Abstract
We report a detailed comparison between RF and microwave (HF) plasmas of N2 and Ar–20 %N2 as well as in the corresponding afterglows by comparing densities of active species at nearly the same discharge conditions of tube diameter (5–6 mm), gas pressure (6–8 Torr), flow rate (0.6–1.0 slm) and applied power (50–150 W). The analysis reveals an interesting difference between the two cases; the length of the RF plasma (~25 cm) is measured to be much longer than that of HF (6 cm). This ensures a much longer residence time (10−2 s) of the active species in the N2 RF plasma [compared to that (10−3 s) of HF], providing a condition for an efficient vibrational excitation of N2(X, v) by (V–V) climbing-up processes, making the RF plasma more vibrationally excited than the HF one. As a result of high V–V plasma excitation in RF, the densities of the vibrationally excited N2(X, v > 13) molecules are higher in the RF afterglow than in the HF afterglow. Destruction of N2(X, v) due to the tube wall is estimated to be very similar between the two system as can be inferred from the γv destruction probability of N2(X, v > 3–13) on the tube wall (2–3 × 10−3 for both cases) obtained from a comparison between the density of N2(X, v > 3–9) in the plasmas to that of the N2(X, v > 13) in the long afterglows. Interestingly enough, densities of N-atoms and N2(A) metastable molecules in the afterglow regions, however, are measured to be very similar with each other. The measured lower density of N2 + ions than expected in the HF afterglow is rationalized from a high oxygen impurity in our HF setup since N2 + ions are very sensitive to oxygen impurity .
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
N2 plasmas and corresponding afterglows have been intensively studied [1–5] with an interest in utilizing excited nitrogen species in various applications such as plasma sterilization [6–8], plasma cleaning [9–11] and surface nitridation [12–14]. Under a controlled flowing condition, an inherent nature of a longer lifetime of a certain excited N2 species makes it possible to obtain an ‘afterglow’ region with a varying density of N-atoms, N2(X, v > 13) and N2(A) metastable molecules and N2 + ions [1]. This condition may provide a chance of developing a ‘damage-free’ plasma process in diverse application fields [7, 14].
In this respect, the exact determination of active species density in the plasma as well as in the afterglow would be a first step toward a successful use of them in application. RF and microwave (HF) plasmas in N2 and Ar–N2 have been previously studied and the detailed densities of active species in the afterglow under a flowing condition have been carefully measured at two different locations of Ajou University and University of Toulouse [1, 2]. The densities of N-atoms, N2(X, v > 13) and N2(A) metastable molecules and N2 + ions were determined using the line ratio method [1, 15] and the calibration of the N-atom density by NO titration [3, 4, 16]. These recent studies revealed important characteristics of the two plasma systems, especially in terms of plasma length, plasma temperatures and the changing densities of active species in the plasmas and the afterglows, which were explained as a result of complex kinetic processes of the active species in the N2 plasmas [1, 2, 4, 5, 16–21]. Recently, we have also reported detailed variation of active species in the RF plasmas and their afterglows of N2 and Ar–20 %N2 [5]. Despite such vast reports on the detailed characteristics of the N2 and Ar–N2 plasmas, a comprehensive comparison of plasma systems with different sources of plasma power (such as RF and HF) is still missing in the literature, while it can be an invaluable information for choosing the right source of plasma power in specific applications such as medical instruments sterilization [7] and iron nitriding [14] in N2 afterglows. Luckily enough, we have accumulated enough experimental data set for a quantitative comparison of the two plasma systems of RF and HF for the case of N2 plasma obtained under very similar flowing conditions. Based on this motivation, here we present our recent analysis on the differences between the two plasmas (RF and HF) and their afterglows in terms of plasma rotational and vibrational temperatures and densities of the active nitrogen species in the respective afterglows of mixed pink afterglow (PA) and late afterglow (LA). The afterglows are compared at the residence times of 7 × 10−2 s for the RF and 10−3 to 6 × 10−2 s for the HF. We also report the destruction probability of vibrationally excited N2(X, v) molecules on the RF and HF afterglow tube walls.
Experimental Setups
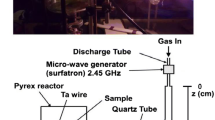
The two experimental setups of RF and HF plasmas in Ajou and Toulouse, respectively, are reproduced in Fig. 1 for comparison.
