Abstract
Metabolic disorders are closely associated with dietary habits and seem to be related to neuroinflammation and neurodegenerative disease in humans. Emblica officinalis (EOT) fruits not only have good nutritional value but also have excellent therapeutic potential. We used a tannins-enriched fraction of EOT fruit with the expectation of controlling diet-induced neuroinflammation and cognitive impairment in rats. A high-salt and cholesterol diet (HSCD) was used to induce neuroinflammation and cognitive impairment in rats. The diet of the rats was then supplemented with EOT (100 and 200 mg/kg b.w.) for 7 weeks. In order to evaluate the neuroprotective effects of EOT; in silico study, neurobehavioral tests, biochemical analyses, and immunohistochemical studies were performed. In silico study of p50 (NF-κB1) receptors with emblicanin (the main constituent of EOT) suggests that EOT has binds to NF-κB. EOT treatment reversed the HSCD-induced behavioral and memory disturbances in a step-down-type passive avoidance test. EOT treatment also inhibited HSCD-induced NF-κB upstream signaling, including the release of Th1, such as TNF-α, and downstream signaling Th2, such as IL-10, by flow cytometer. In addition, EOT treatment attentuated the HSCD-induced increase in the level of cognitive impairment markers, such as amyloid β. Furthermore, immunohistochemical results demonstrated that EOT modulated neuronal cell death by inhibiting the overexpression of NF-kB in brain. This study confirms that EOT may be a promising therapy in ameliorating the neurotoxicity of HSCD; however further studies are warranted to elucidate the exact mechanism of action of EOT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive function is one of the key determinants of quality of life in older age. The metabolic disorder seems to be closely associated with neuroinflammation and cognitive impairment in humans. Accumulating evidence has confirmed a link between the metabolic disorder and the onset of cardiovascular disease (Pistell et al. 2010). Despite an increasing awareness that cardiovascular risk factors exaggerate the risk of cognitive impairment and dementia, the detailed relationship between metabolic disorders and cognitive impairment has not been well defined. Metabolic disorders are the main cause of the cognitive impairment in the elderly (Yaffe et al. 2007). Moreover, blood pressure-related pathophysiological processes adversely affecting the brain may begin earlier in the adult lifespan than previously thought, indicating that earlier prevention of metabolic disorders may be necessary to improve quality of life in the elderly (Kivipelto et al. 2002). Metabolic disorders seems to be closely associated with the daily diet, changes in cognitive function in rats fed with a high-salt intake for hypertension and a high-cholesterol diet for hyperlipidemia have not been well defined.
Nuclear factor kappaB (NF-kB) signaling in the cerebrospinal fluid (CSF) and/or the serum and brain mediates autoimmune responses and induces neuroinflammation that results in neurodegenerative diseases, such as cognitive impairment. The NF-κB group consists of p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), c-Rel (Rel), and RelB. The NF-κB family forms homo- or heterodimers which are present in inactive form in the cytoplasm (Zhu et al. 2015). The activated NF-κB translocates to the nucleus, activating expression of a number of inflammatory genes such as tumor necrosis factor-a (TNF-α), interleukins (IL-1β, IL-6, and IL-18), inducible nitric oxide synthase and cyclooxygenase-2 (Lu et al. 2011). In particular, inflammation has been described as the culprit of disease or an attempt by the immune system to contain accumulation of amyloid-beta (Aβ) in the brain. The extracellular aggregates of Aβ are one of the neuropathological hallmarks of cognitive impairment. Aβ peptide is cleaved from amyloid precursor protein (APP) by proteolytic actions of β-secretase (BACE1) and γ-secretase. NF-κB plays a central role in regulating expressions of inflammatory genes. It also induces decomposition of APP by up-regulating BACE1 transcription. In our previous studies, we confirmed that HSCD impaired spatial learning and that memory performance increased Aβ production via activating NF-κB signaling (Husain et al. 2017b). Therefore, inhibiting NF-κB pathways could interrupt the generation of Aβ as well as neuroinflammation. Together, it is suggested that activation of NF-κB plays an important role in mediating neuroinflammation in cognitive impairment (Capuron and Miller 2011). Diet-associated neuroinflammation is a potential contributor to the accumulation of beta-amyloid (Aβ) and other risk factors for cognitive impairment (Thirumangalakudi et al. 2008).
