Abstract
Chronic cerebral hypoxia (CCH) is caused by a reduction in cerebral blood flow, and cognitive impairment has been the predominant feature that occurs after CCH. Recent reports have revealed that melatonin is proficient in neurodegenerative diseases. However, the molecular mechanism by which melatonin affects CCH remains uncertain. In this study, we aimed to explore the role and underlying mechanism of melatonin in inflammation and blood‒brain barrier conditions in rats with CCH. Male Wistar rats were subjected to permanent bilateral common carotid artery occlusion (BCCAO) to establish the VAD model. Rats were randomly divided into four groups: Sham, BCCAO, BCCAO treated with melatonin (10 mg/kg), and BCCAO treated with resveratrol (20 mg/kg). All drugs were administered once daily for 4 weeks. Our results showed that melatonin attenuated cognitive impairment, as demonstrated by the Morris water maze tests. Furthermore, melatonin reduced the activation of inflammation by attenuating the phosphorylated nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha (pIκBα), causing the suppression of proteins related to inflammation and inflammasome formation. Moreover, immunohistochemistry revealed that melatonin reduced glial cell activation and proliferation, which were accompanied by Western blotting results. Additionally, melatonin also promoted the expression of sirtuin-1 (SIRT1), peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α), and peroxisome proliferator-activated receptor-gamma (PPARγ), causing attenuated blood‒brain barrier (BBB) disruption by increasing tight junction proteins. Taken together, our results prove that melatonin treatment modulated inflammation and BBB disruption and improved cognitive function in VaD rats, partly by activating the SIRT1/PGC-1α/PPARγ signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is a serious public health problem that causes cognitive impairment in aged adults (Hugo and Ganguli 2014). Cerebral hypoperfusion is a risk factor for cognitive decline and vascular dementia (VaD), and chronic cerebral hypoperfusion (CCH) is a suitable model of VaD (Du et al. 2017; Venkat et al. 2015). A decrease in cerebral blood flow (CBF) causes cerebral hypoxia and subsequently generates inflammation (Daulatzai 2017; Venkat et al. 2015). The burst of inflammation promotes inflammatory mediators and induces pyroptosis cell death (Xue et al. 2019). The characteristics of pyroptosis are associated with inflammasome formation via the assembly of NLR family pyrin domain containing 3 (NLRP3), apoptosis-associated speck-like protein containing a CARD (ASC), and caspase 1 to allow formation of the N-terminus of gasdermin D (GSDMD-NT) in the cytosolic membrane, after which cellular substances and inflammatory mediators are released into the extracellular matrix (ECM) (Xue et al. 2019). Thereafter, these cytosolic substances and inflammatory mediators activate glial cells and increase blood‒brain barrier (BBB) permeability, eventually inducing cognitive impairment (Fang et al. 2020).
Sirtuin 1 (SIRT1), a member of the family of NAD+-dependent proteins, acts as a metabolic regulator (Li 2013). SIRT1 has been shown to regulate various physiological functions, including cell stress and metabolism (Hubbard et al. 2013; Li 2013). A previous study reported that SIRT1 was promoted via the activation of AMP-dependent kinase (AMPK) and encouraged peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) to regulate energy metabolism (Price et al. 2012). The involvement of SIRT1 and PGC-1α was excessively observed (Cantó and Auwerx 2009; Ren et al. 2019; Zhou et al. 2018). PGC-1α actuation acts as a comodulator of nuclear receptors such as peroxisome proliferator-activated receptor-gamma (PPARγ) (Liang and Ward 2006). Interestingly, in the central nervous system (CNS), the activation of PGC-1α promoted PPARγ activity and highlighted this as a therapeutic target of neuronal diseases (Corona and Duchen 2015; Govindarajulu et al. 2018). A previous study found that SIRT1 signaling modulates inflammation and NLRP3 inflammasome activation in Parkinson’s disease (Li et al. 2016). Moreover, PPARγ was observed to inhibit inflammation and inflammasome formation by inhibiting NLRP3-ASC and NLRP3-NLRP3 interactions (Yang et al. 2021). CCH is mainly generated after a decrease in CBF, disrupted cell metabolism, and increased neuroinflammation. Therefore, the SIRT1/PGC-1α/PPARγ signaling pathway might play an important role during CCH. Thus, these pathways may offer viable targets for preventing and reducing neurodegeneration in VaD.
Resveratrol, also known as the SIRT1 activator, has been found to activate SIRT in both direct and indirect mechanisms (Kleszcz et al. 2015). Resveratrol has been recognized to increase the ability of SIRT1 proteins to exert neuroprotective effects (Shen et al. 2016). However, the specific mechanisms of resveratrol mediated by SIRT1 are still unclear.
Melatonin is mainly synthesized by pinealocytes in the pineal gland (Amaral̄ and Cipolla-Neto 2018). Due to the physiological actions of melatonin, it easily passes through the BBB and acts to mediate melatonin receptors or its own properties (Amaral and Cipolla-Neto 2018; Yeleswaram et al. 1997). Melatonin is known to exert antioxidant and anti-inflammatory effects. Our previous study demonstrated that melatonin improved cognitive impairment in a 2VO model by reducing oxidative stress, ER stress, and apoptosis (Thangwong et al. 2022). A previous study proved that stimulation of melatonin receptors directly enhances SIRT1 expression to suppress inflammatory signaling (Zhao et al. 2017). Additionally, it has been widely reported that melatonin exerts anti-inflammatory effects by activating SIRT1 and exerting neuroprotective effects (Amaral and Cipolla-Neto 2018; Ma et al. 2021; Sanderson and Wider 2013; Yeleswaram et al. 1997). However, the mechanism by which melatonin regulates anti-inflammatory effects in CCH conditions via molecular signaling remains unclear.
