Abstract
Poor working memory functioning is commonly found in schizophrenia. A number of studies have now tested whether non-invasive brain stimulation can improve this aspect of cognitive functioning. This report used meta-analysis to synthesise the results of these studies to examine whether transcranial electrical stimulation (tES) or repetitive transcranial magnetic stimulation (rTMS) can improve working memory in schizophrenia. The studies included in this meta-analysis were sham-controlled, randomised controlled trials that utilised either tES or rTMS to treat working memory problems in schizophrenia. A total of 22 studies were included in the review. Nine studies administered rTMS and 13 administered tES. Meta-analysis revealed that compared to sham/placebo stimulation, neither TMS nor tES significantly improved working memory. This was found when working memory was measured with respect to the accuracy on working memory tasks (TMS studies: Hedges’ g = 0.112, CI95: −0.082, 0.305, p = .257; tES studies Hedges’ g = 0.080, CI95: −0.117, 0.277, p = .427) or the speed working memory tasks were completed (rTMS studies: Hedges’ g = 0.233, CI95: −0.212, 0.678, p = .305; tES studies Hedges’ g = −0.016, CI95: −0.204, 0.173, p = .871). For tES studies, meta-regression analysis found that studies with a larger number of stimulation sessions were associated with larger treatment effects. This association was not found for TMS studies. At present, rTMS and tES is not associated with a reliable improvement in working memory for individuals with schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Working memory impairments are common in people with schizophrenia (Forbes, Carrick, McIntosh, & Lawrie, 2009; Heinrichs & Zakzanis, 1998). There is evidence indicating that the level of working memory impairment is related to functional outcomes in this group (Green, 1996; Green, Kern, & Heaton, 2004; Zaragoza Domingo, Bobes, Garcia-Portilla, Morralla & EPICOG-SCH Study Group, 2015). Given this, considerable research has been conducted to examine whether this aspect of cognitive functioning can be improved (Hasan, Strube, Palm, & Wobrock, 2016b; Marder, 2006; Wykes, Huddy, Cellard, McGurk, & Czobor, 2011). To date, psychotropic medications have not been found to be effective in improving working memory (Manschreck & Boshes, 2007; Nielsen et al., 2015). Cognitive remediation therapy has also been examined, but appears to have a modest effect (Kambeitz-Ilankovic et al., 2019; Wykes et al., 2011). More recently, non-invasive brain stimulation has been explored as a treatment option (Brunoni & Vanderhasselt, 2014; Hill, Fitzgerald, & Hoy, 2016; Jiang et al., 2019; Martin, McClintock, Forster, & Loo, 2016; Mervis, Capizzi, Boroda, & MacDonald, 2017). This report summarises research that has examined whether non-invasive brain stimulation can improve working memory in individuals with schizophrenia.
Working Memory in Schizophrenia
Working memory can be conceptualised as a memory system that temporarily stores and processes verbal and visuo-spatial information (Baddeley, 2003; D’Esposito, 2007). Functional neuroimaging studies have examined the neural activation patterns associated with working memory (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009; Rottschy et al., 2012; Wager & Smith, 2003). From this research, the dorsolateral prefrontal cortex (DLPFC) has been identified as a key structure supporting working memory processes (Minzenberg et al., 2009; Rottschy et al., 2012; Wager & Smith, 2003). The evidence suggests that the DLPFC plays a role in manipulating verbal and visuo-spatial information by controlling attention, inhibiting interfering stimuli and integrating information (Barbey, Koenigs, & Grafman, 2013; D’Esposito & Postle, 2015; Lara & Wallis, 2015). Generally, the left hemisphere of the DLPFC shows lateralisation for verbal information, and the right for visuo-spatial information (Banich, 1998; Belger & Banich, 1998; Nagel, Herting, Maxwell, Bruno, & Fair, 2013; Reuter-Lorenz et al., 2000). However, when the demands of working memory are high during verbal and visuo-spatial tasks, bilateral activation is observed in the DLPFC to cope with task demands (Höller-Wallscheid, Thier, Pomper, & Lindner, 2017).
Poor working memory in schizophrenia has been linked to abnormal DLPFC activation (Callicott et al., 2003; Jiang et al., 2015; Minzenberg et al., 2009; Potkin et al., 2009; Royer et al., 2009). In schizophrenia, hypo-activation of the DLPFC during tasks of working memory is commonly reported in the literature (Minzenberg et al., 2009). Hypo-activation of the DLPFC appears to be related to encoding and the maintenance of information, especially as the number of items to be remembered increases (Anticevic, Repovs, & Barch, 2013; Metzak et al., 2012). Hyper-activation of the DLPFC has also been reported in schizophrenia (Callicott et al., 2003; Jiang et al., 2015; Potkin et al., 2009; Royer et al., 2009). Hyper-activation of the DLPFC is typically observed when participants with schizophrenia show working memory performance equivalent to healthy controls (Callicott et al., 2003; Jiang et al., 2015; Royer et al., 2009). The higher levels of DLPFC activiation in this instance, likely reflects inefficient neural processing of the information (Callicott et al., 2003; Potkin et al., 2009).
The nature of working memory problems in schizophrenia has also been examined using electroencephalography (Barr et al., 2010; Basar-Eroglu et al., 2007; Chen et al., 2014; Haenschel et al., 2009). When neuronal populations fire synchronously, they generate brain waves or oscillations (Gandal, Edgar, Klook, & Siegel, 2012). This brain wave activity can be extracted from an electroencephalogram (Gandal et al., 2012). Evidence from this research suggests prefrontal neural oscillatory activity is atypical in schizophrenia, particularly in the gamma band frequency range (> 30 Hz) (Haenschel & Linden, 2011; Uhlhaas & Singer, 2010, 2015). Gamma oscillations are thought to have a key role in supporting working memory functions (Gandal et al., 2012; Haenschel & Linden, 2011; Uhlhaas & Singer, 2013). In schizophrenia, altered GABAergic neural transmission in the DLPFC, which is responsible for generating and modulating gamma activity, may lead to impaired gamma oscillatory activity and to working memory deficits in this population (Chen et al., 2014; Lewis, 2000; Lewis, Curley, Glausier, & Volk, 2012; Sun et al., 2011).
In schizophrenia, a small number of studies have demonstrated that gamma oscillations are not optimally regulated during completion of working memory tasks. One finding in this area is that, gamma oscillatory power does not increase as the demands on short-term storage and processing also increase (Barr et al., 2010; Basar-Eroglu et al., 2007; Chen et al., 2014; Haenschel et al., 2009). For example, on the n-back task a sequence of targets is presented; the participant’s task is to determine whether the target on a current trial is the same as the target presented 1, 2 or 3 (or more) trials ago. In healthy controls, gamma power increases as the distance between the target on the current and past trial increases (Barr et al., 2011; Basar-Eroglu et al., 2007). However, in schizophrenia, frontal gamma power remains high, regardless of the demands of the task (Barr et al., 2011; Basar-Eroglu et al., 2007). In addition to working memory tasks, gamma oscillations in schizophrenia are also atypical at rest and during other mental tasks, although findings are inconsistent across studies (Gandal et al., 2012; Uhlhaas & Singer, 2013).
Can Non-Invasive Brain Stimulation Improve Working Memory in Schizophrenia?
An outstanding question is whether non-invasive brain stimulation, specifically, transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES), over the DLPFC can improve working memory in individuals with schizophrenia (Brunoni & Vanderhasselt, 2014; Hill et al., 2016; Jiang et al., 2019; Mervis et al., 2017). TMS delivers brief, high-intensity magnetic pulses close to the surface of the scalp. This is facilitated through the use of a coil that induces electrical currents in a focal area underneath the region of the cortex stimulated (Hallett, 2007). The frequency of TMS pulses can have either an excitatory or inhibitory effect on the neural activity of the targeted brain region. Low frequency TMS (e.g., 1 Hz stimulation) can decrease excitability, while high frequency TMS (e.g., > 5 Hz stimulation) can increase excitability (Hallett, 2007). The application of repeated TMS pulses is referred to as repetitive transcranial magnetic stimulation (rTMS). Compared to a single pulse of TMS, rTMS is able to modulate neural activity beyond the stimulation period (Klomjai, Katz, & Lackmy-Vallée, 2015). Another form of rTMS is theta burst stimulation (TBS). This type of stimulation uses gamma frequency trains applied at a theta rhythm (Demeter, 2016).
In tES, a weak electrical current is administered to the brain via two or more electrodes, an anode and a cathode, that are placed on the scalp. The electrical current passes between the anode and cathode (Tortella et al., 2015). In transcranial direct current stimulation (tDCS), an electrical current is administered at a constant rate over time (Antal & Paulus, 2013; Nitsche et al., 2008). In transcranial alternating current stimulation (tACS), an alternating current is applied to the brain with the goal of entraining neural oscillations at a given frequency range (Herrmann, Rach, Neuling, & Strüber, 2013). Compared to TMS, the weak electrical current from tES does not induce or inhibit action potentials in the underlying neuronal populations (Chase, Boudewyn, Carter, & Phillips, 2020). Instead, tES may modulate neural activity by influencing the direction of electrical fields, to induce a change in the resting trans-membrane potential at the site of stimulation, as well as between the electrodes (Rawji et al., 2018; Ye & Steiger, 2015). Anodal stimulation is typically associated with the enhancement of motor and cognitive functioning, whereas cathodal stimulation is associated with the inhibition of motor and cognitive functioning (Coffman, Clark, & Parasuraman, 2014). However, the effects of stimulation on neuronal excitability are nuanced by several factors, including cellular morphology, intensity of stimulation and electrode placement (Chase et al., 2020; Liu et al., 2018; Rawji et al., 2018; Ye & Steiger, 2015).
