Abstract
Previous studies found that electroacupuncture (EA) at the Shenting (DU24) and Baihui (DU20) acupoints alleviates cognitive impairment in cerebral ischemia–reperfusion (I/R) injury rats. Nonetheless, the mechanisms of the anti-inflammatory effects of EA are unclear. Cerebral I/R injury was induced in rats by middle cerebral artery occlusion (MCAO). Following I/R injury, the rats underwent EA therapy at the Shenting (DU24) and Baihui (DU20) acupoints for seven successive days. The Morris water maze test, magnetic resonance imaging (MRI) and molecular biology assays were utilized to assess the establishment of the rat stroke model with cognitive impairment and the therapeutic effect of EA. EA treatment of rats subjected to MCAO showed a significant reduction in infarct volumes accompanied by cognitive recovery, as observed in Morris water maze test outcomes. The possible mechanisms by which EA treatment attenuates cognitive impairment are by regulating endogenous melatonin secretion through aralkylamine N-acetyltransferase gene (AANAT, a rate-limiting enzyme of melatonin) synthesis in the pineal gland in stroke rats. Simultaneously, through melatonin regulation, EA exerts neuroprotective effects by upregulating mitophagy-associated proteins and suppressing reactive oxygen species (ROS)-induced NLRP3 inflammasome activation after I/R injury. However, melatonin receptor inhibitor (luzindole) treatment reversed these changes. The findings from this research suggested that EA ameliorates cognitive impairment through the inhibition of NLRP3 inflammasome activation by regulating melatonin-mediated mitophagy in stroke rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high incidence and high disability of stroke have created a burdensome possession for society [1], and ischemic stroke remains the most common type of several other stroke types because of its high prevalence attacking the brain [1, 2]. Stroke often leads to persistent poststroke cognitive impairment (PSCI). PSCI has a high incidence, and PSCI can cause various symptoms, which is an essential factor limiting the extensive rehab of stroke patients [3, 4]. As a result, the prevention and treatment of stroke with cognitive impairment have actually come to be an important issue in medical institutions worldwide.
Numerous clinical data and experimental studies have revealed that acupuncture is a practical approach for curing ischemic stroke in the acute period, convalescent period, or sequela period [5, 6]. The Shenting (DU24) and Baihui (DU20) acupoints are located on the Du meridian on the head. These acupoints have been clinically used to treat cognitive problems for thousands of years in China. Our previous research studies have validated the effectiveness of electroacupuncture (EA) at the Shenting and Baihui acupoints in the rehabilitation of cognitive impairment in experimental studies [7,8,9]. Nevertheless, the specific mechanism of EA therapy at the Shenting and Baihui acupoints has yet to be determined.
The NLRP3 inflammasome has been verified to mediate poststroke brain injury [10,11,12]. NLRP3 inflammasome activation triggers the proinflammatory cell death pathway known as pyroptosis, accompanied by the maturation and secretion of IL-1β and IL-18 [13]. It has been reported that inhibition or knockdown of the NLRP3 inflammasome can decrease mature IL-1β and IL-18 secretion, which exerts a neuroprotective effect on a stroke rat model [14, 15]. Of note, a study revealed that the NLRP3 inflammasome is a crucial modifier of neuropathology in PSCI [16]. These studies demonstrate that inhibition of NLRP3 inflammasome activation can be an effective target for the treatment of PSCI.
Reactive oxygen species (ROS) are reported to be a vital factor in inducing NLRP3 inflammasome activation [17], and damaged mitochondria are the leading site of intracellular ROS generation in the injured area of the brain [18]. Therefore, the inhibition of ROS generation and the removal of dysfunctional mitochondria are potential therapeutic targets for stroke [19,20,21]. Mitophagy, a selective form of autophagy that specifically removes damaged mitochondria, is also regarded as an effective way to maintain mitochondrial homeostasis and cellular survival [22]. Moreover, studies have demonstrated that melatonin-mediated mitophagy protects against brain injury through the inhibition of NLRP3 [14]. Melatonin has been verified in the treatment of various neurological diseases due to its antioxidative, antiapoptotic, and anti-inflammatory properties [23]. Additionally, some studies have shown that melatonin ameliorates the neuroinflammation and cognitive impairment caused by ischemia/reperfusion (I/R) injury[24, 25]. Thus, the inhibition of the NLRP3 inflammasome through the regulation of melatonin-mediated mitophagy could be a target for PSCI treatment. Interestingly, a study showed that oral administration of melatonin did not significantly increase serum melatonin levels in rats [26], suggesting that the biological effects of melatonin on the body are mainly through endogenous regulatory mechanisms. EA, as a nonpharmaceutical therapy, may play a crucial role through the regulation of endogenous melatonin to exert its multitarget and multipathway protective effects in the treatment of stroke.
To date, the effects of EA on melatonin-mediated mitophagy and its underlying mechanism with the NLRP3 inflammasome in rat stroke models have remained elusive. The present study thus investigated whether EA at the DU24 and DU20 acupoints alleviates cognitive impairment via the inhibition of NLRP3 inflammasome activation by regulating melatonin-mediated mitophagy in cerebral I/R-injured rats.
Materials and Methods
Animals
Sixty specific pathogen-free (SPF) adult male Sprague–Dawley rats weighing 300 ± 20 were provided by Fujian University of Traditional Chinese Medicine Laboratory Animal Center (Fuzhou, China; Laboratory Animal Use Certificate no. SYXK (FJ) 2019–0007). To reduce variance and bias within group, female rats were not included as estradiol levels may complicate interpretation of melatonin effects because of their interaction [27]. All rats were housed in an SPF animal laboratory at a temperature of 22 ± 1 °C with 50% humidity under a 12‑h light/dark cycle with free access to food and water. All experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (Fuzhou, China).
