Abstract
Afterglows of R/x(N2–5%H2) (R = Ar or He) flowing microwave discharges are characterized by optical emission spectroscopy. Absolute densities of N atoms and N2(A) and N2(X,v > 13) molecules and estimated densities of NH and H atoms are determined after calibration of the N atom density by NO titration. It is determined the variations of active species densities along the post-discharge tube from the early afterglow to the late afterglow region. Conditions allowing to obtain a high concentration of H-atoms in dominant N-atoms have been found in the early afterglow region of the R/x(N2–5%H2) mixture for x < 5%. From these densities, the destruction probabilities of the H-atoms on the afterglow quartz tube wall is found to be: \(\gamma_{H}^{He}\) = (2–3) × 10−4 and \(\gamma_{H}^{Ar}\) = (3–4) × 10−3. The interest of these results concerns the enhancement of surface nitriding by combined effects of N and H atoms inclusion in afterglow conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Afterglows of N2 flowing microwave discharges have previously been studied at medium gas pressures (1–20 Torr) for the sterilization of medical instruments by N-atoms [1, 2]. Recently [3, 4], it has been observed an enhanced nitriding of TiO2 films in the flowing afterglow of N2/2%H2 microwave and RF plasmas, which was attributed to the presence of H and N atoms. Passivation of InGaN/GaN nanowires [5] and treatment of graphene films [6, 7] have also been studied in N2 microwave afterglows.
In the present study, flowing afterglows produced by R/x%(N2–5%H2) (R = Ar or He) microwave plasmas have been studied by emission spectroscopy. Intensities emitted by the 11–7 band of the N2 first positive system (1+) at 580 nm (I580) and by the 1–0 band of the N2 second positive system (2+) at 316 nm (I316) were measured to obtain the absolute concentrations of N-atoms, N2(A) and N2(X,v > 13) after NO titration to calibrate the N-atom density. From these measurements, NH and H-atom densities have been estimated by choosing the appropriated kinetic reactions at the origin of the NH 336 nm emission.
Experimental Set-Up
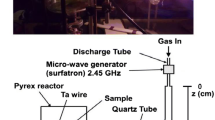
The used experimental setup is reported in Fig. 1 [1, 2, 8,9,10].
Ar/N2 and He/N2 microwave discharges are produced by a surfatron cavity operating at 2.45 GHz. In these mixtures, the plasma length was found to extend between 2 and 20 cm after the surfatron gap, depending on the N2 amount and on the HF power.
With a quartz discharge tube of 5 mm i.d. and 20 cm in length, connected to a bent post-discharge tube of 18 mm i.d. and 50 cm in length, the residence time of the afterglow at z = 3 cm after the beginning of the 18 mm post-discharge tube is 10−3 s.
A pre-mixed N2–5%H2 gas can be introduced instead of N2 to produce NH radicals and H-atoms in addition to the N2 active species.
In a previous work [11], first results were presented for the same R/x(N2–5%H2) (R = Ar or He) gas mixtures with x ≥ 20%. The present paper focuses on early afterglow conditions (z = 3 cm) and low N2–5%H2 percentages (x = 2–10%).
Constant operating conditions were used, with a total gas flow rate Qtotal = 1.0 slpm, a pressure of 8 Torr and an injected microwave power PMW = 150 W, that previously allow obtaining high concentrations of active species in the late afterglows of N2/He and N2/H2 mixtures [11].
TiO2 samples can eventually be exposed (heated or not) in a 5 l Pyrex reactor following the afterglow quartz tube (results not shown in this paper).
Optical emission spectroscopy (OES) along the afterglow tubes was performed using an optical fibre connected to an Acton Spectra Pro 2500i spectrometer (grating 600 g/mm), equipped with a Pixis 256E CCD detector (front illuminated 1024 × 256 pixels).
Active Species Densities in R/2-10%(N2–5%H2) Afterglows (R = Ar or He)
Table 1 gathers the kinetic reactions used in this work, with the corresponding rate coefficients and their origin in the literature.
