Abstract
Nicotinamide adenine dinucleotide (NAD) is a critical cosubstrate for enzymes involved in supplying energy to the brain. Nicotinamide riboside (NR), an NAD+ precursor, emerges as a neuroprotective factor after chronic brain insults. However, researchers have not determined whether it improves cognition after acute ischemia. In the present study, mice with middle cerebral artery occlusion were treated with NR chloride (NRC, 300 mg/kg, IP., 20 min after reperfusion). The results of the Morris water maze test revealed better recovery of learning and memory function in the NRC-treated group. Acute NRC treatment decreased hippocampal infarct volume, reduced neuronal loss and apoptosis in the hippocampus. Western blot and high-performance liquid chromatography assays of hippocampal tissues revealed that the activation of Sirtin-1 and adenosine 5′ monophosphate-activated protein kinase was increased, the NAD content was elevated, and the production of adenosine triphosphate was strengthened by NRC. Collectively, acute NRC treatment increased the energy supply, reduced the neuronal loss and apoptosis, protected the hippocampus and ultimately promoted the recovery of cognitive function after brain ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the leading causes of morbidity and mortality worldwide [1]. Ischemic stroke accounts for 72% of all stroke cases. Acute ischemic stroke is characterized by a loss of or alteration in neurological function resulting from decreased blood perfusion to the brain. Dysfunctions include sensorimotor and cognitive deficits, among others. Hippocampal infarction is correlated with cognitive deficits, such as impairments in spatial learning and memory retention. These deficits are commonly observed in middle cerebral artery occlusion (MCAO) models [2]. Protecting the hippocampus from the consequences of ischemic insults and reversing damage should therefore be a therapeutic target for vascular cognitive disorders [3].
Supported by previous studies, nicotinamide adenine dinucleotide (NAD) was confirmed to be effective at improving cognitive function after insults [4,5,6]. NAD+ is essential for many mitochondrial enzymatic reactions and appropriate bioenergetic metabolism. Under normal conditions, the loss of NAD+ inhibits cellular respiration, resulting in reduced mitochondrial adenosine triphosphate (ATP) production and potentially cell death [6]. NAD+ is used as a substrate by several NAD+-dependent enzymes, including poly (ADP ribose) polymerase 1 (PARP1), Sirtuin-1 (Sirt1), and ADP ribosyl cyclase (CD38). In mammals, NAD+ is synthesized from four precursors: nicotinamide, nicotinic acid, tryptophan, and nicotinamide riboside (NR) [7].
As a component of vitamin B3, NR is a natural NAD+ precursor. NR has better metabolic properties and works well in models of Alzheimer’s disease [8, 9]. NR treatment has been shown to improve cognitive function in Tg2576 transgenic mice [10]. It also supports hippocampal function, reduces brain inflammation and improves cognitive function in diabetic mice [11]. By activating Sirt1, NR restores brain bioenergetics and reduces inflammation [12]. NR also enhances cell functions by activating Sirt1/ adenosine 5′ monophosphate-activated protein kinase (AMPK) [13]. NAD+ biosynthesis and Sirt1 function exert synergistic effects on regulating metabolism; Sirt1 and AMPK are energy-sensing systems that work cooperatively to regulate mitochondrial biogenesis and fuel metabolism [13].
Researchers have not clearly determined whether NR protects the hippocampus and reduces the cognitive impairment caused by acute ischemic insults. Here, we hypothesized that acute NR treatment would reduce ischemic hippocampal deficits by increasing the Sirt1/AMPK-mediated energy supply. Cognitive impairment, hippocampal infarct volume, neuronal damage, Sirt1/AMPK activation, and ischemic energy dysregulation were evaluated using an MCAO mouse model in the present study.
Methods
Mouse MCAO Model and Groups
Male C57BL/6 mice were provided by the Hubei Laboratory Animal Service Center and maintained under specified pathogen free conditions on a standard light–dark cycle with sufficient water and food. The animal use and experimental procedures were performed in accordance with protocols approved by the Governmental Animal Care Committee at Tongji Medical College (TJH-202001003). 192 adult male mice (weighing 25 ± 2 g) were randomly divided into groups for use in subsequent tests. After dissolution in saline, 300 mg/kg nicotinamide riboside chloride (NRC, GIHI Chemicals Co., Limited, China) was administered by intraperitoneal injection 20 min after blood reperfusion. Time points of the measurements, and the number of samples, are shown in Fig. 1.
