Abstract
Nicotinamide adenine dinucleotide (NAD) supplementation to repair the disabled mitochondria is a promising strategy for the treatment of Alzheimer’s disease (AD) and other dementia. Nicotinamide ribose (NR) is a safe NAD precursor with high oral bioavailability, and has beneficial effects on aging. Here, we applied NR supplied food (2.5 g/kg food) to APP/PS1 transgenic AD model mice and aged mice for 3 months. Cognitive function, locomotor activity and anxiety level were assessed by standard behavioral tests. The change of body weight, the activation of microglia and astrocytes, the accumulation of Aβ and the level of serum nicotinamide phosphoribosyltransferase (NAMPT) were determined for the evaluation of pathological processes. We found that NR supplementation improved the short-term spatial memory of aged mice, and the contextual fear memory of AD mice. Moreover, NR supplementation inhibited the activation of astrocytes and the elevation of serum NAMPT of aged mice. For AD model mice, NR supplementation inhibited the accumulation of Aβ and the migration of astrocyte to Aβ. In addition, NR supplementation inhibit the body weight gain of aged and APP/PS1 mice. Thus, NR has selective benefits for both AD and aged mice, and the oral uptake of NR can be used to prevent the progression of dementia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advancing age is a primary risk factor for the development of dementia, and Alzheimer’s disease (AD) is one of the most common dementia (Schneider 2017). All kinds of dementia are characterized by progressive cognitive impairment, and the pathological processes associated with chronic neuroinflammation including the activation of microglia and astrocytes. AD is also characterized by the accumulation of amyloid beta (Aβ) plaques and intracellular phosphorylated Tau protein (Schneider 2017). In light of recently failed clinical trials for the treatment of AD and other dementia (Anderson et al. 2017; Lao et al. 2018), it is imperative to explore novel therapeutic targets and strategies (Cummings et al. 2018). Among them, the supplementation of nicotinamide adenine dinucleotide (NAD) to replenish mitochondria can be a promising one (Bachurin et al. 2018; Ruan et al. 2018).
The progressive decline of mitochondria function is a hallmark of aging and of multiple neurodegenerative diseases, which can result in NAD depletion (Zhang et al. 2016; Zhang and Ying 2018). NAD is not only critical for the energy and substance metabolism, but also an essential cofactor for sirtuin deacetylases, for DNA repair protein PARP1, and for cyclic ADP ribose hydrolases CD38 and CD157 (Mendelsohn and Larrick 2017; Johnson and Imai 2018). The high-energy demand by cells like neuron and cancer cells make them sensitive to NAD depletion. For the synthesis of NAD, the main pathway in mammalian cells is the salvaging synthesis pathway (Braidy et al. 2018; Johnson and Imai 2018). In this pathway, the nicotinamide is metabolized to nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyltransferase (NAMPT), the key rate-limiting enzyme. Subsequently, NMN is metabolized to NAD by NMN adenylyltransferases (NMNATs). In addition, NAD can be synthesized de novo from tryptophan, which only take place in limited types of cells.
NAD level can decline upon aging and during many age-related diseases, which results from the decrease of NAD synthesis and the increase of NAD consumption (Johnson and Imai 2018; Zhang et al. 2018). Thus, the restoration of the cellular NAD level is important to repair the cellular functions (Mouchiroud et al. 2013). Supplementation of NAD by using NMN, nicotinic acid and nicotinamide ameliorates some age-related pathological phenotypes of neurodegenerative diseases (Liu et al. 2013; Long et al. 2015; de Picciotto et al. 2016; Wang et al. 2016; Kirkland and Meyer-Ficca 2018). However, nicotinic acid has the side effects including undesirable flushing under therapeutic dose, and nicotinamide may cause liver damage and be associated with the inhibition of sirtuin activity (Bitterman et al. 2002; Bogan and Brenner 2008). NMN may also be toxic, as it has been reported that the accumulation of NMN may accelerate axonal degeneration (Di Stefano et al. 2015).
Evidences show that oral uptake of nicotinamide ribose (NR) also increases the level of NAD and prevents various pathological disorders in animal models. Importantly, NR is more orally bioavailable than nicotinamide and nicotinic acid (Trammell et al. 2016a, b). NR supplementation at 1000 mg/day for 6 weeks is well tolerated in healthy middle-aged and older adults, and is effective for stimulating NAD metabolism in humans (Martens et al. 2018). NR is a safe Vitamin B3 precursor of NAD and naturally exist in cow milk, and thus has been developed as a dietary ingredient (Bogan and Brenner 2008, Trammell et al. 2016a, b). These make NR extremely attractive, especially for the treatment of aging-related diseases (Mendelsohn and Larrick 2017; Johnson and Imai 2018; Yoshino et al. 2018). It has been proposed that NR treatment can mimic calorie restriction (CR), the only known method to enhance life span to date (Sato et al. 2017). NR has been shown to rejuvenate stem cells in both muscle and brain (Ryu et al. 2016), and induce neurogenesis and enhance life span in mice (Zhang et al. 2016). NR treatment can also prevent neuroinflammation, pTau, DNA damage, synaptic dysfunction and neuronal degeneration in an AD mouse with introduced DNA repair deficiency (Hou et al. 2018), and restore the declined cognition of AD mice (Gong et al. 2013). NR treatment prevents excitotoxicity-induced axonal degeneration (Vaur et al. 2017). In short, NR has neuroprotective effects and has benefits on AD.