Middle Cerebral Artery Occlusion Model (MCAO) and Grouping
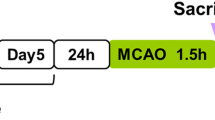
Sixty rats were randomly divided into a sham operation group (n = 15) and an operation group (n = 45) by the random number table method. All rats were fasted for 12 h before surgery, and middle cerebral artery occlusion (MCAO) was applied to establish a cerebral I/R-injured rat model according to the method of Longa et al. [28]. The rats were anesthetized by isoflurane /O2 (with 3% for a 5-min induction followed by 1.2–1.5% for maintaining the rats in a state of deep anesthesia) and fixed in the supine position. The skin was cut in the middle of the neck, and the tissue layers were bluntly separated to expose the left common carotid artery (CCA). The internal carotid artery (ICA) and external carotid artery (ECA) were separated after the bifurcation. The internal and common carotid arteries were clipped, and the proximal and distal ends of the external carotid artery were ligated. Subsequently, 22 mm of nylon thread was inserted through the external carotid artery and ligated with no. 0 surgical silk thread to prevent bleeding. The artery clip at the internal carotid artery was opened, nylon thread was inserted into the internal carotid artery and continued to be inserted into the skull at a depth of 18.5 ± 0.5 mm to achieve slight resistance, the ICA was ligated, and the wound was sutured. The thread was removed after 90 min of occlusion to induce reperfusion injury. Regional cerebral blood flow (CBF) was measured by laser Doppler flowmetry (BIOPAC Systems, Goleta, CA, USA), and a ≥ 80% reduction in CBF was considered a successful model. In this experiment, the success rate of the operation group was approximately 69%. A total of 31 rats were successfully modeled. Considering the principle of balance, the rats in the operation group were further randomly divided into the cerebral I/R control group (I/R group, I/R with EA treatment group (I/R + EA group) and inhibitor (luzindole) group (I/R + EA + Luz group), with 10 rats in each group. Ten rats in the sham operation group were also included as the sham operation control group (sham group).
Morris Water Maze Test
The effects of EA treatment on spatial learning ability and memory were assessed by the Morris water maze test from the 3rd day to the 7th day after I/R injury [8, 9]. The Morris water maze test mainly includes orientation navigation and space exploration trials. In the orientation navigation trial (days 3–6), each rat was arranged to swim for 90 s to find the platform. The swimming route and swimming time of each rat in the four quadrants per day were evaluated. The space exploration trial was conducted on the 7th day. Each rat was allowed to swim freely for 90 s after the platform was removed, and the number of times each rat crossed the platform during this period was recorded.
EA Treatment and Drug Administration
The sham and I/R groups grasped only under the same conditions and did not receive any EA intervention. EA treatment and drug administration were started after 24 h of reperfusion. Rats in the I/R + EA group and I/R + EA + Luz group were treated with EA at the Baihui (GV 20, located in the center of the parietal bone) and Shenting (DU 24, located in the anterior median line) acupoints for 30 min per day for 7 days. Acupuncture needles (0.3 mm) were inserted 30° oblique into the DU24 and DU20 acupoints on the heads of the rats at a depth of 0.2 cm. An electroacupuncture instrument (Model SDZ-V; Suzhou Hua Tuo Medical Instrument Co., Ltd., Suzhou, China) was used for electrical stimulation. The stimulation parameters were set as follows: set the waveform mode as a denser wave/denser wave, in which the sparse wave was 4 Hz, the dense wave was 20 Hz, the output voltage was 2 V, and the output current was 0.5 mA. In the I/R + EA + Luz group, the stroke rats were intraperitoneally administered 30 mg/kg/day melatonin receptor antagonists (luzindole, Abcam, Cambridge, MA, USA).
Magnetic Resonance Imaging
On the seventh day, rats were anesthetized with 1.5% isoflurane (Sigma, Missouri, USA) and subjected to T2-weighted (T2w) imaging by 7.0 T Bruker Scanner Biospin MR imaging system (Pharmascan, microMRI, Bruker Medizintechnik, Germany) to evaluate the infarct area. The parameters were set as follows: echo time, 33 ms; recovery time, 2738 ms; scan time, 5.85 min; field of view, 30 × 30 mm; and 24 contiguous slices of 0.8 mm thickness. Infarct volume of the whole brain and the left hippocampus was measured from T2w images by manually tracing the hyperintense regions on each slice using ImageJ software (National Institutes of Health, Bethesda, MD, USA) as a percentage of the total brain volume.
Enzyme-Linked Immunosorbent Assay
Blood samples were collected at 12:00 and 24:00 (under 5 watts red-light conditions to ensure that melatonin secretion was not affected by lights) on days 1, 3, 5, and 7 after MCAO. Repeated blood collections from the tail vein of nonanesthetized rats were performed with a vacuum blood collection system as previously described [29]. Blood samples were stored at room temperature for 10–20 min to allow clotting. Then, the samples were centrifuged (20 min, 2000–3000 rpm), and the obtained serum was poured into microtubes with a lid and then transferred into − 20 °C freezers so that all samples could be collected and sent to the laboratory for enzyme-linked immunosorbent assay (ELISA) testing. Serum melatonin levels were assessed in triplicate using a rat melatonin ELISA kit (Cusabio, Wuhan, China).
Electron Microscopy
Left hippocampal tissues were prefixed in 3% glutaraldehyde for 24 h at 4 °C and postfixed for 2 h in 1% osmium tetroxide. Then, the tissues were rinsed three times, gradient dehydrated and flat-embedded in Araldite resin at the following conditions: 35 °C for 12 h, 45 °C for 12 h and 60 °C for 48 h. Subsequently, the tissues were sliced into ultrathin sections of 70–80 nm. After double staining with uranyl acetate and lead citrate, the sections were observed with an electron microscope (H‑7650; Hitachi, Tokyo, Japan) at a magnification of × 10,000.