The MCAO model was generated as described previously [14]. During surgery, mice were anesthetized with 2.5% isoflurane and placed on a warming pad to maintain body temperature at 37.0 ± 0.5 °C. Silicon rubber-coated nylon filament (602212PK10, Doccol Corporation, USA) was used to occlude the middle cerebral artery. Occlusion was confirmed by a greater than 80% reduction in cerebral blood flow monitored using laser Doppler flowmetry (moorVMS-LDF1, Moor Instruments, Devon, UK). After 45 min of occlusion, the filament was removed for reperfusion. Mice in the sham group underwent the same surgical procedures without insertion of the filament.
Morris Water Maze Test
The Morris water maze test was employed to assess mouse spatial learning and memory [15]. Twenty-four mice were randomly and equally divided into the sham, vehicle and NRC groups. A digital video camera was positioned directly above the water maze to enable the measurement of activity using the DigBehav System (Jiliang Software Co., Shanghai, China) software. The maze is a circular pool (150 cm in diameter, 45 cm in height) filled with water (30 cm in depth, 19–22 °C). A painted white platform (10 cm in diameter) was submerged and placed in the center of the target quadrant 1 cm below the surface of water, providing the escape area. Mice performed 4 trials starting from a fixed location in different quadrants. The visual platform task was conducted on day 29 after the operation, while the hidden platform task was performed over the next 5 days. Water was made opaque with milk but surrounded by fixed extramaze cues. Each mouse was allowed less than 60 s to find the hidden platform. After 4 trials, the total escape latency and path length were recorded. Finally, the platform was removed, and a 60 s probe trial was performed to observe the crossing times and the percentage of spent time in the target quadrant.
Calculation of the Infarct Volume
Forty mice were randomly and equally divided into vehicle and NRC groups to compare the infarct volumes. Three days after MCAO, the animals were deeply anesthetized by pentobarbital sodium salt for sacrifice. Using a mouse brain matrix slicer, the brain was carefully cut into 1 mm coronal sections, followed by an incubation with a 2% triphenyltetrazolium chloride solution (Sigma-Aldrich, 298-96-4, USA) at 37 °C for 10 min. After visualization (Fig. 3a), the size of the infarct (uncolored area) in the hippocampus were measured with ImageJ software (NIH). The infarct volume was calculated by adding the values from all sections and multiplying by the slice thickness (1 mm).
Tissue Processing and Staining
On day 3 after ischemia, randomly classified mice (n = 6) were anesthetized and transcardially perfused with saline, followed by ice-cold 4% paraformaldehyde. The frozen brains were cut at a 10 μm thickness for staining. Coronal brain sections obtained from bregma -2.0 to -2.5 mm were used for staining. The brain sections obtained from the mice sacrificed after the Morris water maze test were prepared similarly.
Nissl staining was used to visualize the basic structure of neurons. Briefly, coronal Sections (3 sections per mouse) were rehydrated using a decreasing gradient of ethanol and then submerged in cresyl violet staining solution (BOSTER Biological Technology Co., Wuhan, China) for 10 min. Three representative fields in the CA1 region of the hippocampus were imaged at 400 × magnification using a digital camera (Leica, Germany), which resulted in an area of 450 × 340 μm2. The number of Nissl-positive cells in the hippocampal CA1 region was analyzed using ImageJ software (NIH) to assess neuronal loss.
Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) staining was performed to detect DNA fragmentation and apoptotic bodies after ischemia. The Apoplert DNA Fragmentation Assay Kit (Clonteh Laboratories Inc., 630,107 630,108, CA, USA) was used according to the manufacturer’s instructions. After visualization, the slices were costained with a NeuN antibody (1:200, Chemicon, MAB377, Temecula, CA, USA) and DAPI and then imaged using a confocal microscope at 400 × magnification (Olympus, USA). The number of positive cells was counted carefully in 3 representative fields from the hippocampal CA1 region in every slice, with 3 sections analyzed per animal. Numbers were averaged to calculate the mean value. The apoptosis index (AI) was calculated using the following formula: AI = (number of TUNEL-positive cells/total number of DAPI-stained cells) × 100%.