The discharge quartz tubes have nearly the same inner diameters (ID) of 6 mm for the RF plasma reactor and 5 mm for the HF plasma reactor and have a length of 30 cm in both cases. In the post-discharge region, the RF discharge tube is connected to a straight quartz tube of 21 mm ID while the HF discharge tube is connected to a bent quartz tube of 18 mm ID itself connected to a 5 L-Pyrex chamber (Fig. 1). For both systems, experiments were conducted at very similar operation conditions of pressure (8 Torr), flow rate (0.5–1.0 slm) and applied power (50–150 W). The emission spectra across the RF reactor are obtained by means of a spectrometer (Monera 500, resolution 0.2–0.8 nm) with a PMT (Hamamatsu R928) using an optical fiber [1]. Across the HF reactor, an optical fiber is connected to an Acton Spectra Pro 2500i spectrometer (grating 600 g/mm) equipped with a Pixis 256E CCD detector (front illuminated 1024 × 256. The above similarities in the geometry of the two plasma systems allow us to compare the two RF and HF plasmas and afterglows to further understand the inherent differences in the type of plasma between the two plasmas. Plasmas are investigated by emission spectroscopy by measuring emission between the two rings of the RF discharge for the case of RF plasma and near the gap of the surfatron cavity (with a spatial resolution estimated to be 5 mm) for the case of HF plasma, respectively. N2 second positive (2nd pos), N2 + first negative (1st neg) and N2 first positive (1st pos) intensities are recorded at these two positions.
The N2 and Ar–N2 RF and HF Plasmas
In the same ranges of gas pressure (6–8 Torr), flow rate (0.6–1.0 slm) and power (50–100 W), it is observed that the plasma region, defined as the most luminous glow downstream of the RF coils and HF gap, respectively, is longer in RF than in HF. For pure N2, the HF plasma length (Lp) at 8 Torr increases from 1 to 6 cm when the power is increased from 50 to 100 W, while in the RF plasma, Lp varies from 18 to 25 cm for the same power variation. Such an increase of Lp of 2.45 GHz microwave plasmas in HF cavity at 100–140 W has been effectively observed in N2 plasma at 30 Torr with Lp increasing from 2.5 to 8 cm [13]. The difference is even more pronounced in Ar–x %N2 gas mixtures in which Lp increases with increasing Ar ratio. Lp is longer than the tube length (30 cm) for the RF plasma in Ar–x %N2 when x is lower than 20 %. With the HF plasma, it is even possible to decrease x down to 2 % since the HF plasma length is still confined in the tube of 30 cm length. Thus, the comparison between RF and HF is presently limited to results in pure N2 and in the Ar–20 %N2 gas mixture.
For low pressure plasmas, it is known that the (minimum) cut-off electron density required to maintain a surface wave discharge depends on the square of the frequency (f2) of the electro-magnetic wave transferring the energy to the gas [22]. It is mentioned that the f2 variation of electron density at the plasma end is given in [22] for Ar surface wave plasma at pressure less than 1 Torr. It is extended here to the present RF (13.6 MHz) and HF (2450 MHz) plasma columns to explain our observation that the length of the RF plasma is longer than the HF one at a same gas pressure and transmitted power.
The plasma length (Lp) is of crucial importance for the N2(X, v) excitation by the electron–vibration (e–V), vibration–vibration (V–V) and vibration–translation (V–T) processes as previously reported and applied to results of a N2 DC flowing discharge [23]. At the end of the RF plasma (Lp = 30 cm, 100 W), the residence time (tR) is 10−2 s, one order of magnitude higher than at the end of the HF plasma where tR = 8 × 10−4 s (Lp = 6 cm, 100 W). As a consequence, the (V–V) processes are more developed in the RF plasma than in HF one.
The Plasma Gas Temperature
The intensity ratio (P1/P2) of the first two rotational sub-bands (labelled as P1 and P2) of the 1st pos N2 band at 775 nm is related to the rotational temperature (TR) of the plasma which is usually between 300 and 1000 K [19]. It is an important characteristic parameter of the N2 plasma since it is directly related to the gas temperature (Tg), due to the efficient rotational-translational energy transfer. It is found that with an applied power between 50 and 100 W, the RF plasma at 6–8 Torr and 0.5–0.6 slm is a little warmer (Tg = 500–800 K) than the HF plasma at 8 Torr, 1 slm where Tg = 500–600 K. The uncertainty of gas temperature measurements is estimated to be 20 % [19].