Despite these studies, the benefits of NR supplementation on aged brain remains unknown. In the present study, we fed the aging mice and the APP/PS1 transgenic AD model mice with NR supplied food (2.5 g/kg food) for 3 months. The effects of NR supplementation on cognitive behavior, locomotor activity, anxiety-like behavior and neuroinflammation and body weight were determined. We found that NR supplementation is beneficial to both aged and AD mice.
Materials and methods
Animals
Sixteen C57BL/6 J female mice in 14 months old were purchased from Zhejiang Academy of Medical Sciences, and used as aged control mice and NR supplied aged mice. However, 2 control mice and 3 NR supplied mice died from unknown cancers with obvious ascites or with obvious subcutaneous mass between 16 and 18 months old. Six C57BL/6 J female mice in 3 months old were purchased from Zhejiang Academy of Medical Sciences, and used as young mice control. The body weight of the young mice was 24.2 ± 1.2 g at the time before sacrifice.
Sixteen B6/JNju-Tg(APPswe,PSEN1dE9)/Nju (APP/PS1) transgenic Alzheimer’s disease model male mice in 3 months old were purchased from Nanjing Institute of Biomedical Research, Nanjing University. Ten C57BL/6 J male mice were purchased from the same institute with similar age, and these mice were used as wild type control.
All mice were handled in accordance with the Guide for the Care and Use of the Laboratory Animals of the National Institutes of Health. The experimental protocols were approved by the Ethics Committee of Laboratory Animal Care and Welfare, Zhejiang University School of Medicine, with the proved number ZJU2015–012-02. The mice had free access to water and food in air-conditioned rooms (20~26 °C, relative humidity ~50%) on a 12-h light/dark cycle.
NR supplementation
NR was purchased from Baikai Chemical Technology Co., Ltd. (Hangzhou, China, CAS: 1341-23-7). NR was mixed into the mice food with the concentration of 2.5 g/kg by Zhejiang Academy of Medical Sciences. We measured the food consumption of mice for 10 days and found that the average food intake was around 160 g/kg. According to the food intake, the daily orally taking of NR is estimated at 400–460 mg/kg.
The NR supplied food was started since 15 month old for C57BL/6 J mice and 4 month-old for APP/PS1 mice, and this food was lasted for 3 months and a week until sacrificed.
Behavioral test
Behavioral tests were started at 18 month-old for C57BL/6 J mice and 7 month-old for APP/PS1 mice. Each mouse received five behavioral tests according to the following illustrated order. Before the open field test, the mice were gently handled for 4 days (5 min/day) to minimize the stress of mice to the handle. Before each experiment, mice were transferred to the experimental room 30 min in advance to adapt the environment. Experiments were started from 9:30 in the morning. Between each individual test, the test equipment was cleaned by using 70% alcohol.

Open field test (Zhang et al. 2006)
This test was performed by using the field test system (ViewPoint Behavior Technology, France). The mice were gently put into the center of the arena of a 45 cm × 45 cm × 45 cm box. The mice were free to move and the movement was tracked and recorded for 10 min by a camera above the box. The center of the bottom was virtually isolated, which has 1/4 of the total area. The total walking distance and the percentage of the distance in center area were calculated, and these were used to evaluate the locomotor activity and the anxious behavior of mice, respectively.
Elevated plus maze (EPM) (Yang et al. 2016)
This test was performed by using an Any-Maze test system (Global Biotech Inc. Shanghai, China). The elevated plus maze has 4 arms including two open arms and two close arms (30 cm × 5 cm × 15 cm for each arm). The apparatus was 45 cm elevated above the floor. Mouse was put in the center of the plus maze with its nose towards an open arm, the movement in the maze was recorded for 5 min. The entries into and the time spent in the open arms was analyzed, and these were used to evaluate the anxious behavior of mice.
Y-maze test (Xiao et al. 2016)
This test was performed by using an Any-Maze test system, and the schematic was presented (Fig. 1a). The three arms of Y-maze (30 cm × 10 cm × 15 cm for each arm) was labeled as start arm, another arm and novel arm. During the 10 min training, the novel arm was closed, and the mouse was put into the start arm. After training, the mouse was returned to home cage. One hour later, the mouse was put into the start arm again for 10 min testing with the novel arm opened. The movement of mouse was recorded and the entries to each arm was analyzed, and this was used to evaluate the spatial memory of mice.
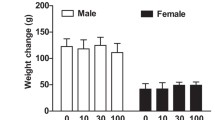
Effects of NR supplementation on the body weight of mice. a Effect of NR supplementation on the body weight of aged mice. N = 5–6. b. Effect of NR supplementation on the body weight of APP/PS1 mice. N = 8–10. *P < 0.05, **P < 0.01, ***P < 0.001, compared with wild type mice, One way ANOVA. #P < 0.05, ##P < 0.01, ###P < 0.001, compared with APP/PS1 mice, One way ANOVA
Novel object recognition (NOR) (Li et al. 2016; Li et al. 2017)
This test was performed by using the field test system, and the schematic was presented (Fig. 1f). The test was carried out in a 45 cm × 45 cm × 45 cm box. During training, two identical objects (5 cm × 5 cm × 5 cm blue cone) was placed diagonally. The mouse was put at the center of the box. The movement of the mouse was recorded for 10 min. Then the mouse was returned to the home cage. Thirty minutes later, the mouse was put to the box again and was recorded for 10 min as testing. Before testing, one of the objects was replaced by an object in distinct color and shape (5 cm × 5 cm × 10 cm yellow cuboid), and the replace object was a novel object. The number of visits to the old and novel objects was analyzed, and this was used to evaluate the spatial memory of the mice.
