a review. Plasma Sour Sci Technol 23:023001. https://doi.org/10.1088/0963-0252/23/2/023001
Verreycken T, van Gessel AFH, Pageau A, Bruggeman P (2011) Validation of gas temperature measurements by OES in an atmospheric air glow discharge with water electrode using Rayleigh scattering. Plasma Sour Sci Technol 20:024002. https://doi.org/10.1088/0963-0252/20/2/024002
Bruggeman P, Ribežl E, Maslani A et al (2008) Characteristics of atmospheric pressure air discharges with a liquid cathode and a metal anode. Plasma Sour Sci Technol 17:025012. https://doi.org/10.1088/0963-0252/17/2/025012
Andre P, Aubreton J, Barinov Y et al (2002) Theoretical study of column of discharge with liquid non-metallic (tap water) electrodes in air at atmospheric pressure. J Phys D Appl Phys 35:1846. https://doi.org/10.1088/0022-3727/35/15/305
Titov VA, Rybkin VV, Smirnov SA et al (2006) Experimental and theoretical studies on the characteristics of atmospheric pressure glow discharge with liquid cathode. Plasma Chem Plasma Process 26:543–555. https://doi.org/10.1007/s11090-006-9014-6
Bobkova ES, Smirnov SA, Zalipaeva YV, Rybkin VV (2014) Modeling chemical composition for an atmospheric pressure DC discharge in air with water cathode by 0-D model. Plasma Chem Plasma Process 34:721–743. https://doi.org/10.1007/s11090-014-9539-z
Sirotkin NA, Khlyustova AV, Maksimov AI (2014) Numerical simulation of the gas phase composition in a glow discharge with an electrolyte cathode. Surf Engin Appl Electrochem 50:323–329. https://doi.org/10.3103/S1068375514040115
Cejas E, Prevosto L, Minotti FO (2024) Numerical simulation of the voltage-current characteristic of an atmospheric pressure discharge: the glow-to-arc transition. Plasma Chem Plasma Process 44:765–784. https://doi.org/10.1007/s11090-023-10438-4
Lukes P, Locke BR, Brisset J-L (2012) Aqueous-phase chemistry of electrical discharge plasma in water and in gas-liquid environments. Plasma Chemistry and Catalysis in Gases and Liquids. John Wiley and Sons, New Jersey, pp 243–308
Voráč J, Synek P, Potočňáková L et al (2017) Batch processing of overlapping molecular spectra as a tool for spatio-temporal diagnostics of power modulated microwave plasma jet. Plasma Sour Sci Technol 26:025010. https://doi.org/10.1088/1361-6595/aa51f0
Voráč J, Synek P, Procházka V, Hoder T (2017) State-by-state emission spectra fitting for non-equilibrium plasmas: OH spectra of surface barrier discharge at argon/water interface. J Phys D Appl Phys 50:294002. https://doi.org/10.1088/1361-6463/aa7570
Voráč J, Kusýn L, Synek P (2019) Deducing rotational quantum-state distributions from overlapping molecular spectra. Rev Sci Instrum 90:123102. https://doi.org/10.1063/1.5128455
Voráč J, Synek P MassiveOES. https://bitbucket.org/OES_muni/massiveoes/src/master/
Staack D, Farouk B, Gutsol A, Fridman A (2005) Characterization of a dc atmospheric pressure normal glow discharge. Plasma Sour Sci Technol 14:700. https://doi.org/10.1088/0963-0252/14/4/009
André P, Barinov YA, Faure G, Shkol’nik SM, (2018) Characteristics of discharge with liquid non-metallic cathode burning in air flow. J Phys D Appl Phys 51:445202. https://doi.org/10.1088/1361-6463/aadfad
Naidis GV (2007) Simulation of convection-stabilized low-current glow and arc discharges in atmospheric-pressure air. Plasma Sour Sci Technol 16:297. https://doi.org/10.1088/0963-0252/16/2/012
Aleksandrov NL, Bazelyan EM (1999) Ionization processes in spark discharge plasmas. Plasma Sour Sci Technol 8:285. https://doi.org/10.1088/0963-0252/8/2/309
Aleksandrov NL, Bazelyan EM, Ponomarev AA, Starikovsky AY (2022) Kinetics of charged species in non-equilibrium plasma in water vapor- and hydrocarbon-containing gaseous mixtures. J Phys D Appl Phys 55:383002. https://doi.org/10.1088/1361-6463/ac7d7c
