Abstract
In this experimental study, Cd (OH)2/CdO nanostructures were produced by pulsed laser ablation method. The beam of a Q-switched Nd:YAG laser with wavelength of 1064 nm at different fluences was employed to irradiate the cadmium target in distilled water. The obtained products at different fluences of laser pulse were characterized by UV–Vis-NIR spectroscopy, X-ray diffraction, scanning electron microscopy, and transmission electron microscopy. Results show that both morphology and chemical bonds of produced Cd nanostructures were changed by increasing the laser fluence. The absorption spectra shows that the Cd(OH)2 nanostructures could be transformed into CdO nanostructures with increasing the laser fluence. XRD analysis showed the presence of crystalline γ-Cd(OH)2, Cd(OH)2 and CdO phases, in the monoclinic, hexagonal and cubic structure, respectively. Results revealed that obtained nanostructures are particle in lowest fluence, while wire shaped nanostructures were formed with increasing the laser fluence. An increase of the laser fluence induced the synthesis of higher aspect ratio nanowires with a smaller tendency of adhesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cadmium oxide (CdO) is an n-type semiconductor with a direct optical bandgap of 2.3 eV, with an indirect bandgap of 1.36 eV (Ghoshal et al. 2009). CdO belongs to the group of II-VI oxides with high exciton binding energy of 75 mV showing unique structural, electronic and optical properties for various applications in many technological areas. CdO is polycrystalline and due to its good optical properties is one of the most widely used nanomaterials for photodiodes, phototransistors, photovoltaic cells, IR detectors, and supercapacitors (Liu et al. 2003; Sonawane et al. 2017; Mane et al. 2006; Salunkhe and Lokhande 2008; Patil et al. 2015). Moreover, due to the unique optical properties and the lowest toxicity in comparison with the other semiconducting nanomaterials, CdO nanostructures can be a promising candidate for bio applications (Salehi et al. 2015).
Up to now there are reports on synthesized CdO nanostructures using different methods such as spray pyrolysis (Afify et al. 2014), sol–gel (Carballeda-Galicia et al. 2000), pulsed laser ablation (Agool et al. 2016) and magnetron sputtering (Gurumurugan et al. 1996). There are also some reports on producing CdO in the form of nanonecklace, based on chemical techniques (Sonawane et al. 2017; Patil et al. 2015; Sonawane et al. 2016). Among these techniques, pulsed laser ablation (PLA) of solids in liquid medium is an effective method for preparing various nanoscale materials (Solati and Dorranian 2016a, b; Mehrani et al. 2015; Solati and Dorranian 2015; Dorranian et al. 2012; Aazadfar et al. 2018; Savadkoohi et al. 2018). Overall, pulsed laser ablation will not generate any poisonous byproduct and the nature of nanomaterials produced by PLA could be controlled by the characteristics of liquid medium (Moradi et al. 2016; Zamiranvari et al. 2017; Solati and Dorranian 2017; Solati et al. 2014) and also by adjusting different features of the used laser, such as its pulse width, wavelength and fluence (Solati et al. 2013, 2014).
The aim of this experimental research is to study effects of the laser fluence on the physical properties of the Cd(OH)2/CdO nanostructures fabricated by pulsed laser ablation method in order to study their structural, optical and morphological behavior, systematically. Nanostructures are prepared using 1064 nm Nd:YAG laser ablation of cadmium target with 99.99% purity immersed in distilled water. The physical properties of products such as structural, optical and morphological properties were investigated using X-ray diffractometer, UV–Vis-NIR spectroscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM), respectively.
2 Experimental
2.1 Synthesis of Cd(OH)2/CdO nanostructures
In this experiment Cd(OH)2/CdO nanostructures were produced by pulsed laser ablation of cadmium target in distilled water. Before experiment, cadmium target was washed and cleaned by alcohol, acetone and distilled water in ultrasonic device respectively. Then, cadmium target was placed on the bottom of a glass vessel containing 30 ml distilled water. Cadmium target was irradiated by the fundamental wavelength of Q-switched Nd:YAG laser of 1064 nm wavelength with 7 ns pulse width and repeating rate of 10 Hz. Laser was focused on the surface of target using a lens with 100 mm focal length. Nanostructures were produced using different fluence of laser pulse. Detail about the samples preparation is presented in Table 1.