N-Atom, N2(A), N2 + and N2(X, v > 13) Densities
In flowing afterglows, absolute N atom densities can be obtained by NO titration, a method extensively described in [8,9,10, 22] and well suited to the study of N2 late afterglows between 5 and 10 Torr. In this pressure range, a good agreement has been found for absolute N-atom densities measured using the NO titration method and using the non-intrusive TALIF (Two-photon Absorption Laser-Induced Fluorescence) method [23].
In full late afterglow conditions, the N2 (1+) (11–7) band emission at 580 nm (I580) is produced by the 3-body recombination process of N atoms:
In the early afterglow, here defined as the afterglow region lying between the pink and the late afterglow, only a fraction (aN+N, with 0 ≤ aN+N ≤ 1) of the I580 emission is due to the process (a), the remaining fraction being caused by collisions between high vibrationally excited levels of the ground molecular state N2(X,v > 13) and metastable states N2(A):
In consequence, in the early afterglow region and neglecting a possible quenching of the N2(B,v′ = 11) level by H2 molecules, the N atom concentration can be related to the aN+N fraction of the observed I580 emission through the proportionality relation:
with \(k_{1} = c_{580} \frac{hc}{580}A_{{{\text{A}},7}}^{{{\text{B}},11}} \frac{{k_{a} [M]}}{{\left( {\upsilon_{{N_{2} \left( {{\text{B}},11} \right)}}^{R} + [R]k_{{N_{2} \left( {{\text{B}},11} \right)}}^{{Q_{R} }} + [N_{2} ]k_{{N_{2} \left( {{\text{B}},11} \right)}}^{{Q_{{N_{2} }} }} } \right)}}\) where \([M] = \frac{p}{kT}\) = 2.6 × 1017 cm−3 at p = 8 Torr and T = 300 K, \(c_{580}\) is the spectral response of the spectral intensity acquisition system at 580 nm, \(A_{{{\text{A}},7}}^{{{\text{B}},11}}\) is the vibrational transition probability of the N2(1+, 11–7) band, \(k_{a}\) is the rate coefficient for reaction (a), \(\upsilon_{{N_{2} \left( {{\text{B}},11} \right)}}^{R}\) and \(k_{{N_{2} \left( {{\text{B}},11} \right)}}^{{Q_{i} }}\) are respectively the radiative loss frequency and the quenching rates of the N2(B,v″ = 11) level by species i (i = R or N2).
As reported by Ricard et al. [11, 13], the N2(A) and N2(X,v > 13) densities can be deduced from the N-atom density by line ratio methods. Assuming that the following reation (c) is the dominant process for the production of the N2(C,v′ = 1) level:
Using the same definitions than above, it comes for the \(I_{316}\) intensity:
with \(k_{2} = c_{316} \frac{hc}{316}A_{{{\text{B}},0}}^{{{\text{C}},1}} \frac{{k_{c} }}{{\left( {\upsilon_{{N_{2} \left( {{\text{C}},1} \right)}}^{R} + [R]k_{{N_{2} \left( {{\text{C}},1} \right)}}^{{Q_{R} }} + [N_{2} ]k_{{N_{2} \left( {{\text{C}},1} \right)}}^{{Q_{{N_{2} }} }} } \right)}}\)
The N2(A) density can then be deduced from the absolute [N] concentration and the measured \(\frac{{I_{580} }}{{I_{316} }}\) band intensity ratio, following:
where \(k_{3} = \frac{{k_{1} }}{{k_{2} }}\) = 2.5(± 0.5) × 10−7 in Ar/> 10%N2 and He/> 10%N2 and \(k_{3}\) = 4 × 10−7 in Ar/(2–5%)N2, with \(\frac{{c_{580} }}{{c_{316} }}\) = 7 [8,9,10].