Emblica officinalis Gaertn. or Phyllanthus emblica Linn. (Euphorbiaceae), commonly known as Indian gooseberry or amla, is used as a medicine and nutritional tonic and possesses vital amino acids and vitamins. The fruit contains several tannins such as emblicanin A, emblicanin B, pedunculagin, and punigluconin, etc. (Variya et al. 2016). In a study by Bhattacharya et al. (2000), it was observed that tannins of Emblica officinalis (EOT) normalized alterations in frontal cortical and striatal superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation induced by chronic stress via chronic unpredictable foot-shock-induced perturbations. Significant antioxidant activity was exhibited by EOT in both in vitro as well as in vivo studies (Bhattacharya et al. 2000). In another study, EOT reversed amnesia caused due to scopolamine administration as well as reduced oxidative stress induced by it. In addition, it also reversed the increased acetylcholinesterase (AchE) levels in the brain. EOT also showed protective activity against neurotoxicity induced by aluminum (Thenmozhi et al. 2016). However, the exact mechanism of the inhibition of HSCD-induced neurotoxicity as well as the possible pharmacodynamic action of EOT via the NF-κB pathway have not been investigated up to now. Therefore, we hypothesized that EOT might interact with the NF-κB receptor complex and inhibit the downstream inflammatory processes.
Materials and methods
Drugs and chemicals
Piracetam (PCT) was obtained as a gift sample from Arbro Pharmaceuticals, New Delhi, India. Griess reagent was procured from Sigma Aldrich, Bangalore, India. The tannin-enriched fraction from EOT was obtained from Indian Herbs Specialties, Uttar Pradesh, India. The tannins were extracted by the method of Ghosal et al. (1996) which involved deactivating the hydrolytic enzymes present in the fresh juice of E. officinalis, following which column chromatography was performed over Sephadex LH-20 employing methanol and methanol–water as the eluent (Ghosal et al. 1996). The concentration of the various tannins was as follows: emblicanin A (37%), emblicanin B (33%), punigluconin (12%), pedunculagin (14%), rutin (3%), and gallic acid (1%). NF-κB protein was purchased from Abcam, Cambridge, MA, USA. The cytokine kits for TNF-α and IL-10 were purchased from BioHouse Solutions, India. An enzyme-linked immunosorbent assay (ELISA) kit for Aß1-42 was purchased from Genxbio Health Sciences, Delhi, India.
In silico study
Molecular docking and MM-GBSA binding free energy
To predict the binding modes of ligands to the receptor by structures, molecular docking studies of the compounds were carried out using the Maestro 10.5 program (Schrodinger, USA). The three-dimensional structure of NF-κB-DNA complexes was retrieved from the protein data bank (PDB code: 1VKX) to be used for the present docking study (Husain et al. 2017b). The test compound (emblicanin) along with the standard (PCT) was used against the NF-κB-DNA complexes. Molecular docking studies mainly involve selection and preparation of the appropriate protein, grid generation, and ligand preparation followed by docking and its analysis. The protein preparation was carried out in three steps, i.e., preprocess, review and modify, followed by refinement using the ‘protein preparation wizard’ in Maestro 10.5. In these steps, water molecules were deleted, and hydrogen atoms were added. The energy of the structure was minimized using the OPLS 2005 force field. Similarly, ligands were prepared, again using Force Field 2005. A receptor grid generation program was run by clicking any atom of the ligand, and the default box was prepared. The ligand was docked into the grid generated from the protein using extra precision. The results were evaluated by docking score: the higher the docking score, the more the binding affinity (Friesner et al. 2004). Alternatively, the prime molecular mechanics-generalized born surface area (MM-GBSA) results can be procured running the MM-GBSA program directly from the file generated by running the docking protocol. It is a tool to calculate ligand binding free energy. The docking score, binding free energy and hydrogen bonds and pi–pi interaction formed with the surrounding amino acids are used to envisage their binding affinities and proper alignment of these compounds at the active site of the NF-κB-DNA (Siddiqui et al. 2016).