A potential factor influencing the effectiveness of non-invasive brain stimulation is the application of stimulation in a single session versus multiple sessions (Baumer et al., 2003; Cirillo et al., 2017; Maeda, Keenan, Tormos, Topka, & Pascual-Leone, 2000; Monte-Silva et al., 2013). A single session of non-invasive brain stimulation can directly modulate neuronal activity to produce acute, but transient changes in brain excitability that typically last between 30 to 120 min post stimulation (Huang et al., 2017). However, to produce enduring changes in neuronal structure and connectivity, the changes in neuronal excitability need to be maintained over time (Cirillo et al., 2017; Monte-Silva et al., 2013). Therefore, the therapeutic effects of stimulation are more likely to occur after the application of multiple sessions (Baumer et al., 2003; Maeda et al., 2000; Monte-Silva et al., 2013). For example, a number of studies have demonstrated that consecutive sessions of non-invasive brain stimulation increase the magnitude and duration of the stimulation effect in the human motor cortex (Baumer et al., 2003; Maeda et al., 2000; Monte-Silva et al., 2013).
Past Meta-Analyses Examining the Effects of Non-Invasive Brain Stimulation on Working Memory in Schizophrenia
A number of studies have examined whether rTMS or tES can improve working memory in schizophrenia (Brunoni & Vanderhasselt, 2014; Hill et al., 2016; Jiang et al., 2019; Martin et al., 2016; Mervis et al., 2017). The rationale for using rTMS or tES to enhance working memory, follows research suggesting non-invasive brain stimulation can modulate irregular gamma activity in schizophrenia (Barr et al., 2011; Hoy, Bailey, Arnold, & Fitzgerald, 2015). To date, data from research specifically examining the effects of rTMS and tES on working memory in schizophrenia has been summarised in three meta-analyses.
Mervis et al. (2017) used meta-analysis to summarise the results of four studies examining the effects of tDCS on working memory in schizophrenia. All studies included in the review, administered tDCS over the left DLPFC. This position was identified using 10–20 EEG system and placing the anodal electrode over F3. There is evidence suggesting that F3 and F4 correspond to the left and right dorsolateral prefrontal cortex respectively (Herwig, Satrapi, & Schonfeldt-Lecuona, 2003). The stimulation protocols did differ between studies. The number of sessions in which stimulation was administered varied from 1 to 28. The stimulation applied varied from 1 mA to 2 mA. Results from the meta-analysis revealed tDCS did not significantly improve working memory compared to sham stimulation. The average standardised mean difference was 0.23 (CI95: −0.31, 0.77, p = .411).Footnote 1
In another meta-analysis, Martin et al. (2016) examined whether rTMS could improve working memory in schizophrenia. The meta-analysis summarised the findings from three studies. In these studies, the DLPFC was identified by placing the rTMS coil over F3/F4 in the 10–20 EEG system. Two studies used unilateral stimulation over F3 and one study used bilateral stimulation over F3/F4. Two studies used 10 Hz stimulation and one study used 20 Hz stimulation. Two studies administered rTMS over 15 sessions at a rate of 1000 pulses per session, and one study administered rTMS over 20 sessions at a rate of 1500 pulses per a session. Results from the meta-analysis showed that rTMS significantly improved working memory in schizophrenia when compared to sham stimulation. The average standardized mean difference was found to be 0.51 (CI95: 0.18, 0.83, p < .001).
Finally, a recent meta-analysis by Jiang et al. (2019) also examined whether rTMS could improve working memory in schizophrenia. This report summarised the results from seven studies that all administered rTMS over the DLPFC. Four studies used unilateral stimulation over F3, and three studies used bilateral stimulation over F3/F4. Five of the studies used 10 Hz stimulation and two used 20 Hz stimulation. The number of sessions in which stimulation was administered varied from 10, 15 and 20 sessions. The total pulses per a session varied from 1000 to 4000. Results from their meta-analysis showed that rTMS significantly improved working memory in schizophrenia relative to sham stimulation. The average standardized mean difference was found to be 0.34 (CI95: 0.08, 0.59, p = .009).
The Current Meta-Analysis
In the current report, meta-analysis was used to further examine whether non-invasive brain stimulation can improve working memory in schizophrenia. This review had two aims. The first was to provide an update concerning the effects of tES and rTMS on working memory in this clinical group. The initial meta-analyses (Mervis et al., 2017) summarising the literature on the effects of tES on working memory in schizophrenia consisted of four studies. To our knowledge, more studies have since been published bringing the total to at least 13 (Chang, Kao, Chao, & Chang, 2019; Göder et al., 2013; Gomes et al., 2018; Hoy, Arnold, Emonson, Daskalakis, & Fitzgerald, 2014; Hoy, Whitty, Bailey, & Fitzgerald, 2016; Jeon et al., 2018; Lindenmayer et al., 2019; Nienow, MacDonald, & Lim, 2016; Palm et al., 2016; Papazova et al., 2018; Rassovsky et al., 2018; Schwippel et al., 2018; Smith et al., 2015). Similarly, for rTMS, at least nine studies have now been published (Barr et al., 2011, 2013; Francis et al., 2019; Guse et al., 2013; Hasan et al., 2016a; Mohr et al., 2006; Rabany, Deutsch, & Levkovitz, 2014; Zheng, Guo, Li, Li, & Wang, 2012; Zhuo et al., 2019). Most studies investigating the effects of non-invasive brain stimulation on working memory in schizophrenia typically have small sample sizes (n < 20), and are underpowered to detect potentially clinically important treatment effects. By pooling these studies using meta-analysis, these effects are more likely to be detected.
The second aim of this report was to use moderator analysis to undertake a “head-to-head test” comparing the efficacy of rTMS and tES to treat working memory in schizophrenia. Based on the meta-analyses undertaken so far, it seems that rTMS but not tES might be able to improve working memory in schizophrenia. However, it is not yet known whether this is still the case given more recent studies.
Method
Literature Search
Studies included in the meta-analysis were identified using MEDLINE, PsycINFO, CINAHL, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) electronic databases. An initial search was conducted in September 2017 and then repeated in June 2019. The search syntax used to identify studies is presented in Appendix Tables 4, 5 and 6.
Selection Criteria
Studies were included in the meta-analysis if they met the following criteria. First, participants were required to have a diagnosis of schizophrenia or schizoaffective disorder confirmed by a psychiatrist or using a structured diagnostic interview. Second, the study was required to have presented a working memory task that involved temporarily storing and processing verbal or non-verbal information. This included tasks such as the digit span task, n-back task, visuospatial working memory task, self-ordered pointing task (SOPT), Brief Assessment of Cognition (BACS), digit sequencing (Keefe et al., 2004), working memory tasks from the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, 2019) and tasks from the MATRICS Consensus Cognitive Battery (MCCB) working memory domain (Kern et al., 2011; Nuechterlein et al., 2008). Studies using verbal fluency tasks (Dlabac-de Lange et al., 2015; Prikryl et al., 2012; Zheng et al., 2012) were excluded from the review since they do not specifically target working memory processes. Third, the study was required to have compared either rTMS or tES to sham stimulation. Fourth, rTMS or anodal tES needed to have been administered over the DLPFC. Only studies published in a peer-review journal were included. The studies could be published in any language.
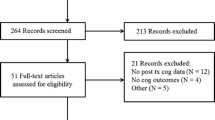
A PRISMA flowchart showing the selection of studies included in the review is presented in Fig. 1. A total of 22 studies were identified and included in this review. Of this total, nine studies examined the effects of rTMS on working memory in schizophrenia (Barr et al., 2011, 2013; Francis et al., 2019; Guse et al., 2013; Hasan et al., 2016a; Mohr et al., 2006; Rabany et al., 2014; Zheng et al., 2012; Zhuo et al., 2019). All these studies used excitatory stimulation protocols. Three studies stimulated the DLPFC sequentially over F3 and F4 in a counterbalanced order (Barr et al., 2011, 2013; Francis et al., 2019). The remaining 13 studies administered tES as the intervention (Chang et al., 2019; Göder et al., 2013; Gomes et al., 2018; Hoy et al., 2014, 2016; Jeon et al., 2018; Lindenmayer et al., 2019; Nienow et al., 2016; Palm et al., 2016; Papazova et al., 2018; Rassovsky et al., 2018; Schwippel et al., 2018; Smith et al., 2015). For studies that administered tDCS, anodal stimulation over F3, F4 or over both F3 and F4 was used. Two studies administered tACS (Göder et al., 2013; Hoy et al., 2016).
Meta-Analytic Approach
Two sets of meta-analyses were undertaken in this review. The first examined the effects of tES and rTMS on accuracy measures of working memory. The second examined the effects of non-invasive brain stimulation on reaction times measured as participants completed a working memory task. For both accuracy and reaction time effect sizes, an overall meta-analysis was undertaken that combined the results of both rTMS and tES studies. The goal of these analyses was to determine whether, overall, non-invasive brain stimulation improved working memory in schizophrenia. Additional meta-analyses were then conducted that summarised the results of rTMS and tES studies separately.
In the studies included in this review, working memory was measured using short-term recall tasks (e.g., digit span, Corsi blocks) or n-back tasks. One of the challenges synthesising effect sizes from n-back tasks was that not all studies used the same n-back ‘load.’ For example, Hoy et al. (2014) and Hoy et al. (2016) presented a 2-back task to participants. In the study by Barr et al. (2011), Papazova et al. (2018) and Schwippel et al. (2018), participants were presented with 1-, 2- and 3-back tasks. It could be that non-invasive brain stimulation is effective at high loads, but not low loads. To take this possibility into account, effect sizes from the n-back task were analysed using two methods. The first involved using meta-analysis to compute an average effect size based on all studies irrespective of the n-back load. The second involved undertaking separate meta-analyses that averaged effect sizes from n-back tasks with the same load. Specifically, meta-analyses were undertaken that averaged effect sizes from studies that administered 0-, 1-, 2 and 3-back tasks. For completeness, we report results at a specific n-back load even if only one effect size was available.
