Abstract
The study was aimed to validate the efficacy of the pulsed Nd:YAG laser on nerve regeneration in a rat sciatic nerve crushed model. 54 Wistar rats were randomly assigned into three groups: shame control, crush control, and laser treated group. For the laser treated group, the pulsed Nd:YAG laser (10 Hz) with 350 mJ per pulse in energy density and 50 J/cm2 in fluence was applied extracorporeally at the lesion site for 12 min to daily deliver 500 J immediately and consecutive 9 days following the crush injury. At week 1, the apoptosis-related activities in the injured nerve were examined (n = 8/each group). The sciatic functional index (SFI) was measured preoperatively and weekly until 4 weeks after the index procedure. The injured nerve and the innervated gastrocnemius muscle histology were assessed at week 4 (n = 10/each group). At week 1, the laser group showed the significant less TUNEL-positive ratio (P < 0.05), and the lower expression of cleaved caspase3/procaspase-3 and beclin-2/beclin-2-associated protein X ratios compared with the crush control. Furthermore, the laser group revealed significantly better SFI since week 1 and throughout the study (P < 0.05, all) compared with the crush control. At week 4, the laser group showed significantly higher axon density, lower myelin g-ratio, and the corresponding higher glycogen expression (P < 0.05, all) in the gastrocnemius muscle compared with those in the crush control. The pulsed Nd:YAG might enhance the injured nerve regeneration via apoptosis inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral nerve injuries often leave patients with substantial sensory or motor deficits, and sometimes with chronic pain [1]. Although the advanced of microsurgical techniques, the functional recovery was not predictable [2,3,4,5,6]. After PNS injury, Wallerian degeneration with injured site apoptosis occurs which the axon degenerates distal to the injury site and leaded to myelin breakdown, or demyelination, and disruption of axonal connections at the lesion site [7, 8]. Several studies showed inhibition of apoptosis is beneficial in nerve regeneration process and nerve function preservation in sciatic nerve injury animal models [9,10,11,12].
Due to the technology improvement, high-intensity laser therapy was developed [13]. The pulsed Nd:YAG laser, one kind of high-intensity laser therapy, was used widely in clinical practice and showed well response in the treatment of neck myofascial pain [14], lumbar spine disorder [15], and knee osteoarthritis [13]. But, there was still lack of the treatment potency of pulsed Nd:YAG in peripheral nerve injury in clinical practice. Alayat et al. had showed the pulsed Nd:YAG laser in improved the sciatic nerve functional recovery after the crush injury in a rat model [16], but, the related mechanism is still unclear and there was a paucity of histology assessment.
Therefore, the aim of this study was to validate the efficacy of pulsed Nd:YAG laser therapy on nerve regeneration in a rat sciatic nerve crushed model and the effect on apoptosis. We hypothesized that pulsed Nd:YAG laser therapy enhanced nerve regeneration via apoptosis inhibition.
Method
Animal Model
54 Male Wistar rats weighing from 400 to 450 g were obtained from Bio-LASCO and housed in the Laboratory Animal Center of National Cheng Kung University in Taiwan. Rats were housed individually in a room with a 12-h light/dark cycle with lights on at 7:00 am and central air conditioning (25 ℃, 70% humidity) with free access to food and water. All procedures used in this study were approved and in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines of National Cheng Kung University (IACUC Approval No. 109263). Anesthesia of the rats was carried out under 3% (flow rate: 5 l/min) of isoflurane anesthesia by using an anesthetic machine (Panion & BF biotech Inc., Taipei, Taiwan). An incision from the right sciatic notch to the distal thigh was made. After bluntly dissecting the biceps femoris muscle, the sciatic nerve was exposed. The sciatic nerve was crushed using a hemostat for 5 min above the bifurcation of tibia nerve and peroneal nerve according to the previous study [17], and then the incision was closed with 4–0 non-absorbable nylon (Ethicon). 54 rats were randomly assigned into three groups: shame control group, which received only nerve exposing without crushing; crush control group, which received crushing without any treatment; pulsed Nd:YAG laser treated group, which received daily pulsed Nd:YAG laser (10 Hz) immediately and consecutive 9 days after crushing. The sciatic functional index (SFI) was examined before injury and weekly until 4 weeks after the index procedure. At week 1, eight rats per group were randomly sacrificed to examine the apoptosis activity via immunofluorescence and western blot analyses. At week 4, ten rats per group were sacrificed for the histological examination of the injured nerve and the innervated gastrocnemius muscle (Fig. 1). The flowchart of the study is presented as Supplementary Fig. 1.