Reverse Transcription-Polymerase Chain Reaction
AANAT mRNA levels in the pineal gland were determined by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted from the rat pineal gland, which was obtained immediately after blood sample collection at 24:00 under anesthesia on day 7, with TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). One microgram of oligo(dT)-primed RNA was used to obtain cDNA samples using SuperScript II Reverse Transcriptase (Fermentas, Ontario, Canada) according to the manufacturer's instructions. mRNA levels of AANAT and β-catenin using Taq DNA polymerase (Fermentas, Ontario, Canada) and β-actin were used as internal controls. The oligonucleotide primers were as follows [30]: AANAT, 5′-TCCTGTGGAGATACCTTCACCA-′3 (forward) and 5′-CACAGTTCAGAAGGCAAGAGGT-3′ (reverse); β-actin, 5′-GACCTGACTGACTACCTCAT-3′ (forward) and 5′-TCGTCATACTCCTGCTTGCT-3′ (reverse). Reactions were conducted in an S1000™ Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The amplification program was set as follows: 95℃ predenaturation for 1 min → 95℃ denaturation for 45 s → 60℃ annealing for 20 s → 72℃ extension for 10 s (35 cycles of denaturation, annealing and extension) → 72℃ final extension for 5 min → 4℃ cooling for 2 min. The amplified fragments were subjected to 1.5% agarose gels in 1 × TBE buffer (all from Beyotime Institute of Biotechnology, Shanghai, China) at 100 V. The DNA bands were examined using a Gel Documentation System (Gel Doc 2000) Bio-Rad Laboratories, Inc., Hercules, CA, USA), and Image Lab 3.0 (both from Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for the analysis of the gray value data.
ROS Assay
The ROS assay was carried out as previously described [31]. Samples from the left rat hippocampal CA1 region were collected 7 d after anesthetization, and total ROS levels were measured with an ROS assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, brain tissues were homogenized in 0.1 mM PBS to prepare a single-cell suspension, followed by centrifugation (1000 × g, 10 min, 4 °C) to obtain the supernatant. The supernatant (190 µl) was incubated with 10 µl DCFH-DA at 37 °C for 30 min in the dark. As a negative control, the supernatant was mixed with PBS. The mixture was measured by spectrofluorophotometry at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. The protein concentration of the supernatant was determined by a BCA protein assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The ROS levels were expressed as fluorescence intensity/mg protein.
Western Blot Analysis
The left hippocampal tissues and pineal gland, which was obtained immediately after blood sample collection at 24:00 under anesthesia on day 7, were lysed with cold cell lysis buffer containing phenylmethanesulfonylfluoride (Beyotime Institute of Biotechnology, Shanghai, China). Subsequently, protein samples (50 µg) were subjected to SDS-PAGE. Proteins were then electrophoretically transferred onto PVDF membranes, which were blocked with 5% milk for 2 h at room temperature, and then exposed to primary antibodies targeted against AANAT (ab3505, 1:500 dilution; Abcam, Cambridge, MA, USA), LC3 (2775, 1:1000, Cell Signaling Technology, Beverly, MA, USA), Parkin (ab15954, 1:1000, Abcam, Cambridge, MA, USA), PINK1 (ab75487, 1:500, Abcam, Cambridge, MA, USA), NLRP3 (ab98151, 1:800, Abcam, Cambridge, MA, USA), ASC (ab155970, 1:1000, Abcam, Cambridge, MA, USA), caspase-1 (ab1872, 1:1000, Abcam, Cambridge, MA, USA), IL-1β (SC-23460, 1:500; Santa Cruz, Dallas, Texas, USA), IL-18 (ab71495, 1:1000; Abcam, Cambridge, MA, USA) and β-actin (1:1000, Cell Signaling Technology, Beverly, MA, USA) at 4 °C overnight, followed by incubation with a HRP-conjugated secondary antibody (1:2500 dilution) for 2 h at room temperature. The specific protein bands were visualized by enhanced eyoECL (Beyotime, Shanghai, China) and scanned with a storm PhosphorImager system (Bio-Rad Laboratories, Hercules, CA, USA).
Immunofluorescence Staining
The paraffin tissue was sectioned, dewaxed, dehydrated in a gradient, and then blocked in a wet box at 37 °C for 30 min by adding blocking serum. The primary antibodies were anti-Beclin-1 (ab16998, 1:200, Abcam, Cambridge, MA, USA), anti-NeuN (MAB377, 1;500, Millipore, Burlington, USA), and Iba-1 (ab178846, 1:2000; Abcam, Cambridge, MA, USA), and the secondary antibodies were Fitc-TSA (G1222, 1:1000, Servicebio, Wuhan, China) and CY3-TSA (G1223, 1:1000, Servicebio, Wuhan, China). The sections were rinsed, stained with DAPI (ab104139, Abcam, Cambridge, MA, USA), rinsed again and mounted with glycerol. A fluorescence microscope (Olympus) was used to analyze the labeling.
Statistical Analysis
All data are expressed as the mean ± standard deviation. The comparison between multiple groups was tested by one-way analysis of variance (ANOVA) or two-way repeated measure (RM) ANOVA followed by Tukey’s post hoc tests. All analyses were performed using GraphPad Prism 9.0 software. P < 0.05 was considered to indicate a statistically significant difference.
Results
EA Reduced Infarct Volume and Attenuated Cognitive Impairment in Cerebral I/R‑Injured Rats
We first evaluated the effect of EA at the DU24 and DU20 acupoints on cerebral infarction evaluated by MRI (Fig. 1). The percentage of the total infarct volume of the whole brain on day 7 was significantly higher in the I/R group (34.5 ± 3.16) than in the Sham group, and EA treatment significantly reduced the cerebral infarct volume to 26.39 ± 2.46% in the I/R + EA group (F (3, 20) = 257.3, P < 0.001). We mainly focused on MRI imaging changes in the left hippocampus (orange contour area: the I/R + EA group had lower signal intensity and area on T2w MRI than the I/R group), and the results showed that EA treatment significantly attenuated the infarct percentage of left hippocampal (3.9 ± 0.97) than in the I/R group(6.2 ± 1.5) (F (3, 20) = 47.24, P < 0.01). The melatonin receptor inhibitor (luzindole) treatment reversed these changes in the I/R + EA + Luz group (F (3, 20) = 21.07, P < 0.01; Fig. 1b). The hippocampal CA1 region is a specific vulnerable region during transient cerebral ischemia (I/R) in the brain and consequently results in neuronal damage [30, 32], and delayed neuronal death in the CA1 region of the hippocampus after I/R injury has been demonstrated [33], thus leading to slow development and consecutive cognitive impairment. Therefore, our study will focus on the molecular biological changes in the CA1 region following EA intervention.