Therefore, we aimed to investigate the effects of melatonin on the suppression of inflammation, inflammasome formation, and BBB disruption in the CCH model. Moreover, we also examined the SIRT1/PGC-1α/PPARγ signaling pathway as a possible molecular target of melatonin.
Materials and methods
Reagents and chemicals
Melatonin and resveratrol were purchased from Sigma‒Aldrich (St. Louis, MO, USA). Antibodies against ionized calcium binding adaptor molecule 1 (Iba1), caspase 1, and ASC were purchased from Affinity Biosciences (Melbourne, Australia). Antibodies against glial fibrillary acidic protein (GFAP), zonula occludens-1 (ZO-1), hypoxia-inducible Factor 1-alpha (HIF-1α), claudin5, and occludin were purchased from Merck (Darmstadt, Germany). Antibodies against phosphorylated nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha (pIκB), nuclear factor kappa light chain enhancer of activated B cells (NFκB), and GSDMD-NT were purchased from Cell Signaling (MA, USA). Antibodies against intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), matrix metallopeptidase 9 (MMP-9), and sirtuin-1 (SIRT1) were purchased from Abcam (Cambridge, UK). Antibodies against PPARγ and PGC-1α were purchased from Thermo Fisher Scientific (MA, USA). An antibody against NLRP3 was purchased from BOSTER (CA, USA).
Animals and chronic cerebral hypoperfusion model
Male Wistar rats (250–300 g) were purchased from Nomura Siam International Co. Ltd, Bangkok, Thailand. All rats were housed at a constant temperature of 24 ± 1 °C, with free access to food and water and a 12 h dark/light cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Faculty of Medicine, Chiang Mai University, and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rats were subjected to sham or bilateral common carotid artery occlusion surgery procedures, as previously described (Sanderson and Wider 2013). Briefly, the rats were anesthetized with Zoletil (30 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection and placed in the supine position. The midline along the neck was gently exposed to identify the common carotid artery (CCA), which was gently separated from the vagus nerve. Then, the common carotid arteries on both sides were gently double ligated using a 5–0 silk suture loop. Sham-operated animals underwent the same procedure without carotid artery ligation. Animals were returned to the normal cage to recover with free access to food and water.
Following surgery day, all rats received either normal saline, as a vehicle, or treatments, which are referred to as Day 1 and continuing until Day 28. During this period, the rats were subjected to behavioral assessment from Days 23 to 28. Then, the rats were sacrificed by decapitation, and brain samples were collected for further analysis.
Drug administration
The rats were randomly divided into four groups (n = 12 in each group): (1) the sham-operated control group (sham), (2) bilateral common carotid artery occlusion (BCCAO)-operated group (BCCAO), (3) BCCAO treated with 10 mg/kg melatonin (BCCAO + Mel) group, and (4) BCCAO treated with 20 mg/kg resveratrol (BCCAO + Res). Melatonin and resveratrol were dissolved in normal saline immediately before administration to the rats. Melatonin and normal saline were then administered orally, while resveratrol was administered intraperitoneally once a day for 28 days. These doses were chosen based on previous studies that showed protection against neuronal damage and improved cognitive function in the CCH model (Shen et al. 2016). After treatment, the behavioral test was performed before the rats were sacrificed by decapitation. The brains were collected for further analysis.
Morris water maze (MWM) test
The MWM was used to behaviorally assess learning and memory in the experimental groups, as previously described (Vorhees and Williams 2006). The MWM consists of a black, circular pool (diameter: 120 cm; depth 50 cm) in an opaque room at 23 ± 2 °C controlled temperature. The tank was divided into four quadrants where a circular platform with a diameter of 10 cm was placed in the middle of the 2nd quadrant, and the platform was submerged 2 cm below the water surface and surrounded by reference objects with different colors and shapes in fixed positions. In the learning trial, all rats underwent four trials for five consecutive days. For these trials, when rats touched or climbed up the platform, this was recorded and interpreted as an escape attempt; swimming paths were also recorded. After five days of training, the platform was removed. Each rat was released in the northeast position for 2 min to search the previous platform location. The time spent in the target quadrant, the number of crossings of the location of the platform, swimming speed, swimming distance, and swimming paths were recorded. The tracking system was analyzed using SMART video tracking software (Panlab Harvard Apparatus, Bioscience Company, Holliston, MA, USA).