The schematic diagram of the animal experiment design. This schematic diagram shows the design of the animal experiments. 54 Wistar rats were randomly assigned into three groups: shame control, crush control, and pulsed Nd:YAG laser treated group. In week 1, western blot and immunofluorescence stain were performed for analysis of the apoptosis activity and cell apoptosis rate separately. In week 4, the histology of sciatic nerve and gastrocnemius muscle were performed. The sciatic functional index was measured preoperatively and weekly until week 4. Shame control group, rats were received wound incision only; crush control group, rats were received sciatic nerve crush only; and Nd:YAG laser group, rats received daily pulsed Nd:YAG laser (500 J) immediately and consecutive 9 days after sciatic nerve crush
Laser Treatment Protocol
The pulsed Nd:YAG laser (HERO-3 laser, ASA, Arcugnano, Vicenza, Italy) was used in the high intensity laser therapy treated group. This Nd:YAG laser is the near infrared laser with 1064 nm in wave length, and 100 μs in pulse duration. The laser probe was perpendicular and close to the skin surface 1 cm in distance without touching. Scanned area was located at and around the injury site about 10 cm2 in area. The treatment parameters were 10 Hz pulsed, 350 mJ per pulse in energy density, 12 min in delivery time, lead to 50 J/cm2 in fluency density and 500 J total energy at lesion site [16].
Immunofluorescence and TUNEL Analyses
The immunofluorescence staining analysis was performed 7 days after injury. For immunofluorescence staining, paraffin-embedded sections receiving similar pretreatment were incubated with the antibody against myelin protein zero (MPZ, Cell Signaling Technology), followed by the Alexa Flour 594-conjugated secondary antibody (ThermoFisher Scientific) and observed by a fluorescence microscope. The DeadEnd™ fluorometric TUNEL system (Promega) was used to detect apoptotic cells in each section according to the standard protocol. DAPI was used for nuclear staining. The TUNEL and DAPI-double positive cells were counted by using Image J software (National Institutes of Health, USA) which presented as the apoptotic cells. The apoptotic cell ratio was shown as the number of apoptotic cells normalized to that of total DAPI-positive cells. In each group, six rats were assigned for this analysis.
Western Blot Analysis
The western blot analyses were performed 7 days after injury. Sciatic nerve tissues were collected and homogenized in tissue-lysis buffer. After centrifugation at 12,000 g for 15 min at 4 °C, the proteins in the supernatant were separated by electrophoresis in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to polyvinylidene difluoride (PVDF) membranes for electroblotting. Membranes were blocked with 5% non-fat skim milk and incubated at 4 °C overnight with the primary antibodies against cleaved caspase3 (C-Cas3, Cell Signaling Technology), procaspase-3 (p-Cas3, Cell Signaling Technology), Beclin-2 (BCL-2, Santa Cruz Biotechnology), and Beclin-2-associated protein X (BAX, Santa Cruz Biotechnology). After washing, membranes were incubated with secondary antibodies (Biolegend, California, USA) and quantitative control anti-actin antibodies (Sigma-Aldrich) at room temperature. Immunoblots were developed using enhanced chemiluminescent solution and analyzed with iBright FL1500 (Invitrogen, MA, USA) and quantified using Image J software.