High-Performance Liquid Chromatography (HPLC)
Eighteen mice were randomly and equally divided into sham, vehicle, and NRC groups to evaluate the energy supply in each timepoint. The hippocampus on the ischemic side was dissociated at set time points and homogenized in 0.4 M perchloric acid (5 μl/mg) using ultrasonication. Levels of NAD and ATP in the hippocampus were determined using an HPLC instrument (Agilent Technologies, CA, USA) equipped with a photodiode array detector (model G1315B). The mobile phase consisted of 20 mM ammonium acetate buffer (pH 5.5) containing 3% (v/v) acetonitrile. The flow rate was 0.9 ml/min, the column temperature was 28 °C, and UV detection was performed at 260 nm. The content of either NAD or ATP in lysates was calculated from a gradient of standard solutions.
Western Blot
Twenty mice were randomly and equally divided into sham, vehicle-6 h, NRC-6 h, vehicle-1 day, and NRC-1 d groups to measure the levels of Sirt1 and AMPK. The hippocampus was extracted and homogenized in RIPA lysis buffer (Thermo Fisher Scientific). Total protein (60 µg) was separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in Tris-buffered saline and then incubated with specific primary antibodies against Sirt1 (1:500, Abcam, ab32441, USA), AMPK/pAMPK (1:100, Cell Signaling Tech. #2532, #2535, USA), or mouse anti-β-actin (1:5000, Sigma, A1978, USA). After an incubation with HRP-conjugated secondary antibodies (1:10,000, BOSTER), the blots were visualized using an ECL kit and finally quantified using ImageJ software (NIH). The levels of Sirt1, pAMPK, and AMPK are presented as the values relative to the control GAPDH.
Sirt1 Deacetylation Assay
Hippocampal tissues were dissected for Sirt1 deacetylation activity assays using the Sirt1 Assay Kit (Sigma). n = 4. Briefly, tissues were homogenized in assay buffer (1:10), and 50 μL of lysate were loaded into the reaction scheme. Reactions were performed according to the manual, and the fluorescence was measured with a fluorimeter (Bio-Rad, USA).
Statistical Analysis
The SPSS 20.0 software package was used to analyze the data. For data with a normal distribution, comparisons between 2 groups were performed using the unpaired Student’s t-test; Differences between 3 groups were compared using one-way ANOVA followed by LSD test. The results are expressed as Mean ± STD. The Mann–Whitney U test was performed to compare the infarct volume between the treated and vehicle groups. The results are presented as medians [interquartile ranges 25%–75%]. Differences were considered significant at P < 0.05.
Results
NRC Ameliorates Ischemia-Induced Cognitive Deficits
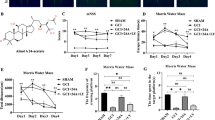
During the Morris water maze test (Fig. 2a), a visual platform task was initiated to screen for underlying motor or visual impairments. No difference in the escape latency was observed between the groups in this step (Fig. 2b1). Coincidently, a significant difference in the swimming speed was not observed between groups during the hidden platform task (Fig. 2b2). In this task, a long escape latency and path length were initially required for mice to find the hidden platform, with both values decreasing after practice. Mice in the vehicle group required a longer latency on days 3 and 4, as well as a longer path length on days 2 to 4, compared with those of the sham-operated group. NRC treatment improved the learning ability of mice, as illustrated by a shorter latency on days 3 and 4 and a decreased path length on day 3 compared with those in the vehicle group (Fig. 2c). In the probe trial, the number of target zone entries and percentage of the time spent in the target quadrant were considered to reflect memory retention. Mice in the vehicle group spent less time in the target quadrant than mice in the sham group; additionally, NRC-treated mice performed much better than those in the vehicle group (Fig. 2d).