The Plasma Vibrational Temperature
The vibrational temperatures can be analyzed from the intensity ratios of the N2 2nd pos. sequence Δv = −2 as detailed in [19]. The vibrational TV(C) and characteristic θ1(X) temperatures, corresponding to the excited N2(C 3Пu) and N2(X 1Σ +g ) ground states, respectively, are connected to each other as can be seen from the following two steps:
First, the f(v) vibrational distribution of N2(X, v) is expressed as follows [24]:
with \({\text{f}}\left( 0 \right) = 1 - \exp^{{ - \frac{{{\text{E}}_{10} }}{{\uptheta_{1} \left( {\text{x}} \right)}}}}\) for v < v T, where v T is the vibrational number of the Treanor minimum given by \(v_{\text{T}} = \frac{{{\text{T}}_{\text{g}} }}{{2\updelta \uptheta_{1} \left( {\text{x}} \right)}} + 0.5\), E10 is the energy difference between the first two vibrational levels E(X, 1)–E(X, 0) (in K) and δ is the anharmonicity constant.
For N2(X), E10 = 3396 K and δ = 6.22 × 10−3.The population of N2(X, v) is then written as [X, v] = f(v) N0, where N0 is the N2 density.
Second, by considering that the N2(C, v′) states are mainly produced by electron collisions on the N2(X, v) ground states, following the Franck–Condon principle (vertical direct excitation by electron collisions), the stationary [C, v′] population is written as follows:
where q(X, v–C, v′) is the Franck–Condon factor [24], ne is the electron density, ke(C) is the electron excitation rate which depends on the electron excitation cross-sections and on the electron energy distribution and ν(C, v′) = νR(C, v′) + N0 kQ(C, v′), (νR(C, v′) and kQ(C, v′) are the radiative loss frequency [25] and the collisional quenching rate by the N2 molecules [26]), respectively. The thresholds between the N2(C, v′ = 0–3) and the N2(X, v = 0–8) varying from 9 to 11.5 eV and the value of ke(C) is approximated to be constant [27]. For the pressure range used in the present work, N0 kQ(C, v′) can be neglected in front of νR(C, v′).
As previously described [19], the experimental relative distribution [C, v′ < 4] is obtained by measuring the band head intensities of the N2 2nd pos. sequence Δv = –2. If a Boltzmann distribution of [C, v′] is verified, the vibrational temperature TV(C) can be obtained.
The vibrational distribution of the N2 ground state [X, v] and the corresponding vibrational temperature θ1(X) are obtained from the experimental values of Tg and of the [C, v′ < 4] distributions by combining Eqs. (1) and (2).
Figure 2 shows the calculated[C, v′ < 4] distributions normalized to [C, v′ = 0] for the θ1(X) values of 5, 8 and 20 × 103 K which are compared with the experimental determined values ([C, v′ < 4]/[C, v′ = 0]) for the RF and HF plasmas. From the comparison, θ1(X) = 8 × 103 K and θ1(X) = 5 × 103 K are chosen for the RF and HF plasmas, respectively. Note that the [C, v′ < 4] calculated distributions have been found to be weakly influenced by the Tg variations between 500 and 800 K. It is observed a high value of the N2(C, v = 1) density of the RF plasma which has been previously interpreted as a result of collisions between electrons and the N2(A) metastable molecules [27, 28].
Table 1 reproduces the values of Tg (see “The plasma gas temperature” section), θ1(X), v T and N2(X, v = v T) for the N2 and Ar–20 %N2 plasmas at 6 Torr, 100 W for RF where [N2]RF = 7 × 1016 cm−3 and at 8 Torr, 100 W for HF where [N2]HF = 1.5 × 1017 cm−3. We find a higher θ1(X) temperature in RF than in HF. This result agrees well with our suggestion of a more vibrationally excited RF plasma than the HF one.
The N2 and Ar–20 %N2 RF and HF Afterglows
Afterglows Characteristics
Characteristics of the afterglows can be studied by measuring the emission bands of N2 1st pos. (chosen band at 580 nm), N2 2nd pos. (chosen band at 316 nm) and N2 + 1st neg. (chosen band at 391.4 nm).
The analysis of the N2 1st pos. vibrational distribution reveals that the N2 afterglows are a mixture of pink (PA) and late (LA) afterglows. In a pure LA, the 1st pos. emission is only due to the 3 body recombination of nitrogen atoms. To evaluate the contribution of the LA emission (aN+N = 1 for pure LA and aN+N = 0 for pure PA), the aN+N value is obtained from a comparison between experimental and theoretical vibrational intensities distributions of the ∆v = −4 sequence (see [1–4, 16]).