Assuming now that in the early afterglow, N2(B,v = 11) levels can also be produced by reactions (b), it comes:
where [N2(X,v > 13)] is the sum of the densities of the N2(X,v) vibrational levels conducing to the exothermicity of reaction (b), happening for v > 13. It is calculated \(k_{4} = \frac{{k_{1} }}{{k_{b} }}\) = 7 × 10−6 in Ar/> 2%N2 and in He/> 80%N2, slowly decreasing to \(k_{4}\) = 5 × 10−6 in He/(60–80%)N2, \(k_{4}\) = 3 × 10−6 in He/(10–40%)N2 and \(k_{4}\) = 2 × 10−6 in He/(2–5%)N2.
Considering that the N2+ (1−) (0–0) band emission at 391 nm (I391) is produced by the following processes:
the N2+ ion concentration can be deduced from the \(\frac{{I_{391} }}{{I_{316} }}\) band intensity ratio, following the equation:
with \(k_{6} = c_{391} \frac{hc}{391}A_{{{\text{X}},0}}^{{{\text{B}},0}} \frac{{k_{d} }}{{\left( {\upsilon_{{N_{2}^{ + } \left( {{\text{B}},0} \right)}}^{R} + [R]k_{{N_{2}^{ + } \left( {{\text{B}},0} \right)}}^{{Q_{R} }} + [N_{2} ]k_{{N_{2}^{ + } \left( {{\text{B}},0} \right)}}^{{Q_{{N_{2} }} }} } \right)}}\) k5 is found to decrease from 8.5 to 4 × 10−2 in Ar/x%N2 and from 12 to 4 × 10−2 in He/x%N2 when x increases from 20 to 100% [11].
Uncertainties in species densities estimation by this line ratio methods are 30% for the N-atoms (only caused by errors made during NO titration, see for example the interesting discussion in [22]) and at least 50% for N2(A), where additional sources of errors are related to the determination of the \(a_{N + N}\) parameter and to uncertainties in the rate coefficients of Table 1. For N2(X,v > 13) and N2+, only the order of magnitude is expected because of the uncertainty increase linked to the use of the \(\frac{{a_{N + N} }}{{1 - a_{N + N} }}\) ratio.
NH Radical and H-Atom Densities
As previously stated in [8,9,10], it is estimated that the NH(A,v = 0) radiative state is produced by the exothermic reaction (e), due to the endothermicity of the 3 body recombination N + H + M → NH(A,v = 0) + M:
The intensity ratio method above developed can be applied to determine the absolute NH radical density, conducing to:
with \(k_{7} = c_{336} \frac{hc}{336}A_{{{\text{X}},0}}^{{{\text{A}},0}} \frac{{k_{e} }}{{\left( {\upsilon_{{NH\left( {{\text{A}},0} \right)}}^{R} + [R]k_{{NH\left( {{\text{A}},0} \right)}}^{{Q_{R} }} + [N_{2} ]k_{{NH\left( {{\text{A}},0} \right)}}^{{Q_{{N_{2} }} }} } \right)}}\)
Using \(\frac{{c_{580} }}{{c_{336} }}\) = 5 [8,9,10], \(A_{{{\text{X}},0}}^{{{\text{A}},0}} = \upsilon_{{NH\left( {{\text{A}},0} \right)}}^{R}\) and \(k_{e}\) = 5 × 10−11 cm3 s−1 (chosen to be equal to the rates of the N2(X,v > 13) + N2(A) → N2 + N2(B,11) and N2(X,v > 13) + N2+ → N2 + N2+(B) exothermic reactions [13, 18]), it is calculated \(k_{8 }\) = 1.3 × 10−7 in pure N2 and in Ar/> 40%N2, \(k_{8}\) = 3.0 × 10−7 in Ar/< 40%N2 and \(k_{8}\) = 2.0 (± 0.5) × 10−7 in He/> 2%N2.