Fear conditioning (Murchison et al. 2004)
The schematic of fear conditioning test was presented (Fig. 2a). During training, the mouse was placed in the apparatus (ACT-100A, Coulbourn Instruments Inc., Lehigh Valley, PA, YSA) for 2 min. Then an 85 db, 3 kHz tone was activated for 30 s. Two seconds before the end of tone, a 2 s foot shock was delivered (1 mA). Thirty seconds after foot shock, the mouse was returned to home cage. Twenty-four hours after training, contextual fear was tested for 5 min in the same apparatus in the absence of the tone. Cued fear was tested by placing the mouse in a context containing distinct visual and olfactory cues. After 2 min in the novel context, the training tone was applied for 3 min. Percent of freezing time was calculated.
Effects of NR supplementation on the short-term spatial memory of mice tested by using Y-maze and novel object recognition (NOR). a. The schematic of the Y-maze experiment. b and c. The time spent in two arms during training (b) and in three arms during testing (c) of aged mice. d and e. The time spent in two arms during training (d) and in three arms during testing (e) of APP/PS1 mice. f. The schematic of NOR experiment. g and h. The discrimination ratio (g) and discrimination index (h) of objection visiting number of aged mice in NOR. I and J. The discrimination ratio (i) and discrimination index (j) of objection visiting number of APP/PS1 mice in NOR. N = 5–6 for aged mice, N = 8–10 for APP/PS1 mice. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA
Histological analysis and immunofluorescence staining
Mice were euthanized by intraperitoneal injection of chloral hydrate (800 mg/kg). After transcardially perfused with 4 °C saline. The brains were removed and quickly separate into right and left hemisphere. The left hemisphere was fixed in 4% paraformaldehyde for 4 days, and were then transferred to 30% sucrose for 3 days. The right hemisphere was used for the Western blotting analysis.
For staining, the fixed hemispheres were sliced into 25 μm thickness slices by using cryomicrotomy (CM1900, Leica, Wezlar, Gemany). For Nissl staining, the slices were incubated in the mixture of acetone and chloroform (1:1) for 15 min, and then sequentially incubated in 100%, 95%, 70% alcohol for 5 min. Then the slices were stained in Nissl staining buffer for 10 min. The Nissl staining buffer include 0.2% purple crystal (Yuanhang Reagent Factory, Shanghai, China, CAS: YHSJ-01-92) and 0.3% acetic acid. Then the slices dehydrate sequentially incubated in 70%, 95%, 100% alcohol for 5 min. Finally, after for 5 min in xylene, and mounted in the mixture of neutral resin and xylene (1:1) mount. The images were taken under a fluorescence microscope (Olympus BX51, Japan). The hippocampus thickness was determined at dorsal hippocampus CA1 region with sections close to Bregma −1.94 mm. For each mouse, 3 brain slices were assessed and averaged.
For immunofluorescence staining, the slices were incubated in 0.1% Triton-X PBS for 30 min, and followed by the incubation of 5% donkey serum for 1 h. Then the slices were incubated with mouse anti-GFAP (glial fibrillary acidic protein) antibody (1:600, Millipore, MAB360, Billerica, MA, USA), rabbit anti Iba1 (ionized calcium binding adapter molecule 1) antibody (1:400, Wako, 019–19,741, Osaka, Japan), Purified anti-β amyloid 1–16 clone: 6E10 (1:600, Biolegend, San Diego, CA, USA) at 4 °C overnight. After washed with PBS (10 min × 3 times), the slices were incubated with Cy3 conjugated donkey anti-rabbit IgG antibody (1:200, Millipore, AP182C) or FITC conjugated goat anti-mouse IgG antibody (1:200, Millipore, AP124F) for 2 h. After washed with PBS (10 min × 3 times), the slices were mounted on slides by using an anti-fade medium containing DAPI (Invitrogen Corp., Carlsbad, CA, USA).
The images of Iba1, GFAP-positive cells were taken under an Olympus FV100 confocal microscope (Olympus, Japan). The density of Iba1-positive cells and of GFAP-positive cells was counted (cells/mm2). The images of Aβ plaques were taken under an VS120 virtual slide microscope (Olympus, Japan). The number of Aβ plaques was counted and the area of Aβ plaques was measured by MetaMorph Offline version7.8.0.0 (Molecular Devices, LLC. USA).
Western blotting analysis
The right hemisphere were separated into two parts, the cortex and sub-cortex. The total protein from brain tissues was extracted by using protein extraction kit (KC-415, Kangchen Bio-tech Inc., Shanghai, China). After adding lysis buffer (0.5 ml/100 mg tissues), brain was thoroughly homogenized with a Heidolph Diax 900 homogenizer (Heidolph Instruments GmbH & CO., Schwabach, Germany) for 1 min at 4 °C. The supernatant of the mixture was collected by centrifugation at 14,000 g for 30 min. The protein concentration was determined by using BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China. P0009).
Protein samples (100 μg) were used for Western blotting and the following antibodies were used: mouse anti-GFAP (1:1000),mouse anti-GAPDH (1:3000, KangChen, KC-5G4),Rabbit anti-CD11b (1:1000, Abcam, ab133357, Cambridge, UK). The secondary antibody was IRDye™ 800 or 680-conjugated affinity-purified anti-rabbit or anti-mouse IgG (1:3000, LI-COR, Bioscience, Lincoln, NE, USA). The immunoblots were then measured by using an Odyssey fluorescent Scanner (LI-COR). The results were normalized to GAPDH (as a loading reference), and then normalized to the control on the same immunoblot membrane.