Immunohistochemistry
The brain tissues were fixed in 4% paraformaldehyde overnight, embedded in paraffin and cut into 4-µm-thick coronal slices. Immunohistochemistry was performed as described previously (Yawoot et al. 2022). The sections were processed in an alcohol gradient for dehydration. After blocking endogenous peroxidase, the slides were incubated with primary antibodies as follows: anti-Iba1 (1:200, DF7217, Affinity biosciences) and anti-GFAP (1:400, MAB360, Merck) in humidified chambers at 4 °C overnight. Thereafter, the slides were washed in PBS followed by incubation with conjugated secondary antibodies in humidified chambers for 1 h at room temperature. Finally, the slides were counterstained with 3,3′-diaminobenzidine (DAP) and hematoxylin. The staining was monitored under a microscope (Olympus BX51; Tokyo, Japan). Iba-1-labeled microglia and GFAP-labeled astrocytes were quantified in six random fields of six coronal sections at 0.2 mm2 in each rat.
Transmission electron microscopy
After animal sacrifice, rat brains were quickly collected and fixed in 4% paraformaldehyde. TEM was performed as described previously (Wicha et al. 2020). The cortex area was selected and cut into 1 × 1 × 1-mm tissue blocks and fixed in 2.5% glutaraldehyde in 0.1 phosphate buffer (pH 7.3) overnight. Then, the tissues were dehydrated, embedded in epoxy resin, double-stained with uranyl acetate to citrate, and dried overnight. Representative area sections were examined as part of the blood‒brain barrier structure (4000x) by TEM (JEM-2200FS, JEOL, Tokyo, Japan).
Western blotting
The total protein in brain tissues was determined using the Bradford assay (Bio-Rad, USA) with bovine serum albumin (BSA) as a standard. Equal protein samples were separated using sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE) gels and transferred to 0.45 µm polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, MA, USA). The membranes were blocked with 5% skim milk and subsequently incubated with primary antibodies as follows: anti-ZO-1 (1:1000, AB2272, Merck), anti-HIF-1α (1:1000, 07–1585, Merck), anti-GFAP (1:1000, #07–1585, Merck), anti-claudin5 (1:1000, ABT45, Merck), anti-occludin (1:1000, ABT146, Merck), anti-pIκB (1:1000, #92,465, Cell signaling), anti-pNF-κB (1:1000, #3033, Cell signaling), NF-κB (1:1000, #3034, Cell signaling), anti-GSDMD (1:1000, #50,928, Cell signaling), anti-ICAM-1 (1:1000, ab171123, Abcam), anti-VCAM-1 (1:1000, ab134047, Abcam), anti-SIRT1 (1:1000, ab189494, Abcam), anti-MMP-9 (1:1000, ab76003, Abcam), anti-Iba1 (1:1000, DF7217, Affinity biosciences), anti-caspase-1 (1:1000, #AF5418, Affinity biosciences), anti-PGC-1α (1:1000, PA5-38,021, Thermo Fisher Scientific), anti-PPARγ (1:1000, PA3-821a, Thermo Fisher Scientific), anti-NLRP3 (1:1000, PA1665, BOSTER), and anti-ASC (1:1000, DF6304, Affinity biosciences) overnight at 4 °C. Then, after incubation, all membranes were washed in TBST followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for 2 h at room temperature. All immunoblots were expressed using enhanced chemiluminescence (ECL) substrate solution (Clarity Max™ Western ECL Substrate; Bio-Rad) before being visualized using an Omega Lum™ W Imaging System 81–12,120-00 (Aplegen Gel Company, Inc., CA, USA), and the band density was analyzed using ImageJ® software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All statistical data were analyzed using GraphPad Prism version 5 (Graph Pad Software, Inc., La Jolla, CA) and expressed as the mean ± standard error of the mean (SEM). The data were analyzed by one-way analysis of variance (ANOVA) to test the differences among multiple groups followed by Dunnett’s post hoc test to compare the differences between two groups. Two-way ANOVA was used to compare escape latency data. A statistically significant difference was considered when the p value < 0.05.
Results
Melatonin alleviated cognitive impairment in BCCAO rats
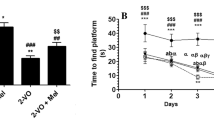
The MWM test was performed to assess cognitive function in rats. Swimming paths displayed swimming tracked for each group in spatial learning trials and memory trials (Fig. 1a). In the spatial learning trial, BCCAO rats had a significantly increased time to reach the platform, as shown by the longer escape latency when compared with the sham group (p < 0.001, Fig. 1b). In addition, in the memory trial, each group showed no significant difference in swimming distance and swimming speed (Fig. 1c, d). However, BCCAO rats displayed memory impairment, as demonstrated by spending less time in the target quadrant and platform crossing when compared with sham rats (p < 0.01 in both, Fig. 1e, f). On the other hand, melatonin and resveratrol improved cognitive functions, as demonstrated by the shorter escape latency time, longer time spent in the target quadrant, and the number of platform crossings when compared with BCCAO rats (p < 0.05 or p < 0.01 or p < 0.001, Fig. 1b, e, f). Our data demonstrate that melatonin or resveratrol treatments were effective in alleviating cognitive functions in BCCAO rats.