T2w MRI imaging in different groups. a T2w MRI imaging indicated the infarct site and area(the red dotted area) in different groups. The orange contour area indicates the infarct site and area in the left hippocampus. b Infarct volume of the whole brain and the left hippocampus was quantified as a percentage of the total brain volume in each group. n = 6 per group, *P < 0.05, **P < 0.01 and ***P < 0.001; ns, nonsignificant. EA, electroacupuncture; I/R, ischemia–reperfusion; Luz, luzindole
We then evaluated the effect of EA on I/R-induced cognitive impairment monitored by a Morris water maze test on the third to the seventh day following MCAO surgery. Our results showed that the I/R group had a longer route and escape latency than the sham group in the orientation navigation test on all time points from day 3 to day 7, and the EA treatment significantly reduced the route and escape latency (compare the I/R + EA group with the I/R group, Fig. 2a–c) (F (3, 80) = 263.9, P < 0.001 for route analysis and F (3, 80) = 331.9 for time analysis, P < 0.001). Furthermore, EA treatment significantly increased the number of times the rats crossed the platform position in the I/R + EA group compared with that in the I/R group (F (3, 20) = 21.07, P < 0.05; Fig. 2d). However, the melatonin receptor inhibitor (luzindole) treatment reversed these changes in the I/R + EA + Luz group (F (3, 20) = 21.07, P < 0.01; Fig. 2d).
Effects of EA on PSCI rats. The effects of EA treatment on spatial learning ability and memory were assessed by the Morris water maze test from the 3rd day to the 7th day after I/R injury. a Tracing images from the Morris water maze test in different groups. b and c The length of the routes and time taken for the rats to find the platform within 90 s. d The number of times the rats crossed the platform’s position on the 7th day after I/R injury. The group data are presented as the mean ± S. D (n = 6), *P < 0.05, **P < 0.01 and ***P < 0.001; ns, nonsignificant. EA, electroacupuncture; I/R, ischemia–reperfusion; Luz, luzindole
EA Regulated Endogenous Melatonin in Cerebral I/R‑Injured Rats
As a melatonin receptor inhibitor (luzindole) reversed the protective effect of EA treatment, melatonin was thought to mediate the protective effect of EA treatment. We further observed the effect of EA on the serum melatonin content in cerebral I/R‑injured rats.
The serum melatonin content in stroke rats was detected by ELISA. The results show that the secretion of melatonin has a circadian rhythm. As shown in Fig. 3a, the serum melatonin content of each group was at a low level at 12:00 noon at all time points, and there was no statistically significant difference among the groups (F (3, 80) = 2.449). The secretion of melatonin increased at 24:00 at night at all time points, which was evidenced by the significant increase in the melatonin content in the sham group, while in the stroke model groups (I/R group, I/R + EA group, I/R + EA + Luz group), under MCAO I/R injury, the serum content of melatonin was significantly lower than that in the sham operation group (F (3, 80) = 461.2, P < 0.001). However, EA intervention in the I/R + EA group promoted melatonin secretion to a certain extent, which was significantly different from the results observed in the I/R group and the sham group (F(3, 80) = 461.2, P < 0.001). However, luzindole treatment was unable to inhibit the secretion of melatonin when accompanied by EA intervention.
EA treatment regulated endogenous melatonin in cerebral I/R-injured rats. a The serum melatonin content in stroke rats was detected by ELISA at 12:00 and 24:00 on days 1, 3, 5 and 7. (b) AANAT mRNA and protein expression levels were detected by RT-PCR and Western blot. The group data are presented as the mean ± S. D (n = 6), *P < 0.05, **P < 0.01 and ***P < 0.001; ns, nonsignificant. EA electroacupuncture, ELISA enzyme-linked immunosorbent assay, I/R ischemia–reperfusion, Luz luzindole
Melatonin is mainly synthesized in the pineal gland; therefore, we further tested the expression of AANAT, the rate-limiting enzyme in melatonin synthesis, in the pineal gland of each group of rats on day 7. As shown in Fig. 3b, RT-PCR and Western blot results showed that the mRNA and protein expression levels of AANAT in the model groups of rats were significantly reduced compared with those in the sham group (F(3, 20) = 77.70, P < 0.001 for mRNA analysis and F(3, 20) = 23.57, P < 0.001 for WB analysis). The AANAT mRNA and protein expression levels in the I/R + EA group were significantly increased compared with those in the I/R group (F(3, 20) = 77.70, P < 0.001 for mRNA analysis and F(3, 20) = 23.57, P < 0.05 for WB analysis). However, luzindole treatment was unable to change the expression of AANAT when accompanied by EA intervention.
EA Regulated Melatonin-Mediated Mitophagy and Reduced ROS Generation after I/R
The mechanism of melatonin-mediated autophagy and mitophagy in neuroprotection has been well illuminated in some studies [14, 34, 35]. To further investigate the mechanism by which EA treatment attenuates hippocampal I/R injury mediated by melatonin, we first evaluated autophagy activation after EA intervention and examined the expression levels of the autophagy-related protein LC3. As shown in Fig. 4a, the LC3-II/I ratio (the conversion of LC3-I to LC3-II) was significantly decreased in the I/R group compared with the sham group on the 7th day after I/R injury (F (3, 20) = 97.33, P < 0.001), indicating autophagy impairment, thus reducing the ability to remove damaged nerve cells. Compared with the I/R group, EA treatment in the I/R + EA group significantly increased the LC3-II/I ratio (F (3, 20) = 97.33, P < 0.001), suggesting enhanced autophagy triggered by EA. However, the melatonin receptor inhibitor luzindole significantly reduced the ratio of LC3-II/LC3-I (F (3, 20) = 97.33, P < 0.001), suggesting that EA promoted melatonin-mediated autophagy. We further verified the autophagy level by double immunostaining in each group on day 7, and the results (Fig. 4b) showed that the neuron (NeuN-positive cells) in the CA1 region of the left hippocampus in sham group were mainly autophagy active cells (Beclin-1-positive), and after 7 days of I/R injury in I/R group, the autophagy level decreased in the hippocampus, as evidenced by the weaker immunofluorescence and colocalization of Beclin-1 in NeuN cells. Importantly, EA intervention in I/R + EA group dramatically promoted autophagy in the hippocampus, as evidenced by the stronger immunofluorescence and colocalization (orange color in Merge column) of Beclin-1 in NeuN cells. However, luzindole treatment I/R + EA + Luz group partly inhibited melatonin-mediated autophagy. These results indicated that autophagy deficiency occurred after 7 days of I/R injury and that EA promoted melatonin-mediated autophagy in the CA1 region of the left hippocampus.