To evaluate the NAMPT protein level in serum, 1 μl serum was used for Western blotting. The rabbit anti-PBEF (1:1000, Pre-B cell enhancing factor, another name of NAMPT) and IRDye™ 800 anti-rabbit IgG was sequentially applied for the immunoblot. The SDS page gel between 45 and 75 kDa was cut, and was stained by using Coomassie brilliant blue. A clear band was selected as loading control.
Statistical analysis
Data are presented as mean ± SD. The GraphPad Prism Software (version 6.0, GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis. “N” represents the number of mice used in each group. The Brown-Forsythe test was performed to assess the equal variances of the data. If the data pass equal variance test, and then we used parametric one-way ANOVA to assess the difference between means. If not, then the nonparametric Dunn’s multiple comparisons test was used. A value of P < 0.05 was considered statistically significant.
Results
NR inhibited the body weight gain of aged and APP/PS1 mice
The body weight of aged mice increased from 28.1 ± 1.0 g at 15 months old to 31.5 ± 2.0 g at 17 months old. However, the body weight of aged mice with NR supplementation kept more constantly, increased from 27.6 ± 1.6 g at 15 months old to 28.8 ± 2.1 g at 17 months old. Seven weeks after the start of NR supplied food, the body weight of NR supplied aged mice became lower than the aged mice, although there was not significant (Fig. 1a).
The body weight of APP/PS1 mice increased from 27.1 ± 1.5 g at 4 months old to 32.6 ± 2.2 g at 6 months old, and the body weight of wild type mice increased from 24.8 ± 1.1 g at 4 months old to 29.1 ± 1.2 g at 6 months old. The body weight increasing of NR treated APP/PS1 mice was similar to that of wild type mice (26.2 ± 1.9 g at 4 months old to 29.1 ± 1.8 g at 6 months old), which is significantly lower than that of wild type mice. The supplementation of NR significantly slow down the body weight gain of APP/PS1 mice at 6 weeks after the start of NR supplementation (Fig. 1b).
NR prevented memory impairment of aged mice
Short-term spatial memory was tested in a Y maze (Fig. 2a). The young mice, the aged mice and the NR supplied aged mice spent similar time in the two arms during training (Fig. 2b). During testing, all mice spent more time in the novel arm (Fig. 2c). The wild type mice, the APP/PS1 mice and the NR supplied APP/PS1 mice spent similar time in the two arms during training (Fig. 2d). And all of these mice spent more time in the novel arm (Fig. 2e).
The short-term spatial memory was further tested by using novel object recognition (NOR) (Fig. 2f). All mice visited similar times to the two objects during training (Fig. 2g-j). During testing, the young mice visited more times to the novel object, indicating these mice recognized the novel object (Fig. 2g, h). The aged mice did not visit more to the novel object when compared the visit number to the old object (Fig. 2g, h). The performance of NR supplied aged mice was similar as the young mice, which visited more times toward the novel object (Fig. 2g, h).
The wild type mice, the APP/PS1 mice and the NR supplied APP/PS1 mice had the similar visit number to two objects during training (Fig. 2i, j). All three kinds of mice had significantly more visits to novel object during testing (Fig. 2)i, j. And there was no difference among these three groups during testing (Fig. 2j).
NR improved the contextual fear memory of APP/PS1 mice
The contextual and cue memory of mice in fear conditioning was determined as illustrated (Fig. 3a). For the aged mice, the freezing time in the same context, which indicate the contextual memory, decreased although there was no significant. The treatment of NR did not increase the freezing time of aged mice (Fig. 3b). The freezing time on tone in a new box of aged mice, which indicate the cue fear memory, significantly decreased. The treatment of NR also failed to improve it (Fig. 3b).
Effects of NR supplementation on the fear memory of mice. a. The schematic of fear conditioning experiment. b and c. The percent of freeze time of aged (b) and APP/PS1 (c) mice. Box A: during training before the tone; Box A*: same context at 24 h after training. Box B: a different box for 2 min without tone at 24 h after training; Tone: a different box for 3 min with tone. N = 5–6 for aged mice, N = 8–10 for APP/PS1 mice. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA
For the APP/PS1 mice, the contextual fear memory significantly decreased when compared to the wild type mice. The treatment of NR improved the contextual fear memory of APP/PS1 mice (Fig. 3c). The APP/PS1 mice showed slightly but not significant decreased cue fear memory, and the treatment of NR failed to affect the cue fear memory of APP/PS1 mice (Fig. 3c).
Effects of NR on the locomotor activity and anxiety behavior of mice
First, the locomotor activity and anxiety behavior of mice was determined in an open field (Fig. 4a, d). The aged mice travelled shorter distance in the open field when compared to the young mice, indicating the less locomotor activity of the aged mice (Fig. 3b). However, the percentage of central distance remained the same between young and aged mice, indicating that the anxious level of the young and aged mice was the similar (Fig. 4c). The NR supplementation did not change the travel distance or the percentage of central distance of the aged mice (Fig. 4b, c). On the contrary, the APP/PS1 mice travelled significantly longer distance in open field when compared to the wild type mice (Fig. 4e), while the percentage of central distance was the same (Fig. 4f). The NR supplementation did not change the total distance and the percentage of central distance of the APP/PS1 mice (Fig. 4e, f).