Melatonin alleviated cognitive decline after BCCAO induction in rats, as assessed by the MWM test a Representative swimming paths of each group in spatial learning and memory trials. b Escape latency changes in different groups from Day 1 to Day 5, c swimming distance at Day 6. d swimming speed at Day 6. e time spent in the target quadrant at Day 6 and f platform crossings during the memory trial at Day 6. All data are expressed as the mean ± SEM (n = 6). **P < 0.01 and ***P < 0.001 versus the sham group, #P < 0.05, ##P < 0.01, and ###P < 0.001 versus the BCCAO group. $$$P < 0.001 BCCAO + Res versus BCCAO group
Melatonin attenuated inflammation in rats subjected to BCCAO
Hypoxic conditions were observed in cerebral hypoperfusion, as demonstrated by increasing HIF-1α expression; this caused an increase in inflammatory mediators (Fig. 2). Our results showed the upregulation of HIF-1α expression in BCCAO rats; however, melatonin and resveratrol did not alter the expression of HIF-1α (p < 0.05 in both, Fig. 2b). At the same time, both melatonin and resveratrol significantly decreased the levels of pI \(\kappa\) Bα (p < 0.05 and p < 0.01, Fig. 2c). Subsequently, the ratio of pNF-\(\kappa\) B to NF-\(\kappa\) B was significantly reduced when compared with the BCCAO group (p < 0.001 in both, Fig. 2d). In addition, both melatonin and resveratrol significantly reduced MMP-9 expression compared with that in the BCCAO group (p < 0.01 and p < 0.001, Fig. 2e). Furthermore, the expression of ICAM-1 and VCAM-1 was significantly reduced in the melatonin group compared with the BCCAO group (p < 0.01 and p < 0.05, Fig. 2f, g). These results implied that although melatonin and resveratrol did not change HIF-1α expression, they attenuated brain inflammation, especially melatonin, with actions that were better than resveratrol, especially following BCCAO in rats.
Melatonin suppresses inflammation reactions induced after BCCAO induction in rats. a Western blot analysis of the expression of HIF-1α, pIκBα, NFκB, MMP-9, VCAM-1, and ICAM-1, b Quantitative analysis of the protein levels of HIF-1α, c Quantitative analysis of the protein levels of p-IκBα, d Quantitative analysis of the protein levels of p-NFκB to NFκB, e Quantitative analysis of the protein levels of MMP-9, f Quantitative analysis of the protein levels of ICAM-1, and g Quantitative analysis of the protein levels of VCAM-1. All data are expressed as the mean ± SEM (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 versus sham group, #P < 0.05, ##P < 0.01, and ###P < 0.001 versus BCCAO group
Melatonin reduced BCCAO-induced inflammasome formation in rats
Our results showed that both melatonin and resveratrol significantly inhibited inflammasome formation, as indicated by the decreased expression of NLRP3 and ASC and the ratio of cleaved caspase 1 to caspase 1 when compared with the BCCAO group (p < 0.05 or p < 0.01 or p < 0.001, Fig. 3a–c). Nevertheless, only melatonin markedly attenuated the expression of the GSDMD-NT protein (p < 0.05), while resveratrol partially decreased GSDMD-NT protein expression (Fig. 3d). These findings suggest that melatonin has the potential to inhibit inflammasome formation and pyroptosis after BCCAO in rats.
Melatonin reduced inflammasome formation and pyroptosis after BCCAO induction in rats. Representative Western blot analysis band and quantification of the relative expression of a NLRP3, b ASC, c cleaved caspase 1 to caspase 1, and d GSDMD-NT. All data are expressed as the mean ± SEM (n = 6). *P < 0.05 and ***P < 0.001 versus the sham group, #P < 0.05, ##P < 0.01, and ###P < 0.001 versus the BCCAO group
Melatonin protected glial cell activation in rats subjected to BCCAO
During pyroptosis, the cytosolic substances released through the GSDMD-NT pore activate the surrounding cells, especially glial cells. The immunohistochemistry results revealed that overactivated microglial cells and astrocytes were observed in BCCAO rats, as shown by the increase in Iba1- and GFAP-positive cells, along with the overexpression of Iba1 and GFAP proteins when compared with the sham group (p < 0.001 in both, Fig. 4a–d). In contrast, both melatonin and resveratrol decreased the number of Iba1- and GFAP-positive cells compared with the BCCAO group (p < 0.01 or p < 0.001, Fig. 4c, d). This result is consistent with immunoblots that showed significantly decreased Iba1 and GFAP expression when compared with the BCCAO group (p < 0.001 and p < 0.05, Fig. 4e, f). Hence, these outcomes indicated that melatonin has the ability to protect glial cell activation after BCCAO induction in rats.
Melatonin attenuated CCH-induced glial cell activation in the rat brain. Representative immunohistochemistry images of an Iba1 b GFAP in the cortex region of rats (indicated by arrow), c Quantitative analysis of Iba1-positive cells in the cortex region, d Quantitative analysis of GFAP-positive cells in the cortex region, e Representative Western blot analysis band and quantification of the relative expression of Iba1, and f Representative Western blot analysis band and quantification of the relative expression of GFAP. All data are expressed as the mean ± SEM (n = 6). ***P < 0.001 versus sham group, #P < 0.05, ##P < 0.01, and ###P < 0.001 versus BCCAO group
Melatonin actions are mediated through the SIRT1/PGC-1α/PPARγ signaling pathway under BCCAO in rats
Under hypoxic conditions, the increased activity of HIF-1α caused the decreased expression of SIRT1. We investigated the possibility that melatonin contributes to the SIRT1/PGC-1α/PPARγ signaling pathway. Immunoblots revealed that SIRT1 was lower in the BCCAO group, with subsequently decreased downstream proteins PGC-1α and PPARγ when compared with the sham group (p < 0.05 or p < 0.01, Fig. 5a–d). In contrast, melatonin and resveratrol significantly increased SIRT1 expression, which caused the upregulation of PGC-1α and PPARγ proteins when compared with the BCCAO group (p < 0.05 or p < 0.01, Fig. 5a–d). These findings indicate that the beneficial effects of melatonin were mediated by activation of the SIRT1/PGC-1α/PPARγ signaling pathway.