Capitelli M, Ferreira C, Gordiets B, Osipov A (2000) Plasma Kinetics in Atmospheric Gases. Springer-Verlag, Berlin, Heidelberg
Sakiyama Y, Graves DB, Chang H-W et al (2012) Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J Phys D Appl Phys 45:425201. https://doi.org/10.1088/0022-3727/45/42/425201
Benilov MS, Naidis GV (2003) Modelling of low-current discharges in atmospheric-pressure air taking account of non-equilibrium effects. J Phys D Appl Phys 36:1834. https://doi.org/10.1088/0022-3727/36/15/314
Ono R, Teramoto Y, Oda T (2009) Effect of humidity on gas temperature in the afterglow of pulsed positive corona discharge. Plasma Sour Sci Technol 19:015009. https://doi.org/10.1088/0963-0252/19/1/015009
Komuro A, Ono R, Oda T (2010) Kinetic model of vibrational relaxation in a humid-air pulsed corona discharge. Plasma Sour Sci Technol 19:055004. https://doi.org/10.1088/0963-0252/19/5/055004
Popov NA (2011) Fast gas heating in a nitrogen–oxygen discharge plasma: I. Kinetic mechanism. J Phys D Appl Phys 44:285201. https://doi.org/10.1088/0022-3727/44/28/285201
Popov NA (2001) Investigation of the mechanism for rapid heating of nitrogen and air in gas discharges. Plasma Phys Rep 27:886–896. https://doi.org/10.1134/1.1409722
Aleksandrov NL, Kindysheva SV, Nudnova MM, Starikovskiy AY (2010) Mechanism of ultra-fast heating in a non-equilibrium weakly ionized air discharge plasma in high electric fields. J Phys D Appl Phys 43:255201. https://doi.org/10.1088/0022-3727/43/25/255201
Pintassilgo CD, Guerra V (2015) On the different regimes of gas heating in air plasmas. Plasma Sour Sci Technol 24:055009. https://doi.org/10.1088/0963-0252/24/5/055009
Aubreton J, Elchinger MF, Vinson JM (2009) Transport Coefficients in Water Plasma: Part I: Equilibrium Plasma. Plasma Chem Plasma Process 29:149–171. https://doi.org/10.1007/s11090-008-9165-8
D’Angola A, Colonna G, Bonomo A et al (2012) A phenomenological approach for the transport properties of air plasmas. Eur Phys J D 66:205. https://doi.org/10.1140/epjd/e2012-30147-8
Akishev Y, Grushin M, Karalnik V et al (2010) On basic processes sustaining constricted glow discharge in longitudinal N2 flow at atmospheric pressure. J Phys D Appl Phys 43:215202. https://doi.org/10.1088/0022-3727/43/21/215202
Lowke JJ (1979) Simple theory of free-burning arcs. J Phys D Appl Phys 12:1873. https://doi.org/10.1088/0022-3727/12/11/016
Schwartz AJ, Ray SJ, Elish E et al (2012) Visual observations of an atmospheric-pressure solution-cathode glow discharge. Talanta 102:26–33. https://doi.org/10.1016/j.talanta.2012.07.096
Sirotkin NA, Titov VA (2017) Transfer of liquid cathode components to the gas phase and their effect on the parameters of the atmospheric pressure DC discharge. Plasma Chem Plasma Process 37:1475–1490. https://doi.org/10.1007/s11090-017-9840-8
Moon DE, Webb MR (2020) Imaging studies of emission and laser scattering from a solution-cathode glow discharge. J Anal At Spectrom 35:1859–1867. https://doi.org/10.1039/D0JA00134A
Sirotkin NA, Titov VA (2018) Experimental study of heating of a liquid cathode and transfer of its components into the gas phase under the action of a DC discharge. Plasma Phys Rep 44:462–467. https://doi.org/10.1134/S1063780X18040086
Hagelaar GJM, Pitchford LC (2005) Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sour Sci Technol 14:722. https://doi.org/10.1088/0963-0252/14/4/011
Komuro A, Ono R (2014) Two-dimensional simulation of fast gas heating in an atmospheric pressure streamer discharge and humidity effects. J Phys D Appl Phys 47:155202. https://doi.org/10.1088/0022-3727/47/15/155202
Silva MG, Crispim LWS, Ballester MY (2022) Modeling spark-plug discharge in humid air. AIP Adv 12:025008. https://doi.org/10.1063/5.0080162
Morgan database, www.lxcat.net.