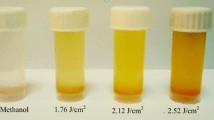
Pictures of nanostructures suspensions prepared at different laser fluences are presented in Fig. 1. Due to the irradiation of the cadmium target immersed in distilled water by laser pulses, the surface of cadmium target was evaporated. At the next stage the energy of laser pulse were absorbed by cadmium vapor, which caused the ionization and finally plasma was produced on the surface of the target. Plasms expansion in the liquid medium leaded to formation of shock waves directed to the center of plasma plume. Chemical reactions which were occurred between cadmium ions and species within the plasma plume were responsible for production of nanostructured materials. Variation of laser fluence changes the plasma plume particles density, plasma plume temperature and pressure, as well as the shock wave intensity during the laser ablation process which in turn may lead to formation of nanostructures with different morphology and chemical structures. As shown in Fig. 1, with increasing the laser fluence, the change in the color of the nanostructures suspensions can be observed, so that the transparent solution becomes a cloudy solution.
2.2 Characterization
Variety of analytical techniques were used to characterize the optical, structural and morphological properties of Cd(OH)2/CdO nanostructures. The optical properties of the samples were examined at room temperature by Varian Cary-500 spectrophotometer. The crystalline structure of Cd(OH)2/CdO nanostructures was analyzed by X-ray diffraction (XRD) with Cu-Kα radiation (λ = 1.54060Å), using an STOE–XRD diffractometer. Zeiss EM10C transmission electron microscopy was employed to investigate the size and form of Cd(OH)2/CdO nanostructures. Scanning electron microscopy micrographs were taken using KYKY-EM3200 systems.
3 Results and discussion
3.1 Absorption studies
The absorption spectra of the Cd(OH)2/CdO nanostructures suspensions are shown in the Fig. 2. The spectra were recorded with distilled water as the baseline reference in quartz cells with thickness of 1 cm. For sample 1, the absorption peak with low intensity dominates the spectrum below 200 nm while a smooth decay towards higher wavelengths is observed. In the absorption spectrum of sample 2, a peak is occurred at 224 nm with a long tail towards a higher wavelength side. The suspensions of nanostructures in sample 3-5 illustrate a wide absorption band with two peaks centered at 240 nm and 290 nm. Absorbance at all the peak positions increases with increasing the laser fluence, showing the rate of ablation was increased with increasing the laser fluence. The absorption peak at 290 nm is assigned to the excitonic absorption feature of CdO (Kumar et al. 2017). In this field Singh and Gopal (2010) reported that the absorption peak around the wavelength range of 230 nm attributes to the interband transition of inner shell electrons to the conduction band, and the absorption peak at 300 nm is due to the coherent oscillations of free electrons along the transverse directions of the nanorods. In our experiment, Cd(OH)2 with structure of nanoparticles were produced at low laser fluence and CdO nanostructure with wire shape were produced at high laser fluence. As can be seen with increasing the laser fluence, absorption peak attribute to the Cd(OH)2 was shifted towards the longer wavelength after its conversion into CdO. Compared with the CdO bulk material, the absorption onset of the CdO nanowires is shifted to lower wavelength due to the quantum confinement effect. The nature of the bandgap energy for semiconductor materials can be determined using the fundamental absorption that is due to electron excitation from the valence band to the conduction band. The bandgap energy of these nanostructures was estimated using the Tauc relation (Solati and Dorranian 2016a, b).
In this equation α is absorption coefficient, hυ is the photon energy, A is a constant that depends on the transition, Eg is the bandgap energy and m = 1/2 for the direct transitions. To calculate the optical bandgap of nanostructures, (αhν) 2 is plotted versus the photon energy \(h\nu\) in Fig. 3.
The extrapolation of the straight line at (αhν) = 0 gives the value of bandgap energy and were obtained from 4.03 to 5.74 eV and 3.00 to 3.17 eV for Cd(OH)2 and CdO, respectively. Detail about the bandgap energy of samples is presented in Table 2. The larger bandgap energies of these Cd(OH)2/CdO nanostructures than the bulks of Cd(OH)2 [with bandgap of 3.2 eV (Salunkhe et al. 2009)] and CdO [with bandgap of 2.3 eV (Ghoshal et al. 2009)] demonstrate their high degree of quantum confinements. The decrease in bandgap energy with increasing the laser fluence may also arise from the change of crystal structure from Cd(OH)2 to CdO (Prakash et al. 2016). The variation in the bandgap energies of CdO nanowires can be attributed to the width of the nanowires. An increase in the bandgap energies of CdO nanowires in samples 3 to 5 can be due to the decrease in the width of the nanowires.