Melatonin upregulated the SIRT1/PGC-1α/PPARγ signaling pathway after BCCAO. a Western blot analysis of the expression of SIRT1, PGC-1α, and PPARγ. b Quantitative analysis of the protein levels of SIRT1. c Quantitative analysis of the protein levels of PGC-1α. d Quantitative analysis of the protein levels of PPARγ. All data are expressed as the mean ± SEM (n = 6). *P < 0.05 and **P < 0.01 versus sham group, #P < 0.05, and ##P < 0.01 versus BCCAO group
Melatonin preserved blood‒brain barrier integrity in rats subjected to BCCAO
The inflammatory cytokines released after CCH gave rise to an increase in BBB permeability. The ultrastructural changes in the morphology of the BBB were determined by TEM. The normal structure of the BBB was displayed in the sham group, which presented a single layer of endothelial cells and an intact endothelium supported by astrocyte foot processes, which are important for modulation of the BBB and controlling the surrounding environment (Fig. 6a). However, a change in the BBB was detected in the BCCAO group. The structure of the BBB showed disrupted capillaries, retracted astrocytes, and increased microvillus formation (Fig. 6a), which correlated with immunoblots indicating that the BCCAO group showed decreased expression of the tight junction proteins claudin-5, occludin, and ZO-1 compared with the sham group (p < 0.05 or p < 0.001, Fig. 6b–e). In addition, the structure of the BBB in the presence of melatonin and resveratrol appeared changed, with the astrocyte foot process covering endothelial cells and decreased microvillus formation in the lumen (Fig. 6a). Accordingly, these results corresponded to immunoblots where melatonin and resveratrol caused improved BBB integrity by increasing expression of the tight junction proteins claudin-5, occludin, and ZO-1 when compared with the BCCAO group (p < 0.05 or p < 0.01 or p < 0.001, Fig. 6b–e). Consequently, our results suggest that melatonin has the ability to preserve BBB integrity under BCCAO in rats.
Melatonin attenuated BBB damage. a Representative TEM microphotograph of the BBB in the cortex region of rats. The black arrow indicates microvillus formation. b Representative Western blot analysis band and quantification of the relative expression of occludin, claudin-5 and zonula occludens-1 (ZO-1) proteins. c Quantitative analysis of occludin protein levels. d Quantitative analysis of claudin-5 protein levels. e Quantitative analysis of ZO-1 protein levels. All data are expressed as the mean ± SEM (n = 6). *P < 0.05 and ***P < 0.001 versus the sham group, #P < 0.05, ##P < 0.01, and ###P < 0.001 versus the BCCAO group. L, lumen; En, endothelial cell; AS, astrocyte footplate, and N, nucleus
Discussion
In this study, we investigated the anti-inflammatory effect of melatonin in the BCCAO model. The major findings are as follows: (1) melatonin improved cognitive functions without altering HIF-1α expression in BCCAO rats, (2) melatonin ameliorated neuroinflammation by downregulating inflammasome formation in the BCCAO model, (3) melatonin suppressed pI \(\kappa\) Bα, pNF \(\kappa\) B, and NLRP3 activation through the SIRT1/PGC-1α/PPARγ signaling pathway, and (4) melatonin prevented BBB damage (Fig. 7).
Schematic representation of the action of melatonin against BCCAO induction. Melatonin exhibits anti-inflammatory properties that exert an effect by activating the SIRT1/PGC-1α/PPARγ signaling pathway, causing the inhibition of pIκBα, a subsequent decrease in NFκB and the consequent attenuation in action, including inflammation, inflammasome formation, glial cell activation, BBB disruption, and cognitive impairment
It has been proven that CCH causes a decrease in CBF and subsequently generates hypoxic conditions in the CNS, thus generating cognitive impairment (Du et al. 2017; Hugo and Ganguli 2014). In this study, BCCAO rats displayed impairments in learning and memory; however, treatment with melatonin and resveratrol improved cognitive functions, as indicated by the lower escape latency, longer time in the target quadrant, and frequency of platform crossing. Our findings were consistent with a previous report showing that melatonin and resveratrol could improve cognitive performance in the BCCAO model (Shen et al. 2016).
Hypoxic conditions promote an inflammatory response and induce cognitive impairment (Daulatzai 2017). Some evidence reveals that the increase in inflammatory mediators during hypoxia is mediated by the increase in HIF-1α (Dehne and Brüne 2009; Watts and Walmsley 2019). Notably, the activation of HIF-1α contributes to inflammation under CCH by modulating pI \(\kappa\) B, which causes the translocation of NF-\(\kappa\) B to the nucleus and promotes the production of various proinflammatory factors (Cummins et al. 2006). Collective data have shown that melatonin effectively inhibits inflammatory actions in various conditions, including aging (Permpoonputtana et al. 2018). Our results showed that melatonin and resveratrol did not alter HIF-1α expression. However, melatonin modulated the phosphorylation of I \(\kappa\) B, subsequently inhibiting the activation of NF-\(\kappa\) B and thereby reducing proinflammatory mediators such as MMP-9, ICAM-1, and VCAM-1, whereas resveratrol did not decrease the expression of ICAM-1 and VCAM-1. These results imply that melatonin ameliorates brain inflammation by decreasing pI \(\kappa\) B activation via HIF-1α-independent actions.