Sciatic Functional Index
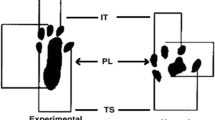
SFI was performed before injury and then weekly until 4 weeks after the index procedure. The hind-limb footprints of each rat were obtained using a walking track with a video-based system (Supplementary Fig. 2) as the current studies [18,19,20,21]. A Plexiglas chamber was made as walkway which 80 cm in length, 6 cm in width, and 12 cm in height, with a 45° tilting mirror placed under the chamber. The LED light was illuminated from the inferioranterior directions of the walkway. The soles on the tract was reflected at the tilted mirror. The reflected footprints can be simultaneously recorded by the digital camera (EX-F1; Casio, Japan) which 1 m in front of and at the same level as the walkway. The horizontal and vertical frames were made of the marked red dots (5 mm in diameter) at the lateral and plantar views of walking track corners respectively. The data were processed by a MATLAB program designed by our laboratory (MathWorks, Natick, MA). The resolution was 0.18 mm in the 50-cm walking track after an initial pixels/mm calibration. Using red circle markers, the system could be calibrated to calculate the pixel-to-distance ratio at any plane. The overall pixel to distance ratios were 0.21 mm/pixel for the bottom views. The SFI was calculated using paired measurements of the print length (PL), toe spread (TS) (first to fifth toe), and intermediary toe spread (IT) (second to fourth toe) [22]. The measurements of the control left foot (CPL, CTS, CIT) and of the corresponding right experimental foot (EPL, ETS, EIT) were recorded at each time point. The measurements were then incorporated into the SFI [22]. The formula is as follows [22]:
From the index calculated from the SFI formula, the 0 is normal sciatic nerve function and the − 100 is totally impairment sciatic nerve function.
Histological Assessments
The rats were sacrificed 4 weeks after injury for the histological examinations. The injured sciatic nerve at previously crushed area was harvested. The ipsilateral gastrocnemius muscle samples were also harvest at the muscle tendon junction. Nerve samples were fixed in 4% paraformaldehyde for 2 to 4 h and stored in 0.2 g glycine in 100 ml PBS prior to tissue embedding. On the day of tissue embedding, the nerves which were used in hematoxylin and eosin stain were immersed in 2% of osmium tetroxide for 2 h. After paraffin embedding, 5-μm of tissue sections were de-waxed in xylene and rehydrated in ethanol gradient and distilled water. The tissue sections were stained with hematoxylin for 4 min, and then stained with eosin for 20 s. The stained samples were rinsed with tap water, dehydrated in an ethanol gradient, and then mounted using xylene. The density of axon density, g-ratio were got from the average in 4 sections of each nerve sample. The myelin g-ratio is the ratio of the inner to the outer radius of the myelin sheath which better myelination showed lower g-ratio [23]. The density of axon density and g-ratio were examined by using Nikon H600L light microscope (Nikon Instruments Inc., NY, USA) at 1000 × magnification and quantified by using Image J 1.46 (National Institutes of Health, USA).
The paraffin sections of gastrocnemius were de-waxed in xylene, rehydrated in an ethanol gradient and distilled water, and then covered with periodic acid for 5 min. After rinsing in distilled water for 5 min, the sections were covered with Schiff reagent for 10 min. Tissue sections were counterstained with hematoxylin and then mounted using a mounting medium. In the periodic acid-Schiff (PAS) stain, the purple stain indicated a rich content of muscle glycogen and a normal function of muscle movement. A lower degree of muscle glycogen content and the decline of muscle movement showed shallow purple cross section, and the most severe impaired movement muscle showed gray appearance. The muscle purple positive area were examined by using Nikon H600L light microscope (Nikon Instruments Inc., NY, USA) at 200 × magnification. The hue value used for purple was measured in all slides, and the average hue “from 195 to 220” was used to evaluate the slides. The default hue width was used to set an adequate threshold for measuring positive PAS stained area which was calculated in a 144′412-pixel photograph and then presented as positive PAS stained area (μm2) as our previous studies quantified the positive trichrome staining area [19]. Furthermore, the positive stained area was analyzed using Image J 1.46 (National Institutes of Health, USA).
Statistical Analysis
Descriptive statistics, including means and standard deviations, were obtained in the three groups. Data were expressed as the mean ± standard deviation (SD). Differences in immunofluorescence and histology analysis among groups were analyzed using one-way ANOVA, followed by Dunnett’s multiple comparison test. Statistical significance was set at P < 0.05. The SFI difference among groups over time was analyzed using a mixed-way ANOVA, followed by Bonferroni post hoc comparisons. Data were analyzed with SPSS for Windows, version 22.0 (SPSS Inc, Chicago, IL, USA).