Effect Size Calculations
The results from each study were summarised using Hedges’ g. Specifically, from each study data from tables, figures, and results from statistical tests were extracted in order to compute Hedges’ g and its variance. For all but one study, Hedges’ g compared the change from baseline in working memory between the rTMS/tES and sham stimulation conditions. That is, the pre-post-test change in working memory functioning. To compute this effect size, both pre- and post-test data reported in each study were used. In one study, Hoy et al. (2014), only post-stimulation data were collected. For all studies, Hedges’ g was computed so that positive values indicated that rTMS/tES improved working memory, relative to sham stimulation. Negative Hedges’ g values indicated that working memory was superior following sham stimulation, compared to non-invasive brain stimulation.
The studies included in this review used either parallel (k = 16) or cross-over designs (k = 6; See Table 1). The tES studies comprised a combination of parallel and cross-over designs. All rTMS studies used parallel designs. In a parallel design, participants are randomly assigned to either stimulation or sham condition. For a cross-over design, participants complete both stimulation and sham conditions (Elbourne, Altman, Higgins, Curtin, & Vail, 2002). When computing an effect size using data extracted from a cross-over trial, the correlation between scores from sham and stimulation conditions needs to be taken into account, as they are from the same participants. In a parallel design, scores from sham and stimulation conditions are treated as independent, since they are from different participants. Given differences in the dependency of the data between parallel design and cross-over studies, separate sets of equations were used to compute Hedges’ g and variance for these different designs.
Equations for Computing Effect Sizes for Parallel and Cross-over Trials
Computing Hedges’ g for parallel- and cross-over trials involved standardising the change in scores from baseline, by the pooled standard deviation of changes for stimulation and sham conditions. Then a correction factor, notated as J, is applied to the standardised mean difference (SMD) and variance. The correction is needed because, the standardised mean difference overestimates the population effect size when sample sizes are small (Hedges & Olkin, 1985). For example, when the sample size is 20, the standardised mean difference overestimates the population effect by 4% (Durlak, 2009). The correction factor J corrects the standardised mean difference using the study’s sample size. Given that the sample sizes of most of the studies included in this meta-analysis were typically between 10 and 20, Hedges’ g was considered the most appropriate effect size for this meta-analysis to avoid overestimating treatment effects. Equations (1) and (2) were used to compute Hedges’ g and variance for parallel trials respectively and, Eqs. (3) and (4) for computing the corresponding values for cross over trials.
Where:
∆MStimulation /∆MSham = Change in working memory score from baseline following rTMS/tES/sham stimulation. For Hoy et al. (2014), ∆MStimulation and ∆MSham comprised post-stimulation data.
- nStimulation/nSham:
-
sample size for rTMS/tES/sham conditions.
- npairs:
-
total number of pairs in cross-over trial.
- ∆SStimulation/∆SSham:
-
Standard deviation of the change score between baseline and rTMS/tES/sham.
- Sdifference:
-
Standard deviation of the differences scores between rTMS/tES and sham conditions in cross over trials.
- r :
-
correlation between rTMS/tES and sham change scores.
For 12 studies, the data required to compute Hedge’s g were obtained from descriptive statistics or results from statistical tests (Francis et al., 2019; Hasan et al., 2016a; Hoy et al., 2014, 2016; Lindenmayer et al., 2019; Mohr et al., 2006; Nienow et al., 2016; Palm et al., 2016; Rabany et al., 2014; Smith et al., 2015; Zheng et al., 2012; Zhuo et al., 2019). However, for the remaining 10 studies (Barr et al., 2011; Barr et al., 2013; Chang et al., 2019; Gomes et al., 2018; Guse et al., 2013; Hoy et al., 2014; Jeon et al., 2018; Papazova et al., 2018; Rassovsky et al., 2018; Schwippel et al., 2018) the necessary information needed to compute the change in standard deviation needed (i.e., ∆S for parallel trials and Sdifference for cross-over trials) for Eqs. 1 or 3 was not available. For these studies, this value was imputed using Eq. 5. However, to compute Eq. 5, the correlation between baseline and stimulation (i.e., pre-post-test) scores is required. This value was not presented in these studies. At present there is no universally agreed upon method for dealing with this missing correlation value in meta-analysis. This value must therefore be estimated (Pearson & Smart, 2018). For this report, following Follmann, Elliott, Suh, and Cutler (1992), the correlation was conservatively set at 0.5.
Where:
- rbaseline, stimulation:
-
correlation between baseline and stimulation scores.
- SBaseline:
-
standard deviation of baseline scores.
- SStimulation:
-
standard deviation of stimulation (i.e., tES, rTMS, sham) scores.
For six cross-over trials, insufficient information was reported to compute Eq. 3 (Göder et al., 2013; Hoy et al., 2014, 2016; Papazova et al., 2018; Rassovsky et al., 2018; Schwippel et al., 2018). This was because the correlation between scores from stimulation and sham conditions was not available (notated as r in Eq. 3). This is a common issue associated with the meta-analysis of cross-over trials (Curtin, Altman, & Elbourne, 2002; Elbourne et al., 2002). To overcome this problem, the value of this correlation was estimated. For this meta-analysis the correlation between stimulation and sham change from baseline scores was therefore set at zero. That is, it is assumed scores in the stimulation and sham conditions are independent. This value has been used in a previous meta-analysis of cross-over trials (Jennings, Davies, Higgins, Anzures-Cabrera, & Broadley, 2012).
Types of Tasks Used to Measure Working Memory
The studies included in this meta-analysis assessed working memory using either n-back or short-term recall tasks (see Table 1). On n-back tasks, performance can be measured using accuracy and reaction times. Thus, separate effect sizes were computed for accuracy and reaction time data. For accuracy data, positive Hedges’ g values indicated that rTMS/tES was associated with higher accuracy scores compared to sham stimulation. Negative Hedges’ g values indicated sham stimulation was associated with higher accuracy scores compared to rTMS/tES. For reaction time data, positive Hedges’ g values indicated that non-invasive brain stimulation was associated with faster reaction times compared to sham stimulation. Negative Hedges’ g values indicated sham stimulation was associated with faster reaction times compared to rTMS/tES.
A total of 13 studies (see Table 1) used short-term recall tasks to measure the effects of stimulation on working memory (Chang et al., 2019; Francis et al., 2019; Göder et al., 2013; Gomes et al., 2018; Hasan et al., 2016a; Jeon et al., 2018; Lindenmayer et al., 2019; Mohr et al., 2006; Palm et al., 2016; Rabany et al., 2014; Smith et al., 2015; Zheng et al., 2012; Zhuo et al., 2019). Common to these tasks is that participants were asked to recall an increasing string consisting of digits, letters or a visuo-spatial sequence. For these studies, working memory performance was measured only in terms of accuracy. That is, the number of items correctly recalled. For studies using these short-term recall tasks, positive Hedges’ g values indicated rTMS/tES was associated with recalling a larger number of elements (i.e., digits, letters, visuo-spatial sequenced) compared to sham stimulation. Negative Hedges’ g values indicated sham stimulation was associated with recalling a larger number of elements compared to rTMS/tES. All individual effect sizes computed from each study are available for download via the Open Science Framework platform (https://osf.io/3darh/?view_only=f2df690695e248628546a9c586f94612).
Studies with Multiple Outcomes or Multiple Comparisons
For five studies, the effects of non-invasive brain stimulation were reported using multiple outcomes (Barr et al., 2011, 2013; Chang et al., 2019; Göder et al., 2013; Mohr et al., 2006). For example, in two studies, the results from an n-back task were reported separately for target and non-target trials (Barr et al., 2011, 2013). In another, results were reported separately for word and picture versions of the n-back task (Nienow et al., 2016). For two studies assessing working memory using a digit span task, results were presented separately for forward and backward trials (Chang et al., 2019; Göder et al., 2013) and in one study, results were presented from verbal and non-verbal variants of the task (Mohr et al., 2006). For these studies, a composite effect size was created. Specifically, separate effect sizes and variances were first computed for each outcome variable and then, an average effect size and variance was computed.
Three studies compared two stimulation interventions to a single sham group. In the study by Zheng et al. (2012), the effects of rTMS at 10 Hz and 20 Hz on working memory were compared to a single sham stimulation group. Hoy et al. (2014) compared the effects of 1 mA and 2 mA tDCS to a single sham stimulation group. Also, Hoy et al. (2016) compared the effects of tDCS and tACS to a single sham stimulation group. For these studies, it was decided to compute separate effect sizes for each different TMS/tES protocols. For example, in the study by Zheng et al. (2012), separate effect sizes were computed that compared the effectiveness of 10 Hz to sham stimulation and 20 Hz to sham stimulation. One limitation with this approach is that summary data from the same sham group are treated as an independent sample, which in turn leads to an underestimation of the standard error when undertaking meta-analysis. However, since this process was undertaken for only three studies, with relatively small sample sizes, the effect on the standard error of the meta-analysis was negligible.
Meta-Analytic Procedures
Study level effect sizes were averaged using a random effects model (Borenstein, Hedges, Higgins, & Rothstein, 2011; Hedges & Olkin, 1985). For all meta-analyses, alpha was set at .05 (two-tailed). Additionally, the amount of ‘true’ heterogeneity or systematic influences between study level effect sizes was measured using the I2 statistic (Thompson & Higgins, 2002). This statistic summarises, as a percentage, differences in study level effect sizes that are due to systematic influence. In this meta-analysis we interpreted I2 values according to the guidelines outlined by Thompson and Higgins (2002) whereby values of 25%, 50% and 75% correspond to low, moderate and high levels of heterogeneity respectively.