A recent study reported that the overexpression of HIF-1α was closely correlated with NLRP3 expression (Huang et al. 2019). Even the increase in NLRP3 was mediated by diverse stimuli; however, NF-\(\kappa\) B has been widely reported as an essential transcription factor of NLRP3 (Govindarajulu et al. 2018). The activation of NLRP3 promoted pyroptosis by assembling with ASC and cleaved caspase 1 to promote the translocation of GSDMD-NT to the cell membrane. Therefore, along with NF-\(\kappa\) B, this promotes NLRP3 expression and causes pyroptosis; the correlation between HIF-1α-induced NLRP3 might be controlled by NF-\(\kappa\) B signaling. Our results demonstrated that melatonin and resveratrol diminished NLRP3, ASC, and the ratio of cleaved caspase 1 to caspase 1, whereas only melatonin decreased GSDMD-NT formation. This finding correlates with our inflammation results, which implies that melatonin treatment regulates inflammasome formation and pyroptosis, particularly by inhibiting inflammatory signaling.
Glial cells are major contributors that respond to hypoxia and enable neurons to function properly. However, under CCH conditions, the expression of HIF-1α triggers inflammation in glial cells, subsequently promoting glial cell activation and proliferation and the production of proinflammatory mediators (Cekanaviciute and Buckwalter 2016; Mojsilovic-Petrovic et al. 2007). Previously published results showed that melatonin could attenuate glial cell activation in CCH conditions (Lee et al. 2016; Tsai et al. 2017). This is consistent with our results, which demonstrated that melatonin protected activation and decreased glial cell proliferation, suggesting that although melatonin does not alter HIF-1α expression, it does exert protective effects against inflammatory responses that are generated downstream of HIF-1α signaling.
Hypoxic conditions cause a gradual drop in NAD+ and the NAD+/NADH ratio; therefore, SIRT1 is downregulated during hypoxia (Braidy et al. 2011; Imai 2011; Lim et al. 2010). Our results show that SIRT1 was reduced in the BCCAO group. Additionally, targeting of the SIRT1, PGC-1α and PPARγ proteins was also decreased in BCCAO rats. Accumulating data suggest that the action of PPARγ modulates neuroinflammation directly by inhibiting pI \(\kappa\) Bα, causing limited NF-\(\kappa\) B-dependent inflammation (Ding et al. 2020; Scirpo et al. 2015). Therefore, we sought to further confirm whether melatonin attenuates inflammation through the SIRT1/PGC-1α/PPARγ signaling pathway. Our findings confirmed that resveratrol, which is a SIRT1 activator, can promote SIRT1, PGC-1α, and PPARγ protein expression. Furthermore, melatonin clearly upregulated the expression of SIRT1, PGC-1α, and PPARγ proteins, similar to resveratrol; however, some effects of melatonin are superior to resveratrol, particularly membrane action proteins such as ICAM1, VCAM1, and GSDMD-NT. For these reasons, the beneficial effects of melatonin and its anti-inflammatory properties against CCH conditions may be mediated through the SIRT1/PGC-1α/PPARγ signaling pathway.
As mentioned above, the activation of glial cells and pyroptosis cause the excessive release of proinflammatory mediators such as MMP-9, ICAM-1, or VCAM-1; subsequently, these proinflammatory factors increase BBB permeability (Wicha et al. 2020). ICAM-1 and VCAM-1 control the rolling and transmigration of white blood cells from the circulation to increase neuroinflammation, whereas MMP-9 causes BBB breakdown by degrading tight junction proteins such as claudin5, occludin, and ZO-1, thus resulting in increased BBB permeability (Weiss et al. 2009). Our results demonstrated for the first time that melatonin could attenuate BBB integrity by increasing the expression of tight junction proteins such as claudin5, occludin, and ZO-1, which caused the intact endothelium to be covered by astrocyte endfeet and reduced microvillus formation, as illustrated by TEM. These results show that inflammation increases in CCH conditions; moreover, accumulated data illustrate that inflammation is one of the hallmarks of BBB disruption (Li et al. 2021). This suggests that melatonin is able to protect the BBB from disruption as a consequence of inhibiting the inflammatory response caused by activation of the SIRT1/PGC-1α/PPARγ signaling pathway.
Additionally, our findings showed for the first time that resveratrol promoted neuroprotective effects following BCCAO induction by affecting the SIRT1/PGC-1α/PPARγ signaling pathway. The increase in SIRT1 by resveratrol not only suppressed neuroinflammation but also inhibited pyroptosis cell death and restored BBB proteins and integrity. Interestingly, our data illustrated that the effects of resveratrol were particularly improved in membrane-bound proteins, especially ICAM1, VCAM1, and GSDMD-NT.