Acute NRC treatment potentiates cognitive recovery after ischemia. Mice were subjected to the Morris water maze test 4 weeks after the operation. a Representative traces recorded on day 3 of the hidden platform test. Charts show the escape latency (b1) and swimming speed (b2) in the visual platform task, the total escape latency (c1) and total path length (c2) in the hidden platform task, as well as the number of entries (d1) and percentage of time spent (d2) in the target quadrant in the probe trial. NRC treatment improved the learning ability of mice, as illustrated by a shorter latency on days 3 and 4, as well as a decreased path length on day 3, compared with those in the vehicle group. Statistical comparisons were performed using one-way ANOVA followed by LSD test. Data are presented as the mean ± STD (n = 8). #P < 0.05 compared with the sham group; *P < 0.05 compared with the vehicle group
NRC Reduces the Infarct Volume and Neuronal Damage in the Hippocampus
The hippocampal infarct volume varies after acute ischemia in MCAO models. Comparisons between the groups indicated that the hippocampal infarct volume of the mice in the acute NRC treatment group decreased significantly compared with that of mice in the vehicle group (median, 0.00 vs. 2.15; interquartile ranges 25%–75%, 0.00–2.25 vs. 0.00–12.65, mm3) (Fig. 3b).
Acute NRC treatment reduces the hippocampal infarct volume. a Representative images of TTC staining in the sham-, vehicle- and NR-treated groups. b Sample distribution and comparison of the infarct volume of hippocampus between the vehicle- and NR-treated groups. The hippocampal infarct volume of the mice in the NRC group was decreased significantly compared with that of the mice in the vehicle group. Mann–Whitney U test, n = 20. *P < 0.05 compared with the vehicle group
In the CA1 region of the sham-operated mice, a dense arrangement and even distribution of pyramidal neurons was visualized using Nissl staining (Fig. 4a1). After ischemia, the damaged pyramidal neurons exhibited deformed cell bodies, condensed nuclear chromatin, increased intracellular cell gaps, loosened cell arrangements and blurred visible staining (Fig. 4a2). NRC treatment partially reversed these changes, as shown by staining on day 3 after ischemia (Fig. 4a3). In addition to the morphological changes observed, reduced numbers of positive cells were noted, which represented local neuronal loss in the CA1 hippocampal area after ischemia. Mice in the NRC treatment group showed an increase in the number of positive cells compared with those in the vehicle group (Fig. 4b).
Acute NRC treatment attenuates neuronal loss in the hippocampus. By Nissl staining, the representative histological appearance of hippocampal CA1 zone from sham (a1), vehicle-treated (a2) and NRC-treated (a3) groups was shown. The number of Nissl positive cells was calculated. Then, the density of positive cells was compared between groups using one-way ANOVA followed by LSD test (b). The mice in the NRC group showed an increase in the number of positive cells, compared with those in the vehicle group. Data are presented as the mean ± STD (n = 6); F = 15.14, P = 0.000. #P < 0.05 compared with the sham group; *P < 0.05 compared with the vehicle group. Scale bar = 100 μm
As suggested by TUNEL staining, apoptotic cells were rarely observed in the sham-operated mice (Fig. 5a); however, abundant TUNEL-positive cells appeared in the CA1 region of the hippocampus after ischemia (Fig. 5b). Statistically, the apoptotic index of pyramidal neurons in the CA1 hippocampal area on day 3 postischemia was 46.6 ± 13.0%. In contrast, NRC treatment significantly suppressed neuronal apoptosis (Fig. 5c), resulting in a reduced apoptotic index of 26.2 ± 9.9% (Fig. 5d).