Effects of NR supplementation on locomotor activity and anxiety behaviors of mice tested by using open field and elevated plus maze. a and d. The representative tracking graphs of aged mice (a) and APP/PS1 mice (d) in open field. d and c. The total distance travelled (b) and the percentage of distance in the center of open field (c) of aged mice. **P < 0.01, compared with the young mice, one-way ANOVA. E and F. The total distance travelled (e) and percentage of distance in the center of open field (F) of APP/PS1 mice. **P < 0.01, ***P < 0.001, compared with wild type mice, one-way ANOVA. g and j. The representative hot maps of aged mice (g) and APP/PS1 mice (j) in elevated plus maze. h and i. The entries to open arms (h) and time spent in open arm (i) in the elevated plus maze of aged mice. k and l. The entries to open arms (k) and time in open arm (l) in the elevated plus maze of APP/PS1 mice. *P < 0.05, compared with wild type mice, Dunn’s multiple comparisons test. N = 5–6 for aged mice, N = 8–10 for APP/PS1 mice
The anxiety behavior of mice was further tested in an elevated plus maze (Fig. 4g, j). The aged mice showed similar entries to and staying time in open arms (Fig. 4h, i). The treatment of NR did not change the entries to and staying time in open arms of the aged mice (Fig. 4h, i). The APP/PS1 mice had the similar entries to and staying time in open arms when compared with the wild type mice (Fig. 4k, l). The treatment of NR did not affect the entries to open arm of APP/PS1 mice (Fig. 4k). However, the treatment of NR decreased the time spent in open arm of APP/PS1 mice when compared with the wild type mice, even though there was no significant difference between the NR supplied and the non-supplied APP/PS1 mice (Fig. 4l).
NR decreased the chronic neuroinflammation of aged mice
The aged mice had thinner layer of neuronal cell body in hippocampus CA1 region when compared with the young mice, and this morphological change could not be rescued by the NR supplementation (Fig. 5a, b). Using immunofluorescence staining, we found that the density of Iba1-positive cells, corresponding to the number of microglia, remained constant for aged mice in the cortex of mouse brains, when compared to the young mice (Fig. 6a, b). However, the density of Iba1-positive cells in hippocampus CA3 region was higher in aged mouse brain than in young mouse brain (Fig. 6c, d). The NR supplementation had no effect on the density of Iba1-positive cells in cortex and in hippocampus CA3 region of aged mice (Fig. 6a-d). The density of GFAP-positive cells, corresponding to number of astrocytes, was higher in hippocampus DG region of aged mouse brain than young mouse brain. The NR supplementation had no effect on the density of GFAP-positive in hippocampus DG region of aged mouse brain (Fig. 6e, f).
Effects of NR supplementation on the neurons in aged and APP/PS1 mice. a and c. representative images of hippocampus CA1 region in aged (a) and APP/PS1 (c) mice stained by using Nissel staining. The brain sections were close to Bregma −1.94 mm. B and D. The thickness of neuron bodies in hippocampal CA1 region in aged (a) and APP/PS1 (c) mice. N = 5–6 for aged mice and N = 8–10 for APP/PS1 mice. *P < 0.05, compared with young mice, one-way ANOVA
Effects of NR supplementation on the CD11b and GFAP expression in aged mice. a and c. Representative immunofluorescent staining of CD11b-positive cells in cortex (a) and hippocampus CA3 region (c). The brain sections were close to Bregma −1.94 mm. b and d. The analyzed density of CD11b-positive cells in cortex (b) and hippocampus CA3 region (d) of aged mice. E. Representative immunofluorescent staining of GFAP-positive cells. F. The analyzed density of GFAP-positive cells in hippocampus DG region. N = 5–6. *P < 0.05, **P < 0.01, compared with young mice, one-way ANOVA
We further analyzed the activation of microglia and astrocyte by determining the expression level of CD11 and GFAP using Western blotting. We found that the expression of CD11b, a microglia marker, remained unchanged in the cortex of aged mice (Fig. 7a), but its expression significantly increased in the sub-cortex of aged mice, when compared with the young mice (Fig. 7b). NR supplementation slightly decreased the expression of CD11b in the sub-cortex of aged mice (Fig. 7b). The GFAP expression was very low in the cortex of young and aged mice (data not shown). Yet, the GFAP expression is high in the sub-cortex, and GFAP level significantly increased in the sub-cortex of aged mice when compared to the young mice (Fig. 7c). The increase of GFAP expression in the sub-cortex of aged mice was significantly decreased upon the supplementation of NR (Fig. 7c).
Effects of NR supplementation on the CD11b, GFAP and NAMPT expression in aged mice. A and B. Expression of CD11b in the cortex (a) and sub-cortex (b) of mice brain. C. Expression of GFAP in the sub-cortex of mice brain. D. The level of NAMPT protein in blood serum. N = 5–6. **P < 0.01, ***P < 0.001, compared with young mice, one-way ANOVA. #P < 0.05, ###P < 0.001, compared with aged mice, one-way ANOVA
The serum level of NAMPT significantly increased in aged mice when compared to the young mice (Fig. 7d). The supplementation of NR abolished the increasing of serum NAMPT in aged mice (Fig. 7d).
NR decreased the chronic neuroinflammation and Aβ accumulation in APP/PS1 mice
The thickness of the neuronal cell body in hippocampus CA1 region remained unchanged for APP/PS1 mice (Fig. 5c, d). Immunofluorescence staining showed that the density of Iba1-positive cells was unchanged in the cortex and hippocampus DG region of APP/PS1 mice when compared with wild type mice, and the treatment of NR had no effect (Fig. 8a-c). The wild type mice had very few GFAP-positive cells in cortex (Fig. 8d). However, there are many GFAP-positive cells in the cortex of APP/PS1 mice with or without the treatment of NR, and these GFAP-positive cells were piled together (Fig. 8d). Surprisingly, the density of GFAP-positive cells decreased in the hippocampus CA1 region of APP/PS1 mice, which was reversed by the supplementation of NR (Fig. 8d, e).