In vivo study
Animals
The experimental protocol was approved by the Institutional Animal Ethics Committee of Jamia Hamdard, New Delhi, India (Approval ID: JH/993/CPCSEA) as per the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines. Female Wistar rats (8–12 weeks old, 150–200 g body weight) were obtained from the Central Animal House Facility, Jamia Hamdard, New Delhi, and housed in standard polypropylene cages (six in each cage) with access to commercial standard pellet diet (Amrut rat feed; Nav Maharashtra Chakan Oil Mills, New Delhi, India) and water ad libitum. The rats were maintained in the animal house under controlled room temperature (20–25 ± 2 °C) and relative humidity (50 ± 15%) with 12 h light/12 h darkness (day/night) cycle.
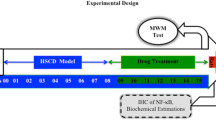
Experimental design
Suspensions of EOT and PCT were prepared by triturating the weighed amount of EOT (100 and 200 mg/kg) and PCT (200 mg/kg) in 0.5% carboxy methyl cellulose (CMC) suspension (w/v) in normal saline (Ghaisas et al. 2010). High-salt saline was freshly prepared by adding 2% w/v NaCl in water. Pellets of the high cholesterol diet were freshly prepared by adding 1.25% cholesterol and 10% coconut oil in standard diet pellets and drying at room temperature (Mogi et al. 2007). Prior to the commencement of the experiments, the animals were fed with standard rat food pellets for 2 days for acclimatization. In this study, PCT and EOT served as the standard and test drugs, respectively. The total duration of the study was 15 weeks. The animals were fed with HSCD ad libitum for 8 weeks to induce neuroinflammation and memory impairment (Liu et al. 2008; Mogi et al. 2007). After that, the rats were treated with EOT (p.o.) and PCT (i.p.) for 7 weeks in different doses. The rats were randomly divided into five groups as described in Table 1. The rats were observed for neurobehavioral parameters and then immediately sacrificed for estimation of biochemical parameters and immunohistochemistry analysis.
Neurobehavioral testing by a step-down type passive avoidance test
After completion of the treatment, the step-down-type passive avoidance test (SDTPAT) was performed as per the method described by Tan et al. (2015). During the beginning of training, rats were placed on the wooden platform to adapt for 2 min. When the rats stepped down from the platform and placed all paws on the grid floor, an electric current was delivered for 15 s. Electrification of grid was maintained for 5 min once the rats had jumped onto the platform to avoid the electric shock. After 24 h, the experiment was repeated and the tendency of the animals to step down from the platform on to the grid for the first time as well as the number of errors subjected to shock in the first 3 min were measured.
Determination of brain nitrite level
The accumulation of nitrite in the brain tissue supernatant, an indicator of the production of nitric oxide (NO), was determined by a colorimetric assay with Griess reagent [0.1% N-(1-naphthyl) ethylene diamine dihydrochloride, 1% sulfanilamide and 2.5% phosphoric acid]. In brief, equal volumes of Griess reagent and supernatant were mixed, after which the mixture was incubated for 10 min at room temperature in the dark. The absorbance of the supernatant was recorded at 540 nm with a Perkin Elmer lambda 20 spectrophotometer. The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and calculated in terms of μg/mL (Ahmad et al. 2016).
Analysis of Th1/Th2 cytokines
The levels of TNF-α (Th1) as well as IL-10 (Th2) cytokines in the serum were estimated using a BD cytometry bead array kit. Serum samples from all the experimental groups were collected and processed for analysis of Th1/Th2 cytokines using a BD LSR II flow cytometer according to the manufacturer’s instructions. FCAP Array software (BD v.3.0) was used to create the standard curves for each cytokine and convert the fluorescent MFI values into cytokine concentrations (Islamuddin et al. 2015).
Determination of Aβ content in brain
Frozen brains were thawed and minced. Then, the tissues of the brain were weighed and homogenized in 50 mM Tris–HCl buffer, pH 8.0, containing a cocktail of protease inhibitors. The homogenates were centrifuged (100,000g, 1 h, 4 ℃), and the supernatants were stored at − 80 ℃ for additional analysis of soluble Aβ. Then, the pellets were sonicated in 5 M guanidine and incubated for 30 min at room temperature, then centrifuged (100,000g, 1 h, 4 ℃) before the supernatants were stored for analysis of insoluble Aβ (Husain et al. 2017a). The concentrations of insoluble and soluble Aβ1-42 were estimated by using Aβ ELISA kits (GenxBio), as per the manufacturers’ instructions.