Acute NRC treatment reduces apoptosis in the hippocampus. Neurons were visualized using NeuN staining (red), DNA fragmentation was detected using TUNEL staining (green), and nuclei were stained with DAPI (blue). Representative photos from the CA1 hippocampal area for sham (a), vehicle-treated (b) and NRC-treated (c) groups are shown. The percentage of TUNEL-positive neurons was compared using one-way ANOVA followed by LSD test (d). NRC treatment significantly suppressed neuronal apoptosis. Data are presented as the mean ± STD (n = 6); F = 35.27, P = 0.000. *P < 0.05 compared with the vehicle group. Scale bar = 100 μm
Brain sections from the mice sacrificed after the Morris water maze test were visualized using Nissl staining (Fig. 6). In the CA1 region, local damaged pyramidal neurons still appeared in the vehicle-treated group, with partially loosened cell arrangements, compared with that in the sham operated mice (Fig. 6a1). However, the NRC treatment reversed these changes. Compared with the vehicle-treated group (Fig. 6a2), NRC restored the local pyramidal neurons in the hippocampal CA1 area (Fig. 6a3/d).
Acute NRC treatment leads to long-term restoration of the hippocampal tissue. On day 34 after MCAO, mice were sacrificed for Nissl staining after Morris water maze tests. The representative photos for sham (a1), vehicle-treated (a2) and NRC-treated (a3) groups were shown. It showed better recovery in the NRC group, while damaged areas persisted in the CA1 region of the vehicle-treated group. After calculating the number of surviving cells (b), mice in the NRC group showed an increased number of neurons with a normal morphology compared with those in the vehicle group. One-way ANOVA; mean ± STD (n = 8); F = 4.93, P = 0.018. #P < 0.05 compared with the sham group; *P < 0.05 compared with the vehicle group. Scale bar = 100 μm
NRC Supports the Energy Supply by Increasing NAD and ATP Levels
The NAD content in the hippocampus was measured, and the results showed that NAD levels were reduced after ischemia in a time-dependent manner. Compared with the sham control group, a significant difference was noted at 3 h after ischemia in the vehicle group. Acute NRC treatment replenished the production of NAD in the hippocampus. At 6 h, 1 day and 3 days after ischemia, the NAD content was significantly increased in NRC-treated mice compared with vehicle-treated mice (Fig. 7a).
Acute NRC treatment increases the energy supply in the hippocampus. The NAD and ATP contents in the hippocampus from the ischemic hemisphere were measured using HPLC. NR treatment potentiated the increases in NAD (a) and ATP (b) levels beginning at 6 h after ischemia compared with those in the vehicle group. One-way ANOVA followed by LSD test, mean ± STD (n = 6); #P < 0.05 compared with the sham group; *P < 0.05 compared with the vehicle group
The ATP content was measured, representing energy loading, and the results revealed that ATP was exhausted quickly after ischemia. The ATP level decreased to 60.7% of the baseline at h 1 postischemia. Acute NRC treatment significantly enhanced the recovery of ATP levels beginning at 6 h postischemia compared with the time-matched results from the vehicle group (Fig. 7b).
NRC Potentiates the Activation of Sirt1 and AMPK in the Hippocampus
The expression and activation of Sirt1 and AMPK in the hippocampus were evaluated at 6 and 24 h after ischemia. Through time-matching comparisons of results between NRC and Vehicle groups, Western blot assays revealed increased Sirt1 levels following acute NRC treatment at 6 h after MCAO (Fig. 8b); an evaluation of Sirt1 deacetylation activity revealed a synchronous increase in Sirt1 activity (Fig. 8a). Although AMPK expression was not changed, acute NRC treatment increased the phosphorylation of AMPK at 6 h after MCAO, as suggested by the western blot analysis (Fig. 8b, c).
Acute NRC treatment increases the activation of Sirt1 and AMPK. Sirt1 activity was measured using a commercial assay kit, while the levels of Sirt1, pAMPK, and AMPK were measured using western blotting at 6 h and 1 day after ischemia (b). NRC potentiated Sirt1 deacetylation (a) and AMPK phosphorylation (c) at 6 h after ischemia compared with the vehicle group. Results in NRC group were compared with that in Vehicle at the matching timepoints using Student’s t-test, and the results were presented as mean ± STD (n = 4); *P < 0.05
Discussion
Stroke is a devastating disease with a complex pathophysiology. After ischemia, energy crisis-induced mitochondrial dysfunction contributes to brain damage. Cell loss and neuronal apoptosis subsequently occur and play a central role in the progression of neurofunctional disorders such as cognitive dysfunction. The results of the present study revealed the effect of NRC on protecting the hippocampus in a mouse MCAO model. By increasing the production of NAD, acute treatment with NRC helped to salvage the energy supply by activating the Sirt1/AMPK signaling pathway. Consequently, neuronal loss in the hippocampus was decreased. Altogether, the results indicate that the hippocampal protection provided by NRC contributed to the recovery of spatial learning and memory after the ischemic insult.