Losev S, Makarov V, Nikolsky V (1994) Thermochemical nonequilibrium kinetic models in strong shock waves on air. In: 6th Joint thermophysics and heat transfer conference. American Institute of Aeronautics and Astronautics
da Silva ML, Guerra V, Loureiro J (2007) Two-temperature models for nitrogen dissociation. Chem Phys 342:275–287. https://doi.org/10.1016/j.chemphys.2007.10.010
Ferziger J, Perić M (2002) Computational Methods for Fluid Dynamics. Springer-Verlag, Berlin, Heidelberg
Mezei P, Cserfalvi T, Csillag L (2005) The spatial distribution of the temperatures and the emitted spectrum in the electrolyte cathode atmospheric glow discharge. J Phys D: Appl Phys 38:2804. https://doi.org/10.1088/0022-3727/38/16/010
Bruggeman P, Liu J, Degroote J et al (2008) Dc excited glow discharges in atmospheric pressure air in pin-to-water electrode systems. J Phys D Appl Phys 41:215201. https://doi.org/10.1088/0022-3727/41/21/215201
Xiong Q, Yang Z, Bruggeman PJ (2015) Absolute OH density measurements in an atmospheric pressure dc glow discharge in air with water electrode by broadband UV absorption spectroscopy. J Phys D Appl Phys 48:424008. https://doi.org/10.1088/0022-3727/48/42/424008
Nikiforov A, Xiong Q, Britun N et al (2011) Absolute concentration of OH radicals in atmospheric pressure glow discharges with a liquid electrode measured by laser-induced fluorescence spectroscopy. Appl Phys Express 4:026102. https://doi.org/10.1143/APEX.4.026102
Lu X, Leipold F, Laroussi M (2003) Optical and electrical diagnostics of a non-equilibrium air plasma. J Phys D Appl Phys 36:2662. https://doi.org/10.1088/0022-3727/36/21/011
Mezei P, Cserfalvi T (2007) Charge densities in the electrolyte cathode atmospheric glow discharges (ELCAD). Eur Phys J Appl Phys 40:89–94. https://doi.org/10.1051/epjap:2007123
Verreycken T, Schram DC, Leys C, Bruggeman P (2010) Spectroscopic study of an atmospheric pressure dc glow discharge with a water electrode in atomic and molecular gases. Plasma Sour Sci Technol 19:045004. https://doi.org/10.1088/0963-0252/19/4/045004
Prevosto L, Kelly H, Mancinelli B et al (2015) On the physical processes ruling an atmospheric pressure air glow discharge operating in an intermediate current regime. Phys Plasmas 22:023504. https://doi.org/10.1063/1.4907661
Aleksandrov NL, Bazelyan EM, Kochetov IV, Dyatko NA (1997) The ionization kinetics and electric field in the leader channel in long air gaps. J Phys D Appl Phys 30:1616. https://doi.org/10.1088/0022-3727/30/11/011
Chernyi G, Losev S, Macheret S, Potapkin B (2002) Plasma chemical reactions (P Models). In: Physical and chemical processes in gas dynamics: cross sections and rate constants, Volume I. American Institute of Aeronautics and Astronautics, pp 237–292
Golubkov GV, Ozerov GK (2014) The near-threshold associative ionization N(2D) + O(3P) → NO+(X1Σ+) + e− reaction. Dokl Phys 59:122–125. https://doi.org/10.1134/S1028335814030094
Ringer G, Gentry WR (1979) A merged molecular beam study of the endoergic associative ionization reaction N(2D) + O(3P) → NO+ + e−. J Chem Phys 71:1902–1909. https://doi.org/10.1063/1.438545