Mixed-effects moderator analysis (Borenstein et al., 2011) was used to undertake a “head-to-head test” comparing the efficacy of rTMS and tES to treat working memory in schizophrenia. That is, whether there was a significant difference in the average effect sizes from rTMS studies compared to tES studies. Mixed-effects moderator analysis was undertaken using Comprehensive Meta-Analysis Software 2.0. Finally, exploratory random effects meta-regression (Thompson & Higgins, 2002) was undertaken to examine the effects of different stimulation protocols on effect sizes.
Risk of Bias
The risk of bias was assessed using the Cochrane Collaboration’s Risk of Bias Tool (Higgins & Altman, 2008). This tool assesses whether a study is at high, low or unclear risk of selection bias, performance bias, detection bias, attrition bias and reporting bias in intervention studies.
Publication Bias
For each meta-analysis, publication bias was assessed using Egger’s Test (Egger, Davey Smith, Schneider, & Minder, 1997). This test assesses publication bias on the basis of the distribution of effect sizes around the weighted average effect size. In the absence of publication bias, effect sizes are expected to be symmetrically distributed around the weighted average effect size. When publication bias is present, effect sizes are asymmetrically distributed around the weighted average effect size. For example, effect sizes reporting null or negative effects may be missing from the distribution. The extent the distribution of effect sizes is asymmetrically distributed was formally tested using Egger’s Test (Egger et al., 1997). A significant result indicates the effect sizes are asymmetrically distributed and publication bias may be present.
Results
Methodological Characteristics of Included Studies
Table 1 presents a summary of the methodological characteristics of the studies included in this review. Seven studies administered rTMS, one study administered both rTMS and theta burst stimulation (TBS), and one study administered rTMS using a deep TMS coil. The sample sizes ranged from 16 to 100 participants. For six rTMS studies, stimulation was administered over the F3 electrode position in the EEG 10–20 system. In three studies, rTMS was administered bilaterally and sequentially over both F3 and F4 electrode positions with the order of administration counterbalanced. Pulses were delivered at 10 Hz, 20 Hz or 50 Hz. There was some variability with respect to the number of pulses administered per session as well as the number of sessions participants completed.
In 13 studies tES was administered. Eleven studies administered tDCS, one study administered tACS and one study administered both tDCS and tACS. The sample sizes ranged from 10 to 60 participants. For 12 of the tES studies, anodal stimulation was applied over the F3 electrode. For one study, anodal stimulation was applied over the F4 electrode. The cathode was most commonly placed on the contralateral supraorbital or orbitofrontal area. In two studies, the cathode was placed over the temporo-parietal junction, midway between the T3 and P3 electrode. One study placed the cathode over the F4 electrode. The stimulation intensity varied from 1 mA or 2 mA. In two studies tACS was applied. There was variability in the number of sessions participants completed, this ranged from a single session to 40 sessions.
Across all studies working memory was most commonly assessed using variants of the n-back or digit span tasks. With respect to the n-back tasks, 0–1-, 2- and 3-back versions of the tasks were administered. On these tasks, participants are asked to indicate whether a presented visual or auditory stimulus matches a previously displayed item shown n-previous positions. In two studies working memory was assessed using variants of the digit span task in which participants aim to temporarily remember a string of digits or spatial locations.
Summary of Participant Characteristics of Studies Included in the Meta-Analyses
Table 2 shows participant characteristics of the studies included in this review that examined the effects of rTMS and tES on working memory in schizophrenia. Of the nine studies using rTMS, the average age of the participants was 39.76 years (SD = 10.82). Of the total sample, 20% were female. The Positive and Negative Syndrome Scale (PANSS) was used in all studies as a clinical measure of symptom severity at baseline. The PANSS total score was reported in the majority of studies except for Barr et al. (2013), Mohr et al. (2006) and Rabany et al. (2014). The mean PANSS total score for the intervention group was 65.95 (SD = 12.57). The mean PANSS total score for the control ‘sham’ group was 66.41 (SD = 11.10). In all studies participants were treated with antipsychotic medication. Most studies except for Barr et al. (2011), Barr et al. (2013) and Mohr et al. (2006) reported that participants remained on a stable dose of antipsychotic medications for the duration of the trial.
Of the 13 tES studies included, the average age of participants was 41.21 years (SD = 7.30). Of the total sample, 30% were female. Most studies provided a measure of the PANSS total score, except for Nienow et al. (2016) and Rassovsky et al. (2018) who assessed clinical severity using the Brief Psychiatric Rating Scale. For the tES studies that used a parallel study design, the mean PANSS score for the intervention group was 78.11 (SD = 6.67) and 76.41 for the control group (SD = 7.31). For the studies that used a cross-over design, the mean PANSS score was 55.66 (SD = 7.69). All participants were treated with antipsychotic medication. All studies except for Gomes et al. (2018), Papazova et al. (2018) and Smith et al. (2015) reported that clients remained on a stable dose of antipsychotic medications.
The Effect of Non-Invasive Brain Stimulation on Accuracy in Schizophrenia
Figure 2 presents a forest plot showing the results of the meta-analyses examining the effect of tES and TMS on working memory. The first notable trend to emerge from the analyses is that all results were non-significant. Neither rTMS or tES were found to reliably improve working memory in schizophrenia, with respect to accuracy. This result was found for short-term recall and n-back tasks. Results from Egger’s test was not significant for all meta-analyses. Thus, publication bias does not appear to be leading to an over-estimation of effect sizes.
Another trend to emerge from these meta-analyses is that in almost all instances, the I2 statistic was close to zero for the rTMS studies. This indicates that despite differences in participant characteristics and stimulation protocols noted above, there appears to be no detectable systematic influence on study-level effect sizes. That is, at present it seems that differences in individual study effect sizes can be attributed to random error (i.e., chance rather than by the influence of one or more independent/predictor variables). However, for the tES studies, there does appear to be one or more systematic influences affecting the magnitude of effect sizes. This is reflected by the small-to-medium levels of heterogeneity observed for the working memory outcome measures. This indicates that differences between study level effect sizes for tES studies may not only be due to chance or random error.
The next set of analyses tested whether tES was superior to rTMS with respect to improving accuracy scores on working memory tasks in schizophrenia. The results from mixed-effects moderator analysis revealed no significant differences between rTMS and tES effect sizes. Specifically, the average effect size computed from ‘All Tasks’ (Q (1) = 0.052, p = .820), ‘short-term recall tasks’ (Q (1) = 0.001, p = .971), ‘n-Back (all loads)’ (Q (1) = 0.003, p = .960), ‘1-Back’ (Q (1) = 0.238, p = .625), ‘2-Back’ (Q (1) = 3.019, p = .082) and ‘3-Back’ (Q (1) = 0.395, p = .530) were all non-significant.
The Effect of Non-invasive Brain Stimulation on Reaction Times in Schizophrenia
Figure 3 presents results from meta-analyses examining the effects of non-invasive brain stimulation on reaction times. All effect sizes were from n-back tasks. In all cases, the average effect size was not found to be significant. This result was found when effect sizes were averaged across all loads from the n-back as well as at specific n-back loads. Another trend to note in Fig. 3 is that for all analyses the I2 statistic was zero. This indicates differences in study level effect sizes can be best explained with respect to random error. For reaction times, publication bias does not appear to be leading to an overestimation of effect sizes. With the exception of three meta-analyses, the results of Egger’s tests were not significant. To estimate the effect of this bias on the significant Egger’s test results, an unbiased estimate of these average effect sizes was computed using Duval and Tweedie’s (2000) trim-and-fill method. In each case the unbiased estimate yielded a comparable result to the observed value (Unbiased Estimate: All Studies, n-back (all loads): Hedges’ g = −.047, p = .568; All studies, 3-Back: Hedges’ g = −.040, p = .657; tES Studies n-Back (all loads): Hedges’ g = −.087, p = .318). Thus, publication bias does not appear to be leading to an over-estimation of effect sizes.
The results from mixed-effects moderator analysis revealed no significant differences between rTMS and tES effect sizes on n-Back (all loads) (Q (1) = 1.015, p = .314), 1-Back (Q (1) = 0.621, p = .431) and 2-Back (Q (1) = 0.687, p = .407). However, on the 3-Back (Q (1) = 5.352, p = .021), the average effect size from TMS studies was significantly larger compared to tES studies.
Using Meta-Regression to Examine the Influence of Stimulation Protocols on Effect Sizes
The next set of analyses examined the effect of varying stimulation protocols on study level effect sizes. As noted previously, Table 1 showed considerable variability with respect to the stimulation protocols in the rTMS and tES studies. For example, for both rTMS and tES studies, differences were present in relation to the type of stimulation (rTMS, deep rTMS, TBS, tDCS, tACS), stimulation site, intensity of the stimulation, duration and number of sessions. Meta-regression was used to test whether one or more of these variables was related to study-level differences in effect sizes.