For the experimental conditions of 8 Torr, 0.5 slm and 100 W in the RF set-up with the tube ID of 21 mm at z = 34 cm (see Fig. 1), a pure N2 PA is observed. It is followed by an increasing contribution of the LA with increasing z. At z = 51 cm (17 cm downstream from the starting point of the 21 mm quartz tube), the aN+N value of 0.5 is obtained at the same experimental conditions.
For the HF afterglow, aN+N of 0.3 is obtained at 3 cm downstream from the starting point of the 18 mm quartz tube (z = 18 cm, Fig. 2) at 8 Torr, 1 slm and 150 W. It increases up to 0.8 at z = 55 cm. In this case, a pure LA is obtained just before the 5 L-reactor (see Fig. 1).
The measured larger z values for the RF afterglows (the mixed PA and LA of 0 < aN+N < 1) than for the HF indicate longer afterglow times. For the RF afterglow, it is calculated to be 7 × 10−2 s at z = 51 cm (Fig. 1) by assuming a laminar flow in the 21 mm quartz tube.
As for the HF afterglow, it is estimated to be 1.5 × 10−3 s at z = 18 cm (Fig. 2) for a post-discharge jet with low radial expansion having the same gas velocity than in the discharge tube of 5 mm ID, as calculated in [29]. At z > 20 cm, a laminar flow is assumed in the tube of dia. 18 mm, giving a residence time of 6 × 10−2 s at z = 55 cm.
Densities of N, N2(A), N2(X, v > 13) and N2 +
In the LA (aN+N = 1), the N-atoms recombine as follows [1–4, 16]:
When aN+N < 1 (that is, a mixture of LA and PA), the aN+N fraction of the measured \({\text{I}}_{580}^{\text{m}}\) intensity can be related to the square of the N-atom density as follows:
The calibration coefficient k3 is obtained by the use of NO titration, which also gives an independent access to the N-atom density.
In the RF N2 plasma, it is found that the N-atom density slowly increases from 1 to 2 × 1015 cm−3 as the RF power increases from 50 to 130 W under the condition of 8 Torr, 0.5 slm and z = 51 cm (residence time of 7 × 10−2 s) where aN+N = 0.5. In Ar–20 %N2, the N-atom density is about 2 × 1015 cm−3 at 10 – 50 W.
For the HF case (see Table 2), the N-atom density slowly increases from 1.0 to 1.2 × 1015 cm−3 for the residence times between 1.5 × 10−3 and 6 × 10−2 s. Thus, it can be concluded that the N-atom density is about the same in the afterglow regions after the RF and HF plasmas considering the uncertainty on N-atom density to be 30 % [4].
Densities of the other active species (N2(A), N2(X, v > 13) and N2 +) are then obtained by the line-ratio method as developed in [1].
For the N2(A) density determination, the main N2(C) excitation is considered from the following pooling reaction:
Then, the following line–ratio is considered:
The k4 coefficient is calculated from the rate coefficients of reactions (a) and (b) as reported in [1–4, 16] for the two RF and HF studied afterglows.
It is concluded that a nearly constant value of [N2(A)] = 1–2 × 1011 cm−3 is obtained as the RF power increases from 50 to 100 W in N2 and from 10 to 50 W in Ar–20 %N2 at the residence time of 7 × 10−2 s.
The N2(A) density at the residence time of 1.5 × 10−3 s is 1–5 × 1011 and 5 × 1012 cm−3, respectively, after the N2 and Ar–20 %N2 HF plasmas under the condition of 8 Torr, 1 slm and 100–150 W. As shown in Table 2, the N2(A) density is nearly constant for the residence times up to 3.5 × 10−2 s. By this method, the N2(A) density is estimated to be in the range 1011–1012 cm−3.
Then, the N2(X, v > 13) density is deduced by considering that the N2(B, v = 11) state in the PA is produced by the following main reaction:
The ratio of aN+N \({\text{I}}_{580}^{\text{m}}\) (LA) and (1 − aN+N) \({\text{I}}_{580}^{\text{m}}\) (PA) is as follows:
Here, the N2(X, v > 13) density is determined from aN+N values, the N-atoms and the N2(A) densities.