Results
Effects of Pulsed Nd:YAG Laser Treated on the Apoptosis in Crushed Sciatic Nerve
To clarify the role of pulsed Nd:YAG laser on the apoptosis in the early phase of the nerve repair process, the TUNEL-positive cell ratio and apoptosis-related proteins, including c-Cas3, p-Cas3, BAX, and BCL2, were examined. Enhanced c-Cas3/p-Cas3 and BAX/BCL2 ratiosand significantly higher TUNEL-positive cell ratio (5.54 ± 2.40% versus 0 ± 0%, P < 0.01) were observed in the crush control compared with the sham control. Following the pulsed Nd:YAG laser treatment, there were the decreased c-Cas3/p-Cas3 and BAX/BCL2 ratios, and the significantly lower TUNEL-positive cell ratio (0.81 ± 0.99%, P < 0.01) in the laser treated group in comparison with the crush control (Fig. 2).
The expression of apoptosis-related proteins and status Expression of apoptosis molecules and TUNEL staining in the sciatic nerve crushing model after pulse Nd:YAG laser treatment. Scale bar, 20 μm. A The apoptotic protein: Cleaved caspase3 (c-Cas3), procaspase3 (p-Cas3), BAX, and BCL2 were determined by immunoblotting from rats with shame control group, crush control group, Nd:YAG laser group. Shame control group, rats were received wound incision only; crush control group, rats were received sciatic nerve crush only; and Nd:YAG laser group, rats treated with pulsed Nd:YAG laser (500 J) daily for 10 days after sciatic nerve crush. B The apoptotic cell ratio: the above groups were subjected to TUNEL (green), and DAPI (blue) staining. The TUNEL and DAPI-positive cells were quantitated. Data are means ± SD. *P < 0.05. c-Cas3: cleaved caspase3, p-Cas3: procaspase3, BCL-2: beclin-2, BAX: beclin-2-associated protein X
Effects of Pulsed Nd:YAG Laser on the Recovery of Nerve Function After Sciatic Nerve Crush
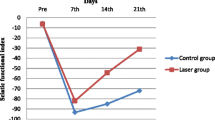
To examine the effects of pulsed Nd:YAG laser on the recovery of impaired sciatic nerve function, the sciatic functional index was assessed. The laser treated group revealed the significantly higher SFI since week 1 and throughout the study as compared with the crush control (− 79.04 ± 4.88 vs − 90.78 ± 5.92, − 72.02 ± 7.13 vs − 87.24 ± 6.28, − 59.05 ± 9.43 vs − 80.63 ± 6.18, − 43.74 ± 6.96 vs − 77.11 ± 6.27; week 1, week 2, week 3, week 4, respectively; all P < 0.005) (Fig. 3).
Sciatic functional index. The sciatic functional index was assessed pre-injury, 1 week, 2 weeks, 3 weeks and 4 weeks after sciatic nerve crush. Shame control group, rats were received wound incision only; crush control group, rats were received sciatic nerve crush only; and Nd:YAG laser group, rats rats received daily pulsed Nd:YAG laser (500 J) immediately and consecutive 9 days after sciatic nerve crush. Data are means ± SD. *P < 0.05, **P < 0.01, ***P < 0.005
Effects of Pulsed Nd:YAG Laser on Nerve Regeneration After Sciatic Nerve Crush and Associated Recovery of Muscle Atrophy
At week 4, significantly decreased axon density (80.17 ± 42.34 versus 131.56 ± 39.82/104μm2, P < 0.05) and significantly increased g-ratio (0.58 ± 0.04, 0.42 ± 0.05, P < 0.05) were observed in the crush control compared with the sham control. In the laser treated group, there were significantly increased axon density (122.01 ± 15.25/104μm2), and the decreased g-ratio (0.42 ± 0.02) in comparison with the crush control (P < 0.05, both; Fig. 4). PAS stain (glycogen expression) in rat gastrocnemius muscle were determined to examine the effects of pulsed Nd:YAG laser on the recovery of muscle atrophy. A significantly lower expression level of glycogen which showed less purple (19.85 × 103 ± 5.44 × 103, 98.02 × 103 ± 27.55 × 103 μm2, P < 0.005) was found in crush control group compared with shame control. The expression level of glycogen in the laser treated group significantly increased (66.77 × 103 ± 10.56 × 103, P < 0.05) compared with the crush control. (Fig. 5).