Immunohistochemical analysis of NF-κB protein
For Immunohistochemical analysis, paraffin sections of the brains were deparaffinized in xylene and then with acetone for 5 min each. Samples were rehydrated with a graded series of ethanol. After washing under running double-distilled water, antigen retrieval was performed with a citrate buffer (pH 6). Three changes of the tissue sections were carried out with TBS buffer solution. These sections were then blocked with 1.5% normal goat serum for 1 h. They were then incubated with purified goat polyclonal antibody raised against a peptide mapping at the N-terminus of NF-κB of human origin (1:200; Santa Cruz Biotechnology) overnight at 4 °C. Immunoreactivity was detected with biotinylated anti-goat rabbit secondary antibodies and the avidin–biotin–peroxidase complex. Immunoreactive signals were developed using diaminobenzidine as a substrate for 2 min. Photomicrographs were taken with a Meiji microscope enabled with a Lumenera camera. The images were analyzed with Lumenera Infinity Analyze 3 software (Raza et al. 2013). All immunohistochemical samples were analyzed in a blinded fashion. For the quantification of the protein expression semi-automatically, ImageJ 1.49 software was used to estimate the volume fraction of immune-reactive cells within the tissue sample. The range of pixel intensities of images was between 0 and 250, with the values 0 and 250 indicating the darkest and lightest shade of the image color, respectively (Nguyen et al. 2013).
Statistical analysis
Results were expressed as the mean ± standard error of the mean (SEM). The statistical significance of differences between the groups was determined using one-way analysis of variance followed by Tukey’s test. p value < 0.05 was considered statistically significant. Error bars represent the standard error of the mean (SEM). All statistical tests were performed using the Prism software package (v.4; GraphPad, San Diego, CA, USA).
Results
Molecular docking and MM-GBSA binding free energy analysis
Docking studies were carried out by using ligand docking to study the binding mode of emblicanin (the main constituent of EOT) with the active site of NF-κB p50 using the Schrodinger programme Maestro 10.5. For the validation, the protein was docked with the standard PCT (Fig. 1). The NF-kB p50/p65 heterodimer is the classic member of the Rel family of transcription factors that regulate diverse cellular functions such as immune response, cell growth, and development. Secondary structures of the subunits are equivalent, apart from a 32-amino acid insert in the N-terminal domain of p50 that adds a second α-helix (Chen et al. 1998). The docking scores, binding free energy and interaction with amino acids of the emblicanin and PCT with the active site of NF-κB p50 are summarized in Table 2.
Representative 2D and 3D ligand interaction of emblicanin (the main constituent of EOT) and piracetam (PCT) in the binding site of NF-κB p50. a D ligand interaction representation of emblicanin showing hydrogen bond interaction with purple arrow line and pi–pi stacking with green line in the binding site of NF-κB p50. b 2D ligand interaction representation of PCT showing hydrogen bond interaction by the purple arrow line and pi–pi stacking by the green line in the binding site of NF-κB p50. c The docked pose of emblicanin represented as sticks in the binding site of NF-κB p50 showing hydrogen bond interaction and pi–pi-stacking. d The docked position of PCT represented as sticks in the binding site of NF-κB p50 showing hydrogen bond interaction and pi–pi-stacking
Effects of EOT and PCT on step-down latency and the number of errors
SDTPAT was performed to assess the effects of HSCD, EOT and PCT on rats’ learning and memory. As shown in Fig. 2a, a significant decline (###p < 0.001) in the step-down latency was observed in the TC group which was fed HSCD as compared to the NC. This was accompanied by a significant increase in the number of errors made in Fig. 2b). Both of these observations indicate memory impairment in the HSCD-fed rats. A significant increase in step-down latency was observed with groups fed EOT100 and EOT200 (***p < 0.001). Similarly, a significant increase in step-down latency was seen in the PCT200 group (***p < 0.001); however, the rats fed EOT200 showed significantly greater step-down latency than the PCT200 group ($$p < 0.01). Similarly, EOT significantly decreased the number of errors made by the animals in a dose-dependent manner (EOT100, *p < 0.05; EOT200, **p < 0.01). A significantly decreased number of errors was also observed with the PCT200 group (*p < 0.05).