A nearly constant [N2(X, v > 13)] density of 1–2 × 1014 cm−3 is obtained at 50–100 W for pure RF N2 and at 10–50 W for the Ar–20 %N2 gas mixture.
At the residence time of 1.5 × 10−3 s after the HF plasma (8 Torr, 1 slm and 100 – 150 W), the [N2(X, v > 13)] density is 5–6 × 1013 cm−3 for both N2 and Ar–20 %N2.
Such [N2(X, v > 13)] density can be considered as an estimated value which can vary depending on the kc rate coefficient.
The measured N-atoms and N2(X, v > 13) densities above are in the same order of magnitude with the values reported in [14] in which [N2(X, v > 4)] = 3 × 1013 cm−3 and [N] = 6 × 1014 cm−3 are obtained in N2 and Ar–N2 afterglow of HF plasmas at 7 Torr, 3 slm and 200 W.
It should be noted that the N2(X, v > 13) densities found in the present RF and HF afterglows are about one order of magnitude lower than the N2(X, v = 7–9) densities in the corresponding plasmas (see Table 1). This result is used to determine the rate of destruction of these vibrational molecules on the tube wall in the following section.
For the N2 + ions, the following dominant reactions in PA are considered [1]:
where reaction (d) is for the Penning ionization in the afterglow and reaction (e) indicates the excitation of N2 +(X) by vibrational molecules N2(X > 12).
The N2 + density is deduced from the N2(X, v > 12) and N2(A) densities by considering the line-ratio I391/I316 from reactions (e) and (b):
The k5 coefficient is calculated from the rate coefficients of reactions (e) and (b) [4, 16].
By taking [N2(X), v > 12] = [N2(X), v > 13], it is found that the N2 + density increases from 5 × 108 to 2 × 109 cm−3 for the case of the RF N2 plasma, when the RF power is raised from 70 to 100 W. In Ar–20 %N2 RF gas mixture, the N2 + ion density remains at 5 × 108 cm−3 at 10–50 W. After the HF afterglows, the N2 + density is in the range (0.2–1.4) × 108 cm−3 as reported in Table 2.
The observed strong variations of the N2 + density originate from the fact that it is very sensitive to air impurity. By applying the intensity line-ratio method in comparison of I320 of the NOβ band to aN+N I580, an O-atom density of 9 × 1012 cm−3 (that is, 30 ppm at 8 Torr) and N2 + density of 109 cm−3 have been previously determined in the RF afterglow [1]. In the HF afterglow, the O-atom density is in the order of 1014 cm−3 (that is, 400 ppm or 4 × 10−4 [O]/[N2] ratio) and the N2 + density is 108 cm−3. These results clearly show that N2 + ions produced in the afterglow by the Penning ionization N2(a′) + N2(a′) reaction are strongly correlated to the amount of O-atom impurity.
Destruction Probability of N2(X, v > 3–13) on the Tube Walls
Densities of the N2(X, v > 3) vibrational levels are found to be of 3 and 4 × 1015 cm−3 in the respective RF and HF plasmas and about 1014 cm−3 for N2(X, v > 13) in the afterglows. By assuming a “plateau” of N2(X, v > v T), which means that a nearly constant vibrational density is reached after the Treanor minimum with v T = 3 and before a drop of high N2(X, v) density by (V–T) vibration–translation reactions, typically at v > 35 as described in [30], we evaluate a γv destruction probability of the N2(X, v > 3–13) vibrational levels on the tube walls.
The same procedure as in [31] for N-atoms is taken here for the calculations of the γv recombination probability of the N2(X, v > 3–13) molecules on the tube wall.
It is written as follows:
where υX,v = γv<c>/2R, <c> is the thermal gas velocity (<c> = 5 × 104 cm s−1) and R the tube radius.
z = 0 is taken at the RF and HF plasma end, which is at z = 25 cm in RF and at z = 6 cm in HF, respectively.
For such positions, the flow velocity in the RF set-up can be directly calculated from the gas flow rate and from the tube i.d. (21 mm) for a laminar flow.
From Eq. 7, it is found γv = 2 × 10−3 after the RF plasma at z = 25 cm (residence time of 7 × 10−2 s). At z > 20 cm after the HF plasma, it is obtained: γv = 5 × 10−3 at the residence time of 2 × 10−2 s and γv = 3 × 10−3 at the residence time of 3.5 × 10−2 s where a laminar flow is expected.