Separate meta-regression analyses were undertaken for rTMS and tES studies. For rTMS studies, separate meta-regression analyses tested whether (1) the type of rTMS used (rTMS, Deep rTMS, TBS), (2) stimulation site (bilateral vs. unilateral), (3) stimulation frequency, (4) number of pulses or the (5) number of stimulation sessions, predicted study level effect sizes. For tES studies, we tested whether (1) the type of tES (tDCS vs tACS), (2) stimulation site (bilateral vs. unilateral), (3) stimulation frequency, (4) current density, (5) stimulation duration, (6) stimulation frequency or the (7) number of sessions, predicted effect sizes. The study-level values for each of these predictor variables is presented in Table 1. The outcome variable used in each study were study level effect sizes. For each study, where applicable, effect sizes were averaged across reaction time and accuracy, as well as across different spans from the n-back task. The results from the meta-regression analyses are summarised in Table 3. The only significant result found was that the number of test sessions for tES studies predicted effect sizes. For illustrative purposes, this result is shown in Fig. 4. For this analysis, the positive beta value indicates studies with more stimulation sessions, observed larger positive effect sizes.
Scatterplot showing the association between the number of stimulations sessions and study level effect sizes for tES study. Broken line shows the regression line computed using parameters estimated from the meta-regression analyses. The size of the data points in the figure reflect the weight of the effect sizes in the model
Sensitivity Analyses: The Effect of Correlation Assumptions on Meta-Analyses
As noted in methods, for a number of studies, assumptions needed to be made about the magnitude of the correlation between (1) pre-post test scores and (2), stimulation and sham scores. For 10 studies, the correlation between pre-post test scores was fixed at 0.5. For six tES cross-sectional studies, the correlation between sham and stimulation scores was set at 0.0. The impact of using these correlation values on the meta-analyses was tested by re-running the meta-analysis and systematically varying correlation values. For the pre-post-test correlation, meta-analyses were re-run with the correlation values of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8 and 0.9. For the stimulation and sham scores correlation used to compute effect sizes for cross-over studies, meta-analyses were run using values of 0.0, 0.5 and 0.9. Sensitivity analyses were run separately for rTMS and tES studies and also for accuracy and reaction time data. The results are presented in Fig. 5. The key result to note from this figure is that the average effect sizes remain stable and all meta-analyses returned a non-significant result irrespective of the correlation value.
Results from the Risk of Bias Assessment
Results from the assessment of bias using Cochrane Collaboration’s Risk for rTMS and tES studies are presented in Figs. 6 and 7 respectively. Amongst rTMS studies and tES studies, high or unclear risk was commonly assessed for selection, performance and reporting bias.
Results from the assessment of bias for rTMS studies. A summary plot is presented that illustrates the percentage of bias for the rTMS studies included in the meta-analysis. The summary plot is colour coded to represent the percentage of low risk, unclear risk or high risk of bias in the following areas, selection bias, performance bias, detection bias, attrition bias and reporting bias
Results from the assessment of bias for tES studies. A summary plot is presented that illustrates the percentage of bias for the tES studies included in the meta-analysis. The summary plot is colour coded to represent the percentage of low risk, unclear risk or high risk of bias in the following areas, selection bias, performance bias, detection bias, attrition bias and reporting bias
Discussion
This report summarised research examining the effectiveness of non-invasive brain stimulation for improving working memory in schizophrenia. The meta-analyses presented in this report summarised results from 22 studies representing data from 737 participants across different measures of working memory. Three key findings emerged. First, the evidence does not indicate that rTMS or tES improves working memory in schizophrenia. This interpretation of the results is forwarded from multiple analyses that examined the effect of non-invasive brain stimulation on different tasks and outcome variables (i.e., accuracy & reaction time). In all cases non-significant treatment effects were observed. In addition, unlike previous meta-analysis, attempts were made to take into account correlated data structures arising from pre-post-test designs and cross-over trials. Even when different correlation values were examined (see Fig. 5), meta-analyses were still non-significant. Second, head-to-head tests did not find evidence that rTMS was more effective at treating working memory compared to tES. The one exception was that for reaction times on the 3-back task. The effect size for rTMS was found to be larger compared to the tES effect sizes. Finally, meta-regression analysis largely indicated that different rTMS and tES stimulation protocols were not systematically influencing effect sizes. However, one meta-regression analysis indicated that, for tES studies, there was a positive association between the number of stimulation sessions and effect sizes. Overall, the results of this review indicate that, at present, neither rTMS or tES improves working memory in schizophrenia.
Results from the meta-analyses showing tES does not improve working memory in schizophrenia are consistent with the preliminary findings of Mervis et al. (2017). Their meta-analysis summarised the results of four tDCS studies. Overall, tDCS was not found to have a significant treatment effect. The current report replicated this result even when synthesising results from 13 studies. However, in the current report, small to moderate degrees of systematic influence were found for the meta-analyses examining the effect of tES on accuracy scores. This result indicates that differences between study level effect sizes are not attributable to random error. Meta-regression analyses revealed that tES studies with more stimulation sessions, were associated with larger treatment effects. That is, applying electrical stimulation to the DLPFC over multiple sessions appears to enhance the therapeutic effects of tES. This finding might suggest that to improve working memory via non-invasive brain stimulation, changes in neural structure and connectivity is required. This in turn requires multiple stimulation sessions (Baumer et al., 2003; Maeda et al., 2000; Monte-Silva et al., 2013). In terms of the number of sessions required, it is noted that in the study by Lindenmayer et al. (2019), participants completed 40 tDCS sessions. That study also observed the largest positive effect size on working memory in schizophrenia. To improve working memory in this clinical group using tDCS, 40 or more sessions may therefore be required.
Our finding that rTMS does not improve working memory in schizophrenia is not consistent with the meta-analyses presented by Martin et al. (2016) and Jiang et al. (2019). In the review by Jiang et al. (2019), which was based on seven studies, rTMS was found to significantly improve working memory. One explanation for the difference in findings may relate to the tasks included in the meta-analysis. Jiang et al. (2019) included studies in their meta-analyses that did not specifically assess working memory. One study included in their review assessed general intelligence (Mittrach et al., 2010). Three studies included in the review assessed the effects of the stimulation on tasks that are typically associated with the assessment of declarative memory (Dlabac-de Lange et al., 2015; Mogg et al., 2007; Wölwer et al., 2014). These tasks largely depend on the hippocampus and temporal lobes (Lezak, Howieson, Loring, Hannay, & Fischer, 2004). Given this, the results of Jiang et al. might provide new evidence that rTMS can be effective for improving declarative memory functioning in schizophrenia.
Another finding to emerge from the current meta-analyses of rTMS studies was the absence of systematic influences on study level effect sizes. The I2 value for accuracy and reaction time data was repeatedly found to be 0%. This value was also found by Jiang et al. (2019). This indicates at least for two meta-analyses, the differences in study findings can be attributed to random error. This result was further confirmed by the meta-regression analyses undertaken in this report, which showed that various stimulation protocols did not predict study effect sizes. However, one avenue for further research might be to examine the number of rTMS sessions on working memory outcomes. As noted above, for tES studies, the number of stimulation sessions was positively related to effect sizes. This result was not found for TMS studies. It could be more TMS sessions are required to improve working memory. As noted above, in the tES literature, 40 stimulation sessions were associated with the largest improvement in working memory. In the rTMS literature reviewed as part of this report, the maximum number of stimulation sessions administered to participants was 20 (see Table 1). It could be that rTMS might also be able to improve working memory in schizophrenia, but substantially more stimulation sessions are needed to modify the neural networks that support this memory system.
An additional aim of this review was to directly compare the effectiveness of tES and rTMS interventions. Results from the moderator analyses revealed non-significant differences between average effect sizes from rTMS and tES studies, for most accuracy and reaction time outcome variables. For these variables, there is insufficient evidence to indicate that rTMS or tES are associated with a superior treatment effect. The one exception to this trend was observed for reaction time effect sizes from 3-back tasks. For this one outcome variable, rTMS was associated with a greater improvement compared to tES. However, this result should be interpreted with caution. Results from the meta-analysis showed that for tES studies, the average effect size for reaction times from 3-back tasks was negative and approached statistical significance (Hedges’ g = −.206, p = .072; See Fig. 3). For TMS studies, a large positive effect size that was also close to significance was observed (Hedges’ g = .497, p = .077). It is difficult to determine whether the significant moderator result was due to superior working memory performance following sham tES, or the positive treatment effects associated with rTMS. This is further compounded by the small number of studies/effect sizes that were represented in this analysis (k = 2 for rTMS studies and k = 4 for tES studies). The superiority of rTMS or tES will need to be investigated in future research.
Limitations and Avenues for Future Research
The results from this review indicate that at present, the evidence does not indicate non-invasive brain stimulation can reliably improve working memory in schizophrenia. Additionally, for the rTMS studies in particular, there does not appear to be a particular stimulation protocol that optimally improves working memory in this group. For the rTMS studies the I2 values were consistently low. A low I2 can be interpreted to indicate that systematic influences may be present, but not detectable in the meta-analysis. This issue may have arisen because of the small sample sizes associated with most all studies. To overcome this problem, multiple studies with larger sample sizes will be required. Given the difficulty recruiting and testing individuals with schizophrenia for non-invasive brain stimulation research, multi-site randomised controlled trials may be more appropriate. Additional considerations about whether other stimulation protocols that vary in their focality, length of treatment, intensity, frequency and number of treatments and inter-treatment intervals will also need to be investigated in future research.
Another issue that needs to be taken into account in future research arises from the assessment of bias undertaken in this report. Overall, the included studies were assessed to be at high risk of selection and performance bias. Most commonly, the randomisation procedure was not outlined and the personnel administering the stimulation were not blinded to the treatment condition. These types of bias are a concern in meta-analyses should significant treatment effects have been observed. This is because selection and performance bias may lead to an overestimation of treatment effects (Higgins & Altman, 2008).
A final limitation of this report is that the majority of studies used rTMS and tDCS over the DLPFC to modulate working memory in schizophrenia. In this review, only two studies used tACS, one study used deep rTMS and one study used TBS. Other forms of non-invasive brain stimulation were included in the search syntax (e.g. single pulse TMS, transcranial random noise stimulation, high definition tDCS) but no studies using these other variants of stimulation were identified for inclusion in the analyses. Therefore, the finding of the current review does not rule out that other forms of stimulation might improve working memory in this clinical population.