The step-down type passive avoidance test for assessment of cognitive function in animal groups. a The average step-down latency. b The average number of errors within 3 min. Data are presented as mean ± SEM for six rats in each group. ###p < 0.001 vs. TC group. ***p < 0.001, **p < 0.01, *p < 0.05 vs. TC group. $$p < 0.01 vs. PCT200 group
Effects of EOT and PCT on brain nitrite level
A significant increase (###p < 0.001) in nitrite level in the TC group was observed when compared with the NC group. This effect was significantly reversed by EOT (EOT100 and EOT200) administration in a dose-dependent manner (**p < 0.01, ***p < 0.001, respectively). This effect was also significantly (***p < 0.001) reversed by PCT200 administration (Fig. 3).
Effects of EOT and PCT on Th1/Th2 cytokines
There was a significant increase (###p < 0.001) in TNF-α (Th1) level in the TC group animals when compared to the NC group animals (Fig. 4). This effect was significantly reversed by EOT (***p < 0.001) administration in a dose-dependent manner. The PCT200 group showed a significant reduction in the TNF-α level (***p < 0.001), but the diminution was less than that observed in the EOT groups. Moreover, EOT affected the production of IL-10, a Th2 cytokine (Fig. 4). EOT-treated levels of IL-10 in the HSCD-fed rats were significantly different (***p < 0.001), although elevated IL-10 production was noted after EOT treatment. Interestingly, HSCD significantly decreased the IL-10 protein expression (###p < 0.001) when compared with the NC group. PCT increased IL-10 levels, but a significant effect was not observed. However, the rats fed EOT100 and EOT200 showed significantly greater IL-10 protein expression than the PCT200 group (EOT100, $$p < 0.01; EOT200, $$$p < 0.001) respectively.
Effect of EOT and PCT on serum levels of cytokines. a Effect of EOT and PCT on serum TNF-α and IL-10 concentration (pg/ml) in different animal groups were measured by bead-based multiplex assay (BD CBA kit). b TNF-α and IL-10 concentration (pg/ml) are presented in bar diagram. Data are represented as mean ± SEM for six rats in each group. ###p < 0.001 vs. TC group. ***p < 0.001, **p < 0.01, *p < 0.05 vs. TC group. $$p < 0.01, $p < 0.05 vs. PCT200 group
Effects of EOT and PCT on Aβ1-42 content in brain
Figure 5 shows the different levels of soluble and insoluble Aβ1-42 in the brain. A significantly (###p < 0.001) increased level of serum Aβ1-42, along with soluble and insoluble Aβ1-42, in the brains of the TC group were observed in compared to NC group. This effect significantly reversed EOT (EOT100 and EOT200) administration dose-dependently (***p < 0.001). PCT200 also decreased the levels of soluble and insoluble Aβ1-42 in the brain but less then EOT. However, the rats treated with EOT100 and EOT200 showed a significantly decreased level of insoluble Aβ1-42 compared with the PCT200 group ($$$p < 0.001), as shown in Fig. 5b. EOT200 also showed a significantly decreased level of soluble Aβ1-42 compared with the PCT200 group ($$$p < 0.001), as shown in Fig. 5a.
Effects of EOT and PCT on NF-κB protein in brain
Further measuring the inflammatory effects of HSCD on the brain of rats, we examined the expression of nuclear trans-localized NF-κB in the cortices and CA1 region of the hippocampus. HSCD-fed rats demonstrated an increased expression of NF-κB as compared to normal control group rats. EOT treatment significantly attenuated HSCD-induced NF-κB nuclear translocation in the brain compared to the toxic control group (Fig. 6).
Immunohistochemistry analysis of NF-κB protein by fluorescent microscope in coronal brain sections at the level of CA1 region of hippocampus and cortex. The profound expression of nuclear NF-κB was observed in the TC group as compared to the NC group, and treatment groups of EOT and PCT show the effects on staining of NF-κB. Black arrows show the positively stained cells at ×10 magnification
In the CA1 region of hippocampus, densitometry results confirmed that a significant increased (###p < 0.001) expression of NF-κB in the TC group was observed when compared with the NC group. This effect was significantly reversed by EOT (EOT100 and EOT200) administration in a dose-dependent manner (**p < 0.01, ***p < 0.001, respectively). This effect was also significantly (***p < 0.001) reversed by PCT200 administration (Fig. 7).