Previous reports support the neuroprotective role of NR in CNS impairment. For example, 3 months of dietary treatment with NR at a dose of 250 mg/kg/day attenuates amyloid toxicity, improves cognitive function and increases synaptic plasticity in AD mouse models [11]; a 10-week oral treatment with NR at a dose of 400 mg/kg/day delays the senescence of neural stem cells and increases the lifespan of mice [16]. NR has also been reported to increase the content of brain-derived neurotrophic factor (BDNF) in the milk of mother mice, which is advantageous to the offspring as it increases adult hippocampal neurogenesis [17]. Oral NR treatment at a dose of 400 mg/kg improves the intestinal microbiota, diminishes the alcohol-induced decrease in BDNF levels and microglial activation in the mouse hippocampus, and ultimately alleviates alcohol-induced depression-like cognitive and behavioral deficits [18]. Six weeks of NR treatment at a dose of 400 mg/kg/day reduces neuroinflammation and amyloidogenesis in the whole brain and attenuates impairments in spatial working memory and recognition memory function [12]. NR has also been reported to prevent mitochondrial defects and neuronal loss in induced pluripotent stem cells (iPSCs) and fly models of Parkinson’s disease [19]. All these studies have focused on chronic CNS injuries and provide evidence supporting the neuroprotective function of NR. The findings from the present study revealed that NR treatment is also effective for acute ischemic insults and improves the outcome of cognitive dysfunction caused by hippocampal infarction.
As shown in the present study, acute NRC treatment decreased neuronal loss and apoptosis in the hippocampus. This change may be attributed to the elevated energy supply. Increased NAD levels have been observed after chronic NR treatment [11, 17], even in humans [20,21,22]. NAD levels were also increased by even one dose of NR administered after ischemia in the present study. NAD is a classic coenzyme required for many critical metabolic pathways, such as glycolysis, the tricarboxylic acid cycle, and mitochondrial oxidative phosphorylation [23]. It is also important for the maintenance of hippocampal functions [24]. NAD+ levels have been shown to decrease in the mouse hippocampus during the aging, and replenishment has been reported to be helpful [25].
Both Sirt1 and AMPK signaling pathways mediate the maintenance of energy homeostasis [26]. Sirt1 and AMPK are fuel-sensing molecules that have coexisted in cells throughout evolution. Sirt1 has been shown to play a major role in counteracting cellular stress and apoptosis [27]. The activation and overexpression of Sirt1 have been reported to diminish lysine acetylation of liver kinase B1 (LKB1) and cause its transport from the nucleus to the cytoplasm, where LKB1 activates AMPK [28]. When an energy crisis occurs, AMPK activation restores the energy balance by stimulating catabolic processes that generate ATP [29]. The present study showed increased ATP levels in the hippocampal tissue 6 h after treatment. The accompanying activation of Sirt1 and AMPK suggested that NRC may function to provide energy through the Sirt1 and AMPK pathways.
NR is not a fast-activating compound that functions immediately after administration. Notably, it might provide neuronal protection through alternate pathways that were not elucidated in the present study [30,31,32,33]. As the converted product of NR, NAD potentiates the synthesis of essential compounds, including nucleoside triphosphates and some amino acids [31]. Other NAD-dependent enzymes, such as PARPs and CD38/CD157, may play certain roles in this process [32, 33]. The protective effect of NAD has been associated with the activation of other Sirtins as well as PGC-1a [11]. NR has also been reported to decrease the levels of the proinflammatory cytokines interleukin (IL)-1, tumor necrosis factor-α, and IL-6 in the brains of diabetic mice [12]. NRCs also protect other brain regions in addition to the hippocampus, which may contribute to the recovery of MCAO mice (data not shown).