EA regulated melatonin-mediated autophagy. a The Western blot relative band density of LC3 in the left hippocampus. b Double immunofluorescence labeling of Beclin-1 with NeuN (neuronal marker) in the CA1 region of the left hippocampus on day 7 (× 20). The group data are presented as the mean ± S. D (n = 6), *P < 0.05, **P < 0.01 and ***P < 0.001; ns, nonsignificant. EA, electroacupuncture; I/R, ischemia–reperfusion; Luz, luzindole
To further determine the specific autophagy types that EA primarily triggers, considering that melatonin-mediated mitophagy protects against brain injury through the inhibition of NLRP3 [14], we investigated the effect of EA on mitophagy in the hippocampus. Mitophagy, as a type of selective autophagy, mediates the clearance of damaged mitochondria via the PINK1/Parkin pathway [36]. Our Western blot analysis showed that the protein expression of PINK1 and Parkin was significantly decreased on the 7th day after I/R injury in the I/R group compared with the sham group (F(3, 20) = 6.99, P < 0.05 for PINK1 and F(3, 20) = 9.38, P < 0.05 for Parkin; Fig. 5a), whereas EA upregulated this expression (F(3, 20) = 6.99, P < 0.01 for PINK1 and F(3, 20) = 9.38, P < 0.05 for Parkin; Fig. 5a). Moreover, treatment with luzindole inhibited the upregulation of these proteins observed in the I/R + EA group (F(3, 20) = 6.99, P < 0.05 for PINK1 and F(3, 20) = 9.38, P < 0.001 for Parkin; Fig. 5a). The upregulation of mitophagy occurred after EA intervention, indicating that EA can promote mitophagy to remove damaged mitochondria and maintain mitochondrial homeostasis as well as cellular survival. Next, we observed ultrastructural changes in the mitochondria in different groups. As shown in Fig. 5b, swollen mitochondria with cristae disarrangement and partial cristolysis were found in the hippocampi of I/R group rats. EA intervention attenuated the morphological changes observed in the mitochondria. However, luzindole treatment reversed the beneficial effects of EA intervention (Fig. 5b). Mitochondria are the leading sites of intracellular ROS generation. EA treatment protected mitochondria, indicating reduced generation of ROS and ultimately reduced NLRP3 inflammasome activation. As expected, ROS levels were significantly increased after I/R injury, and EA intervention markedly reduced ROS levels compared with those of the sham group (F(3, 20) = 167.3, P < 0.001). However, luzindole treatment promoted ROS generation after I/R and EA intervention (F(3, 20) = 167.3, P < 0.001; Fig. 5c). These results suggested that EA intervention increased mitophagy activity and reduced ROS generation after I/R injury induction mediated by melatonin.
Effect of EA on mitophagy and ROS generation. a Western blot relative band density of Parkin and PINK1. b Ultrastructural changes in mitochondria in the left hippocampus. c Quantification of ROS levels in the left hippocampus. Green arrows indicate normal mitochondria, and red arrows indicate damaged mitochondria (× 10,000). The group data are presented as the mean ± S. D (n = 6). *P < 0.05, **P < 0.01 and ***P < 0.001; ns, nonsignificant. EA, electroacupuncture; I/R, ischemia–reperfusion; ROS, reactive oxygen species; Luz, luzindole
EA Inhibited NLRP3 Inflammasome Activation and Reduced Proinflammatory Cytokine Secretion Mediated by Melatonin after I/R
The accumulation of ROS is reported to be a vital factor to trigger NLRP3 inflammasome activation, which promotes proinflammatory cytokine secretion and amplifies the inflammatory response [37, 38]. Our above results showed that EA intervention reduced ROS generation after I/R injury; consequently, EA treatment reduced NLRP3 inflammasome-related proteins. As expected, our Western blot results (Fig. 6a–d) further showed that EA treatment significantly reduced the expression of NLRP3 (F(3, 20) = 191.4, P < 0.001), ASC(F(3, 20) = 133.5, P < 0.001), and caspase-1c (F(3, 20) = 205.7, P < 0.001) compared with that of the I/R group, indicating that EA treatment inhibited NLRP3 inflammasome activation triggered by ROS generation. However, luzindole treatment reversed the effects of EA on the expression of NLRP3 inflammasome-related proteins, which indicated that blockade of endogenous melatonin biological effects regulated by EA treatment promoted NLRP3 inflammasome activation. Additionally, Western blot results showed that the expression of mature IL-1βand IL-18 (, P < 0.001) was increased significantly in the I/R group, whereas EA treatment significantly reduced the expression of IL-1β and IL-18 compared with that of the I/R group (F(3, 20) = 205.7, P < 0.001for IL-1β and F(3, 20) = 119.2, P < 0.001 for IL-18; Fig. 6a, e, f), indicating that EA treatment can reduce proinflammatory cytokine secretion. We further assessed microglial activation in the CA1 region of the left hippocampus on day 7 (Fig. 7b and c) by morphology to determine CA1 macrophages in response to I/R injury and inflammasomes. As shown in Fig. 7a, a loss of ramification and a thickening of processes were observed in the I/R group compared with the sham group but were reversed significantly by EA intervention (Fig. 7a and d), indicating that microglial activation was obviously observed after I/R injury and EA treatment showed significantly attenuated microglial activation in the CA1 region of the left hippocampus compared with the I/R group, as evidenced by the quantification of Iba-1-positive cells (F (3, 20) = 317.1, P < 0.001; Fig. 7d). Moreover, luzindole treatment inhibited the beneficial effects of EA intervention on proinflammatory cytokine secretion and microglial activation (F (3, 20) = 317.1, P < 0.001; Fig. 7a and d). These results indicated that EA can inhibit NLRP3 inflammasome activation and reduce proinflammatory cytokine secretion mediated by melatonin after I/R.