Conclusion
BCCAO rats exhibited neuroinflammation, consequently generating inflammasome formation, glial cell activation, BBB disruption, and cognitive impairment. Interestingly, melatonin suppressed neuroinflammation by attenuating inflammasome formation, glial cell activation, BBB disruption, and cognitive impairment. Moreover, our results show for the first time that melatonin action is mediated through the SIRT1/PGC-1α/PPARγ signaling pathway, which directly controls the inflammatory process. Therefore, melatonin acts as an anti-inflammatory mediator to exert neuroprotective benefits in neurodegenerative disorders.
Availability of data and materials
All data are available on request from authors.
References
Amaral FGd, Cipolla-Neto J (2018) A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab 62:472–479. https://doi.org/10.20945/2359-3997000000066
Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R (2011) Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One 6:e19194. https://doi.org/10.1371/journal.pone.0019194
Cantó C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20:98. https://doi.org/10.1097/MOL.0b013e328328d0a4
Cekanaviciute E, Buckwalter MS (2016) Astrocytes: integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics 13:685–701. https://doi.org/10.1007/s13311-016-0477-8
Corona JC, Duchen MR (2015) PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem Res 40:308–316. https://doi.org/10.1007/s11064-014-1377-0
Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J (2006) Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc Natl Acad Sci 103:18154–18159. https://doi.org/10.1073/pnas.0602235103
Daulatzai MA (2017) Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J Neurosci Res 95:943–972. https://doi.org/10.1002/jnr.23777
Dehne N, Brüne B (2009) HIF-1 in the inflammatory microenvironment. Exp Cell Res 315:1791–1797. https://doi.org/10.1016/j.yexcr.2009.03.019
Ding Y, Kang J, Liu S, Xu Y, Shao B (2020) The protective effects of peroxisome proliferator-activated receptor gamma in cerebral ischemia-reperfusion injury. Front Neurol 11:1469. https://doi.org/10.3389/fneur.2020.588516
Du SQ, Wang XR, Xiao LY, Tu JF, Zhu W, He T, Liu CZ (2017) Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol. Neurobiol 54:3670–3682. https://doi.org/10.1007/s12035-016-9915-1
Fang Y, Gao S, Wang X, Cao Y, Lu J, Chen S, Zhang J (2020) Programmed cell deaths and potential crosstalk with blood–brain barrier dysfunction after hemorrhagic stroke. Front Cell Neurosci 14:68. https://doi.org/10.3389/fncel.2020.00068
Govindarajulu M, Pinky PD, Bloemer J, Ghanei N, Suppiramaniam V, Amin R (2018) Signaling mechanisms of selective PPARγ modulators in Alzheimer’s disease. PPAR Res. https://doi.org/10.1155/2018/2010675
Huang JJ, Xia J, Huang LL, Li YC (2019) HIF-1α promotes NLRP3 inflammasome activation in bleomycin-induced acute lung injury. Mol Med Rep 20:3424–3432. https://doi.org/10.3892/mmr.2019.10575
Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, Lamming DW, Pentelute BL (2013) Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339:1216–1219. https://doi.org/10.1126/science.1231097
Hugo J, Ganguli M (2014) Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 30:421–442. https://doi.org/10.1016/j.cger.2014.04.001
Imai S-i (2011) Dissecting systemic control of metabolism and aging in the NAD World: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett 585:1657–1662. https://doi.org/10.1016/j.febslet.2011.04.060
Kleszcz R, Paluszczak J, Baer-Dubowska W (2015) Targeting aberrant cancer metabolism–The role of sirtuins. Pharmacol Rep 67:1068–1080. https://doi.org/10.1016/j.pharep.2015.03.021
Lee CH, Park JH, Ahn JH, Won MH (2016) Effects of melatonin on cognitive impairment and hippocampal neuronal damage in a rat model of chronic cerebral hypoperfusion. Exp Ther Med 11:2240–2246. https://doi.org/10.3892/etm.2016.3216
Li X (2013) SIRT1 and energy metabolism. Acta Biochim Biophys Sin 45:51–60. https://doi.org/10.1093/abbs/gms108
Li Y, Wang P, Yang X, Wang W, Zhang J, He Y, Zhang W, Jing T, Wang B, Lin R (2016) SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol Immunol 77:148–156. https://doi.org/10.1016/j.molimm.2016.07.018
Li J, Zheng M, Shimoni O, Banks WA, Bush AI, Gamble JR, Shi B (2021) Development of novel therapeutics targeting the blood-brain barrier: from barrier to carrier. Adv Sci. https://doi.org/10.1002/advs.202101090
Liang H, Ward WF (2006) PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. https://doi.org/10.1152/advan.00052.2006
Lim J-H, Lee Y-M, Chun Y-S, Chen J, Kim J-E, Park J-W (2010) Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol Cell 38:864–878. https://doi.org/10.1016/j.molcel.2010.05.023
Ma J, Yang H, Liu L, Wan Y, Wang F (2021) Melatonin alleviated oxidative stress induced by energy restriction on sheep Leydig cells through Sirt1/Sod2 pathway. Theriogenology 173:83–92. https://doi.org/10.1016/j.theriogenology.2021.07.011
Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W (2007) Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation 4:1–15. https://doi.org/10.1186/1742-2094-4-12