Padellec AL (2005) Partial near threshold cross sections for the associative ionization to form CO+, NO+ and O2+. Phys Scr 71:621. https://doi.org/10.1088/0031-8949/71/6/008
Matveyev AA, Silakov VP (1999) Theoretical study of the role of ultraviolet radiation of the non-equilibrium plasma in the dynamics of the microwave discharge in molecular nitrogen. Plasma Sour Sci Technol 8:162. https://doi.org/10.1088/0963-0252/8/1/019
Brunet H, Rocca-Serra J (1985) Model for a glow discharge in flowing nitrogen. J Appl Phys 57:1574–1581. https://doi.org/10.1063/1.334473
Kossyi IA, Kostinsky AY, Matveyev AA, Silakov VP (1992) Kinetic scheme of the non-equilibrium discharge in nitrogen–oxygen mixtures. Plasma Sour Sci Technol 1:207. https://doi.org/10.1088/0963-0252/1/3/011
Hellberg F, Rosén S, Thomas R et al (2003) Dissociative recombination of NO+: dynamics of the X 1Σ+ and a 3Σ+ electronic states. J Chem Phys 118:6250–6259. https://doi.org/10.1063/1.1557917
Mintoussov EI, Pendleton SJ, Gerbault FG et al (2011) Fast gas heating in nitrogen–oxygen discharge plasma: II. Energy exchange in the afterglow of a volume nanosecond discharge at moderate pressures. J Phys D Appl Phys 44:285202
Neau A, Al Khalili A, Rosén S et al (2000) Dissociative recombination of D3O+ and H3O+: absolute cross sections and branching ratios. J Chem Phys 113:1762–1770. https://doi.org/10.1063/1.481979
Tsang W, Hampson RF (1986) Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds. J Phys Chem Ref Data 15:1087–1279. https://doi.org/10.1063/1.555759
Baulch DL, Cobos CJ, Cox RA et al (1992) Evaluated kinetic data for combustion modelling. J Phys Chem Ref Data 21:411. https://doi.org/10.1063/1.555908
Javoy S, Naudet V, Abid S, Paillard CE (2003) Elementary reaction kinetics studies of interest in H2 supersonic combustion chemistry. Exp Thermal Fluid Sci 27:371–377. https://doi.org/10.1016/S0894-1777(02)00241-8
Pontiga F, Soria C, Castellanos A, Skalny JD (2002) A study of ozone generation by negative corona discharge through different plasma chemistry models. Ozone Sci Eng 24:447–462. https://doi.org/10.1080/01919510208901634
Baulch DL, Cox RA, Crutzen PJ et al (1982) Evaluated kinetic and photochemical data for atmospheric chemistry: supplement I CODATA task group on chemical kinetics. J Phys Chem Ref Data 11:327–496. https://doi.org/10.1063/1.555664
Peyrous R, Pignolet P, Held B (1989) Kinetic simulation of gaseous species created by an electrical discharge in dry or humid oxygen. J Phys D Appl Phys 22:1658. https://doi.org/10.1088/0022-3727/22/11/015
Tsang W, Herron JT (1991) Chemical kinetic data base for propellant combustion I. Reactions involving NO, NO2, HNO, HNO2, HCN and N2O. J Phys Chem Ref Data 20:609–663. https://doi.org/10.1063/1.555890
Varga T, Olm C, Nagy T et al (2016) Development of a joint hydrogen and syngas combustion mechanism based on an optimization approach. Int J Chem Kinet 48:407–422. https://doi.org/10.1002/kin.21006
Baulch DL, Cobos CJ, Cox RA et al (1994) Evaluated kinetic data for combustion modeling. supplement I. J Phys Chem Ref Data 23:847–848. https://doi.org/10.1063/1.555953