EA inhibits NLRP3 inflammasome activation and proinflammatory cytokine secretion. a Representative Western blot bands of NLRP3, ASC, IL-1β, IL-18, and cleaved caspase-1. b–f The relative band densities of NLRP3, ASC, IL-1β, IL-18, and cleaved caspase-1. The group data are presented as the mean ± S. D (n = 6), *P < 0.05, **P < 0.01 and ***P < 0.001; ns, nonsignificant. EA, electroacupuncture; I/R, ischemia–reperfusion; Luz, luzindole
EA inhibits microglial activation in the CA1 region of the left hippocampus. a Representative micrographs showing microglial activation in the CA1 area of the left hippocampus, with immunofluorescence for Iba-1 (× 40). b, c) Comparation between inactivated microglia activated microglia in the CA1 region of the left hippocampus (× 100). d Quantification of Iba-1-positive cells. The group data are presented as the mean ± S. D (n = 6), *P < 0.05, **P < 0.01 and ***P < 0.001; ns nonsignificant, EA electroacupuncture, I/R ischemia–reperfusion, Luz luzindole
Discussion
Numerous research studies have shown the clinical efficacy and therapeutic benefits of acupuncture in stroke/PSCI patients as well as in animal stroke models [5,6,7,8,9]. The Baihui and Shenting acupoints are located on the Du meridian, and their stimulation is beneficial to memory function and human health. Our previous studies have revealed the efficacy of EA at the Shenting and Baihui acupoints in the rehabilitation of cognitive impairment in experimental settings [7,8,9], and the specific mechanism is related to the inhibition of oxidative stress [9] and improvements in the ultrastructural changes in mitochondria in the cerebral cortex [8]. These results suggested that EA may play a multitarget and multipathway role in neuroprotection by regulating some neuroendocrine substances related to oxidative stress, mitochondrial dysfunction, inflammation, and so forth.
Melatonin is a neurohormone secreted by pineal and extrapineal tissues and has been verified in the treatment of various neurological diseases due to its antioxidative, antiapoptotic, and anti-inflammatory properties [23]. The biological processes of melatonin are mediated via two G-protein-coupled membrane receptors called MT1 and MT2. Of note, melatonin is considered a potent therapeutic for stroke and stroke-related dementia [24, 25, 39]. Additionally, studies have demonstrated that EA can improve cognitive function in aged insomnia patients, and its mechanism may be related to the regulation of serum melatonin [40]. From the above, we hypothesized that EA might regulate the secretion of melatonin after stroke and, through its effects on melatonin, mediate a variety of biological neuroprotective effects, including anti-inflammatory, antioxidative, and anti-pyroptosis effects and the regulation of autophagy.
As expected, the present study showed that EA treatment at the DU24 and DU20 acupoints regulated endogenous melatonin in cerebral I/R‑injured rats. The secretion of melatonin showed a circadian rhythm in sham group rats. However, this circadian rhythm was disrupted by cerebral I/R‑injury. These results are similar to those of some clinical studies and animal stroke models [41,42,43]. For the first time, we reveal that EA treatment at DU24 and DU20 can promote the secretion of melatonin and improve the circadian rhythm of melatonin by regulating AANAT (a rate-limiting enzyme of melatonin) synthesis in the pineal gland in stroke rats. Melatonin mediates a variety of neuroprotective effects, including a reduction in infarct volume (particularly in the CA1 region of the hippocampus) and an attenuation of cognitive impairment. We further used luzindole, an MT2 melatonin receptor inhibitor, to validate the neuroprotective effects of EA mediated by melatonin. Our results indicated that luzindole treatment reversed the neuroprotective changes induced by melatonin without changing the expression of AANAT when accompanied by EA intervention. This demonstrates that EA can regulate endogenous melatonin secretion in the pineal gland and probably exert its neuroprotective effect partially through MT2.
Studies have demonstrated that melatonin-mediated mitophagy protects against brain injury through the inhibition of NLRP3 [14]. Therefore, we further investigated the mechanisms underlying the effects of EA on mitophagy and NLRP3 inflammasome activation mediated by melatonin in a rat stroke model. We made the following observations: (1) EA upregulated mitophagy-associated proteins (PINK1/Parkin) and reduced the mitochondrial damage and ROS generation observed after I/R; (2) following ROS inhibition through the activation of mitophagy and the protection of mitochondria, EA exerted a neuroprotective effect by inhibiting NLRP3 inflammasome activation and microglial activation after I/R injury and thereby attenuated the inflammatory response mediated by melatonin.
The NLRP3 inflammasome is activated by ROS [14, 17], and mitochondria play a critical role in the overproduction of ROS. The inhibition of ROS-induced oxidative stress and inflammatory responses was beneficial for stroke rats [44, 45]. Mitophagy is a selective form of autophagy that specifically removes damaged mitochondria and plays a vital role in maintaining mitochondrial homeostasis and cellular survival [22]. One study revealed deficient mitophagy in ischemic neurons [46]. Our study consistently showed that deficient autophagy and mitophagy in hippocampal neurons led to the inability to remove damaged mitochondria and ROS production, thus triggering NLRP3 inflammasome activation. Interestingly, we found that EA enhanced autophagy and mitophagy to protect against I/R-induced injury by protecting mitochondria and inhibiting ROS production through the regulation of melatonin, which demonstrated that EA upregulated mitophagy-associated proteins, eliminated damaged mitochondria, and reduced ROS generation by regulating melatonin, which is linked to the inhibition of NLRP3 inflammasome activation. The neuroinflammatory response contributes to the pathogenesis of ischemic stroke [47]. Extensive studies have shown that inflammatory cells and proinflammatory cytokines are strongly associated with brain injury and neurological dysfunction after stroke [10, 12, 13]. More importantly, research shows that the NLRP3 inflammasome is an essential modifier of neuropathology in PSCI [16]. The findings of our study indicated that EA promotes mitophagy and suppresses ROS-induced NLRP3 inflammasome activation to attenuate the inflammatory response after I/R injury by regulating melatonin secretion, which dominates the modifier of neuropathology and ameliorates neuroinflammation-mediated cognitive deficits in PSCI rat models.