The H-atom and NH radical densities are related by the following kinetics:
Using the pseudo-stationary approximation and considering the kf rate coefficient independent of the third body (\(k_{f}^{{N_{2} }} = k_{f}^{Ar} = k_{f}^{He} )\), it comes at 8 Torr:
Figure 2 reproduces spectra measured at z = 3 cm in the Ar/2%(N2–5%H2) mixture at 8 Torr, 1 slpm and 150 W.
In Fig. 2a, the NH (0–0) and (1–1) bands at respectively 336 and 337 nm are the most intense emissions. It is also observed the N2 2+ (1–0) band at 316 nm, the NO β (0–8) band at 320 nm and the N2+ (0–0) band at 391 nm, close to the CN violet bands between 385 and 388 nm. For data treatment, the NH (1–1) band at 337 nm has been discarded because of possible mixing with the N2 2+ (0–0) band at 337 nm. The I336 intensity is thus measured from the half intensity of the I336 and I337 band junction.
As previously shown by considering the I11/I9 ratio, the aN+N coefficient can be deduced from the vibrational distribution observed in Fig. 2b [11]. With the experimental conditions of Fig. 2, it is found the value aN+N = 0.35 and after calibration by NO titration, it is deduced [N] = 1.0 × 1015 cm−3.
In previous studies [11], the NH and H densities were obtained in N2/2.5%H2, Ar/50%(N2–5%H2) and He/80%(N2–5%H2) gas mixtures. For N2/2.5%H2 and Ar/50%(N2–5%H2), it was found a [H]/2[H2] dissociation rate of about 0.3%. In the He/80%(N2–5%H2) mixture, the hydrogen dissociation rate was found to be higher (1.0%) and it was obtained a [H]/[N] ratio of about 30%.
According to Eq. (7), the accuracy of the determination of the [NH] density largely depends on the accuracy of the [N2(X,v > 13)] density (limited to the order of magnitude) and on the choice of the ke rate coefficient for reaction (e), chosen to be similar to the one of the N2(X,v > 13) + N2(A) → N2 + N2(B,11) and N2(X,v > 13) + N2+ → N2 + N2+(B) exothermic reactions [11].
As the H density is related to the NH density through Eq. (8) and to the kf rate coefficients of the 3-body recombination N + H + M → NH + M, it is clear that the presented absolute densities of NH radicals and H atoms are highly speculative. Nevertheless, their relative variations with R(He or Ar) and with the dilution x of the (N2–5%H2) mixture in the rare gas remain significant.
Table 2 compares the aN+N coefficients and the absolute densities obtained at z = 3 cm using the developed line ratio methods in the He/x(N2–5%H2) and the Ar/x(N2–5%H2) mixtures, for x varying between 2 and 10% (8 Torr, 1 slpm, 150 Watt).
With He and for x < 20%, the accuracy of the abacus given in Fig. 3a [7] and showing the variation of the I11/I9 ratio is too low and it has been chosen to use instead the I11/I10 ratio, given in Fig. 3b.
At high dilutions (2% and 5%), N-atom, N2(A), N2+ and N2(X,v > 13) densities are higher in Ar/x(N2–5%H2) mixtures than in He/x(N2–5%H2) mixtures. At lower dilutions (20–50%), densities of N2+ and N2(X,v > 13) are also higher in the Ar mixture than in the He mixture.
Whatever the buffer gas (R = Ar or He), nitrogen and hydrogen dissociation rates are the highest in highly diluted mixtures (x = 2%). The nitrogen dissociation rate, which is usually less than 1% in pure N2 afterglows [8,9,10], increases by one order of magnitude with high dilution in Ar, to reach 10% in the Ar/2%(N2–5%H2) mixture.
The H-atom concentration is minimum in medium dilution mixtures (5–10%), while the N-atom concentration is less dilution dependent. As produced by the 3-body reaction (f), the NH density also shows a minimum for (5–10%) mixtures.