3.2 XRD and morphological characterization
Figure 4 shows a typical XRD pattern of the Cd(OH)2/CdO nanostructures prepared at different laser fluence. For XRD measurement, the suspensions of nanostructures were dried on silicon substrate. Diffraction peaks at 2θ = 16.4°, and 18.8°, are assigned to the (001), and (220) Miller indices of the monoclinic, end-centered Cm (8) phase (JCPDS Card No. 40-0760) of the γ-Cd(OH)2 (Solati et al. 2013; Singh and Gopal 2010). These peaks indicate the hexagonal crystal structure of Cd(OH)2. The XRD peaks at 2θ = 37.9°, 44.5°, and 77.1° are attributed to (111), (200), and (311) indices for the cubic symmetry of CdO nanocrystals (JCPDS Card No. 05-0640) (Lu et al. 2008; Singh et al. 2006).
The XRD patterns indicated the formation of well crystallined products at low laser fluence and with increasing the laser flunece the intensity of X-ray diffraction peaks were decreased.
Figure 5 shows the surface morphology of the nanostructures using emission scanning electron microscopy. SEM micrograph of sample 1 reveals images of Cd(OH)2 synthesized by PLA method at lowest laser fluence with structure of spherical nanoparticles. SEM micrographs of sample 2–5 exhibit large quantity of CdO nanowires. In these samples the nanowires were of few micrometers in length and bending of the nanowires was not sharp enough. As can be seen with increasing the laser fluence adhesion of nanowires was decreased. Nanowires with small diameter were also seen from these micrographs lying below the big diameter nanowires. Most of the nanowires were formed with uniform diameters through their entire lengths. Furthermore, with increasing the laser fluence the surfaces of the nanowires became smooth. For nano device applications, the smoothness of the nanowires influences the efficiency of the device (Eskizeybek et al. 2011). Nanowires in samples produced at low laser fluence consisted of ensembles of particles stacked along the length of the nanowire. The presence of these particles along with CdO nanowires is due to the presence of Cd(OH)2.
In PLA method, interaction between pulsed laser and cadmium target produce local high temperature and high pressure plasma plumes above the surface of cadmium target. Several chemical reactions and physical processes occur between the ablated cadmium target and the surrounding liquid molecules. The result of these reactions and processes leads to the formation of nanostructures in the aqueous environment.
Cadmium ions exist in the plasma created by laser radiation to cadmium target. High pressure in front of expanding plume provides better way for reaction of ablated species with that dissolved in the liquid media such as oxygen and OH− ions (Singh et al. 2009). Cd2+ ions can easily react with OH− ions. Local high temperature applied to solid materials leads to generate a thermal decomposition or phase transformation (Eskizeybek et al. 2011). In the high temperature, the Cd(OH)2 decomposed into CdO nanostructures. The chemical reactions can be described in detail as follows (Eskizeybek et al. 2011):
In this process, Cd(OH)2 nanoparticles were changed to CdO with the formation of nanowires structure. Thus, the morphologies of nanostructures produced by PLA method can be controlled by the laser fluecne.
Figure 6 reveals TEM micrographs of Cd(OH)2/CdO nanostructures synthesized by PLA in distilled water. According to the TEM micrograph, the Cd(OH)2 in sample 1 are irregular particles. The diameters of the Cd(OH)2 nanoparticles ranged from 20 nm to 40 nm. TEM micrographs of sample 2–5 depicted in Fig. 6 have a large number of isolated CdO nanowires. The diameters of the CdO nanowires varied in the range between 10 and 20 nm. As can be seen all the CdO nanowires synthesized by PLA method at different laser fluence have smooth surfaces. The diameter of the nanowires along them is almost uniform.
Characteristics of generated plasma on the surface of the target during laser ablation play an important role on the morphology of produced nanostructures. There are several report on the effect of laser fluence on controlling the size of nanoparticles produced by laser ablation method (Solati and Dorranian 2017; Solati et al. 2013, 2014). In the current experiment not only the laser fluence but the chemical elements in nanostructures were changed. With increasing the laser fluence Cd(OH)2 molecules were changed to CdO leaded to variation of plasma plume pressure and density. Variation of these parameters simultaneously with variation of nanoproducts chemical bonds is the main responsible for changing the morphology of products from nanoparticle to nanowire.