Cheskis SG, Nadtochenko VA, Sarkisov OM (1981) Study of the HNO+HNO and HNO+NO reactions by intracavity laser spectroscopy. Int J Chem Kinet 13:1041–1050. https://doi.org/10.1002/kin.550131005
Su M-C, Kumaran SS, Lim KP et al (2002) Rate constants, 1100≤T≤2000 K, for H+NO2→OH+NO using two shock tube techniques: comparison of theory to experiment. J Phys Chem A 106:8261–8270. https://doi.org/10.1021/jp0141023
Karkach SP, Osherov VI (1999) Ab initio analysis of the transition states on the lowest triplet H2O2 potential surface. J Chem Phys 110:11918–11927. https://doi.org/10.1063/1.479131
Tochikubo F, Uchida S, Watanabe T (2004) Study on decay characteristics of oh radical density in pulsed discharge in Ar/H2O. Jpn J Appl Phys 43:315. https://doi.org/10.1143/JJAP.43.315
Smith CA, Molina LT, Lamb JJ, Molina MJ (1984) Kinetics of the reaction of OH with pernitric and nitric acids. Int J Chem Kinet 16:41–55. https://doi.org/10.1002/kin.550160107
Yang H, Gardiner WC, Shin KS, Fujii N (1994) Shock tube study of the rate coefficient of H+O2→OH+O. Chem Phys Lett 231:449–453. https://doi.org/10.1016/0009-2614(94)01288-1
Dean AM, Bozzelli JW (2000) Combustion Chemistry of Nitrogen. In: Gardiner WC (ed) Gas-Phase Combustion Chemistry. Springer, New York, NY, pp 125–341
Krivonosova O, Losev S, Nalivaiyko V (1987) Advisable data on rate constants of chemical reactions between molecules consisting from N and O atoms. In: Smirnov B (ed) Plasma Chemistry. Energoatomizdat, Moscow, p 3
Piper LG (1988) State-to-state N2(A3∑+u) energy pooling reactions. II. The formation and quenching of N2(B3Πg, v′=1–12). J Chem Phys 88:6911–6921. https://doi.org/10.1063/1.454388
Herron JT (1999) Evaluated Chemical Kinetics Data for Reactions of N(2D), N(2P), and N2(A 3Σ+u) in the Gas Phase. J Phys Chem Ref Data 28:1453–1483. https://doi.org/10.1063/1.556043
Smirnov SA, Shutov DA, Bobkova ES, Rybkin VV (2015) Physical parameters and chemical composition of a nitrogen dc discharge with water cathode. Plasma Chem Plasma Process 35:639–657. https://doi.org/10.1007/s11090-015-9626-9
Gordiets BF, Ferreira CM, Guerra VL et al (1995) Kinetic model of a low-pressure N2–O2 flowing glow discharge. IEEE Trans Plasma Sci 23:750–768. https://doi.org/10.1109/27.467998
Piper LG (1987) Quenching rate coefficients for N2(a′1Σ−u). J Chem Phys 87:1625–1629. https://doi.org/10.1063/1.453223
Shkurenkov I, Burnette D, Lempert WR, Adamovich IV (2014) Kinetics of excited states and radicals in a nanosecond pulse discharge and afterglow in nitrogen and air*. Plasma Sour Sci Technol 23:065003. https://doi.org/10.1088/0963-0252/23/6/065003
Pancheshnyi SV, Starikovskaia SM, Starikovskii AYu (2000) Collisional deactivation of N2(C3Πu, v=0, 1, 2, 3) states by N2, O2, H2 and H2O molecules. Chem Phys 262:349–357. https://doi.org/10.1016/S0301-0104(00)00338-4
Baulch DL, Cox RA, Hampson RF Jr et al (1980) Evaluated kinetic and photochemical data for atmospheric chemistry. J Phys Chem Ref Data 9:295–472. https://doi.org/10.1063/1.555619
Filseth SV, Stuhl F, Welge KH (1970) Collisional Deactivation of O(1S). J Chem Phys 52:239–243. https://doi.org/10.1063/1.1672671