NR does not bind to the GPR109A receptor and is therefore considered to exert fewer unfavorable side effects [21]. Several studies have examined NR as a possible therapeutic agent for chronic CNS diseases [22, 34]. In addition to chronic diseases, the findings of the present study suggest that NR is still a valuable treatment for acute injuries such as ischemia. Acute NRC treatment improved the energy supply, protected the hippocampus, and ultimately promoted the recovery of cognitive function. Of course, there are limitations of the present study. For example, only young male adult mice were employed; data are not well interpreted using only a MCAO model; and further research will be needed to reveal other mechanisms involved and to identify the best NRC treatment protocol for acute CNS injuries.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Abbreviations
- NAD:
-
Nicotinamide adenine dinucleotide
- NR:
-
Nicotinamide riboside
- NRC:
-
Nicotinamide riboside chloride
- HPLC:
-
High-performance liquid chromatography
- Sirt1:
-
Sirtin-1
- AMPK:
-
Adenosine 5′ monophosphate-activated protein kinase
- ATP:
-
Adenosine triphosphate
- MCAO:
-
Middle cerebral artery occlusion
- PARP1:
-
(ADP ribose) polymerase 1
- PGC1α:
-
Proliferator-activated receptor-γ coactivator 1α
- AI:
-
Apoptosis index
- BDNF:
-
Brain-derived neurotrophic factor
- iPSC:
-
Induced pluripotent stem cells
- LKB1:
-
Liver kinase B1
References
Katan M, Luft A (2018) Global burden of stroke. Semin Neurol. https://doi.org/10.1055/s-0038-1649503
Kathner-Schaffert C, Karapetow L, Günther M et al (2019) Early stroke induces long-term impairment of adult neurogenesis accompanied by hippocampal-mediated cognitive decline. Cells. https://doi.org/10.3390/cells8121654
Guo X, Qun Xue Q, Zhao J et al (2020) Clinical diagnostic and therapeutic guidelines of stroke neurorestoration (2020 China version). J Neurorestorat. https://doi.org/10.26599/JNR.2020.9040026
Braidy N, Grant R, Sachdev PS (2018) Nicotinamide adenine dinucleotide and its related precursors for the treatment of Alzheimer’s disease. Curr Opin Psychiatry
Hosseini L, Mahmoudi J, Pashazadeh F, et al (2021) Protective effects of nicotinamide adenine dinucleotide and related precursors in alzheimer’s disease: a systematic review of preclinical studies. J Mol Neurosci
Hosseini L, Vafaee MS, Mahmoudi J, Badalzadeh R (2019) Nicotinamide adenine dinucleotide emerges as a therapeutic target in aging and ischemic conditions. Biogerontology
Covarrubias AJ, Perrone R, Grozio A, Verdin E (2021) NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol
Xie X, Gao Y, Zeng M et al (2019) Nicotinamide ribose ameliorates cognitive impairment of aged and Alzheimer’s disease model mice. Metab Brain Dis. https://doi.org/10.1007/s11011-018-0346-8
Braidy N, Liu Y (2020) Can nicotinamide riboside protect against cognitive impairment? Curr Opin Clin Nutr Metab Care. https://doi.org/10.1097/MCO.0000000000000691
Vaur P, Brugg B, Mericskay M et al (2017) Nicotinamide riboside, a form of Vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. https://doi.org/10.1096/fj.201700221RR
Gong B, Pan Y, Vempati P et al (2013) Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging 34:1581–1588. https://doi.org/10.1016/j.neurobiolaging.2012.12.005
Lee HJ, Yang SJ (2019) Supplementation with nicotinamide riboside reduces brain inflammation and improves cognitive function in diabetic mice. Int J Mol Sci. https://doi.org/10.3390/ijms20174196
Cantó C, Gerhart-Hines Z, Feige JN et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060. https://doi.org/10.1038/nature07813
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91. https://doi.org/10.1161/01.STR.20.1.84
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. https://doi.org/10.1016/0165-0270(84)90007-4
Zhang H, Ryu D, Wu Y et al (2016) NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. https://doi.org/10.1126/science.aaf2693