We note that the same range of γv values are obtained after the RF and HF plasmas (γv = 2–5 × 10−3) at long times (7 and 3.5 × 10−3 s) when the parts of LA and PA are in equality.
The present γv values are about 2–5 times higher than the previous published value [32] for the first N2(X, v = 1) state: γ1 = 10−3 on Pyrex and quartz tube walls. They are largely higher than the γN values of N-atoms recombination on quartz and Pyrex tube walls: γN = 10−5–10−4 [31]. Such results could come from vibrational relaxation of the N2(X, v) molecules on the tube wall by N2(X, v) + wall → N2(X, v–n) +wall, with n ≥ 1, faster than for the N-atoms with N + wall → 1/2 N2 + wall.
The loss frequency of the N2(X, v > 3–13) on the tube wall is in the range of 50–150 s−1.
It can be compared to the following volume losses: 10 s−1 for the reaction N2(X, v > 13) + O with a rate of 10−13 cm3 s−1 [33] with [O] = 1014 cm−3; 1–5 s−1 for the reaction N2(X, v) + N → N2(X, w) + N, with a rate k(v = 10–15, w = 0,15) = 1–5 × 10−15 cm3 s−1 [34] and [N] = 1015 cm−3.
As for the (V–V) and (V–T) collisions, it is given in [35] the characteristic frequencies at 5 Torr, 500 K: υ(V–V) = 2 103s−1 for N2(1) + N2(0) → N2(0) + N2(1) and υ(V–T) = 4 10−3 s−1 for N2(1) + N2 → N2(0) + N2.
Outside the high V–V frequency, these frequencies are low in comparison to the N2(X, v > 3–13) loss frequency on the tube wall: 50–150 s−1.
Conclusion
To conclude, the presently analyzed N2 RF and HF flowing plasmas with nearly the same discharge parameters (tube diameters, gas pressure, flow rates and electrical power) produce long time afterglows with about the same N atoms and N2(A) densities. The main difference between RF and HF appears in the plasma length, which is longer in RF than in HF. As a consequence, the residence time of the active species is increased in the RF plasma, allowing in comparison to HF plasmas an enhancement of N2(X, v) excitation by (V–V) collisions. Such an effect is detected as a higher density of vibrationally excited N2(X, v) molecules in the RF plasma and particularly in the afterglow where a pure pink afterglow is observed in RF, ‘not in HF’.
If about equal densities of N and N2(A) are found in the long time RF and HF afterglows, it appears a higher N2(X, v > 13) and N2 + densities in the RF afterglow which can be related to high N2(V–V) excitation until the plasma end. The present results concern first the N-atom density calibrated by NO titration with an accuracy of 30 %. Then the other active species densities: N2(A), N2(X, v > 13) and N2 + are obtained by a line-ratio method, giving the order of magnitude of density, depending on kinetic rates of the chosen dominant reactions.
A new result obtained with these RF and HF afterglows concerns the γv destruction probability of the N2(X, v ≥ 3–13) vibrational levels on the quartz tube wall, which is more than one order of magnitude higher than for the N-atoms, certainly as the result of a more easy climbing down of N2(X, v) on the tube wall.