Smirnov B (1978) Negative ions. Atomizdat, Moscow
Howard CJ, Fehsenfeld FC, McFarland M (1974) Negative ion-molecule reactions with atomic hydrogen in the gas phase at 296 K. J Chem Phys 60:5086–5089. https://doi.org/10.1063/1.1681027
Schofield K (1973) Evaluated Chemical Kinetic Rate Constants for Various Gas Phase Reactions. J Phys Chem Ref Data 2:25–84. https://doi.org/10.1063/1.3253112
Botner M, Baurer T (1979) Defense Nuclear Agency Reaction Rate Handbook. Second Edition. Revision Number 7. https://apps.dtic.mil/sti/citations/ADA062474.
Ikezoe Y, Matsouka S, Takebe M, Viggiano A (1987) Gas phase ion-molecule reaction rate constants through 1986. Maruzen Company Ltd, Tokyo
Anicich VG (1993) Evaluated bimolecular ion-molecule gas phase kinetics of positive ions for use in modeling planetary atmospheres, cometary comae, and interstellar clouds. J Phys Chem Ref Data 22:1469–1569. https://doi.org/10.1063/1.555940
Eichwald O, Yousfi M, Hennad A, Benabdessadok MD (1997) Coupling of chemical kinetics, gas dynamics, and charged particle kinetics models for the analysis of NO reduction from flue gases. J Appl Phys 82:4781–4794. https://doi.org/10.1063/1.366336
Sieck LW, Heron JT, Green DS (2000) Chemical kinetics database and predictive schemes for humid air plasma chemistry. Part I: positive ion-molecule reactions. Plasma Chem Plasma Process 20:235–258
Gaens WV, Bogaerts A (2013) Kinetic modelling for an atmospheric pressure argon plasma jet in humid air. J Phys D Appl Phys 46:275201. https://doi.org/10.1088/0022-3727/46/27/275201
Acknowledgements
This work was supported by grants from the Universidad Tecnológica Nacional (PID 8460 and PID 8461) and CONICET (PIP CONICET 11220200100459CO). L. P. and B. J. G. are members of the CONICET. E. C. and J. C. C. thanks CONICET for their postdoctoral fellowship. M. G. F. and B. S. thanks CONICET for their doctoral fellowships.
Funding
Universidad Tecnológica Nacional, PID 8460 and PID 8461, Consejo Nacional de Investigaciones Científicas y Técnicas, PIP 11220200100459CO.
Author information
Authors and Affiliations
Contributions
M.G.F., B.S., and L.P. conceived, planned, and carried out the electrical experiments; M.G.F., J.C.C, and B.J.G. conceived, planned, and carried out the optical experiments; M.G.F, E.C., and L.P. developed the theoretical formalism; M.G.F., E.C., and B.S. developed, performed, and carried out the numerical simulations; M.G.F., E.C. and L.P., contributed to the interpretation of the results; writing—original draft preparation, M.G.F, and E.C.; writing—review and editing, M.G.F., E.C., and L.P.; project administration, L.P.; funding acquisition, L.P.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
See Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferreyra, M.G., Cejas, E., Santamaría, B. et al. Numerical Simulation of the Ionic Composition and Ionization Phenomena in the Positive Column of a Millisecond DC-Pulsed Glow-Type Discharge in Atmospheric Pressure Air with a Water-Cathode. Plasma Chem Plasma Process (2024). https://doi.org/10.1007/s11090-024-10496-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11090-024-10496-2