Conclusions
The meta-analyses indicate that rTMS and tES when applied over the DLPFC are not associated with a reliable improvement of working memory in schizophrenia. This interpretation of the results was forwarded from multiple meta-analyses that all revealed non-significant treatment effects. There is also insufficient evidence to suggest whether rTMS or tES is superior as a treatment for working memory problems. An interesting finding to emerge from this report is that increasing the number of stimulation sessions might lead to greater treatment effects. However, this was only observed for the tES studies. Due to an absence of systematic influence on study level effect sizes for the rTMS studies, it is unclear whether particular stimulation protocols are able to have a greater effect on working memory functioning. Additional research investigating the effects of different stimulation protocols using larger sample sizes is needed.
Notes
p value computed from confidence intervals.
References
Antal, A., & Paulus, W. (2013). Transcranial alternating current stimulation (tACS). Frontiers in Human Neuroscience, 7, 317. https://doi.org/10.3389/fnhum.2013.00317
Anticevic, A., Repovs, G., & Barch, D. M. (2013). Working memory encoding and maintenance deficits in schizophrenia: Neural evidence for activation and deactivation abnormalities. Schizophrenia Bulletin, 39(1), 168–178. https://doi.org/10.1093/schbul/sbr107
Baddeley, A. (2003). Working memory: Looking back and looking forward. Nature Reviews Neuroscience, 4(10), 829–839. https://doi.org/10.1038/nrn1201
Banich, M. T. (1998). The missing link: The role of interhemispheric interaction in attentional processing. Brain and Cognition, 36(2), 128–157. https://doi.org/10.1006/brcg.1997.0950
Barbey, A. K., Koenigs, M., & Grafman, J. (2013). Dorsolateral prefrontal contributions to human working memory. Cortex, 49(5), 1195–1205. https://doi.org/10.1016/j.cortex.2012.05.022
Barr, M. S., Farzan, F., Arenovich, T., Chen, R., Fitzgerald, P. B., & Daskalakis, Z. J. (2011). The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS One, 6(7), e22627. https://doi.org/10.1371/journal.pone.0022627
Barr, M. S., Farzan, F., Rajji, T., Voineskos, A., Blumberger, D., Arenovich, T., … Daskalakis, Z. (2013). Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biological Psychiatry, 73(6), 510–517. https://doi.org/10.1016/j.biopsych.2012.08.020
Barr, M. S., Farzan, F., Tran, L. C., Chen, R., Fitzgerald, P. B., & Daskalakis, Z. J. (2010). Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophrenia Research, 121(1–3), 146–152. https://doi.org/10.1016/j.schres.2010.05.023
Basar-Eroglu, C., Brand, A., Hildebrandt, H., Karolina Kedzior, K., Mathes, B., & Schmiedt, C. (2007). Working memory related gamma oscillations in schizophrenia patients. International Journal of Psychophysiology, 64(1), 39–45. https://doi.org/10.1016/j.ijpsycho.2006.07.007
Baumer, T., Lange, R., Liepert, J., Weiller, C., Siebner, H. R., Rothwell, J. C., & Munchau, A. (2003). Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. NeuroImage, 20(1), 550–560. https://doi.org/10.1016/s1053-8119(03)00310-0
Belger, A., & Banich, M. T. (1998). Costs and benefits of integrating information between the cerebral hemispheres: A computational perspective. Neuropsychology, 12(3), 380–398.
Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2011). Introduction to meta-analysis. Hoboken: Wiley.
Brunoni, A. R., & Vanderhasselt, M. A. (2014). Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain and Cognition, 86(1), 1–9. https://doi.org/10.1016/j.bandc.2014.01.008
Callicott, J. H., Mattay, V. S., Verchinski, B. A., Marenco, S., Egan, M. F., & Weinberger, D. R. (2003). Complexity of prefrontal cortex dysfunction in schizophrenia: More than up or down. American Journal of Psychiatry, 160(12), 2209–2215. https://doi.org/10.1176/appi.ajp.160.12.2209
Cambridge Cognition. (2019). CANTAB (Cognitive assessment software). Retrieved from www.cantab.com
Chang, C. C., Kao, Y. C., Chao, C. Y., & Chang, H. A. (2019). Enhancement of cognitive insight and higher-order neurocognitive function by fronto-temporal transcranial direct current stimulation (tDCS) in patients with schizophrenia. Schizophrenia Research, 208, 430–438. https://doi.org/10.1016/j.schres.2018.12.052
Chase, H. W., Boudewyn, M. A., Carter, C. S., & Phillips, M. L. (2020). Transcranial direct current stimulation: A roadmap for research, from mechanism of action to clinical implementation. Molecular Psychiatry, 25(2), 397–407. https://doi.org/10.1038/s41380-019-0499-9
Chen, C. M., Stanford, A. D., Mao, X., Abi-Dargham, A., Shungu, D. C., Lisanby, S. H., … Kegeles, L. S. (2014). GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage. Clinical, 4, 531–539. https://doi.org/10.1016/j.nicl.2014.03.007
Cirillo, G., Di Pino, G., Capone, F., Ranieri, F., Florio, L., Todisco, V., … Di Lazzaro, V. (2017). Neurobiological after-effects of non-invasive brain stimulation. Brain Stimulation, 10(1), 1–18. https://doi.org/10.1016/j.brs.2016.11.009
Coffman, B. A., Clark, V. P., & Parasuraman, R. (2014). Battery powered thought: Enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. NeuroImage, 85(3), 895–908. https://doi.org/10.1016/j.neuroimage.2013.07.083
Curtin, F., Altman, D. G., & Elbourne, D. (2002). Meta-analysis combining parallel and cross-over clinical trials. I: Continuous outcomes. Statistics in Medicine, 21(15), 2131–2144. https://doi.org/10.1002/sim.1205
D’Esposito, M. (2007). From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society of London, 362(1481), 761–772. https://doi.org/10.1098/rstb.2007.2086
D’Esposito, M., & Postle, B. R. (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66(1), 115–142. https://doi.org/10.1146/annurev-psych-010814-015031
Demeter, E. (2016). Enhancing cognition with theta burst stimulation. Current Behavioral Neuroscience Reports, 3(2), 87–94. https://doi.org/10.1007/s40473-016-0072-7
Dlabac-de Lange, J. J., Bais, L., van Es, F. D., Visser, B. G., Reinink, E., Bakker, B., … Knegtering, H. (2015). Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: Results of a multicenter double-blind randomized controlled trial. Psychological Medicine, 45(6), 1263–1275. https://doi.org/10.1017/S0033291714002360
Durlak, J. A. (2009). How to select, calculate, and interpret effect sizes. Journal of Pediatric Psychology, 34(9), 917–928. https://doi.org/10.1093/jpepsy/jsp004
Duval, S., & Tweedie, R. (2000). Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463.
Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal, 315(7109), 629–634. https://doi.org/10.1136/bmj.315.7109.629
Elbourne, D. R., Altman, D. G., Higgins, J. P. T., Curtin, F., Worthington, H. V, & Vail, A. (2002). Meta-analyses involving cross-over trials: Methodological issues. International Journal of Epidemiology, 31(1), 140–149. https://doi.org/10.1093/ije/31.1.140
Follmann, D., Elliott, P., Suh, I., & Cutler, J. (1992). Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology, 45(7), 769–773. https://doi.org/10.1016/0895-4356(92)90054-q
Forbes, N. F., Carrick, L. A., McIntosh, A. M., & Lawrie, S. M. (2009). Working memory impairments in schizophrenia: A meta-analysis. Psychological Medicine, 39(6), 889–905. https://doi.org/10.1017/S0033291708004558
Francis, M. M., Hummer, T. A., Vohs, J. L., Yung, M. G., Visco, A. C., Mehdiyoun, N. F., … Breier, A. (2019). Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: A pilot study. Brain Imaging and Behavior, 13(3), 852–861. https://doi.org/10.1007/s11682-018-9902-4
Gandal, M. J., Edgar, J. C., Klook, K., & Siegel, S. J. (2012). Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology, 62(3), 1504–1518. https://doi.org/10.1016/j.neuropharm.2011.02.007
Göder, R., Baier, P. C., Beith, B., Baecker, C., Seeck-Hirschner, M., Junghanns, K., & Marshall, L. (2013). Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophrenia Research, 144(1–3), 153–154. https://doi.org/10.1016/j.schres.2012.12.014, 153
Gomes, J. S., Trevizol, A. P., Ducos, D. V., Gadelha, A., Ortiz, B. B., Fonseca, A. O., … Dias, A. M. (2018). Effects of transcranial direct current stimulation on working memory and negative symptoms in schizophrenia: A phase II randomized sham-controlled trial. Schizophrenia Research Cognition, 12, 20–28. https://doi.org/10.1016/j.scog.2018.02.003
Green, M. F. (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? The American Journal of Psychiatry, 153(3), 321–330. https://doi.org/10.1176/ajp.153.3.321
Green, M. F., Kern, R. S., & Heaton, R. K. (2004). Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophrenia Research, 72(1), 41–51. https://doi.org/10.1016/j.schres.2004.09.009
Guse, B., Falkai, P., Gruber, O., Whalley, H., Gibson, L., Hasan, A., … Wobrock, T. (2013). The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls-a randomized placebo-controlled, double-blind fMRI study. Behavioural Brain Research, 237, 300–307. https://doi.org/10.1016/j.bbr.2012.09.034
Haenschel, C., Bittner, R. A., Waltz, J., Haertling, F., Wibral, M., Singer, W., … Rodriguez, E. (2009). Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. The Journal of Neuroscience, 29(30), 9481–9489. https://doi.org/10.1523/JNEUROSCI.1428-09.2009
Haenschel, C., & Linden, D. (2011). Exploring intermediate phenotypes with EEG: Working memory dysfunction in schizophrenia. Behavioural Brain Research, 216(2), 481–495. https://doi.org/10.1016/j.bbr.2010.08.045
Hallett, M. (2007). Transcranial magnetic stimulation: A primer. Neuron, 55(2), 187–199. https://doi.org/10.1016/j.neuron.2007.06.026
Hasan, A., Guse, B., Cordes, J., Wölwer, W., Winterer, G., Gaebel, W., … Wobrock, T. (2016a). Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: Results from a multicenter-randomized sham-controlled trial. Schizophrenia Bulletin, 42(3), 608–618. https://doi.org/10.1093/schbul/sbv142
Hasan, A., Strube, W., Palm, U., & Wobrock, T. (2016b). Repetitive noninvasive brain stimulation to modulate cognitive functions in schizophrenia: A systematic review of primary and secondary outcomes. Schizophrenia Bulletin, 42(Suppl 1), S95–S109. https://doi.org/10.1093/schbul/sbv158
Hedges, L. V., & Olkin, I. (1985). Statistical methods for meta-analysis. New York: Academic Press.