Histological study of the sciatic nerve A Morphology of the sciatic nerve. Scale bar, 10 μm. B The number of axons per 10,000 μm2. C The g-ratio of sciatic nerve. Shame control group, rats were received wound incision only; crush control group, rats were received sciatic nerve crush only; and Nd:YAG laser group, rats received daily pulsed Nd:YAG laser (500 J) immediately and consecutive 9 days after sciatic nerve crush. Data are means ± SD. *P < 0.05
Histological study for muscle atrophy of gastrocnemius muscle A The periodic acid-schiff stain of gastrocnemius muscle. Scale bar, 50 μm. B The integrated optical purple positive area of periodic acid-schiff stain. Shame control group, rats were received wound incision only; crush control group, rats were received sciatic nerve crush only; and Nd:YAG laser group, rats received daily pulsed Nd:YAG laser (500 J) immediately and consecutive 9 days after sciatic nerve crush. Data are means ± SD. *P < 0.05
Discussion
The current study evaluated the therapeutic effects of the pulsed Nd:YAG laser on peripheral nerve injury based on the functional and histological results, and the possible therapeutic mechanism in a rat model. We found that the pulsed Nd:YAG laser significantly improved the SFI since one week after the crush injury and throughout the study. At week 4, Nd:YAG laser improved the axon density and the myelin g-ratio in the injured nerve and the glycogen expression in the innervated gastrocnemius muscle. At week 1, the western blot and immunofluorescence stain showed the reduced apoptosis activity in the laser group compared with the crush control. Therefore, the pulsed Nd:YAG laser might enhance nerve regeneration via anti-apoptosis.
Some research showed there was no significant improvement in SFI after laser therapy in the rat sciatic nerve injury models [24, 25]. But, some showed SFI improvement after laser therapy [16, 26,27,28,29]. The conflict result might be due to due to different laser settings including power, wavelength, laser material, energy density, dose and type of laser [16, 30]. The Nd:YAG laser in the present study, differed from the traditional photobiomodulation which previously known as low level laser therapy, is one kind of high intensity laser with 1064 nm in wave length [31]. High intensity laser is more powerful and allow for deeper tissue penetration compared with photobiomodulation [32]. Alayat et al. reported that in a rat sciatic nerve crush injury model, the use of Nd:YAG laser at a fluence of 50 J/cm2 resulted in a better SFI compared to those at 4 and 10 J/cm2. [16]. In our work, we followed his protocol and found the laser group presenting the significantly better SFI since week 1 and throughout the study (week 4). At week 4, the crushed sciatic nerve histology in laser group showed significantly increased axon density and decreased g-ratio which hint better axon regeneration and myelination. The higher expression of PAS stain in gastrocnemius muscle in laser group represents better physiological function of muscle. Therefore, the pulsed Nd:YAG laser therapy reveals its therapeutic effects on the peripheral nerve injury according to our results. However, the laser setting in clinical practice still need further clinical studies to validate.
Thus, in our literature review, there were still no studies evaluate the effect of laser therapy in the injured site apoptosis. After peripheral nerve injury, Schwann and neuron cells die due to apoptosis which lead to myelin breakdown, or demyelination, and disruption of axonal connections [33].Therefore, the positive effect on nerve function recovery via apoptosis inhibition has been reported in several studies [9,10,11,12]. To date, the possible therapeutic mechanism of high intensity laser on peripheral nerve injury is still unclear. Our results suggested that pulsed Nd:YAG laser treatment can promote the nerve function recovery by inhibiting axon apoptosis by affecting the expression of apoptosis-related proteins. Following the sciatic nerve crushed injury, We found a remarked increase in the ratio of BAX/BCL-2 and c-Cas3/p-Cas3 in the crush control group, which is consistent to those reported by Wang et al. [10]. BCL-2 and BAX, key regulators of apoptosis, are members of the BCL-2 family. BCL-2 is an anti-apoptotic factor, while BAX is a pro-apoptotic factor [34]. Programmed cell death will be inhibited after BCL-2 binding to BAX whereas the c-Cas3 was the factors involved in the mitochondrial controlled cell apoptosis [35]. Thus, increased of BAX/BCL-2 and c-Cas3/p-Cas-3 represent the high expression of apoptosis [34, 35]. In present study, the ratio of BAX/BCL-2, c-Cas3/p-Cas3, and TUNEL-positive cell ratio decreased following laser treatment. Our results hint the therapeutic effect of the pulsed Nd:YAG laser might result from the apoptosis inhibition. Furthermore, we analyzed the apoptosis related proteins and the apoptotic cell ratio in week 1 of nerve crush injury based on previous researches finding which showed the apoptosis inhibition would present significantly after 5–7 days of nerve injury [9, 11, 12].