Brenner C (2019) Maternal nicotinamide riboside enhances postpartum weight loss, juvenile offspring development, and neurogenesis of adult offspring (P11–003-19). Curr Dev Nutr. https://doi.org/10.1093/cdn/nzz048.p11-003-19
Jiang Y, Liu Y, Gao M et al (2020) Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. https://doi.org/10.1039/c9fo01780a
Schöndorf DC, Ivanyuk D, Baden P et al (2018) The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and Fly models of parkinson’s disease. Cell Rep. https://doi.org/10.1016/j.celrep.2018.05.009
Trammell SAJ, Schmidt MS, Weidemann BJ et al (2016) Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. https://doi.org/10.1038/ncomms12948
Martens CR, Denman BA, Mazzo MR et al (2018) Chronic nicotinamide riboside supplementation is well-Tolerated and elevates NAD+ in healthy middle-Aged and older adults. Nat Commun. https://doi.org/10.1038/s41467-018-03421-7
Airhart SE, Shireman LM, Risler LJ et al (2017) An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE. https://doi.org/10.1371/journal.pone.0186459
Cantó C, Menzies KJ, Auwerx J (2015) NAD+ Metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab
Imai S, Guarente L (2014) NAD+ and sirtuins in aging and disease. Trends Cell Biol 24:464–471. https://doi.org/10.1016/j.tcb.2014.04.002
Johnson S, Wozniak DF, Imai S (2018) CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ Aging Mech Dis. https://doi.org/10.1038/s41514-018-0029-z
Guo JM, Shu H, Wang L et al (2017) SIRT1-dependent AMPK pathway in the protection of estrogen against ischemic brain injury. CNS Neurosci Ther. https://doi.org/10.1111/cns.12686
Cattelan A, Ceolotto G, Bova S et al (2015) NAD+-dependent SIRT1 deactivation has a key role on ischemia-reperfusion-induced apoptosis. Vascul Pharmacol. https://doi.org/10.1016/j.vph.2015.02.004
Ruderman NB, Xu XJ, Nelson L, et al (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab
Ke R, Xu Q, Li C, et al (2018) Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol Int
Wang Y, Guo X, Liu J et al (2020) Olfactory ensheathing cells in chronic ischemic stroke: A phase 2, double-blind, randomized, controlled trial. J Neurorestorat. https://doi.org/10.26599/JNR.2020.9040019
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. https://doi.org/10.1146/annurev-cellbio-092910-154237
Alano CC, Garnier P, Ying W et al (2010) NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. https://doi.org/10.1523/JNEUROSCI.5552-09.2010
Klimova N, Kristian T (2019) Multi-targeted effect of nicotinamide mononucleotide on brain bioenergetic metabolism. Neurochem Res. https://doi.org/10.1007/s11064-019-02729-0
Mehmel M, Jovanović N, Spitz U (2020) Nicotinamide riboside—the current state of research and therapeutic uses. Nutrients 12(6):1616. https://doi.org/10.3390/nu12061616
Funding
This work was supported by the Natural Science Foundation of China (81400977 to Wen-sheng Qu and 81771341 to Xiang Luo), Fund for Returnees of Tongji Hospital (2018hgry013 to Wen-sheng Qu), and the open project of Henan Key Laboratory of Neurorestoratology (HNSJXF-2018-012 to Wen-sheng Qu).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YHC performed the HPLC and staining experiments. WFZ performed the western blot assay; JHZ prepared the reagents. XJW, ZX and YY prepared the mice and performed the Morris water maze test under the instruction of YHC. YFJ generated the animal model. XL and WW supervised the experiments and obtained financial support, and WSQ performed the analysis and prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, Yh., Zhao, Jh., Zong, Wf. et al. Acute Treatment with Nicotinamide Riboside Chloride Reduces Hippocampal Damage and Preserves the Cognitive Function of Mice with Ischemic Injury. Neurochem Res 47, 2244–2253 (2022). https://doi.org/10.1007/s11064-022-03610-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03610-3