References
Ricard A, Oh SG, Guerra V (2013) Line-ratio determination of atomic oxygen and N2 metastable absolute densities in an RF nitrogen late afterglow. Plasma Sources Sci Technol 22(3):035009. doi:10.1088/0963-0252/22/3/035009
Zerrouki H, Ricard A, Sarrette JP (2013) Determination of N and O-atom and N2(A) metastable molecule densities in the afterglows of N2 and N2–O2 microwave discharges. Contrib Plasmas Phys 53(8):599–604. doi:10.1002/ctpp.201300008
Ricard A, Oh SG (2014) Densities of active species in N2 and N2–H2 RF pink afterglow. Plasma Sources Sci Technol 23(4):045009. doi:10.1088/0963-0252/23/4/045009
Zerrouki H, Ricard A, Sarrette JP (2014) Determination of N and O-atoms and N2(A) metastable molecule densities in the afterglows of N2–H2, Ar–N2–H2 and Ar–N2–O2 microwave discharges. Contrib Plasmas Phys 54(10):827–837. doi:10.1002/ctpp.201400001
Ricard A, Oh SG, Jang J, Kim YK (2015) Quantitative evaluation of the densities of active species of N2 in the afterglow of Ar-embedded N2 RF plasma. Curr Appl Phys 15(11):1453–1462. doi:10.1016/j.cap.2015.08.013
Philip N, Saoudi B, Crevier MC, Moisan M, Barbeau J, Pelletier J (2002) The respective roles of UV photons and oxygen atoms in plasma sterilization at reduced gas pressure: the case of N2–O2 mixtures. IEEE Trans Plasma Sci 30(4):1429–1436. doi:10.1109/TPS.2002.804203
Villeger S, Sarrette JP, Ricard A (2005) Synergy between N and O atom action and substrate surface temperature in a sterilization process using a flowing N2–O2 microwave post discharge. Plasma Process Polym 2(9):709–714. doi:10.1002/ppap.200500040
Pointu A-M, Ricard A, Dodet B, Odic E, Larbre J, Ganciu M (2005) Production of active species in N2–O2 flowing post-discharges at atmospheric pressure for sterilization. J Phys D Appl Phys 38(12):1905–1909. doi:10.1088/0022-3727/38/12/009
Ricard A, Jaoul C, Gaboriau F, Gherardi N, Villeger S (2004) Production of N, H, O, and C atoms in flowing microwave discharges. Surf Coat Technol 188–189:287–293. doi:10.1016/j.surfcoat.2004.08.171
Ricard A, Monna V (2002) Reactive molecular plasmas. Plasma Sources Sci Technol 11(3A):A150. doi:10.1088/0963-0252/11/3A/322
Molchan IS, Thompson GE, Skeldon P, Trigoulet N, Chapon P, Tempez A, Malherbe J, Lobo Revilla L, Bordel N, Belenguer P, Nelis T, Zahri A, Therese L, Guillot P, Ganciu M, Michler J, Hohl M (2009) The concept of plasma cleaning in glow discharge spectrometry. J Anal At Spectrom 24(6):734–741. doi:10.1039/B818343K
Kaluri SR, Hess DW (1996) Nitrogen incorporation in thin oxides by constant current N2O plasma anodization of silicon and N2 plasma nitridation of silicon oxides. Appl Phys Lett 69(8):1053–1055. doi:10.1063/1.116928
Ricard A, Hubert J, Michel H (1992) Correlations between active plasma species and steel surface nitriding in microwave post-discharge reactors. In: Capitelli M, Gorse C (eds) Plasma technology: fundamentals and applications. Springer, Boston, pp 125–142. doi:10.1007/978-1-4615-3400-6_9
Ricard A, Czerwiec T, Belmonte T, Bockel S, Michel H (1999) Detection by emission spectroscopy of active species in plasma–surface processes. Thin Solid Films 341(1–2):1–8. doi:10.1016/S0040-6090(98)01529-6
Zhu X-M, Pu Y-K (2010) Optical emission spectroscopy in low-temperature plasmas containing argon and nitrogen: determination of the electron temperature and density by the line-ratio method. J Phys D Appl Phys 43(40):403001. doi:10.1088/0022-3727/43/40/403001
Zerrouki H, Ricard A, Sarrette JP (2014) Determination of N and O-atoms, of N2 (A) and N2 (X, v > 13) metastable molecules and N2+ ion densities in the afterglows of N2–H2, Ar–N2–H2 and Ar–N2–O2 microwave discharges. J Phys Conf Ser 550(1):012045. doi:10.1088/1742-6596/550/1/012045
Kang N, Lee M, Ricard A, S-g Oh (2012) Effect of controlled O2 impurities on N2 afterglows of RF discharges. Curr Appl Phys 12(6):1448–1453. doi:10.1016/j.cap.2012.04.009
Boudam MK, Saoudi B, Moisan M, Ricard A (2007) Characterization of the flowing afterglows of an N2–O2 reduced-pressure discharge: setting the operating conditions to achieve a dominant late afterglow and correlating the NO β UV intensity variation with the N and O atom densities. J Phys D Appl Phys 40(6):1694–1711. doi:10.1088/0022-3727/40/6/019