Conclusions
In conclusion, the results of this study expand our understanding of the neuroprotective effects of EA on I/R-induced cognitive impairment and reveal potential underlying mechanisms. We demonstrated that EA-upregulated endogenous melatonin played a multiprotective role in stroke and PSCI via the inhibition of NLRP3 inflammasome activation and reduced inflammatory responses. However, the multiprotective effect of EA treatment on the DU24 and DU20 acupoints still needs to be further determined.
Data Availability
The data used to support the findings of this study are included in the article.
Change history
31 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11064-022-03590-4
References
Liu J, Zheng L, Cheng Y, Zhang S, Wu B, Wang D, Zhang S, Tao W, Wu S, Liu M (2019) Trends in outcomes of patients with ischemic stroke treated between 2002 and 2016: insights from a Chinese cohort. Circ Cardiovasc Qual Outcomes 12:e005610. https://doi.org/10.1161/circoutcomes.119.005610
Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG (2009) Classification of stroke subtypes. Cerebrovasc Dis 27:493–501. https://doi.org/10.1159/000210432
Pasi M, Poggesi A, Salvadori E, Pantoni L (2012) Post-stroke dementia and cognitive impairment. Front Neurol Neurosci 30:65–69. https://doi.org/10.1159/000333412
Zheng G, Liu F, Li S, Huang M, Tao J, Chen L (2015) Tai Chi and the protection of cognitive ability: a systematic review of prospective studies in healthy adults. Am J Prev Med 49:89–97. https://doi.org/10.1016/j.amepre.2015.01.002
Liu F, Li ZM, Jiang YJ, Chen LD (2014) A meta-analysis of acupuncture use in the treatment of cognitive impairment after stroke. J Altern Complement Med 20:535–544. https://doi.org/10.1089/acm.2013.0364
Zhou L, Wang Y, Qiao J, Wang QM, Luo X (2020) Acupuncture for improving cognitive impairment after stroke: a meta-analysis of randomized controlled trials. Front Psychol 11:549265. https://doi.org/10.3389/fpsyg.2020.549265
Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L (2013) Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep 7:1516–1522. https://doi.org/10.3892/mmr.2013.1392
Lin R, Li X, Liu W, Chen W, Yu K, Zhao C, Huang J, Yang S, Peng H, Tao J, Chen L (2017) Electro-acupuncture ameliorates cognitive impairment via improvement of brain-derived neurotropic factor-mediated hippocampal synaptic plasticity in cerebral ischemia-reperfusion injured rats. Exp Ther Med 14:2373–2379. https://doi.org/10.3892/etm.2017.4750
Lin R, Lin Y, Tao J, Chen B, Yu K, Chen J, Li X, Chen LD (2015) Electroacupuncture ameliorates learning and memory in rats with cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting p-CREB expression in the hippocampus. Mol Med Rep 12:6807–6814. https://doi.org/10.3892/mmr.2015.4321
Fann DY, Lee SY, Manzanero S et al (2013) Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis 4:e790. https://doi.org/10.1038/cddis.2013.326
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75:209–219. https://doi.org/10.1002/ana.24070
Zhao S, Li X, Wang J, Wang H (2021) The role of the effects of autophagy on NLRP3 inflammasome in inflammatory nervous system diseases. Front Cell Dev Biol 9:657478. https://doi.org/10.3389/fcell.2021.657478
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832. https://doi.org/10.1016/j.cell.2010.01.040
Cao S, Shrestha S, Li J, Yu X, Chen J, Yan F, Ying G, Gu C, Wang L, Chen G (2017) Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci Rep 7:2417. https://doi.org/10.1038/s41598-017-02679-z
Sun R, Peng M, Xu P, Huang F, Xie Y, Li J, Hong Y, Guo H, Liu Q, Zhu W (2020) Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J Neuroinflammation 17:330. https://doi.org/10.1186/s12974-020-01988-x
Li YQ, Chen JX, Li QW, Xiao ZJ, Yuan T, Xie ZH (2020) Targeting NLRP3 inflammasome improved the neurogenesis and post-stroke cognition in a mouse model of photothrombotic stroke. NeuroReport 31:806–813. https://doi.org/10.1097/wnr.0000000000001489
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225. https://doi.org/10.1038/nature09663
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13. https://doi.org/10.1042/bj20081386
He J, Liu J, Huang Y, Tang X, Xiao H, Hu Z (2021) Oxidative stress, inflammation, and autophagy: potential targets of mesenchymal stem cells-based therapies in ischemic stroke. Front Neurosci 15:641157. https://doi.org/10.3389/fnins.2021.641157
Jia J, Jin H, Nan D, Yu W, Huang Y (2021) New insights into targeting mitochondria in ischemic injury. Apoptosis 26:163–183. https://doi.org/10.1007/s10495-021-01661-5
Poznyak AV, Melnichenko AA, Wetzker R, Gerasimova EV, Orekhov AN (2020) NLPR3 inflammasomes and their significance for atherosclerosis. Biomedicines 8:205. https://doi.org/10.3390/biomedicines8070205
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9–14. https://doi.org/10.1038/nrm3028
Andrabi SS, Parvez S, Tabassum H (2015) Melatonin and ischemic stroke: mechanistic roles and action. Adv Pharmacol Sci 2015:384750. https://doi.org/10.1155/2015/384750
Lee CH, Park JH, Ahn JH, Won MH (2016) Effects of melatonin on cognitive impairment and hippocampal neuronal damage in a rat model of chronic cerebral hypoperfusion. Exp Ther Med 11:2240–2246. https://doi.org/10.3892/etm.2016.3216
Tsai TH, Lin CJ, Chua S, Chung SY, Yang CH, Tong MS, Hang CL (2017) Melatonin attenuated the brain damage and cognitive impairment partially through MT2 melatonin receptor in mice with chronic cerebral hypoperfusion. Oncotarget 8:74320–74330. https://doi.org/10.18632/oncotarget.20382