4 Conclusion
The present study indicates that the method of pulsed laser ablation in distilled water medium can by successfully employed for the preparation Cd(OH)2/CdO nanostructures.
With increasing the laser fluence the absorption peak was clearly shifted to longer wavelength and this result is due to the conversion of Cd(OH)2 to CdO nanostructures. Optical bandgap obtained for the samples confirms the size of the nanostructures. XRD measurements revealed that prepared samples possess monoclinic, hexagonal and cubic structure for γ-Cd(OH)2, Cd(OH)2 and CdO phases respectively and overall polycrystalline in nature. The laser fluence affects the morphology of synthesized nanostructures.
In general it can be concluded that the variation of laser fluence in PLA method can be provide an alternative way, without pollution, to synthesize nanostructures of cadmium oxide and cadmium hydroxide with different morphologies. Specifically laser fluence is an effective and strong tool to control the chemical structure and morphology of laser ablation produced nanoproduct.
References
Aazadfar, P., Solati, E., Dorranian, D.: Properties of Au/Copper oxide nanocomposite prepared by green laser irradiation of the mixture of individual suspensions. Opt. Mater. 78, 388–395 (2018)
Afify, H.H., Ahmed, N.M., Tadros, M.Y., Ibrahim, F.M.: Temperature dependence growth of CdO thin film prepared by spray pyrolysis. J. Electr. Syst. Inf. Technol. 1, 119–128 (2014)
Agool, I.R., Abd, A.N., Dawood, M.O.: Preparation and study of colloidal CdO nanoparticles by laser ablation in polyvinylpyrrolidone. Int. J. Eng. Technol. 6, 1–7 (2016)
Carballeda-Galicia, D.M., Castanedo-Perez, R., Jimenez-Sandoval, O., Jimenez-Sandoval, S., Torres-Delgado, G., Zuniga-Romero, C.I.: High transmittance CdO thin films obtained by the sol-gel method. Thin Solid Films 371, 105–108 (2000)
Dorranian, D., Solati, E., Dejam, L.: Photoluminescence of ZnO nanoparticles generated by laser ablation in deionized water. Appl. Phys. A 109, 307–314 (2012)
Eskizeybek, V., Avcı, A., Chhowalla, M.: Structural and optical properties of CdO nanowires synthesized from Cd(OH)2 precursors by calcination. Cryst. Res. Technol. 46, 1093–1100 (2011)
Ghoshal, T., Kar, S., De, S.K.: Morphology controlled solvothermal synthesis of Cd (OH) 2 and CdO micro/nanocrystals on Cd foil. Appl. Surf. Sci. 255, 8091–8097 (2009)
Gurumurugan, K., Mangalaraj, D., Narayandass, S.K., Nakanishi, Y.: DC reactive magnetron sputtered CdO thin films. Mater. Lett. 28, 307–312 (1996)
Kumar, S., Ahmed, B., Ojha, A.K., Das, J., Kumar, A.: Facile synthesis of CdO nanorods and exploiting its properties towards supercapacitor electrode materials and low power UV irradiation driven photocatalysis against methylene blue dye. Mater. Res. Bull. 90, 224–231 (2017)
Liu, X., Li, C., Han, S., Han, J., Zhou, C.: Synthesis and electronic transport studies of CdO nanoneedles. Appl. Phys. Lett. 82, 1950–1952 (2003)
Lu, H.B., Liao, L., Li, H., Tian, Y., Wang, D.F., Li, J.C., Fu, Q., Zhu, B.P., Wu, Y.: Fabrication of CdO nanotubes via simple thermal evaporation. Mater. Lett. 62, 3928–3930 (2008)
Mane, R.S., Pathan, H.M., Lokhande, C.D., Han, S.H.: An effective use of nanocrystalline CdO thin films in dye-sensitized solar cells. Sol. Energy 80, 185–190 (2006)
Mehrani, A., Dorranian, D., Solati, E.: Properties of Au/ZnO nanocomposite prepared by laser irradiation of the mixture of individual colloids. J. Cluster Sci. 26, 1743–1754 (2015)
Moradi, M., Solati, E., Darvishi, S., Dorranian, D.: Effect of aqueous ablation environment on the characteristics of ZnO nanoparticles produced by laser ablation. J. Cluster Sci. 27, 127–138 (2016)