Discussion
The present study has demonstrated that HSCD is significantly associated with neuroinflammation and cognitive impairment in rats, which is supported by previous studies (Husain et al. 2017a; Mogi et al. 2007). Cognitive impairment is one of the strongest determinants of quality of life in elderly persons. HSCD activated the NF-κB pathway which is associated with cognitive impairment in the rat (Husain et al. 2017b). The tannin-enriched fraction of EOT fruits showed anti-inflammatory and memory-recovering effects in HSCD-induced neuroinflammation and cognitive impairment.
The docking studies were performed by positioning the emblicanin (the main constituent of EOT) in the same binding site as that of PCT. The results for the binding mode of the compounds emblicanin and PCT are presented in Fig. 1. As shown in the figure, emblicanin and PCT can nearly overlap in the binding mode where C=O of the pyrrole-2-one ring of PCT bonded to Arg356, similar to –SO2 of the emblicanin. Another hydrogen bond was observed between the amide group of PCT with Hie364 and Gly365 similar to the SO2 group of the drug emblicanin. These interactions underscore the importance of the sulfonyl group present in the compound emblicanin. The higher docking and binding score of the drug emblicanin could be because of the additional hydrogen bond interaction between Lys414 and Cys416 of the hydroxyl group and Gly419 and Asp418 with C=O of the carboxylic acid group. Thus, in silico study gives us a new direction to predict EOT to be a good candidature to decrease neuroinflammation and cognitive impairments inhibiting the NF-kB signaling pathway similar to the drug PCT.
HSCD treatment increased the body weight significantly of all the animals as compared to the normal control rats. Increase in body weight is correlated with cognitive impairment (Mogi et al. 2007). EOT treatment in rats significantly prevented the rise in body weight induced by HSCD (Husain et al. 2017a).
The passive avoidance model is a popular neurobehavioral test to evaluate learning and memory in rodents as well as to identify compounds that alter cognitive developments (Nagel and Kemble 1976). We performed the SDTPAT to evaluate the efficacy of EOT in ameliorating cognitive impairment induced by HSCD. The step-down latency was significantly less in the TC group as compared with the NC group, which suggests the impairment of learning and memory in the TC group and is consistent with earlier findings (Pinton et al. 2013). The latency increased linearly with the increase in EOT dose, which indicates that EOT attenuated HSCD-induced cognitive impairment in a dose-dependent manner. A similar trend was observed when the numbers of errors of each group in 180 s were compared. PCT also showed a significant effect, but less than the higher dose of EOT. These observations indicate that EOT has the potential to renovate HSCD-induced cognitive impairment.
Neuroinflammation is known to be associated with the pathology of neurodegenerative diseases including cognitive impairment (Wyss-Coray and Mucke 2002). The transcription factor, NF-κB, regulates the transcription of several cytokines that play an important role in neuroinflammation-mediated neurodegenerative disease. Several studies have confirmed that over-activation of proinflammatory genes have been observed in cognitive impairment patients as well as in animal models of cognitive impairment (Pannu and Singh 2006). In the current study, HSCD increased NF-κB protein (Fig. 7) expression in the CA1 region of the hippocampus as compared with the control group animals. NF-κB protein expression was significantly attenuated by EOT in a dose-dependent manner. Similar results were obtained from the densitometric analysis. These immunohistochemistry results support the role of EOT in improving the cognitive function by inhibiting NF-κB expression.