To the best of our knowledge, this is the first study to evaluate the antiapoptotic effects of high-intensity laser therapy in a peripheral nerve crush injury model. In previous research, the therapeutic effects of laser therapy were based on the premise that cytochrome C oxidase acts as a photoacceptor and transducer of photosignals within the light spectrum, subsequently inducing photobiological processes within cells after laser therapy [36, 37]. Furthermore, these photobiological processes increase the availability of electrons and activate the respiratory chain, thereby enhancing the production of adenosine triphosphate (ATP). This increase in ATP levels leads to improved neuron metabolism, subsequently promoting axon myelination and proliferation following the injury [36, 37]. Therefore, further research is necessary to analyze the relationship between the antiapoptotic effects and cytochrome C oxidase.
There were some limitations in this research. Firstly, we examined apoptosis activity during the early stage (week 1) of the nerve recovery process rather than throughout the entire study duration. Previous studies have reported that apoptosis following peripheral nerve injury occurs at different times and typically peaks within 2 weeks after the injury [36, 37]. Therefore, it is common practice to assess apoptosis-related expressions during the early stage, which is typically defined as within the first 2 weeks after injury, following various treatments [9, 10]. Second, there was lack of the apoptosis agonist following the laser treatment to confirm the therapeutic mechanism of Nd:YAG laser. However, in our model, there was the remarkably increased expression of apoptosis including c-Cas3/p-Cas3 and bel-2/BAX2 ratios, and TUNEL-positvie cell ratio in the crush control compared with the sham control, and the increased apoptosis-related expression was suppressed following the laser treatment. Therefore, the apoptosis should play a role in the pulsed Nd:YAG laser treatment. Further studies using overexpression of the molecules involved in the intrinsic or extrinsic apoptosis pathway are necessary to confirm the therapeutic mechanism of Nd:YAG laser.
Conclusion
The present study demonstrated that the pulsed Nd:YAG laser treatment promoted the nerve functional and histological recovery after peripheral nerve injury in a rat crushed sciatic nerve model. Furthermore, the beneficial effects were associated with apoptosis inhibition. These results indicate that the pulsed Nd:YAG laser might enhance nerve regeneration via apoptosis inhibition. Further studies are needed to verify the therapeutic effects of the pulsed Nd:YAG laser in the human peripheral nerve injury.
Data Availability
All post analyzed data were showed in the main manuscript. The raw data generated during the current study are not publicly available due to the huge foot print data were collected, stored on the MATLAB program designed by our laboratory (MathWorks, Natick, MA) but are available from the corresponding author on reasonable request.