Effects of NR supplementation on the CD11b and GFAP expression in APP/PS1 mice. a. Representative immunofluorescent staining of CD11b-positive cells in cortex (upper panel) and hippocampus DG region (lower panel) of APP/PS1 mice. The brain sections were close to Bregma −1.94 mm. b and c. The analyzed density of CD11b-positive cells in cortex (b) and hippocampus DG region (c) of APP/PS1 mice. D. Representative immunofluorescent staining of GFAP-positive cells in cortex (upper two panels) and hippocampus CA1 region (lower panel) of APP/PS1 mice. E. The analyzed density of GFAP-positive cells in hippocampus CA1 region. N = 8–10. ***P < 0.001, compared with wild type mice, ###P < 0.001, compared with APP/PS1 mice, one-way ANOVA
The expression level of CD11b in the cortex and sub-cortex of APP/PS1 mice was similar as that of the wild type mice, and was not affected by the supplementation of NR (Fig. 9a, b). The expression level of GFAP significantly increased in the cortex and sub-cortex of APP/PS1 mice when compared to the wild type. The supplementation of NR did not inhibit the increase of GFAP expression (Fig. 9c, d). The level of NAMPT in blood serum significantly increased, which was not affected by the supplementation of NR (Fig. 9e).
Effects of NR on the CD11b, GFAP and NAMPT expression in APP/PS1 mice. A and B. Expression of CD11b in the cortex (a) and sub-cortex (b) of mice brain. C and D. Expression of GFAP in the cortex (c) and sub-cortex (d) of mice brain. E. The level of NAMPT protein in blood serum. N = 8–10. *P < 0.05, **P < 0.01, ***P < 0.001, compared with wild type mice, one-way ANOVA
Fluorescence immunostaining showed that there were many Aβ plaques accumulated in the cortex and sub-cortex of APP/PS1 mice, but not in the brain of wild type mice (Fig. 10a). The supplementation of NR did not change the number and total area of Aβ plaques in hippocampus of APP/PS1 mice (Fig. 10b, c). Surprisingly, the supplementation of NR significantly decreased the number and total area of Aβ plaques in cortex of APP/PS1 mice (Fig. 10d, e).
Effects of NR on Aβ formation in APP/PS1 mice. a. Representative images of immune-fluorescence staining of Aβ. The brain sections were close to Bregma −1.94 mm. b and c. The number of plaques (b) and total area of plaques (c) in the hippocampus. d and e. The number of plaques (d) and total area of plaques (e) in the cortex. N = 8–10. *P < 0.05, unpaired t-test
Discussion
We have shown here that the supplementation of NR ameliorated selective cognitive impairment and the chronic brain neuroinflammation in aged mice, and ameliorated selective cognitive impairment and Aβ accumulation in AD model mice. Further, the supplementation of NR decreased the gain of body weight for aged and APP/PS1 mice.
The beneficial effects of NR regiment on AD model mice has been reported, for example in a DNA repair-deficient 3xTgAD/Polbeta(± ) mouse (Hou et al. 2018) and in a Tg2576 mouse (Gong et al. 2013). The later study shows that 250 mg/kg/day of NR for 3 months significantly attenuates cognitive deterioration, which coincides with an increase in the steady-state levels of NAD in the cerebral cortex (Gong et al. 2013). Consistently, we also showed that the supplementation of NR improved the contextual fear memory of APP/PS1 mice. We found that NR also inhibited the accumulation of Aβ, similar to the previous reports (Hou et al. 2018). In addition, the AD mice showed a decreasing density of astrocyte in hippocampus, and miraculously NR supplementation prevented such decreasing. For this density counting, we deliberately avoided the piled astrocyte, since the piles were similar as the Aβ accumulation zone. Since astrocytes can migrate and surround amyloid plaques in AD brain (Lai et al. 2013), the decreasing of astrocytes may be due to the migration of astrocytes to the Aβ accumulation zones. The supplementation of NR decreased Aβ accumulation, which may result in the inhibition of the migration of astrocyte.
However, the APP/PS1 mice showed intact short-term spatial memory in Y maze and NOR experiments. This may be due to the short intervals between training and testing, which makes the task much easier for the AD model mice. In comparison, the APP/PS1 mice showed impairment in fear memory with a 24-h interval between training and testing. The supplementation of NR only rescued the contextual fear memory but not cue fear memory. It has been known that the contextual fear memory is hippocampus dependent, and the cued fear memory is non-hippocampus dependent (Murchison et al. 2004). This may imply that the oral uptake of NR has selective effects on distinct neurons or brain regions with unknown reasons. We do not know whether this is due to the different permeability of blood-brain barrier in different brain region for the transport of NR, or due to the penetration and metabolism of NR in selective neurons. In addition, the APP/PSA1 mice increased the locomotor activity in the open-field experiment, and this may cover the performance of APP/PS1 mice in Y maze and NOR performance. The supplementation of NR slightly decreased the time spend in open arm in the EPM, indicating the possible increasing of anxiety-like behavior, which may also counter act the effect of NR on cognitive performance.
The protective effects of NAD supplementary have been reported in aged organs (Yoshino et al. 2018), such as non-alcoholic fatty liver (Zhou et al. 2016), skeletal muscle (Zhang et al. 2016), blood vessels (de Picciotto et al. 2016). Here we found that the supplementation of NR also has beneficial effects on aged brain. The supplementation of NR improved the novel object recognition, and inhibited the chronic neuroinflammation of aged mice. It should be noted that NR did not affect the anxiety-like behavior of aged mice. However, the supplementation of NR only improved the short memory with 30–60 min interval between the training and testing, but failed to rescue the fear memory, which has 24 h interval between training and testing. The effects of NR supplementation on memory seems dissociate with the low locomotor activity of aged mice. Since, NR supplementation did not change the locomotor activity of aged mice. And in general, the sedative effect of aging may increase the freezing time (Ritter et al. 2014), however, we found that the freezing time significantly decreased in aged mice with/without NR supplementation. Thus, NR did not show sedative effect, and the sedative situation of aged mice did not affect the result of NR supplementation on cognition.