Cytokines, which are important immunomodulators in the normal functioning of the central nervous system, can be released, among others, by the microglial cells (Lakhan et al. 2009). Cytokines can also be harmful: previous studies have shown that neurodegeneration originating through neuroinflammation is often elicited by activated microglia through the release of different Th1 cytokines. High levels of Th1 could be observed, for instance, in the vicinity of amyloid plaques of cognitive impairment patients, where activated microglia accumulate (Wang et al. 2015). As expected, Th1 cytokines (TNF-α) were significantly increased in TC group rats as compared to the NC group rats. EOT strongly inhibited levels of the TNF-α when tested in HSCD-fed rats. Additionally, Th2 cytokines levels (IL-10) were significantly reduced in the TC group and administration of EOT led to a dose-dependent surge in anti-inflammatory IL-10 levels. Thus, EOT showed a marked decrease in TNF-α as well as significantly increasing the IL-10, which could be responsible for its potent anti-inflammatory action.
Nitrite estimation in biological material is gradually being used as a marker for NO production (Moshage et al. 1995). NO levels were increased in HSCD-fed rat brain as previous reported in which an increased level of NO is implicated in neurological and aging-related disorders (Li et al. 2015). Hence, the regulation of NO release may contribute in reducing the inflammatory damage. The results of the present study suggest that EOT exerts its anti-inflammatory effects by inhibiting NF-κB nuclear translocation and the subsequent release of cytokines and activation of NO in HSCD-fed rats. Previous studies have also suggested that EOT has reduced the level of inflammatory markers (Thenmozhi et al. 2016). Such am endogenously defensive mechanism broadens the way for EOT to exhibit its neuroprotective outcomes.
According to the amyloid cascade evidence, the accumulated and oligomerized Aβ1-42 causes progressive and neuritic injury, thereby inducing oxidative injury, which may be responsible for the synaptic dysfunction and cognitive impairment in Alzheimer disease (AD) patients (Bayer and Wirths 2010). Therefore, measuring the quantity of Aβ1-42 in the brains of our HSCD-fed rats could indicate the therapeutic effects of EOT. On the basis of these results, we found decreases in the levels of the soluble Aβ1-42, as well as insoluble Aβ1-4,2 in the brain, featuring a remarkable reduction in surface area of senile plaque deposits after treatment with EOT, which halted the deposition of Aβ1-42, thereby reducing the formation of senile plaques (Thenmozhi et al. 2016).
Conclusion
The findings of the current study support the use of EOT as a novel therapeutic agent for the treatment of HSCD-associated neuroinflammation and cognitive impairment, through anti-inflammatory responses by inhibiting the NF-kB pathway. However, further research is required to evaluate the mechanistic role of EOT in various neurodegenerative disorders such as AD.
References
Ahmad M, Najmi A, Mujeeb M, Akhtar M (2016) Protective effect of guggulipid in high fat diet and middle cerebral artery occlusion (MCAO) induced ischemic cerebral injury in rats. Drug Res (Stuttg) 66:407–414
Bayer TA, Wirths O (2010) Intracellular accumulation of amyloid-Beta-a predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front Aging Neurosci 2:8
Bhattacharya A, Ghosal S, Bhattacharya SK (2000) Antioxidant activity of tannoid principles of Emblica officinalis (Amla) in chronic stress-induced changes in rat brain. Indian J Exp Biol 38:877–880
Capuron L, Miller AH (2011) Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130:226–238
Chen FE, Huang D-B, Chen Y-Q, Ghosh G (1998) Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA. Nature 391:410–413
Friesner RA et al (2004) Glide: a new approach for rapid, accurate docking and scoring. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Ghaisas MM, Dandawate PR, Zawar SA, Ahire YS, Gandhi SP (2010) Antioxidant, antinociceptive and anti-inflammatory activities of atorvastatin and rosuvastatin in various experimental models. Inflammopharmacology 18:169–177
Ghosal S, Tripathi VK, Chauhan S (1996) Active constitutents of Emblica officinalis: part 1—the chemistry and antioxidant effects of two immunohydrolysable tannins, Emblicanin A and B. Indian J Chem 35:941
Husain I, Akhtar M, Abdin MZ, Islamuddin M, Shaharyar M, Najmi A (2017a) Rosuvastatin ameliorates cognitive impairment in rats fed with high-salt and cholesterol diet via inhibiting acetylcholinesterase activity and amyloid beta peptide aggregation. Hum Exp Toxicol. https://doi.org/10.1177/0960327117705431.