Destruction of H-Atoms on the Quartz Tube Wall
From values reported in Table 3 for the Ar/2%(N2–5%H2) afterglow, it is deduced that the N–atom density remains practically constant along the dia.18 mm quartz afterglow tube between 3 and 35 cm (10−3 to 10−2 s). At the contrary, the H-atom density shows a sensitive decrease, which is the result of a significant destruction on the tube wall.
Assuming that at 8 Torr, H-atoms losses in the afterglow are only due to wall recombination through H + H + wall → H2 + wall and H + N + wall → NH + wall processes, the global \(\gamma_{H}\) destruction probability on the quartz tube walls can be calculated, as demonstrated in [2] for the N atoms. It is written:
with \(\nu_{H} = \gamma_{H} \frac{\left\langle v \right\rangle }{2r}\), where \(\left\langle v \right\rangle\) is the thermal gas velocity (\(\left\langle v \right\rangle\) = 5 × 104 cm s−1 at 300 K) and r is the tube radius
z = 0 being taken at the beginning of the afterglow in the dia.18 mm tube (Fig. 1), it is obtained \(\gamma_{H}^{Ar}\) = 4 × 10−3
As reported in Table 4 for 2% ≤ x ≤ 10% in He/x(N2–5%H2) mixtures, the N-atom density also shows a nearly constant value between the early and the late afterglow.
[N2(A)] and [N2+] densities decrease sharply between z = 3 cm and z = 40 cm while the [N2(X,v > 13)] density increases. The high concentrations of [N2(X,v > 13)] obtained for z ≥ 20 cm are related to low densities of [N2(A)], resulting of dominant loss frequencies of N2(X,v > 13) and N2(A) densities produced by reaction (b) where kb = 4 × 10−11 cm3 s−1 [12].
Using Eq. (8), a global destruction probability of \(\gamma_{H}^{He}\) = 2.5(± 1.0) × 10−4 is obtained in He/x(N2–5%H2) mixtures. It has been observed that \(\gamma_{H}^{He}\) kept a nearly constant value for x = 2–90%. The obtained order of magnitude is in good agreement with values \(\gamma_{H}^{{H_{2} }}\) = 7 × 10−4 and \(\gamma_{H}^{{N_{2} /H_{2} }}\) = 4 × 10−4 previously reported in H2 and a N2–90%H2 gas mixture [24]. It appears that the \(\gamma_{H}^{Ar}\) value in the Ar/2%(N2–5%H2) gas mixtures is higher by about one order of magnitude. In comparison, the \(\gamma_{N}^{{N_{2} }}\) value of the N-atoms destruction probability on quartz walls in pure N2 is in the range 10−5–10−4 [2, 23], decreased by a factor 2 when 1% H2 is introduced into N2 [24].
The \(\gamma_{N}^{{N_{2} }}\) value is thus about one order of magnitude lower than \(\gamma_{H}^{He}\) and two orders of magnitude lower than \(\gamma_{H}^{Ar}\).
Conclusion
Early afterglows of R/x%(N2–5%H2) (R = Ar or He) gas mixtures have been studied to obtain the absolute densities of N-atoms, N2(A) and N2(X,v > 13) metastable molecules by line intensity ratio methods, after calibration of the N-atom density by NO titration. The line intensity ratio method also allowed estimating the density of NH radicals and H-atoms.
For this evaluation, a rate coefficient of 5 × 10−11 cm3 s−1 has been considered for the reaction N2(X,v > 13) + NH → N2 + NH(A,v = 0) and a rate coefficient of 5 × 10−32 cm6 s−1 for the N + H + M → NH + M 3-body recombination.
Such values conduce to high dissociation rates of H2 and N2 in R/x%(N2–5%H2) early afterglows with R = Ar or He ([H]/2[H2] = 15% with Argon and 6% with He) for x < 5% at 8 Torr, 1 slpm, 150 W.
Using H-atoms density profiles along the afterglow quartz tubes, the global H-atom destruction probability \(\gamma_{H}^{R}\) was calculated in the different R/x%(N2–5%H2) gas mixtures.