Britun N, Gaillard M, Ricard A, Kim YM, Kim KS, Han JG (2007) Determination of the vibrational, rotational and electron temperatures in N2 and Ar–N2 RF discharge. J Phys D Appl Phys 40(4):1022. doi:10.1088/0022-3727/40/4/016
Nassar H, Pellerin S, Musiol K, Martinie O, Pellerin N, Cormier JM (2004) N2 +/N2 ratio and temperature measurements based on the first negative N2 + and second positive N2 overlapped molecular emission spectra. J Phys D Appl Phys 37(14):1904–1916. doi:10.1088/0022-3727/37/14/005
Sadeghi N, Foissac C, Supiot P (2001) Kinetics of N2 (A 3Σ +u ) molecules and ionization mechanisms in the afterglow of a flowing N2 microwave discharge. J Phys D Appl Phys 34(12):1779. doi:10.1088/0022-3727/34/12/304
Chaker M, Moisan M, Zakrzewski Z (1986) Microwave and RF surface wave sustained discharges as plasma sources for plasma chemistry and plasma processing. Plasma Chem Plasma Process 6:79–96
Massabieaux B, Plain A, Ricard A, Capitelli M, Gorse C (1983) Excitation of vibrational and electronic states in a glow discharge column in flowing N2. J Phys B At Mol Phys 16(10):1863. doi:10.1088/0022-3700/16/10/021
Gordiets B, Hamedov SS, Shelepin IA (1975) Vibrational kinetics of harmonic oscillators under essentially nonequilibrium conditions. Sov Phys JETP 40:640
Gilmore FR, Laher RR, Espy PJ (1992) Franck–Condon factors, r-centroids, electronic transition moments, and Einstein coefficients for many nitrogen and oxygen band systems. J Phys Chem Ref Data 21(5):1005–1107. doi:10.1063/1.555910
Pancheshnyi SV, Starikovskaia SM, Starikovskii AY (2000) Collisional deactivation of N2(C 3Πu, v = 0, 1, 2, 3) states by N2, O2, H2 and H2O molecules. Chem Phys 262(2–3):349–357. doi:10.1016/S0301-0104(00)00338-4
De Benedictis S, Dilecce G (1995) Vibrational relaxation of N2(C, v) state in N2 pulsed RF discharge: electron impact and pooling reactions. Chem Phys 192(2):149–162. doi:10.1016/0301-0104(94)00370-P
Mavadat M, Ricard A, Sarra-Bournet C, Laroche G (2011) Determination of ro-vibrational excitations of N2 (B, v′) and N2 (C, v′) states in N2 microwave discharges using visible and IR spectroscopy. J Phys D Appl Phys 44(15):155207. doi:10.1088/0022-3727/44/15/155207
Blanchard H, Sarrette JP, Villeger S, Baudel P, Ricard A (2004) Density of oxygen atoms in high pressure (10–50 torr) flowing microwave post-discharges for elastomer treatments. Eur Phys J Appl Phys 26(03):221–226. doi:10.1051/epjap:2004036
Capitelli M, Gorse C, Ricard A (1982) Non equilibrium dissociation and ionization of N2 in decaying plasmas. J Phys (Paris) 43:L417–L423
Villeger S, Sarrette JP, Rouffet B, Cousty S, Ricard A (2008) Treatment of flat and hollow substrates by a pure nitrogen flowing post discharge. Eur Phys J Appl Phys 42(01):25–32. doi:10.1051/epjap:2007177
Marinov D, Lopatik D, Guaitella O, Hubner M, Ionikh Y, Ropcke J, Rousseau A (2012) Surface vibrational relaxation of N2 studied by CO2 titration with time-resolved quantum cascade laser absorption spectroscopy. J Phys D Appl Phys 45(175201):33
Guerra V, Loureiro J (1997) Self-consistent electron and heavy particle kinetics in a low-pressure N2–O2 glow discharge. Plasmas Sources Sci Technol 6:373–385
Armenise I, Capitelli M, Garcia E, Gorse C, Lagana A, Longo S (1992) Deactivation dynamics of vibrationally excited nitrogen molecules by nitrogen atoms. Chem Phys Lett 200:597–604
Capitelli M, Gorse C, Ricard A (1986) Coupling of vibrational and electronic energy distributions in discharge and post-discharge conditions. In: Topics in current chemistry, vol 39. Non equilibrium vibrational kinetics. Chapter 11, pp 315–337
Acknowledgments
Y.K.K. acknowledges a financial support from the International Research and Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015K1A3A1A21000248). This work was supported by the Franco-Korean project PHC STAR 2015 (34306TK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ricard, A., Sarrette, JP., Oh, SG. et al. Comparison of the Active Species in the RF and Microwave Flowing Discharges of N2 and Ar–20 %N2 . Plasma Chem Plasma Process 36, 1559–1570 (2016). https://doi.org/10.1007/s11090-016-9739-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9739-9