Interestingly, the treatment of NR inhibited the increase of NAMPT in serum of aged-mice. NAMPT can be secreted by multiple cells, serving as a cytokine (Montecucco et al. 2013), and the serum NAMPT level is elevated in inflammatory diseases (Gesing et al. 2017), age-related diseases (Imai and Kiess 2009; Moschen et al. 2010) and aging (Liu et al. 2012). Thus, NR may show anti-inflammatory effect of aged and APP/PS1 mice via the inhibition of the elevation of serum NAMPT. And NR may also protect the peripheral organs in aged mice and ameliorate chronic inflammation by inhibit NAMPT release. Yet, the serum NAMPT also increased in APP/PS1 mice, which was not inhibited by the supplementation of NR. This may imply that the sources of NAMPT or the mechanism of NMAPT secretion can be different, yet both remains unknown. Another surprised benefit of NR supplementation is the inhibition of body weight gain of aged and APP/PS1 mice. It has been reported that the increase of NAD/SIRT1 is associated with weight loss (Rappou et al. 2016). And NR supplementation significantly attenuated high-fat diet induced body weight gain, which was related with the NAD/SIRT1 pathway (Canto et al. 2012).
We can see that the effects of NR supplementation on aged mouse and APP/PS1 mice were different, which may arise from the distinct mechanisms. Aging is a natural process that is accompanied with the depletion of NAD and dysfunction of mitochondria, which may result in dementia (Zhang et al. 2016; Johnson and Imai 2018). The supplementation of NR is a direct replenish of NAD and can restore the function of mitochondria (Zhang et al. 2016; Yoshino et al. 2018). APP/PS1 mouse is a transgenic AD model mouse, which is characterized by the accumulation of Aβ and the followed disorders, such as neuroinflammation and cognition impairment (Yan et al. 2009; Lagadec et al. 2012). The supplementation of NR may enhance the function of mitochondria via the replenish of NAD, which can be result in the stronger clearance of Aβ (Long et al. 2015; Hou et al. 2018).
In summary, the 3 months supplementation of NR had benefits to both the aged brain and the brain of AD mice. This implies that NR may possess beneficial effects to multiple dementia with particular mechanisms. Thus, the oral uptake of NR can be a promising stratagem for the prevention of dementia due to AD and the others.
References
Anderson RM, Hadjichrysanthou C, Evans S, Wong MM (2017) Why do so many clinical trials of therapies for Alzheimer's disease fail? Lancet 390(10110):2327–2329
Bachurin SO, Gavrilova SI, Samsonova A, Barreto GE, Aliev G (2018) Mild cognitive impairment due to Alzheimer disease: contemporary approaches to diagnostics and pharmacological intervention. Pharmacol Res 129:216–226
Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA (2002) Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 277(47):45099–45107
Bogan KL, Brenner C (2008) Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 28:115–130
Braidy N, Berg J, Clement J, Khorshidi F, Poljak A, Jayasena T, Grant R, Sachdev P (2018) Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7269
Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J (2012) The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15(6):838–847
Cummings J, Lee G, Ritter A, Zhong K (2018) Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement (N Y) 4:195–214
de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR (2016) Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15(3):522–530
Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, Tickle J, Patrick J, Webster JR, Marangoni M, Carpi FM, Pucciarelli S, Rossi F, Meng W, Sagasti A, Ribchester RR, Magni G, Coleman MP, Conforti L (2015) A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ 22(5):731–742
Gesing J, Scheuermann K, Wagner IV, Loffler D, Friebe D, Kiess W, Schuster V, Korner A (2017) NAMPT serum levels are selectively elevated in acute infectious disease and in acute relapse of chronic inflammatory diseases in children. PLoS One 12(8):e0183027
Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM (2013) Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models. Neurobiol Aging 34(6):1581–1588
Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O'Connell JF, Baptiste BA, Stevnsner TV, Mattson MP, Bohr VA (2018) NAD(+) supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A 115(8):E1876–E1885
Imai S, Kiess W (2009) Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front Biosci (Landmark Ed) 14:2983–2995
Johnson S, Imai SI (2018) NAD (+) biosynthesis, aging, and disease. F1000Res 7:132
Kirkland JB, Meyer-Ficca ML (2018) Niacin. Adv Food Nutr Res 83:83–149
Lagadec S, Rotureau L, Hemar A, Macrez N, Delcasso S, Jeantet Y, Cho YH (2012) Early temporal short-term memory deficits in double transgenic APP/PS1 mice. Neurobiol Aging 33(1):203. e201–203. e211
Lai W, Wu J, Zou X, Xie J, Zhang L, Zhao X, Zhao M, Wang Q, Ji J (2013) Secretome analyses of Abeta(1-42) stimulated hippocampal astrocytes reveal that CXCL10 is involved in astrocyte migration. J Proteome Res 12(2):832–843
Lao K, Ji N, Zhang X, Qiao W, Tang Z, Gou X (2018) Drug development for Alzheimer's disease: review. J Drug Target:1–10
Li C, Yan Y, Cheng J, Xiao G, Gu J, Zhang L, Yuan S, Wang J, Shen Y, Zhou YD (2016) Toll-like receptor 4 deficiency causes reduced exploratory behavior in mice under approach-avoidance conflict. Neurosci Bull 32(2):127–136