Abbreviations
- c-Cas3:
-
Cleaved caspase3
- p-Cas3:
-
Procaspase3
- BCL-2:
-
Beclin-2
- BAX:
-
Beclin-2-associated protein X
- SFI:
-
Sciatic functional index
- PL:
-
Print length
- TS:
-
Toe spread
- IT:
-
Intermediary toe spread
References
Isaacs J (2010) Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am 35:491–497
Garozzo D (2019) Peripheral nerve injuries and their surgical treatment: new perspectives on a changing scenario. Neurol India 67:S20–S22
Harhaus L, Hirche C, Giunta RE, Aszmann O, Siemers F, Kneser U, Lehnhardt M (2017) Strategies on the treatment of nerve injuries accompanied by severe soft tissue damage—Consensus statement of the German-speaking society for microsurgery of peripheral nerves and vessels. Handchir Mikrochir Plast Chir 49:257–266
Kaiser R (2016) Surgical treatment of lower extremity peripheral nerve injuries. Cas Lek Cesk 155:16–20
Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L (2014) Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys 68:449–454
Topuz AK, Eroglu A, Atabey C, Cetinkal A (2013) Surgical treatment outcomes in peripheral nerve lesions due to gunshot injuries: assessment of 28 cases. Ulus Travma Acil Cerrahi Derg 19:235–240
Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martinez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR (2015) Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol 210:153–168
Koeppen AH (2004) Wallerian degeneration: history and clinical significance. J Neurol Sci 220:115–117
Liu X, Cui X, Guan G, Dong Y, Zhang Z (2020) microRNA-192-5p is involved in nerve repair in rats with peripheral nerve injury by regulating XIAP. Cell Cycle 19:326–338
Wang JB, Zhang Z, Li JN, Yang T, Du S, Cao RJ, Cui SS (2020) SPP1 promotes Schwann cell proliferation and survival through PKCalpha by binding with CD44 and alphavbeta3 after peripheral nerve injury. Cell Biosci 10:98
Zhao Z, Li X, Li Q (2017) Curcumin accelerates the repair of sciatic nerve injury in rats through reducing Schwann cells apoptosis and promoting myelinization. Biomed Pharmacother 92:1103–1110
Liu CY, Yin G, Sun YD, Lin YF, Xie Z, English AW, Li QF, Lin HD (2020) Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci Ther 26:189–196
Kheshie AR, Alayat MS, Ali MM (2014) High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: a randomized controlled trial. Lasers Med Sci 29:1371–1376
Alayat MS, Mohamed AA, Helal OF, Khaled OA (2016) Efficacy of high-intensity laser therapy in the treatment of chronic neck pain: a randomized double-blind placebo-control trial. Lasers Med Sci 31:687–694
Alayat MSM, Alshehri MA, Shousha TM, Abdelgalil AA, Alhasan H, Khayyat OK, Al-Attar WS (2019) The effectiveness of high intensity laser therapy in the management of spinal disorders: a systematic review and meta-analysis. J Back Musculoskelet Rehabil 32:869–884
Alayat MSM, Basalamah MA, Elbarrany W, El-Sawy NAM, Abdel-Kafy EM, El-Fiky AA (2020) Dose-dependent effect of the pulsed Nd:YAG laser in the treatment of crushed sciatic nerve in Wister rats: an experimental model. Lasers Med Sci. https://doi.org/10.1007/s10103-020-02999-z
Lan SM, Yang CC, Lee CL, Lee JS, Jou IM (2017) The effect of molecular weight and concentration of hyaluronan on the recovery of the rat sciatic nerve sustaining acute traumatic injury. Biomed Mater 12:045024
Hong CK, Yeh ML, Chang CH, Chiang FL, Jou IM, Wang PH, Su WR (2019) Comparison of changes in shoulder functions between biceps tenotomy and tenodesis in an animal model. Asia Pac J Sports Med Arthrosc Rehabil Technol 15:17–22
Ko PY, Yang CC, Kuo YL, Su FC, Hsu TI, Tu YK, Jou IM (2018) Schwann-cell autophagy, functional recovery, and scar reduction after peripheral nerve repair. J Mol Neurosci 64:601–610
Lee HY, Hsieh TH, Liang JI, Yeh ML, Chen JJ (2012) Quantitative video-based gait pattern analysis for hemiparkinsonian rats. Med Biol Eng Comput 50:937–946
Liang JI, Chen MY, Hsieh TH, Liu CY, Lam CF, Chen JJ, Yeh ML (2012) Video-based gait analysis for functional evaluation of healing achilles tendon in rats. Ann Biomed Eng 40:2532–2540
Bain JR, Mackinnon SE, Hunter DA (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83:129–138
Rushton WA (1934) A physical analysis of the relation between threshold and interpolar length in the electric excitation of medullated nerve. J Physiol 82:332–352