It is found a value \(\gamma_{H}^{He}\) = 2.5(± 1.0) × 10−4 in the He/(2–90)%(N2–5%H2) mixtures, one order of magnitude lower than the \(\gamma_{H}^{Ar}\) = 4 × 10−3 value obtained in the Ar/2%(N2–5%H2) mixture. The \(\gamma_{H}^{He}\) probability is in good agreement with data published by Gordiets et al. and is higher by one order of magnitude when compared with the \(\gamma_{N}^{{N_{2} }}\) recombination probability.
Appearing to be a rich source of NH radicals and H atoms, accompanying dominant N-atoms, highly diluted R/2%(N2–5%H2) (R = Ar or He) early afterglows are of interest for surface treatments, as already observed for selective surface nitriding of TiO2 films [3, 4] in pure N2 afterglow.
In future works, the N and H atoms absolute densities will be determined by TALIF to compare with the present results obtained by NO titration and the line-ratio intensity method.
Change history
17 July 2019
Unfortunately, the original publication contains errors. The authors would like to correct the errors as given.
References
Villeger S, Sarrette JP, Ricard A (2005) Plasma Process Polym 2:709
Villeger S, Sarrette JP, Rouffet B, Cousty S, Ricard A (2008) Eur Phys J Appl Phys 42:25
Ryu S, Wang Y, Ricard A, Sarrette JP, Kim A, Kim A, Kim YK (2019) Accepted Surf Coat Technol
Wang Y, Ricard A, Sarrette JP, Kim A, Kim YK (2017) Surf Coat Technol 324:243
Ferreira JA, Nguyen HTP, Mi Z, Leonelli R, Stafford L (2014) Nanotechnology 25:435606
Bigras GR, Glad X, Martel R, Sarkissian A, Stafford L (2018) Plasma Sources Sci Technol 27:124004
Bigras GR, Glad X, Vandsburger L, Charpin C, Levesque P, Martel R, Stafford L (2019) Carbon 144:532
Zerrouki H, Ricard A, Sarrette JP (2013) Contrib Plasma Phys 53:599
Zerrouki H, Ricard A, Sarrette JP (2014) Contrib Plasma Phys 54:827
Zerrouki H, Ricard A, Sarrette JP (2014) J Phys Conf Ser 550:012045
Ricard A, Sarrette JP (2019) J Phys Conf Ser. (accepted for publication)
Piper LG (1994) J Chem Phys 101:10229
Ricard A, Oh SG, Guerra V (2013) Plasma Sources Sci Technol 22:035009
Gat E, Gherardi N, Lemoing S, Massines F, Ricard A (1999) Chem Phys Lett 306:263
Anketell J (1977) Can J Phys 55:1134
Laux CO, Kruger CH (1992) JQSRT 48:9
Tellinghuisen JB, Winkler CA, Freeman CG, McEwan MJ, Phillips LF (1972) J Chem Soc Faraday Trans II 68:833
Ricard A, Oh SG (2014) Plasma Sources Sci Technol 23:045009
Hofzumahaus A, Stuhl F (1985) J Chem Phys 82:3152
Brown RL (1973) Int J Chem Kinet 5:663
Tatarova E, Dias FM, Gordiets B, Ferreira CM (2005) Plasma Sources Sci Technol 14:19
Vasina P, Kudrle V, Talsky A, Botos P, Mrazkova M, Mesko M (2004) Plasma Sources Sci Technol 13:668
Rouffet B, Gaboriau F, Sarrette JP (2010) J Phys D Appl Phys 43:185203
Gordiets B, Ferreira CM, Pinheiro MJ, Ricard A (1998) Plasmas Sources Sci Technol 7:363
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ricard, A., Sarrette, J.P. Densities of Active Species in R/x%(N2–5%H2) (R = Ar or He) Microwave Flowing Afterglows. Plasma Chem Plasma Process 39, 1103–1114 (2019). https://doi.org/10.1007/s11090-019-09992-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-09992-7