Heinrichs, R. W., & Zakzanis, K. K. (1998). Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology, 12(3), 426–445. https://doi.org/10.1037/0894-4105.12.3.426
Herrmann, C. S., Rach, S., Neuling, T., & Strüber, D. (2013). Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Frontiers in Human Neuroscience, 7, 279. https://doi.org/10.3389/fnhum.2013.00279
Herwig, U., Satrapi, P., & Schonfeldt-Lecuona, C. (2003). Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography, 16(2), 95–99. https://doi.org/10.1023/B:BRAT.0000006333.93597.9d
Higgins, J. P. T., & Altman, G. A. (2008). Assessing risk of bias in included studies. In J. P. T. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions: Cochrane book series (pp. 187–235). West Sussex: John Wiley & Sons. https://doi.org/10.1002/9780470712184.ch8
Hill, A. T., Fitzgerald, P. B., & Hoy, K. E. (2016). Effects of anodal transcranial direct current stimulation on working memory: A systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimulation, 9(2), 197–208. https://doi.org/10.1016/j.brs.2015.10.006
Höller-Wallscheid, M. S., Thier, P., Pomper, J. K., & Lindner, A. (2017). Bilateral recruitment of prefrontal cortex in working memory is associated with task demand but not with age. Proceedings of the National Academy of Sciences, 114(5), E830–E839. https://doi.org/10.1073/pnas.1601983114
Hoy, K. E., Arnold, S. L., Emonson, M. R., Daskalakis, Z. J., & Fitzgerald, P. B. (2014). An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophrenia Research, 155(1–3), 96–100. https://doi.org/10.1016/j.schres.2014.03.006
Hoy, K. E., Bailey, N. W., Arnold, S. L., & Fitzgerald, P. B. (2015). The effect of transcranial direct current stimulation on gamma activity and working memory in schizophrenia. Psychiatry Research, 228(2), 191–196. https://doi.org/10.1016/j.psychres.2015.04.032
Hoy, K. E., Whitty, D., Bailey, N., & Fitzgerald, P. B. (2016). Preliminary investigation of the effects of γ-tACS on working memory in schizophrenia. Journal of Neural Transmission, 123(10), 1205–1212. https://doi.org/10.1007/s00702-016-1554-1
Huang, Y. Z., Lu, M. K., Antal, A., Classen, J., Nitsche, M., Ziemann, U., … Rothwell, J. (2017). Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clinical Neurophysiology, 128(11), 2318–2329. https://doi.org/10.1016/j.clinph.2017.09.007
Jennings, A. L., Davies, A. N., Higgins, J. P. T., Anzures-Cabrera, J., & Broadley, K. E. (2012). Opioids for the palliation of breathlessness in advanced disease and terminal illness. The Cochrane Database of Systematic Reviews, 7, 1465–1858. https://doi.org/10.1002/14651858.CD002066.pub2
Jeon, D. W., Jung, D. U., Kim, S. J., Shim, J. C., Moon, J. J., Seo, Y. S., … Kim, Y. N. (2018). Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: A double-blind 12-week study. Schizophrenia Research, 197, 378–385. https://doi.org/10.1016/j.schres.2017.12.009
Jiang, S., Yan, H., Chen, Q., Tian, L., Lu, T., Tan, H. Y., … Zhang, D. (2015). Cerebral inefficient activation in schizophrenia patients and their unaffected parents during the N-back working memory task: A family fMRI study. PLoS One, 10(8), e0135468. https://doi.org/10.1371/journal.pone.0135468
Jiang, Y., Guo, Z., Xing, G., He, L., Peng, H., Du, F., … Mu, Q. (2019). Effects of high-frequency transcranial magnetic stimulation for cognitive deficit in schizophrenia: A meta-analysis. Frontiers in Psychiatry, 10, 135. https://doi.org/10.3389/fpsyt.2019.00135
Kambeitz-Ilankovic, L., Betz, L. T., Dominke, C., Haas, S. S., Subramaniam, K., Fisher, M., … Kambeitz, J. (2019). Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: Revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neuroscience and Biobehavioral Reviews, 107, 828–845. https://doi.org/10.1016/j.neubiorev.2019.09.031
Keefe, R., Goldberg, T. E., Harvey, P. D., Gold, J. M., Poe, M. P., & Coughenour, L. (2004). The brief assessment of Cognition in schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research, 68(2–3), 283–297. https://doi.org/10.1016/j.schres.2003.09.011
Kern, R. S., Gold, J. M., Dickinson, D., Green, M. F., Nuechterlein, K. H., Baade, L. E., … Marder, S. R. (2011). The MCCB impairment profile for schizophrenia outpatients: Results from the MATRICS psychometric and standardization study. Schizophrenia Research, 126(1–3), 124–131. https://doi.org/10.1016/j.schres.2010.11.008
Klomjai, W., Katz, R., & Lackmy-Vallée, A. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Annals of Physical and Rehabilitation Medicine, 58(4), 208–213. https://doi.org/10.1016/j.rehab.2015.05.005
Lara, A. H., & Wallis, J. D. (2015). The role of prefrontal cortex in working memory: A mini review. Frontiers in Systems Neuroscience, 9, 173. https://doi.org/10.3389/fnsys.2015.00173
Lewis, D. A. (2000). GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Research Reviews, 31(2–3), 270–276. https://doi.org/10.1016/S0165-0173(99)00042-9
Lewis, D. A., Curley, A. A., Glausier, J. R., & Volk, D. W. (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences, 35(1), 57–67. https://doi.org/10.1016/j.tins.2011.10.004
Lezak, M. D., Howieson, D. B., Loring, D. W., Hannay, H. J., & Fischer, J. S. (2004). Neuropsychological assessment (4th ed.). New York: Oxford University Press.
Lindenmayer, J. P., Kulsa, M. K. C., Sultana, T., Kaur, A., Yang, R., Ljuri, I., … Khan, A. (2019). Transcranial direct-current stimulation in ultra-treatment-resistant schizophrenia. Brain Stimulation, 12(1), 54–61. https://doi.org/10.1016/j.brs.2018.10.002
Liu, A., Vöröslakos, M., Kronberg, G., Henin, S., Krause, M. R., Huang, Y., … Buzsáki, G. (2018). Immediate neurophysiological effects of transcranial electrical stimulation. Nature Communications, 9(1), 5092. https://doi.org/10.1038/s41467-018-07233-7
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., & Pascual-Leone, A. (2000). Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 111(5), 800–805. https://doi.org/10.1016/s1388-2457(99)00323-5
Manschreck, T. C., & Boshes, R. A. (2007). The CATIE schizophrenia trial: Results, impact, controversy. Harvard Review of Psychiatry, 15(5), 245–258. https://doi.org/10.1080/10673220701679838
Marder, S. R. (2006). The NIMH-MATRICS project for developing cognition-enhancing agents for schizophrenia. Dialogues in Clinical Neuroscience, 8(1), 109–113.
Martin, D. M., McClintock, S. M., Forster, J., & Loo, C. K. (2016). Does therapeutic repetitive transcranial magnetic stimulation cause cognitive enhancing effects in patients with neuropsychiatric conditions? A systematic review and meta-analysis of randomised controlled trials. Neuropsychology Review, 26(3), 295–309. https://doi.org/10.1007/s11065-016-9325-1
Mervis, J. E., Capizzi, R. J., Boroda, E., & MacDonald, A. W. (2017). Transcranial direct current stimulation over the dorsolateral prefrontal cortex in schizophrenia: A quantitative review of cognitive outcomes. Frontiers in Human Neuroscience, 11, 44. https://doi.org/10.3389/fnhum.2017.00044
Metzak, P. D., Riley, J. D., Wang, L., Whitman, J. C., Ngan, E. T. C., & Woodward, T. S. (2012). Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophrenia Bulletin, 38(4), 803–813. https://doi.org/10.1093/schbul/sbq154
Minzenberg, M. J., Laird, A. R., Thelen, S., Carter, C. S., & Glahn, D. C. (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry, 66(8), 811–822. https://doi.org/10.1001/archgenpsychiatry.2009.91
Mittrach, M., Thünker, J., Winterer, G., Agelink, M. W., Regenbrecht, G., Arends, M., … Cordes, J. (2010). The tolerability of rTMS treatment in schizophrenia with respect to cognitive function. Pharmacopsychiatry, 43(3), 110–117. https://doi.org/10.1055/s-0029-1242824
Mogg, A., Purvis, R., Eranti, S., Contell, F., Taylor, J. P., Nicholson, T., … McLoughlin, D. M. (2007). Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: A randomized controlled pilot study. Schizophrenia Research, 93(1–3), 221–228. https://doi.org/10.1016/j.schres.2007.03.016
Mohr, P., Rodriguez, M., Novák, T., Kopeček, M., Horáček, J., Hendrychová, Y., … Seifertova, D. (2006). Repetitive transcranial magnetic stimulation and rehabilitation of cognitive functions in schizophrenia. Psychiatrie, 10(1), 7–15.