Husain I, Akhtar M, Vohora D, Abdin MZ, Islamuddin M, Akhtar MJ, Najmi AK (2017b) Rosuvastatin attenuates high-salt and cholesterol diet-induced neuroinflammation and cognitive impairment via preventing nuclear factor kappab pathway. Neurochem Res 42:2404–2416
Islamuddin M, Chouhan G, Farooque A, Dwarakanath BS, Sahal D, Afrin F (2015) Th1-biased immunomodulation and therapeutic potential of Artemisia annua in murine visceral leishmaniasis. PLoS Negl Trop Dis 9:e3321
Kivipelto M, Laakso MP, Tuomilehto J, Nissinen A, Soininen H (2002) Hypertension and hypercholesterolaemia as risk factors for Alzheimer’s disease. CNS Drugs 16:435–444
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97
Li W-C, Zou Z-J, Zhou M-G, Chen L, Zhou L, Zheng Y-K, He Z-J (2015) Effects of simvastatin on the expression of inducible NOS in acute lung injury in septic rats. Int J Clin Exp Pathol 8:15106–15111
Liu RY et al (2008) Decreased nicotinic receptors and cognitive deficit in rats intracerebroventricularly injected with beta-amyloid peptide (1–42) and fed a high-cholesterol diet. J Neurosci Res 86:183–193
Lu J, D-m Wu, Zheng Y-l HuB, Cheng W, Z-f Zhang, Shan Q (2011) Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav Immun 25:1658–1667
Mogi M et al (2007) Inhibition of cognitive decline in mice fed a high-salt and cholesterol diet by the angiotensin receptor blocker, olmesartan. Neuropharmacology 53:899–905
Moshage H, Kok B, Huizenga JR, Jansen P (1995) Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 41:892–896
Nagel JA, Kemble ED (1976) Effects of amygdaloid lesions on the performance of rats in four passive avoidance tasks. Physiol Behav 17:245–250
Nguyen DH, Zhou T, Shu J, Mao JH (2013) Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes 2(1):e. https://doi.org/10.1038/protex.2013.097
Pannu R, Singh I (2006) Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int 49:170–182
Pinton S, Brüning CA, Oliveira CES, Prigol M, Nogueira CW (2013) Therapeutic effect of organoselenium dietary supplementation in a sporadic dementia of Alzheimer’s type model in rats. J Nutr Biochem 24:311–317
Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ (2010) Cognitive impairment following high-fat diet consumption is associated with brain inflammation. J Neuroimmunol 219:25–32
Raza S, Khan M, Ahmad A, Ashafaq M, Islam F, Wagner A, Safhi M (2013) Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience 230:157–171
Siddiqui N, Alam MS, Ali R, Yar MS, Alam O (2016) Synthesis of new benzimidazole and phenylhydrazinecarbothiomide hybrids and their anticonvulsant activity. Med Chem Res 25:1390–1402
Tan X et al (2015) Ginseng improves cognitive deficit via the RAGE/NF-κB pathway in advanced glycation end product-induced rats. J Ginseng Res 39:116–124
Thenmozhi TA, Divya Bharathi M, William Raja TR, Manivasagam T, Essa MM (2016) Tannoid principles of Emblica officinalis renovate cognitive deficits and attenuate amyloid pathologies against aluminum chloride induced rat model of Alzheimer’s disease. Nutr Neurosci 19:269–278
Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 106:475–485
Variya BC, Bakrania AK, Patel SS (2016) Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol Res 111:180–200
Wang W-Y, Tan M-S, Yu J-T, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3:136
Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease—a double-edged sword. Neuron 35:419–432
Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N (2007) Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging Study. J Am Geriatr Soc 55:758–762
Zhu S et al (2015) Quetiapine attenuates glial activation and proinflammatory cytokines in APP/PS1 transgenic mice via inhibition of nuclear factor-κB pathway. Int J Neuropsychopharmacol 18:pyu022
Acknowledgements
This research work was supported by the Grant funded by the Department of Science and Technology (DST INSPIRE FELLOW, IF130014), Government of India, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Husain, I., Akhtar, M., Shaharyar, M. et al. High-salt- and cholesterol diet-associated cognitive impairment attenuated by tannins-enriched fraction of Emblica officinalis via inhibiting NF-kB pathway. Inflammopharmacol 26, 147–156 (2018). https://doi.org/10.1007/s10787-017-0437-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0437-x