Li D, Zhang L, Huang X, Liu L, He Y, Xu L, Zhang Y, Zhao T, Wu L, Zhao Y, Wu K, Wu Y, Fan M, Zhu L (2017) WIP1 phosphatase plays a critical neuroprotective role in brain injury induced by high-altitude hypoxic inflammation. Neurosci Bull 33(3):292–298
Liu LY, Wang F, Zhang XY, Huang P, Lu YB, Wei EQ, Zhang WP (2012) Nicotinamide phosphoribosyltransferase may be involved in age-related brain diseases. PLoS One 7(10):e44933
Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, Kawamoto EM, Mattson MP (2013) Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging 34(6):1564–1580
Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA (2015) Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer's disease-relevant murine model. BMC Neurol 15:19
Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR (2018) Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun 9(1):1286
Mendelsohn AR, Larrick JW (2017) The NAD+/PARP1/SIRT1 Axis in aging. Rejuvenation Res 20(3):244–247
Montecucco F, Cea M, Cagnetta A, Damonte P, Nahimana A, Ballestrero A, Del Rio A, Bruzzone S, Nencioni A (2013) Nicotinamide phosphoribosyltransferase as a target in inflammation- related disorders. Curr Top Med Chem 13(23):2930–2938
Moschen AR, Gerner RR, Tilg H (2010) Pre-B cell colony enhancing factor/NAMPT/visfatin in inflammation and obesity-related disorders. Curr Pharm Des 16(17):1913–1920
Mouchiroud L, Houtkooper RH, Auwerx J (2013) NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol 48(4):397–408
Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA (2004) A distinct role for norepinephrine in memory retrieval. Cell 117(1):131–143
Rappou E, Jukarainen S, Rinnankoski-Tuikka R, Kaye S, Heinonen S, Hakkarainen A, Lundbom J, Lundbom N, Saunavaara V, Rissanen A, Virtanen KA, Pirinen E, Pietilainen KH (2016) Weight loss is associated with increased NAD(+)/SIRT1 expression but reduced PARP activity in white adipose tissue. J Clin Endocrinol Metab 101(3):1263–1273
Ritter AM, Ames FQ, Otani F, de Oliveira RM, Cuman RK, Bersani-Amado CA (2014) Effects of anethole in nociception experimental models. Evid Based Complement Alternat Med 2014:345829
Ruan Q, Ruan J, Zhang W, Qian F, Yu Z (2018) Targeting NAD(+) degradation: the therapeutic potential of flavonoids for Alzheimer's disease and cognitive frailty. Pharmacol Res 128:345–358
Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mazala DA, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM, Bellemin S, Iyer SR, Wang X, Gariani K, Sauve AA, Canto C, Conley KE, Walter L, Lovering RM, Chin ER, Jasmin BJ, Marcinek DJ, Menzies KJ, Auwerx J (2016) NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med 8(361):361ra139
Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, Brenner C, Masri S, Benitah SA, Sassone-Corsi P (2017) Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170(4):664–677 e611
Schneider L (2017) Alzheimer's disease and other dementias: update on research. Lancet Neurol 16(1):4–5
Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C (2016a) Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7:12948
Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C (2016b) Nicotinamide riboside is a major NAD+ precursor vitamin in cow Milk. J Nutr 146(5):957–963
Vaur P, Brugg B, Mericskay M, Li Z, Schmidt MS, Vivien D, Orset C, Jacotot E, Brenner C, Duplus E (2017) Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J 31(12):5440–5452
Wang X, Hu X, Yang Y, Takata T, Sakurai T (2016) Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res 1643:1–9
Xiao J, Huang Y, Li X, Li L, Yang T, Huang L, Yang L, Jiang H, Li H, Li F (2016) TNP-ATP is beneficial for treatment of neonatal hypoxia-induced Hypomyelination and cognitive decline. Neurosci Bull 32(1):99–107
Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, Gonzales E, Holtzman DM, Lee JM (2009) Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci 29(34):10706–10714
Yang L, Shi LJ, Tang B, Han QQ, Yu J, Wu GC, Zhang YQ (2016) Opposite sex contact and isolation: a novel depression/anxiety model. Neurosci Bull 32(1):92–98
Yoshino J, Baur JA, Imai SI (2018) NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab 27(3):513–528
Zhang M, Ying W (2018) NAD(+) deficiency is a common central pathological factor of a number of diseases and aging: mechanisms and therapeutic implications. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017
Zhang Q, Wei EQ, Zhu CY, Zhang WP, Wang ML, Zhang SH, Yu YP, Chen Z (2006) Focal cerebral ischemia alters the spatio-temporal properties, but not the amount of activity in mice. Behav Brain Res 169(1):66–74
Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J (2016) NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352(6292):1436–1443
Zhou CC, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF, Wang P, Miao CY (2016) Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol 173(15):2352–2368
Acknowledgments
The authors are grateful to the Core Facilities of Zhejiang University Institute of Neuroscience for technical assistance.
This work was supported by grants from the National Key R&D Program of China (2018YFA0507700), the National Natural Sciences Foundation of China (81573400), the Zhejiang Provincial Natural Science Foundation of China (LY18H170001), and Public Technology Application Research of Zhejiang Province (2016F82G2010036).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, X., Gao, Y., Zeng, M. et al. Nicotinamide ribose ameliorates cognitive impairment of aged and Alzheimer’s disease model mice. Metab Brain Dis 34, 353–366 (2019). https://doi.org/10.1007/s11011-018-0346-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0346-8