Monte-Silva, K., Kuo, M. F., Hessenthaler, S., Fresnoza, S., Liebetanz, D., Paulus, W., & Nitsche, M. A. (2013). Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimulation, 6(3), 424–432. https://doi.org/10.1016/j.brs.2012.04.011
Nagel, B. J., Herting, M. M., Maxwell, E. C., Bruno, R., & Fair, D. (2013). Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain and Cognition, 82(1), 58–68. https://doi.org/10.1016/j.bandc.2013.02.007
Nielsen, R. E., Levander, S., Kjaersdam Telleus, G., Jensen, S., Østergaard Christensen, T., & Leucht, S. (2015). Second-generation antipsychotic effect on cognition in patients with schizophrenia-a meta-analysis of randomized clinical trials. Acta Psychiatrica Scandinavica, 131(3), 185–196. https://doi.org/10.1111/acps.12374
Nienow, T. M., MacDonald, A. W., & Lim, K. O. (2016). TDCS produces incremental gain when combined with working memory training in patients with schizophrenia: A proof of concept pilot study. Schizophrenia Research, 172(1–3), 218–219. https://doi.org/10.1016/j.schres.2016.01.053
Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori, A., Lang, N., Antal, A., … Pascual-Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1(3), 206–223. https://doi.org/10.1016/j.brs.2008.06.004
Nuechterlein, K. H., Green, M. F., Kern, R. S., Baade, L. E., Barch, D. M., Cohen, J. D., … Marder, S. R. (2008). The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. The American Journal of Psychiatry, 165(2), 203–213. https://doi.org/10.1176/appi.ajp.2007.07010042
Palm, U., Keeser, D., Hasan, A., Kupka, M. J., Blautzik, J., Sarubin, N., … Padberg, F. (2016). Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: A double-blind, sham-controlled proof-of-concept study. Schizophrenia Bulletin, 42(5), 1253–1261. https://doi.org/10.1093/schbul/sbw041
Papazova, I., Strube, W., Becker, B., Henning, B., Schwippel, T., Fallgatter, A. J., … Hasan, A. (2018). Improving working memory in schizophrenia: Effects of 1 mA and 2 mA transcranial direct current stimulation to the left DLPFC. Schizophrenia Research, 202, 203–209. https://doi.org/10.1016/j.schres.2018.06.032
Pearson, M. J., & Smart, N. A. (2018). Reported methods for handling missing change standard deviations in meta-analyses of exercise therapy interventions in patients with heart failure: A systematic review. PLoS One, 13(10), e0205952. https://doi.org/10.1371/journal.pone.0205952
Potkin, S. G., Turner, J. A., Brown, G. G., McCarthy, G., Greve, D. N., Glover, G. H., … FBIRN. (2009). Working memory and DLPFC inefficiency in schizophrenia: The FBIRN study. Schizophrenia Bulletin, 35(1), 19–31. https://doi.org/10.1093/schbul/sbn162
Prikryl, R., Mikl, M., Prikrylova Kucerova, H., Ustohal, L., Kasparek, T., Marecek, R., … Vanicek, J. (2012). Does repetitive transcranial magnetic stimulation have a positive effect on working memory and neuronal activation in treatment of negative symptoms of schizophrenia? Neuro Endocrinology Letters, 33(1), 90–97.
Rabany, L., Deutsch, L., & Levkovitz, Y. (2014). Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. Journal of Psychopharmacology, 28(7), 686–690. https://doi.org/10.1177/0269881114533600
Rassovsky, Y., Dunn, W., Wynn, J. K., Wu, A. D., Iacoboni, M., Hellemann, G., & Green, M. F. (2018). Single transcranial direct current stimulation in schizophrenia: Randomized, cross-over study of neurocognition, social cognition, ERPs, and side effects. PLoS One, 13(5), e0197023. https://doi.org/10.1371/journal.pone.0197023
Rawji, V., Ciocca, M., Zacharia, A., Soares, D., Truong, D., Bikson, M., … Bestmann, S. (2018). tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimulation, 11(2), 289–298. https://doi.org/10.1016/j.brs.2017.11.001
Reuter-Lorenz, P. A., Jonides, J., Smith, E. E., Hartley, A., Miller, A., Marshuetz, C., & Koeppe, R. A. (2000). Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience, 12(1), 174–187.
Rottschy, C., Langner, R., Dogan, I., Reetz, K., Laird, A. R., Schulz, J. B., … Eickhoff, S. B. (2012). Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage, 60(1), 830–846. https://doi.org/10.1016/j.neuroimage.2011.11.050
Royer, A., Schneider, F. C., Grosselin, A., Pellet, J., Barral, F. G., Laurent, B., … Lang, F. (2009). Brain activation during executive processes in schizophrenia. Psychiatry Research: Neuroimaging, 173(3), 170–176. https://doi.org/10.1016/j.pscychresns.2009.02.009
Schwippel, T., Papazova, I., Strube, W., Fallgatter, A. J., Hasan, A., & Plewnia, C. (2018). Beneficial effects of anodal transcranial direct current stimulation (tdcs) on spatial working memory in patients with schizophrenia. European Neuropsychopharmacology, 28(12), 1339–1350. https://doi.org/10.1016/j.euroneuro.2018.09.009
Smith, R. C., Boules, S., Mattiuz, S., Youssef, M., Tobe, R. H., Sershen, H., … Davis, J. M. (2015). Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophrenia Research, 168(1–2), 260–266. https://doi.org/10.1016/j.schres.2015.06.011
Sun, Y., Farzan, F., Barr, M. S., Kirihara, K., Fitzgerald, P. B., Light, G. A., & Daskalakis, Z. J. (2011). γ oscillations in schizophrenia: Mechanisms and clinical significance. Brain Research, 1413, 98–114. https://doi.org/10.1016/j.brainres.2011.06.065
Thompson, S. G., & Higgins, J. P. T. (2002). How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine, 21(11), 1559–1573. https://doi.org/10.1002/sim.1187
Tortella, G., Casati, R., Aparicio, L. V. M., Mantovani, A., Senço, N., D’Urso, G., … Brunoni, A. R. (2015). Transcranial direct current stimulation in psychiatric disorders. World Journal of Psychiatry, 5(1), 88–102. https://doi.org/10.5498/wjp.v5.i1.88
Uhlhaas, P. J., & Singer, W. (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews. Neuroscience, 11(2), 100–113. https://doi.org/10.1038/nrn2774
Uhlhaas, P. J., & Singer, W. (2013). High frequency oscillations and the neurobiology of schizophrenia. Dialogues in Clinical Neuroscience, 15(3), 301–313.
Uhlhaas, P. J., & Singer, W. (2015). Oscillations and neuronal dynamics in schizophrenia: The search for basic symptoms and translational opportunities. Biological Psychiatry, 77(12), 1001–1009. https://doi.org/10.1016/j.biopsych.2014.11.019
Wager, T. D., & Smith, E. E. (2003). Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(4), 255–274.
Wölwer, W., Lowe, A., Brinkmeyer, J., Streit, M., Habakuck, M., Agelink, M. W., … Cordes, J. (2014). Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimulation, 7(4), 559–563. https://doi.org/10.1016/j.brs.2014.04.011
Wykes, T., Huddy, V., Cellard, C., McGurk, S. R., & Czobor, P. (2011). A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. American Journal of Psychiatry, 168(5), 472–485. https://doi.org/10.1176/appi.ajp.2010.10060855
Ye, H., & Steiger, A. (2015). Neuron matters: Electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. Journal of Neuroengineering and Rehabilitation, 12(1), 65. https://doi.org/10.1186/s12984-015-0061-1
Zaragoza Domingo, S., Bobes, J., Garcia-Portilla, M. P., Morralla, C., & EPICOG-SCH Study Group. (2015). Cognitive performance associated to functional outcomes in stable outpatients with schizophrenia. Schizophrenia Research Cognition, 2(3), 146–158. https://doi.org/10.1016/j.scog.2015.03.002
Zheng, L. N., Guo, Q., Li, H., Li, C. B., & Wang, J. J. (2012). Effects of repetitive transcranial magnetic stimulation with different paradigms on the cognitive function and psychotic symptoms of schizophrenia patients. Journal of Peking University Health Science, 44(5), 732–736.
Zhuo, K., Tang, Y., Song, Z., Wang, Y., Wang, J., Qian, Z., … Liu, D. (2019). Repetitive transcranial magnetic stimulation as an adjunctive treatment for negative symptoms and cognitive impairment in patients with schizophrenia: A randomized, double-blind, sham-controlled trial. Neuropsychiatric Disease and Treatment, 15, 1141–1150. https://doi.org/10.2147/NDT.S196086
Funding
PGE is supported by a Future Fellowship from the Australian Research Council (FT160100077).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Sloan, N.P., Byrne, L.K., Enticott, P.G. et al. Non-Invasive Brain Stimulation Does Not Improve Working Memory in Schizophrenia: A Meta-Analysis of Randomised Controlled Trials. Neuropsychol Rev 31, 115–138 (2021). https://doi.org/10.1007/s11065-020-09454-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-020-09454-4