Patil, S., Raut, S., Gorea, R., Sankapal, B.: One-dimensional cadmium hydroxide nanowires towards electrochemical supercapacitor. New J. Chem. 39, 9124–9131 (2015)
Prakash, T., Bonavita, A., Neri, G., Kumar, E.R.: Microwave-assisted synthesis of Cd (OH) 2/CdO nanorods: effect of irradiation time. Superlattices Microstruct. 90, 117–123 (2016)
Salehi, B., Mortaz, E., Tabarsi, P.: Comparison of antibacterial activities of cadmium oxide nanoparticles against Pseudomonas aeruginosa and Staphylococcus aureus bacteria. Adv. Biomed. Res. 4, 105 (2015)
Salunkhe, R.R., Lokhande, C.D.: Effect of film thickness on liquefied petroleum gas (LPG) sensing properties of SILAR deposited CdO thin films. Sens. Actuators B Chem. 129, 345–351 (2008)
Salunkhe, R.R., Patil, U.M., Gujar, T.P., Lokhande, C.D.: Synthesis and characterization of cadmium hydroxide nano-nest by chemical route. Appl. Surf. Sci. 255, 3923–3926 (2009)
Savadkoohi, M., Dorranian, D., Solati, E.: Using silicon nanoparticles to modify the surface of graphene nanosheets. Mater. Sci. Semicond. Process. 75, 75–83 (2018)
Singh, S.C., Gopal, R.: Nanoarchitectural evolution from laser-produced colloidal solution: growth of various complex cadmium hydroxide architectures from simple particles. J. Phys. Chem. C 114, 9277–9289 (2010)
Singh, N., Charan, S., Patil, K.R., Viswanath, A.K., Khanna, P.K.: Unusual formation of nano-particles of CdO and Cd(OH)2 from the reaction of dimethyl cadmium with DMF. Mater. Lett. 60, 3492–3498 (2006)
Singh, S.C., Swarnkar, R.K., Gopal, R.: Laser ablative approach for the synthesis of cadmium hydroxide–oxide nanocomposite. J. Nanopart. Res. 11, 1831–1838 (2009)
Solati, E., Dorranian, D.: Comparison between silver and gold nanoparticles prepared by pulsed laser ablation in distilled water. J. Cluster Sci. 26, 727–742 (2015)
Solati, E., Dorranian, D.: Nonlinear optical properties of the mixture of ZnO nanoparticles and graphene nanosheets. Appl. Phys. B 122, 1–10 (2016a)
Solati, E., Dorranian, D.: Effect of temperature on the characteristics of ZnO nanoparticles produced by laser ablation in water. Bull. Mater. Sci. 39, 1677–1684 (2016b)
Solati, E., Dorranian, D.: Estimation of lattice strain in ZnO nanoparticles produced by laser ablation at different temperatures. J. Appl. Spectrosc. 84, 490–497 (2017)
Solati, E., Mashayekh, M., Dorranian, D.: Effects of laser pulse wavelength and laser fluence on the characteristics of silver nanoparticle generated by laser ablation. Appl. Phys. A 112, 689–694 (2013)
Solati, E., Dejam, L., Dorranian, D.: Effect of laser pulse energy and wavelength on the structure, morphology and optical properties of ZnO nanoparticles. Opt. Laser Technol. 58, 26–32 (2014)
Sonawane, N.B., Baviskar, P.K., Ahire, R.R., Ojha, V.H., Sankapal, B.R.: Nanonecklace of CdO through simple solution chemistry. Mater. Sci. Semicond. Process. 49, 81–83 (2016)
Sonawane, N.B., Baviskar, P.K., Ahire, R.R., Sankapal, B.R.: CdO necklace like nanobeads decorated with PbS nanoparticles: room temperature LPG sensor. Mater. Chem. Phys. 191, 168–172 (2017)
Zamiranvari, A., Solati, E., Dorranian, D.: Effect of CTAB concentration on the properties of graphene nanosheets produced by laser ablation. Opt. Laser Technol. 97, 209–218 (2017)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghoranneviss, P., Dorranian, D. & Sari, A.H. Effects of laser fluence on the Cd(OH)2/CdO nanostructures produced by pulsed laser ablation method. Opt Quant Electron 51, 88 (2019). https://doi.org/10.1007/s11082-019-1809-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-019-1809-9