Farhadi N, Gharghani M, Farhadi Z (2016) Effects of long-term light, darkness and oral administration of melatonin on serum levels of melatonin. Biomed J 39:81–84. https://doi.org/10.1016/j.bj.2015.09.003
Tai SH, Hung YC, Lee EJ, Lee AC, Chen TY, Shen CC, Chen HY, Lee MY, Huang SY, Wu TS (2011) Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: the impact of circulating estrogen on its hormetic dose-response. J Pineal Res 50:292–303. https://doi.org/10.1111/j.1600-079X.2010.00839.x
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91. https://doi.org/10.1161/01.str.20.1.84
Zou W, Yang Y, Gu Y, Zhu P, Zhang M, Cheng Z, Liu X, Yu Y, Peng X (2017) Repeated blood collection from tail vein of non-anesthetized rats with a vacuum blood collection system. J Vis Exp. https://doi.org/10.3791/55852
Park OK, Yoo KY, Lee CH, Choi JH, Hwang IK, Park JH, Kwon YG, Kim YM, Won MH (2010) Arylalkylamine N-acetyltransferase (AANAT) is expressed in astrocytes and melatonin treatment maintains AANAT in the gerbil hippocampus induced by transient cerebral ischemia. J Neurol Sci 294:7–17. https://doi.org/10.1016/j.jns.2010.04.013
Li J, Chen J, Mo H, Chen J, Qian C, Yan F, Gu C, Hu Q, Wang L, Chen G (2016) Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol Neurobiol 53:2668–2678. https://doi.org/10.1007/s12035-015-9318-8
Horiguchi T, Shima H, Suga S, Ogino M, Shimizu K, Toya S, Nagao M, Kawase T (2002) Transient forebrain ischemia induces expression of serine/threonine protein phosphatase 1 mRNA in the vulnerable regions of gerbil brain. Neurosci Lett 325:115–118. https://doi.org/10.1016/s0304-3940(02)00244-6
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69. https://doi.org/10.1016/0006-8993(82)90833-2
Luo F, Sandhu AF, Rungratanawanich W, Williams GE, Akbar M, Zhou S, Song BJ, Wang X (2020) Melatonin and autophagy in aging-related neurodegenerative diseases. Int J Mol Sci 21:7174. https://doi.org/10.3390/ijms21197174
Wu X-L, Lu S-S, Liu M-R et al (2020) Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury. Acta Pharmacol Sin 41:1016–1024. https://doi.org/10.1038/s41401-020-0361-2
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524:309–314. https://doi.org/10.1038/nature14893
Jiang Q, Geng X, Warren J, Eugene Paul Cosky E, Kaura S, Stone C, Li F, Ding Y (2020) Hypoxia inducible factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience 448:126–139. https://doi.org/10.1016/j.neuroscience.2020.09.036
Minutoli L, Puzzolo D, Rinaldi M et al (2016) ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev 2016:2183026. https://doi.org/10.1155/2016/2183026
Sadanandan N, Cozene B, Cho J, Park YJ, Saft M, Gonzales-Portillo B, Borlongan CV (2020) Melatonin-A potent therapeutic for stroke and stroke-related dementia. Antioxidants (Basel) 9:672. https://doi.org/10.3390/antiox9080672
Wang XQ, Qin S, Wu WZ, Liu CY, Shang HT, Wan QY, Zhao YN, Xi HQ, Zheng SY, Li JH, Wang Y (2021) Effect of electroacupuncture on serum melatonin and dopamine in aged insomnia. Zhongguo Zhen Jiu 41:501–504. https://doi.org/10.13703/j.0255-2930.20200404-k0001
Adamczak-Ratajczak A, Kupsz J, Owecki M, Zielonka D, Sowinska A, Checinska-Maciejewska Z, Krauss H, Michalak S, Gibas-Dorna M (2017) Circadian rhythms of melatonin and cortisol in manifest Huntington’s disease and in acute cortical ischemic stroke. J Physiol Pharmacol 68:539–546
Benot S, Molinero P, Soutto M, Goberna R, Guerrero JM (1998) Circadian variations in the rat serum total antioxidant status: correlation with melatonin levels. J Pineal Res 25:1–4. https://doi.org/10.1111/j.1600-079x.1998.tb00378.x
Meng H, Liu T, Borjigin J, Wang MM (2008) Ischemic stroke destabilizes circadian rhythms. J Circadian Rhythms 6:9. https://doi.org/10.1186/1740-3391-6-9
Mi Y, Jiao K, Xu JK et al (2021) Kellerin from Ferula sinkiangensis exerts neuroprotective effects after focal cerebral ischemia in rats by inhibiting microglia-mediated inflammatory responses. J Ethnopharmacol 269:113718. https://doi.org/10.1016/j.jep.2020.113718
Zhou X, Lu W, Wang Y, Li J, Luo Y (2020) A20-Binding Inhibitor of NF-κB 1 Ameliorates neuroinflammation and mediates antineuroinflammatory effect of electroacupuncture in cerebral ischemia/reperfusion rats. Evid Based Complement Alternat Med 2020:6980398. https://doi.org/10.1155/2020/6980398
Wu X, Zheng Y, Liu M et al (2021) BNIP3L/NIX degradation leads to mitophagy deficiency in ischemic brains. Autophagy 17:1934–1946. https://doi.org/10.1080/15548627.2020.1802089
Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A (2020) Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci 21:6454. https://doi.org/10.3390/ijms21186454
Acknowledgements
We would like to thank AJE (www.aje.cn) for English language editing.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81803889 and 81804175), the Natural Science Foundation of Fujian Province (Grant Nos. 2018J01329, 2019J01497, and 2020J011043), and the National Traditional Chinese Medicine (TCM) Clinical Research Base Foundation of Fujian (Grant No. JDZX2019043).
Author information
Authors and Affiliations
Contributions
XZ Conceptualization, Methodology, Performed the experiment, Writing- Original draft preparation, analysis, Reviewing and Editing. BC, ZL and RL Performed the experiment, data analyses. HL, SR and FW Project administration, Data Curation and analysis. JT Conceptualization, Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, X., Chen, B., Li, Z. et al. Electroacupuncture Ameliorates Cognitive Impairment Through the Inhibition of NLRP3 Inflammasome Activation by Regulating Melatonin-Mediated Mitophagy in Stroke Rats. Neurochem Res 47, 1917–1930 (2022). https://doi.org/10.1007/s11064-022-03575-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03575-3