Permpoonputtana K, Tangweerasing P, Mukda S, Boontem P, Nopparat C, Govitrapong P (2018) Long-term administration of melatonin attenuates neuroinflammation in the aged mouse brain. EXCLI J 17:634. https://doi.org/10.17179/excli2017-654
Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J (2019) The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett 24:1–10. https://doi.org/10.1186/s11658-019-0158-9
Sanderson TH, Wider JM (2013) 2-vessel occlusion/hypotension: a rat model of global brain ischemia. J vis Exp. https://doi.org/10.3791/50173
Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M (2015) Stimulation of nuclear receptor PPAR-γ limits NF-kB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 62:1551. https://doi.org/10.1002/hep.28000
Shen D, Tian X, Sang W, Song R (2016) Effect of melatonin and resveratrol against memory impairment and hippocampal damage in a rat model of vascular dementia. NeuroImmunoModulation 23:318–331. https://doi.org/10.1159/000454681
Thangwong P, Jearjaroen P, Govitrapong P, Tocharus C, Tocharus J (2022) Melatonin improves cognitive impairment by suppressing endoplasmic reticulum stress and promoting synaptic plasticity during chronic cerebral hypoperfusion in rats. Biochem Pharmacol 198:114980. https://doi.org/10.1016/j.bcp.2022.114980
Tsai TH, Lin CJ, Chua S, Chung SY, Yang CH, Tong MS, Hang CL (2017) Melatonin attenuated the brain damage and cognitive impairment partially through MT2 melatonin receptor in mice with chronic cerebral hypoperfusion. Oncotarget 8:74320. https://doi.org/10.18632/oncotarget.20382
Venkat P, Chopp M, Chen J (2015) Models and mechanisms of vascular dementia. Exp Neurol 272:97–108. https://doi.org/10.1016/j.expneurol.2015.05.006
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858. https://doi.org/10.1038/nprot.2006.116
Watts ER, Walmsley SR (2019) Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol Med 25:33–46. https://doi.org/10.1016/j.molmed.2018.10.006
Weiss N, Miller F, Cazaubon S, Couraud P-O (2009) The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Et Biophys Acta Biomembr 1788:842–857. https://doi.org/10.1016/j.bbamem.2008.10.022
Wicha P, Tocharus J, Janyou A, Jittiwat J, Chaichompoo W, Suksamrarn A, Tocharus C (2020) Hexahydrocurcumin alleviated blood–brain barrier dysfunction in cerebral ischemia/reperfusion rats. Pharmacol Rep. https://doi.org/10.1007/s43440-019-00050-9
Xue Y, Tuipulotu DE, Tan WH, Kay C, Man SM (2019) Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol 40:1035–1052. https://doi.org/10.1016/j.it.2019.09.005
Yang CC, Wu CH, Lin TC, Cheng YN, Chang CS, Lee KT, Tsai PJ, Tsai YS (2021) Inhibitory effect of PPARγ on NLRP3 inflammasome activation. Theranostics 11:2424. https://doi.org/10.7150/thno.46873
Yawoot N, Sengking J, Wicha P, Govitrapong P, Tocharus C, Tocharus J (2022) Melatonin attenuates reactive astrogliosis and glial scar formation following cerebral ischemia and reperfusion injury mediated by GSK-3β and RIP1K. J Cell Physiol 237:1818–1832. https://doi.org/10.1002/jcp.30649
Yeleswaram K, McLaughlin LG, Knipe JO, Schabdach D (1997) Pharmacokinetics and oral bioavailability of exogenous melatonin in preclinical animal models and clinical implications. J Pineal Res 22:45–51. https://doi.org/10.1111/j.1600-079x.1997.tb00302.x
Zhao L, Liu H, Yue L, Zhang J, Li X, Wang B, Lin Y, Qu Y (2017) Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF-κB signaling pathway following subarachnoid hemorrhage in mice. Mol Neurobiol 54:1612–1621. https://doi.org/10.1007/s12035-016-9776-7
Zhou Y, Wang S, Li Y, Yu S, Zhao Y (2018) SIRT1/PGC-1α signaling promotes mitochondrial functional recovery and reduces apoptosis after intracerebral hemorrhage in rats. Front Mol Neurosci 10:443. https://doi.org/10.3389/fnmol.2017.00443
Funding
This work was supported by grants from Functional Food Research Center for Wellbeing, Chiang Mai University, Thailand Science Research and Innovation (TSRI) (grant number 64/004) and the Development and Promotion of Science and Technology Talents Project Royal Government of Thailand scholarship (grant number 572109).
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used. All authors were involved in the study design. PT performed writing–original draft, methodology, investigation, and formal analysis. PJ performed the investigation. PG performed resources. CT performed methodology, writing–review, and editing. JT performed writing–review and editing, conceptualization, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Compliance with ethical standards
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Faculty of Medicine, Chiang Mai University, (Permit number: 32/2563) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thangwong, P., Jearjaroen, P., Tocharus, C. et al. Melatonin suppresses inflammation and blood‒brain barrier disruption in rats with vascular dementia possibly by activating the SIRT1/PGC-1α/PPARγ signaling pathway. Inflammopharmacol 31, 1481–1493 (2023). https://doi.org/10.1007/s10787-023-01181-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01181-5