Akgul T, Gulsoy M, Gulcur HO (2014) Effects of early and delayed laser application on nerve regeneration. Lasers Med Sci 29:351–357
dos Reis FA, Belchior AC, de Carvalho PT, da Silva BA, Pereira DM, Silva IS, Nicolau RA (2009) Effect of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24:741–747
Bae CS, Lim SC, Kim KY, Song CH, Pak S, Kim SG, Jang CH (2004) Effect of Ga-as laser on the regeneration of injured sciatic nerves in the rat. In Vivo 18:489–495
Shen CC, Yang YC, Huang TB, Chan SC, Liu BS (2013) Neural regeneration in a novel nerve conduit across a large gap of the transected sciatic nerve in rats with low-level laser phototherapy. J Biomed Mater Res A 101:2763–2777
Shen CC, Yang YC, Liu BS (2011) Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury 42:803–813
Zhang LX, Tong XJ, Yuan XH, Sun XH, Jia H (2010) Effects of 660-nm gallium-aluminum-arsenide low-energy laser on nerve regeneration after acellular nerve allograft in rats. Synapse 64:152–160
Diker N, Aytac D, Helvacioglu F, Oguz Y (2020) Comparative effects of photobiomodulation therapy at wavelengths of 660 and 808 nm on regeneration of inferior alveolar nerve in rats following crush injury. Lasers Med Sci 35:413–420
Elsodany AM, Alayat MSM, Ali MME, Khaprani HM (2018) Long-term effect of pulsed Nd:YAG laser in the treatment of patients with rotator cuff tendinopathy: a randomized controlled trial. Photomed Laser Surg 36:506–513
Dundar U, Turkmen U, Toktas H, Solak O, Ulasli AM (2015) Effect of high-intensity laser therapy in the management of myofascial pain syndrome of the trapezius: a double-blind, placebo-controlled study. Lasers Med Sci 30:325–332
Gaudet AD, Popovich PG, Ramer MS (2011) Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 8:110
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Glushakova OY, Glushakov AO, Borlongan CV, Valadka AB, Hayes RL, Glushakov AV (2018) Role of caspase-3-mediated apoptosis in chronic caspase-3-cleaved tau accumulation and blood-brain barrier damage in the corpus callosum after traumatic brain injury in rats. J Neurotrauma 35:157–173
Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA (2005) Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci 25:3478–3487
Yang DP, Zhang DP, Mak KS, Bonder DE, Pomeroy SL, Kim HA (2008) Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis. Mol Cell Neurosci 38:80–88
Acknowledgements
The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) guidelines of National Cheng Kung University (IACUC Approval No. 109263). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments. No AI software was used in this manuscript writing. We are grateful to the Skeleton Materials and Bio-compatibility Core Lab, Clinical Medicine Research Center, National Cheng Kung University Hospital for the assistance in this study. We also wish to thank Ms. Yu-Ying Chen for her valuable assistance.
Funding
This study was supported by grant MOST108-2314-B-006-011-MY2, NSTC 112-2314-B-006-065, NSTC 112-2622-E-006-013, MOST 111-2314-B-006-054 from the National Science and Technology Council, Taiwan, and grant NCKUH-10904016, NCKUH-11004043 from the National Cheng Kung University Hospital, Tainan, Taiwan.
Author information
Authors and Affiliations
Contributions
P-YK, I-MJ, P-TW: conceptualization P-YK, C-CH, I-MJ, P-TW: methodology S-YC, C-LL: software C-CH, P-TW: validation P-YK, C-CH, S-YC, C-LL, I-MJ, P-TW: formal analysis P-YK, C-CH, P-TW, I-MJ: investigation S-YC, C-LL, I-MJ, P-TW: resources P-YK, C-CH, I-MJ, P-TW: data curation P-YK, C-CH, S-YC, C-LL, P-TW: writing—original draft preparation P-YK, C-CH, P-TW: writing—review and editing S-YC, C-LL, I-MJ: visualization P-YK, I-MJ, P-TW: supervision S-YC, C-LL, I-MJ, P-TW: project administration P-YK, I-MJ, P-TW: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
This research involving animals and the experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) guidelines of National Cheng Kung University (IACUC Approval No. 109263). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ko, PY., Hsu, CC., Chen, SY. et al. The Pulsed Nd:YAG Laser Therapy Enhanced Nerve Regeneration via Apoptosis Inhibition in a Rat Crushed Sciatic Nerve Model. Neurochem Res 49, 949–958 (2024). https://doi.org/10.1007/s11064-023-04068-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04068-7